Abstract
Background
Pyruvate dehydrogenase (PDH) activity is altered in many human disorders. Current methods require tissue samples and yield inconsistent results. We describe a modified method for measuring PDH activity from isolated human peripheral blood mononuclear cells (PBMCs).
Results/Methodology
We found that PDH activity and quantity can be successfully measured in human PBMCs. Freeze-thaw cycles cannot efficiently disrupt the mitochondrial membrane. Processing time of up to 20 h does not affect PDH activity with proteinase inhibitor addition and a detergent concentration of 3.3% showed maximum yield. Sample protein concentration is correlated to PDH activity and quantity in human PBMCs from healthy subjects.
Conclusion
Measuring PDH activity from PBMCs is a novel, easy and less invasive way to further understand the role of PDH in human disease.
Pyruvate dehydrogenase (PDH) is a large mitochondrial enzyme that plays an essential role in aerobic energy metabolism through the conversion of pyruvate to acetyl coenzyme A (AcCoA). Because of this essential role, the function of PDH is of interest in both acute and chronic human illnesses [1–5]. Primary PDH deficiency is a rare but devastating disorder with a diverse clinical manifestations including neurological abnormalities and often lactic acidosis [2]. Apart from primary deficiency, disease may manifest in situations when PDH activity is compromised. Furthermore, increased attention has been paid to co-factors of PDH such as thiamine [3–9] and dichloroacetic acid [10–12] in their relation to many human disease states.
PDH oxidatively decarboxylates pyruvate in the presence of nicotinamide adenine dinucleotide (NAD+) and coenzyme A, forming carbon dioxide (CO2), NADH and AcCoA. PDH is the regulated ‘gateway’ for oxidation of energy derived from all carbohydrate sources, producing NADH for oxidation by the electron transport chain and AcCoA for further oxidation in the tricarboxylic acid cycle. The PDH enzyme is a large and complex molecule that consists of a number of subunits including pyruvate dehydrogenase (E1), dihydrolipoamide transacetylase (E2) and dihydrolipoamide dehydrogenase (E3) [13]. Regulation of PDH depends on the state of phosphorylation of E1, which is inactive in the phosphorylated form and active in the dephosphorylated form [14]. These changes are catalyzed by specific kinases and phosphatases that are naturally inhibited or stimulated by physiological mediators and other regulators (including thiamine and dichloroacetic acid).
Several methods are available for measuring overall PDH activity [15], the most common of which is the arylamine acetyltransferase- coupled (ArAT) assay [16] and the CO2 release isotopic assay [17]. However, these methods have significant disadvantages and yield inconsistent results both between and within assay types [15–16,18]. PDH activity has also been assessed in vivo using magnetic resonance spectroscopy. In this method, hyperpolarized magnetic resonance spectroscopy is used to assess PDH flux by monitoring the rate of production of a byproduct of the PDH reaction (bicarbonate) [19]. In this study, the results of in vivo PDH flux and ex vivo PDH flux measurements were different and this method requires advanced magnetic equipment limiting the applicability of the method. A new method has been established by Lib et al. to measure the activity and quantity of the PDH complex by immunocapture and microplate-based activity measurement of mammalian PDH [18]. However, this assay is developed to be used on cell cultures and tissue samples. It is generally problematic to obtain tissue samples in humans, making this method less useful. Thus, the objective of the current study was to establish a practical protocol for a PDH microplate assay yielding consistent results for PDH activity and quantity using peripheral blood mononuclear cells (PBMCs) isolated from human whole blood.
In introducing a modified technique to measure PDH activity and quantity from human whole blood, we are providing a method that could prove useful in the study of PDH and its role in acute and chronic human diseases.
Materials & methods
Preparation of peripheral blood mononuclear cells
Human blood samples were obtained from subjects who had no significant acute illness and various degrees of coexisting medical conditions. Ten samples obtained from patients with no significant past medical history were used for the reference standard curve. We also obtained blood samples from 10 patients with severe sepsis in the intensive care unit. Severe sepsis were defined according to the 2001 Surviving Sepsis Guidelines [20]. All participants or their legally authorized surrogate provided written informed consent and the study was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center, Boston, MA. Blood was collected via venipuncture or from an already established intravenous line into 10 ml tubes containing ethylenediaminetetraacetic acid. Plasma was separated by centrifugation at 800 g for 15 min at 4°C and saved without disturbing the buffy coat. The plasma was then replaced with the same volume of phosphate buffered saline (PBS). The cell pellets were then mixed by gently pipetting up and down several times to disperse the cells. Next, PBMCs were isolated from the PBS substituted blood samples or fresh blood using a density gradient, Ficoll-Paque premium (GE Healthcare Bio-Sciences Corp, NJ, USA), according to the manufacture’s instruction. PDH activity was preserved by adding 100× proteinase inhibitors (Bioproduct, MA, USA) to the blood sample along with 5 mM dichloroacetate (TCI America, OR, USA) and 20 mM sodium fluoride (Fisher Chemical, PA, USA) to the PBS buffer. PBMC pellets were stored in a −80°C freezer immediately after isolation.
Disruption of the mitochondria membrane
The procedure was completed on ice in the presence of protease inhibitors. First, at least 2 × 107 PBMCs were resuspended in 50–100 µl PBS. Protein concentration of each sample was determined by the BCA (bicinchoninic acid) assay (Thermo Fisher Scientific, MA, USA) and adjusted to a protein concentration at 15 mg/ml. Then intact, functional PDH were solubilized in the presence of detergent (lauryl maltoside, Abcam, Inc., MA, USA). Incubation was performed on ice for 10 min with intermittent mixing, after which samples were centrifuged at 1000 g for 10 min at 4°C. The supernatant was immediately used for PDH activity and quantity measurement.
Determination of PDH activity & quantity
PDH activity and quantity was measured using the PDH combo microplate assay kit (Abcam, Inc., MA, USA) following a revised protocol. Briefly, 200 µl of PBMC mitochondria extract, which contains 1–2 mg protein, were added to each well. Solubilized PDH enzyme was immunocaptured within each well by PDH antibodies after 3 h of incubation on an orbital plate shaker at 500 rpm at room temperature. After washing it two times with 1× stabilizer, fresh assay solution was added to each well and the absorbance of each well was measured at 37°C by a kinetic program at 450 nm for 20 min with a 20 s reading interval (spectra- MAX190, molecular devices, CA, USA). PDH activity (mini-units/min) was expressed as the initial reaction rate determined from the slopes of the curves generated. Immediately after the activity assay, wells were washed twice with 1× stabilizer and incubated with a secondary detection antibody, at room temperature for 1 h. Next, the wells were washed two times without 1× stabilizer. Horseradish peroxidase label was added to each well and incubated for another hour. A final wash was performed three times after incubation and horseradish peroxidase development solution was rapidly added to each empty well. The absorbance of each well was measured at room temperature by a kinetic program at 600 nm for 15 min with a 20 s reading interval. The PDH quantity (mini-units/min) was expressed as the initial reaction rate determined from the slopes of the curves generated. The PDH specific activity was expressed as PDH activity/ Ln(PDH quantity). The final PDH activity and quantity were normalized by per mg of protein.
Additional tests to address the effect of PBS substitution of plasma, processing time & different methods of mitochondria disruption on PDH activity & quantity
Processing of human PBMCs from the same subject was either done with fresh blood samples or with samples where plasma were replaced with the same volume of PBS.
To test the effect of processing time, PBMCs from the same subject were either isolated right away (0 h) or delayed for 1.5 h, 3 h, 6 h or 20 h after blood sample collection. Proteinase inhibitors were added to the blood samples immediately after collection and the samples were kept at 4°C.
To test the concept utilized by the ArAT method that mitochondria can be disrupted by freeze–thaw cycles, PBMCs from the same subject were frozen and thawed for up to three freeze–thaw cycles to test the efficiency of mitochondria disruption and effect on PDH activity. In each freeze–thaw cycle, samples were placed on ice until completely thawed and then put back in a −80°C freezer for at least 1 h before starting the next freeze–thaw cycle.
To find the optimum detergent concentration for lysing the mitochondria of PBMCs without decreasing PDH activity, four different concentrations of detergent were used to test the efficiency of mitochondria disruption and effect of PDH activity: 10% (1:9, detergent volume: sample volume), 5% (1:19), 3.3% (1:29) and 2.5% (1:39).
To determine if the PDH quantity assay is affected by the PDH activity assay, PBMCs from the same subject were randomly assigned to three testing groups as follows: Immunocaptured PDH was directly incubated with the secondary antibody for the quantity assay without doing the activity assay; the PDH quantity assay was conducted right after performing the PDH activity assay and the PDH quantity assay was conducted after overnight incubation after the PDH activity assay.
Reference standard curves
PBMCs were obtained from ten healthy subjects to generate reference standard curves of PDH activity and quantity.
PDH activity & quantity in human cell cultures
Human skeletal muscle cells (HSkMC), human cardiac myocytes (HCM) and human coronary artery smooth muscle cells (HCASMC) were purchased from PromoCell (PromoCell, Heidelberg, Germany). All cells were maintained and subcultivated according to the manufacturer’s instructions. All cells were incubated at 37°C in an atmosphere of 5% CO2. Cells were harvested by cell scraper in ice cold PBS supplemented with proteinase inhibitor. The cell pellets were stored in a −80°C freezer immediately until assayed. PDH activity and quantity was measured using the PDH combo microplate assay kit (Abcam, Inc., MA, USA) following the manufacture’s protocol.
PDH activity & quantity in mouse muscle, liver & brain tissue
Muscle, liver and brain tissue from male, 8–12 weeks old C57 mice were kindly provided by Dr. Evan D. Rosen, Beth Israel Deaconess Medical Center. All animals were handled in accordance with the principles and guidelines established by the National Institutes of Health. The animal facilities and protocols reported were approved by the BIDMC Institutional Animal Care and Use Committee. After collection, samples were immediately frozen in liquid nitrogen, and stored at −80°C until assayed. On the day of the assay, tissues were homogenized in ice cold PBS supplemented with proteinase inhibitor. PDH activity and quantity were then measured using the PDH combo microplate assay kit (Abcam, Inc.) following the manufacture’s protocol.
Statistical analysis
Means ± standard error (SE) were determined for the descriptive statistics. All variables were tested for normal distribution, and logarithmically transformed if not normally distributed. Paired t-tests and repeated measures analyses were used to evaluate differences among all groups. SAS (version 9.3, SAS Institute, NC, USA) was used for the statistical analysis and statistical significance was set at p < 0.05 (two-tailed).
Results
The PDH activity and quantity was successfully measured with our modified protocol using the immunocapture assay on isolated PBMCs from human blood samples. The results are outlined below showing that our modified method can measure PDH activity and quantity under different conditions and how each of these conditions affects PDH activity and quantity.
PBMCs isolated from PBS substituted blood samples compared with fresh blood samples
PDH activity and quantity were measured from PBMCs isolated from PBS substituted blood and fresh blood from the same subject for ten subjects. We found that the PDH activity (p = 0.17), PDH enzyme quantity (p = 0.68) and PDH specific activity (p = 0.39) were not significantly different from the PBMCs isolated from PBS substituted blood samples compared with PBMCs isolated from fresh blood (Table 1, n = 10).
Table 1.
Comparison of pyruvate dehydrogenase measurements within the same subject using peripheral blood mononuclear cells isolated from phosphate-buffered saline substituted blood and peripheral blood mononuclear cells isolated from fresh blood.
| Measurement | Fresh blood (reference) | PBS substituted blood | p-value |
|---|---|---|---|
| Relative PDH activity | 100 ± 0.0 | 126.6 ± 17.7 | 0.17 |
| Relative PDH quantity | 100 ± 0.0 | 105.7 ± 13.4 | 0.68 |
| Relative PDH specific activity | 100 ± 0.0 | 111.5 ± 12.7 | 0.39 |
Values are mean ± SE; n = 10/group.
PBS: Phosphate buffered saline; PDH: Pyruvate dehydrogenase
PBMCs isolated 0 to 20 h after collection
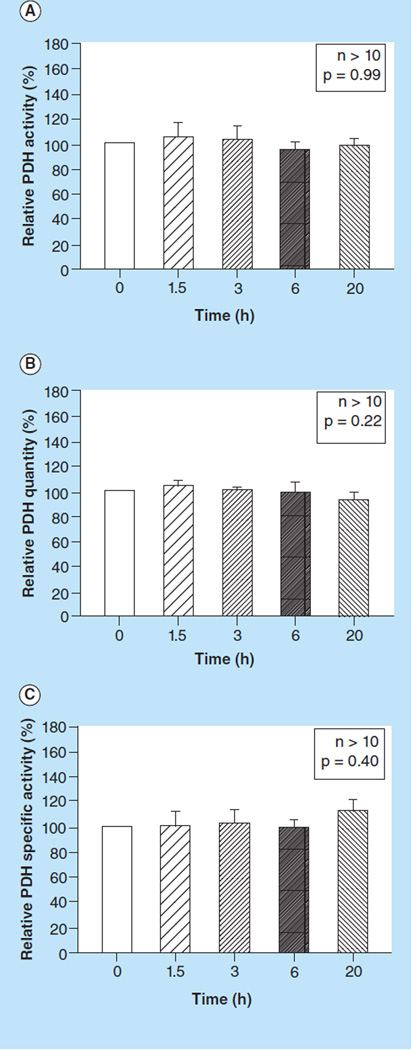
There was no significant difference between PDH activity (p = 0.99, Figure 1A), PDH quantity (p = 0.22, Figure 1B) or PDH specific activity (p = 0.40, Figure 1C) in PBMCs isolated 0, 1.5, 3, 6 or 20 h after blood sample collection.
Figure 1. The effect of processing time on pyruvate dehydrogenase measurements.
(A) Relative PDH activity, (B) quantity, and (C) specific activity measured in peripheral blood mononuclear cells isolated 0, 1.5, 3, 6 and 20 h after blood sample collection. Values are means +SE; n >10/group.
PDH: Pyruvate dehydrogenase.
Effect of freeze–thaw cycles
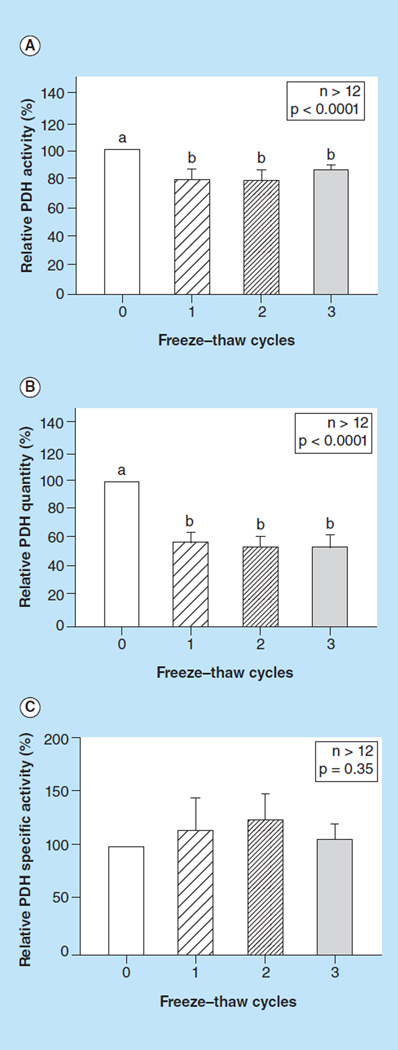
Contrary to the mechanism proposed in the ArAT method, we found that up to three freeze–thaw cycles cannot sufficiently disrupt mitochondria membranes. This method produced significantly lower PDH activity (p < 0.0001, Figure 2A) and PDH enzyme quantity (p < 0.0001, Figure 2B), but did not affect PDH specific activity (p = 0.35, Figure 2C).
Figure 2. The effect of freeze–thaw cycles on pyruvate dehydrogenase (PDH) measurements.
(A) Relative PDH activity, (B) quantity, and (C) specific activity, measured in peripheral blood mononuclear cells with detergent (0) or subjected to one to three freeze–thaw cycles. Values are means +SE; n >12/group. Means with different letters are significantly different (p < 0.05).
PDH: Pyruvate dehydrogenase.
Effects of detergent concentrations
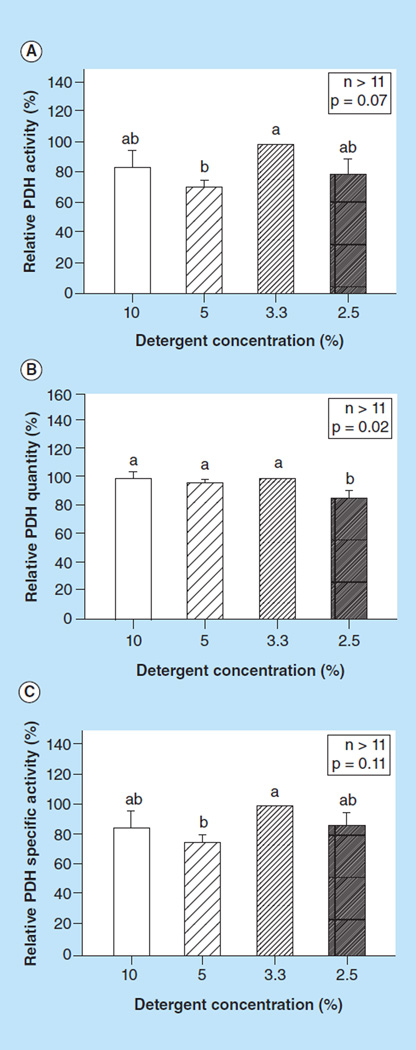
We found that a detergent concentration of 2.5% had significantly lower PDH quantity compared with all other higher detergent concentrations (p = 0.02, Figure 3B), suggesting that this concentration cannot sufficiently lyses the mitochondria. We also found that a 3.3% detergent concentration had highest yield of PDH activity (Figure 3A) and specific activity (Figure 3C).
Figure 3. The effect of detergent concentration on pyruvate dehydrogenase measurements.
(A) Relative PDH activity; (B) quantity and (C) specific activity, measured in peripheral blood mononuclear cells using different detergent concentration to lyse the mitochondria. Values are means + SE; n >11/group. Means with different letters are significantly different (p < 0.05). Means that share the same letters are not significantly different.
PDH: Pyruvate dehydrogenase.
Effects of the PDH activity assay on the PDH quantity assay
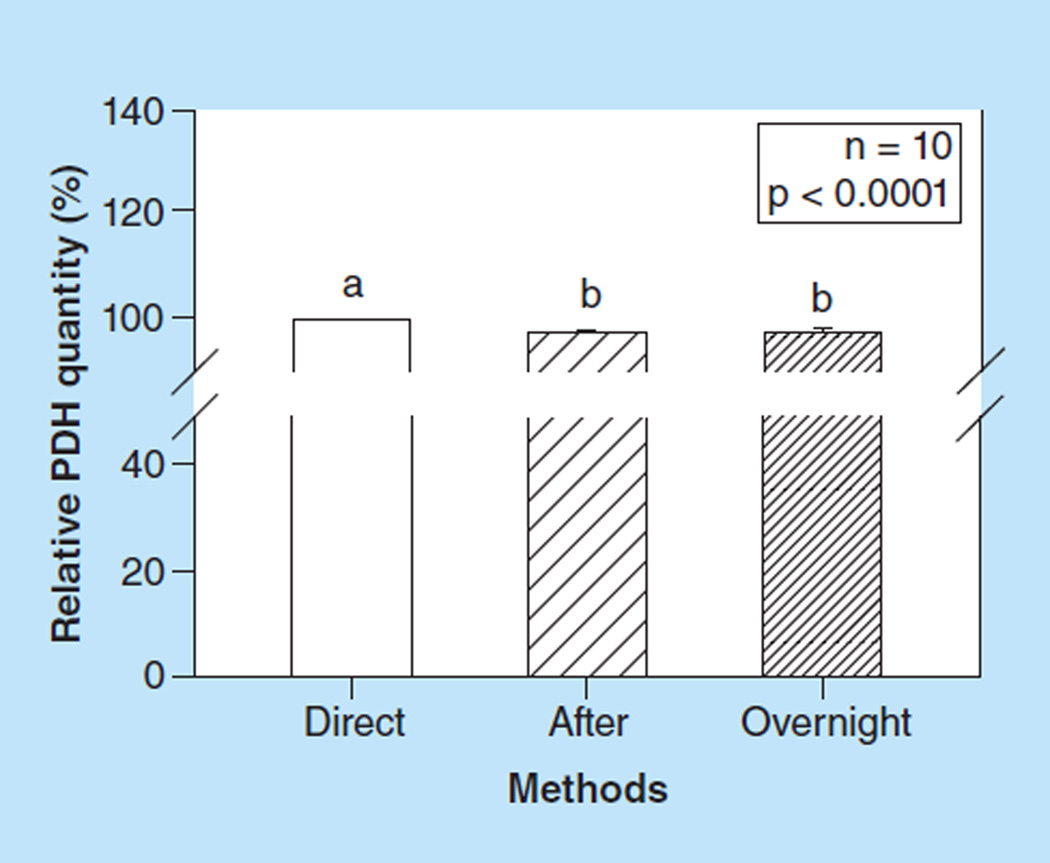
We found that measuring PDH quantity right after the PDH activity assay was consistently approximately 2% lower compared with measuring PDH quantity directly after the first antibody incubation (p < 0.0001, Figure 4). We also found that overnight incubation after the PDH activity assay did not further decrease the PDH quantity compared with be quantity measured right after the PDH activity assay.
Figure 4. The effect of the pyruvate dehydrogenase activity assay on the pyruvate dehydrogenase quantity assay.
Values are means +SE; n =10 /group. Means with different letters are significantly different (p < 0.05). Data were generated from ten healthy subjects.
PDH: Pyruvate dehydrogenase.
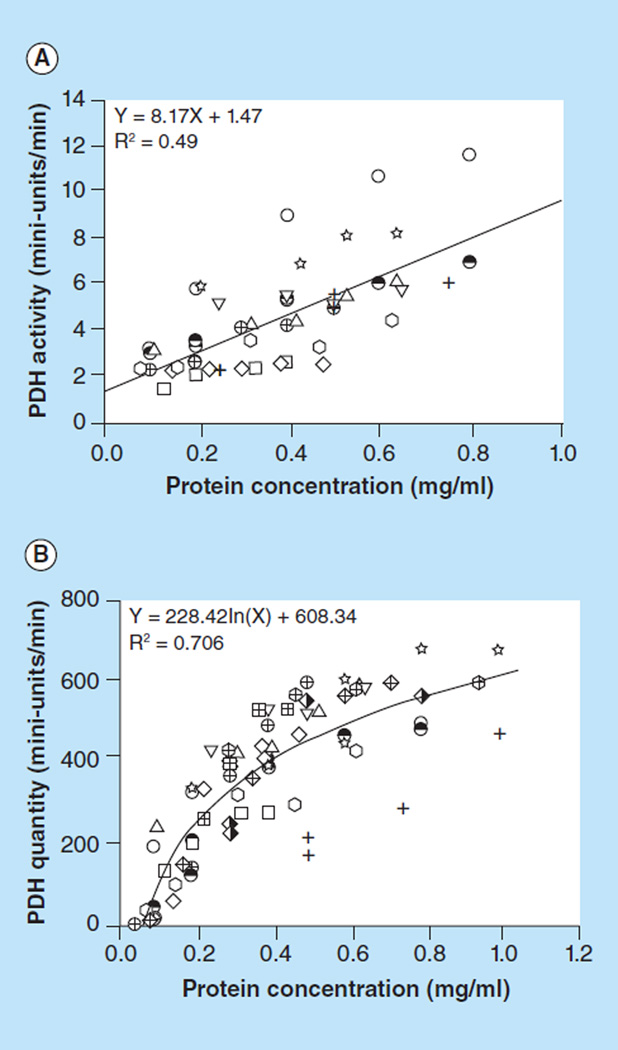
Reference standard curves
Reference standard curves of PDH activity and quantity for human PBMCs were generated from ten healthy subjects. A linear relationship (y = 8.17× + 1.47, R2 = 0.49) of PDH activity between signal and sample load at different protein concentrations is shown in Figure 5A. The rates shown were determined as change in absorbance over time, and these are best represented as change in absorbance per second. A logarithmic relationship (y = 228.42ln(x) + 608.34, R2 = 0.706) of PDH enzyme quantity between signal and sample load at different protein concentration is shown in Figure 5B. Thus, the PDH specific activity was expressed as PDH activity/ln(PDH quantity).
Figure 5. Reference standard curves.
(A) PDH activity; and (B) quantity, generated from ten healthy subjects.
PDH: Pyruvate dehydrogenase.
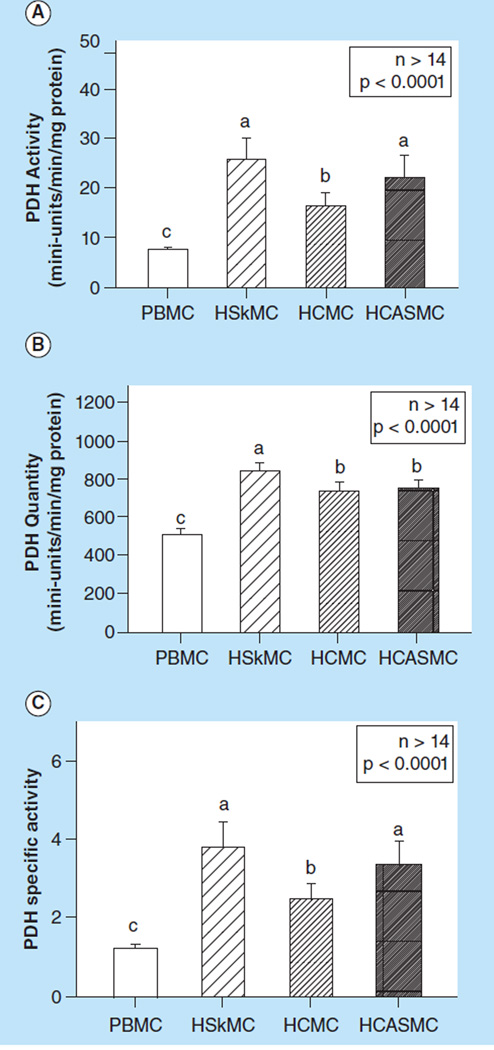
PDH activity comparison between PBMCs & human muscle cells
We validated the kit by measuring the PDH activity and quantity from three human primary cell lines. We also found that human PBMCs had significantly lower PDH activity (p < 0.0001, Figure 6A), quantity (p < 0.0001, Figure 6B) and specific activity (p < 0.0001, Figure 6C) compared with HSkMC, HMC and HCASMC.
Figure 6. Comparison of (A) pyruvate dehydrogenase activity, (B) quantity and (C) specific activity between peripheral blood mononuclear cells and human primary muscle cell lines including human skeletal muscle cells, human cardiac myocytes and human coronary Artery smooth muscle cells.
Values are means +SE; n >14/group. Means with different letters are significantly different (p < 0.05).
HCASMC: Human coronary artery smooth muscle cell; HCMC: Human cardiac myocyte; HSkMC: Human skeletal muscle cell; PBMC: Peripheral blood mononuclear cell; PDH: Pyruvate dehydrogenase.
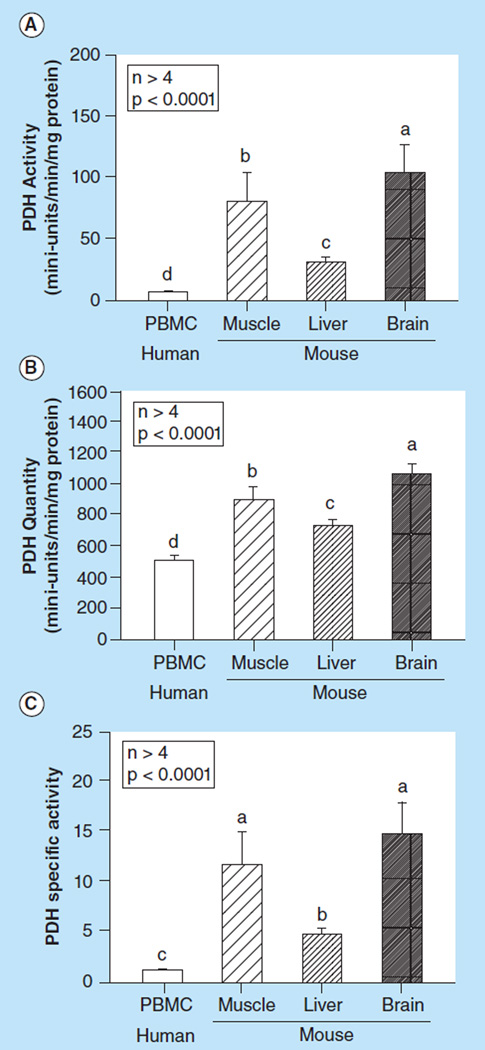
PDH activity comparison between human PBMCs & mouse muscle, liver & brain tissues
We compared the PDH activity and quantity between human PBMCs and mouse tissue from muscle, liver and brain. We found that human PBMCs had significantly lower PDH activity (p < 0.0001, Figure 7A), quantity (p < 0.0001, Figure 7B) and specific activity (p < 0.0001, Figure 7C) compared with mouse muscle, liver and brain tissue.
Figure 7. Comparison of (A) Pyruvate dehydrogenase activity, (B) quantity, and (C) specific activity between human peripheral blood mononuclear cells and mouse muscle, liver and brain tissue.
Values are means +SE; n > 4/group. Means with different letters are significantly different (p < 0.05).
PBMC: Peripheral blood mononuclear cell; PDH: Pyruvate dehydrogenase.
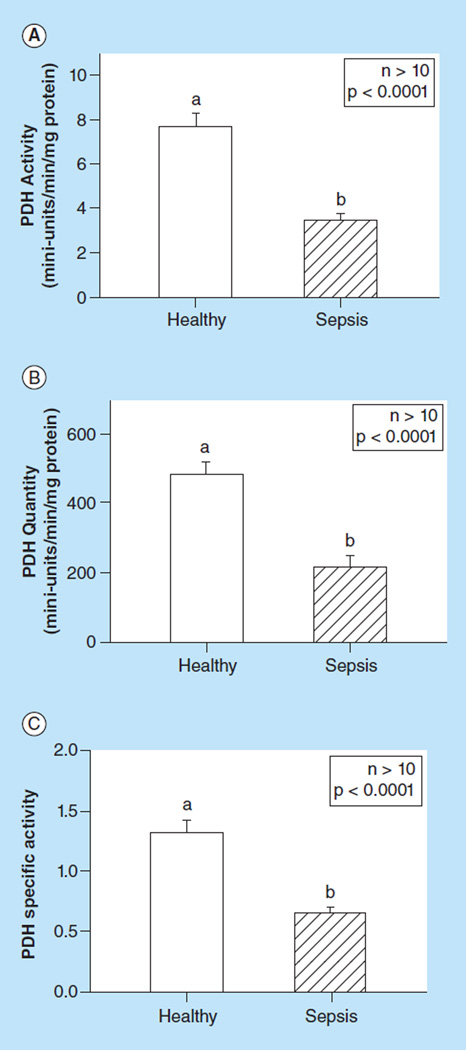
PDH activity in healthy individuals & patients with severe sepsis
PDH activity and quantity were measured from 30 healthy subjects and 10 patients with severe sepsis. The PDH activity form healthy subjects ranged from 3.78 to 14.82, median 6.88 (mini-units/min/mg protein, Figure 8A). The PDH quantity ranged from 235.9 to 839.3, median 488.7 (mini-units/min/mg protein, Figure 8B). The PDH activity from patients with severe sepsis ranged from 2.24 to 5.55, median 3.42 (mini-units/min/mg protein, Figure 8A); PDH quantity ranged from 114.3 to 464.6, median 181.4 (mini-units/min/mg protein, Figure 8B). We found that patients with severe sepsis had significantly lower PDH activity, quantity and specific activity compared with the healthy subjects (all p < 0.0001).
Figure 8. Comparison of (A) Pyruvate dehydrogenase activity, (B) quantity, and (C) specific activity between peripheral blood mononuclear cells from healthy human subjects and patients with severe sepsis.
Values are means +SE; n >10/group. Means with different letters are significantly different (p < 0.05).
PDH: Pyruvate dehydrogenase.
Discussion
The current study provides a modified and practical method for measuring PDH activity and quantity using PBMCs obtained from human whole blood. This is done by immunocapturing the active PDH complex, after disruption of mitochondrial membranes, from isolated human PBMCs from whole blood.
A number of methods have previously been used to measure PDH activity. The most commonly used methods include the CO2 release isotopic assay [17] and the ArAT assay [16,21]. However, as described previously by Lib et al. these methods have significant disadvantages [18]. In the CO2 method, the level of CO2 is radioactively monitored, hence bringing many limitations as it cannot be performed in all locations [17]. ArAT results are dependent on the success of multiple freeze-thaw cycles to lyse the mitochondria, which, as previously shown [15] and reported here, are inconsistent. Moreover, both methods yield inconsistent results both between and within assay types [15,17,18,21]. Lib et al [18] introduced a new method utilizing immunocapture and microplate- based activity measurements as a more reliable method for measuring PDH activity and quantity. This method has significant advantages including user-friendliness and the fact that the method allows for immune-purification of the PDH complex. This method allows for other pyruvate metabolizing enzymes or NADH-utilizing enzymes that can cause interference to be disregarded [18]. We validated this method by measuring PDH activity and quantity from human skeletal muscle cells, cardiac myocytes and coronary artery smooth muscle cells. This method, as well as the CO2 release isotopic assay and ArAT assay, is performed on cell cultures and tissue cultures, thereby decreasing the utility of these methods in assessing PDH activity and quantity in human illnesses, especially when studying acute and critical illnesses, where obtaining tissue samples is rarely feasible. We established a practical protocol for a PDH microplate assay, based on Lib et al., yielding consistent results for PDH quantity and activity using PBMCs isolated from human whole blood.
We measured PDH activity and quantity under a number of conditions and found that our method is both highly practical and reproducible. For example, we found that there was no difference in the PDH quantity or activity in samples processed within the first 20 h of blood sampling. Thus, even a sample processed after 20 h (with the addition of proteinase inhibitor) can be efficiently used to measure PDH activity and quantity. This provides a practical approach for studying PDH, especially in the setting of acute illness, since advanced laboratory support is rarely available 24 h a day. However, we found that freeze–thaw cycles should be avoided for measurement of PDH activity and quantity. Lastly, we found that the PBS substituted PBMC isolation had no impact on the PDH activity and quantity assay. Thus, the plasma samples from patients can be saved for additional assays.
As expected, we found that in human PBMCs the PDH activity and quantity were significantly lower when compared with tissue samples from mice. PDH activity was significantly higher in brain tissue compared with liver and muscle. The PDH activity, quantity and specific activity from healthy subjects were significantly higher compared with the patients with severe sepsis. The implications of decreased PDH activity and quantity in those with severe sepsis are unknown and warrant further research.
The primary limitation of this assay is that there is no confirmation to get an accurate standard curve for PDH activity. Unlike DNA and many other proteins, the PDH enzyme is unstable and its activity varies in different species, tissues and under different testing conditions. We recommend generating reference standards based on specific species and tissues. Here, a reference standard for human PBMCs is generated based on 10 healthy subjects and can be applied to the same assay conditions. Furthermore, it is unclear if the isolated PBMCs accurately represent the activity and quantity of PDH in organ-specific tissues. However, ongoing research is addressing this issue.
Conclusion
In conclusion, this modified method provides a rapid, consistent and practical approach to measure PDH activity and quantity using PMBCs isolated from human whole blood. The assay and protocol can be a helpful tool in measuring the variation in PDH activity in different human diseases in which obtaining a tissue sample may not be feasible.
Future perspective
Mitochondrial pathology, including deficiencies and defects in key mitochondrial enzymes, continues to be elucidated in many acute and chronic human illnesses. As the field expands it will be important that reliable, less invasive and practical bioanalytic methods are developed such that clinical studies can be conducted in a feasible and safe way. We consider the method described within this manuscript such a method for pyruvate dehydrogenase, one of many important mitochondrial enzymes. We believe that the method presented will be validated and integrated, and serve as a tool for assessing pyruvate dehydrogenase activity in both observational and interventional clinical trials. It is our hope that similar methods will be developed for other mitochondrial enzymes and components such that the role of mitochondrial pathology in human diseases can be further described and pharmacological interventions can be developed and tested.
Key terms.
Immunocapture: Technique which is able to isolate large enzyme complex in their fully intact, fully active states from small samples of tissue or cells by capture antibodies.
Sepsis: Systemic inflammatory reaction to a severe infection. Severe sepsis is characterized by abnormal vital signs and organ failure and caries a high mortality. Elevated lactate levels are a common finding in patients with severe sepsis which have been postulated to be partly related to decreased PDH activity [1].
Buffy coat: Fraction of an anticoagulated blood sample that contains the majority of the white blood cells.
Ficoll-Paque: Density gradient used to separate blood cells based on their densities.
Homogenized: Cells are homogenized, meaning that all fractions of the sample are equal in composition and thus are uniform.
Executive summary.
Current methods for measuring pyruvate dehydrogenase activity require tissue samples and are therefore often not feasible in humans.
We describe a modified method to measure pyruvate dehydrogenase activity and quantity in peripheral blood mononuclear cells isolated from human whole blood.
We found that repeated freeze–thaw cycles, as previously utilized in older methods, cannot efficiently disrupt the mitochondrial membrane.
Processing time up to 20 h does not affect pyruvate dehydrogenase activity with proteinase inhibitor addition making the current method highly practical.
Sample protein concentration is correlated to pyruvate dehydrogenase activity and quantity from healthy human peripheral blood mononuclear cells and reference standard curves from healthy volunteers are presented.
Acknowledgement
The authors would like to acknowledge the assistance of Francesca Montillo in preparation of the manuscript.
This work was conducted in part with support from NHLBI (1K02HL107447-01A1) and NIH (R21AT005119-01)..
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
• of interest, •• of considerable interest
- 1.Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit. Care. 2014;18(5):503–514. doi: 10.1186/s13054-014-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cross JH, Connelly A, Gadian DG, et al. Clinical diversity of pyruvate dehydrogenase deficiency. Pediatr. Neurol. 1994;10(4):276–283. doi: 10.1016/0887-8994(94)90122-8. • Described some of the important clinical manifestations of primary pyruvate dehydrogenase (PDH) deficiency.
- 3.Donnino M. Gastrointestinal beriberi: a previously unrecognized syndrome. Ann. Intern. Med. 2004;141(11):898–899. doi: 10.7326/0003-4819-141-11-200412070-00035. [DOI] [PubMed] [Google Scholar]
- 4.Kuriyama M, Yokomine R, Arima H, Hamada R, Igata A. Blood vitamin B1, transketolase and thiamine pyrophosphate (TPP) effect in beriberi patients, with studies employing discriminant analysis. Clin. Chim. Acta. 1980;108(2):159–168. doi: 10.1016/0009-8981(80)90001-7. [DOI] [PubMed] [Google Scholar]
- 5.Donnino MW, Vega J, Miller J, Walsh M. Myths and misconceptions of Wernicke’s encephalopathy: what every emergency physician should know. Ann. Emerg. Med. 2007;50(6):715–721. doi: 10.1016/j.annemergmed.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Page GL, Laight D, Cummings MH. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int. J. Clin. Pract. 2011;65(6):684–690. doi: 10.1111/j.1742-1241.2011.02680.x. [DOI] [PubMed] [Google Scholar]
- 7.Donnino MW, Carney E, Cocchi MN, et al. Thiamine deficiency in critically ill patients with sepsis. J. Crit. Care. 2010;25(4):576–581. doi: 10.1016/j.jcrc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Donnino MW, Cocchi MN, Smithline H, Carney E, Chou PP, Salciccioli J. Coronary artery bypass graft surgery depletes plasma thiamine levels. Nutrition. 2010;26(1):133–136. doi: 10.1016/j.nut.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moskowitz A, Graver A, Giberson T, et al. The relationship between lactate and thiamine levels in patients with diabetic ketoacidosis. J. Crit. Care. 2013;29(2):6. doi: 10.1016/j.jcrc.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnet S, Archer SL, Allalunis-Turner J, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11(1):37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 11.McMurtry MS, Bonnet S, Wu X, et al. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ. Res. 2004;95(8):830–840. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 12.Michelakis ED, McMurtry MS, Wu XC, et al. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105(2):244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 13. Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem. Soc. Trans. 2006;34(Pt 2):217–222. doi: 10.1042/BST20060217. • Gave a detailed description of the regulation of the PDH complex.
- 14.Linn TC, Pettit FH, Reed LJ. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc. Natl Acad. Sci. USA. 1969;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas RH, Thompson G, Morris B, Conright K, Andrews T. Pyruvate dehydrogenase activity in osmotically shocked rat brain mitochondria: stimulation by oxaloacetate. J. Neurochem. 1988;50(3):673–680. doi: 10.1111/j.1471-4159.1988.tb02966.x. [DOI] [PubMed] [Google Scholar]
- 16.Ksiezak-Reding H, Blass JP, Gibson GE. Studies on the pyruvate dehydrogenase complex in brain with the arylamine acetyltransferase-coupled assay. J. Neurochem. 1982;38(6):1627–1636. doi: 10.1111/j.1471-4159.1982.tb06643.x. [DOI] [PubMed] [Google Scholar]
- 17.Schofield PJ, Griffiths LR, Rogers SH, Wise G. An improved method for the assay of platelet pyruvate dehydrogenase. Clin. Chim. Acta. 1980;108(2):219–227. doi: 10.1016/0009-8981(80)90008-x. [DOI] [PubMed] [Google Scholar]
- 18. Lib M, Rodriguez-Mari A, Marusich MF, Capaldi RA. Immunocapture and microplate-based activity measurement of mammalian pyruvate dehydrogenase complex. Anal. Biochem. 2003;314(1):121–127. doi: 10.1016/s0003-2697(02)00645-0. •• The immunocapture assay described by Lib et al. is used to measure PDH in cell cultures and tissue. We further developed this methodology and approach to measure PDH in human blood cells.
- 19.Atherton HJ, Schroeder MA, Dodd MS, et al. Validation of the in vivo assessment of pyruvate dehydrogenase activity using hyperpolarised 13C MRS. NMR Biomed. 2011;24(2):201–208. doi: 10.1002/nbm.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 21.Brooks SP, Storey KB. An improvement in the pyruvate dehydrogenase complex assay: a high-yield method for purifying arylamine acetyltransferase. Anal. Biochem. 1993;212(2):452–456. doi: 10.1006/abio.1993.1354. [DOI] [PubMed] [Google Scholar]