Abstract
Background/Purpose
The genomes of different Aggregatibacter actinomycetemcomitans strains contain many strain-specific genes and genomic islands (defined as DNA found in some but not all strains) of unknown functions. Genetic analysis for the functions of these islands will be constrained by the limited availability of genetic markers and vectors for A. actinomycetemcomitans. In this study we tested a novel genetic approach of gene deletion and restoration in a naturally competent A. actinomycetemcomitans strain D7S-1.
Methods
Specific genes’ deletion mutants and mutants restored with the deleted genes were constructed by a markerless loxP/Cre system. In mutants with sequential deletion of multiple genes loxP with different spacer regions were used to avoid unwanted recombinations between loxP sites.
Results
Eight single-gene deletion mutants, four multiple-gene deletion mutants, and two mutants with restored genes were constructed. No unintended non-specific deletion mutants were generated by this protocol. The protocol did not negatively affect the growth and biofilm formation of A. actinomycetemcomitans.
Conclusion
The protocol described in this study is efficient and specific for genetic manipulation of A. actinomycetemcomitans, and will be amenable for functional analysis of multiple genes in A. actinomycetemcomitans.
Keywords: genomic islands, aggressive periodontitis, gene deletion, genetic analysis
Introduction
Gram-negative facultative Aggregatibacter actinomycetemcomitans is recognized as an etiology of periodontitis1. There are six serotypes of A. actinomycetemcomitans based on the structural distinction of O-antigen of lipopolysaccharide2, 3. Each serotype represents a distinct clonal lineage that shows little recombination with strains of other serotypes. Moreover, different serotypes or genotypes of A. actinomycetemcomitans may display distinct disease-association patterns4–6. However, little detailed information has been known of the underlying genomic variation among strains.
Recent studies from our laboratory have revealed remarkable genomic differences among A. actinomycetemcomitans strains7, 8. For example, 0.4–19.5% of the total protein-coding genes in each genome could differ between strains. Cumulatively among the 14 A. actinomycetemcomitans there are more than 1,200 accessory genes (ie, genes that are not shared by all strains), many of which reside in genomic islands and have no known functions. Approaches to assess the functions of these accessory genes need to be efficient, able to monitor multiple genes if necessary, and easily adaptable to assays in a variety of experimental conditions.
The genetic tools for A. actinomycetemcomitans are limited. The most common genetic markers used for A. actinomycetemcomitans are the resistance gene for spectinomycin, tetracycline, kanamycin or chloramphenicol9–14. In order to study the functions of multiple genes, more than one marker are required for deletion or complementation. This may pose some technical difficulties. This study was initiated to test a genetic protocol that is amenable for complex genetic analysis that involved multiple genes. Our future goal is to examine the functions of accessory genes (such as those carried on genomic islands) of A. actinomycetemcomitans. A markerless gene deletion protocol using loxP with different spacer regions was developed for single or sequential deletions of multiple DNA in A. actinomycetemcomitans. Both the accessory genes and core genes (ie, genes shared by all A. actinomycetemcomitans strains) were tested in deletion experiments. The results demonstrated that the protocol is highly efficient and specific in gene deletion and restoration. The protocol for genetic manipulation has not led to unintended deleterious effects to the growth and biofilm formation of A. actinomycetemcomitans.
Materials and Methods
Bacterial strains, plasmids and growth conditions
The bacteria strains and plasmids used in this study are listed in Table 1 and Table 2. Bacteria were grown either in a solid Trypticase Soy Broth agar (sTSB agar) containing 3% trypticase soy broth, 0.3% yeast extract, 5% horse serum and 1.5% agar, or Modified Trypticase Soy Broth (mTSB) composed of 3% trypticase soy broth and 0.6% yeast extract at 37°C in air supplemented with 5% CO2. The pBluescript II KS plasmids (Stratagene, La Jolla, CA) were replicated in Escherichia coli host strain DH5α by standard methods 15. For selection of transformants or mutants, spectinomycin (Spe, 50μg/ml), tetracycline (Tc, 4μg/ml) or ampicillin (Amp, 100μg/ml) were added to the media.
Table 1.
Bacterial strains
| Strains | Feature descriptions | Annotation of affected gene(s); deletion size in bp |
|---|---|---|
| D7S-1 | Wildtype, fimbriated | N/A |
| D7SΔltxA::Spe | Deletion of ltxA replaced with a loxPW-Spe-loxPW | LtxA; 934 |
| D7SΔltxA | Derivative of D7SΔltxA::Spe; Spe removed leaving a loxPW | |
| D7SΔcdtB::Spe | Deletion of cdtB replaced with a loxP6-Spe-loxP6 | CdtB; 289 |
| D7SΔcdtB | Derivative of D7SΔcdtB::Spe; Spe removed leaving a loxP6 | |
| D7SΔacrB::Spe | Deletion of acrB replaced with a loxP1-Spe-loxP1 | acriflavine resistance protein; 566 |
| D7SΔacrB | Derivative of D7SΔAcrB::Spe; Spe removed leaving a loxP1 | |
| D7SΔpulG::Spe | Deletion of pulG replaced with a loxP1-Spe-loxP1 | pseudopilin; 617 |
| D7SΔpulG | Derivative of D7SΔpulG::Spe; Spe removed leaving a loxP1 | |
| D7SΔp2579::Spe | Deletion of gene “p2579” replaced with a loxP3-Spe-loxP3 | PTS system; 989 |
| D7SΔp2579 | Derivative of D7SΔp2579::Spe; Spe removed leaving a loxP3 | |
| D7SΔp2639::Spe | Deletion of gene “p2639” replaced with a loxP1-Spe-loxP1 | oligopeptide transport system permease protein (p2639 ); 662 |
| D7SΔp2639 | Derivative of D7SΔp2639::Spe; Spe removed leaving a loxP1 | |
| D7SΔ16::Spe | Deletion of the 16-island replaced with a loxP5-Spe-loxP5 | helicase, hypothetical protein, type III DNA modification methylase, protein of unknown function and hypothetical protein; 7,845 |
| D7SΔ16 | Derivative of D7SΔ16::Spe; Spe cassette removed leaving a loxP5 | |
| D7SΔ285::Spe(R) | Deletion of the 285-island replaced with a loxP1-Spe-loxP1 | integral membrane protein, MarR-family transcriptional regulator, AcrA protein, hypothetical protein, SecA-related protein and acriflavine resistance protein; 5,087 |
| D7SΔ285(R) | Derivative of D7SΔ285::Spe(R); Spe cassette removed leaving a loxP1 | |
| D7SΔltxAΔcdtB::Spe | Derivative of D7SΔltxA with a deletion in cdtB replaced with a loxP6-Spe-loxP6 | See above; 1,223 |
| D7SΔltxAΔcdtB | Derivative of D7SΔltxAΔcdtB::Spe; Spe removed leaving a loxP6 at cdtB and a prior loxPW at ltxA | |
| D7SΔltxAΔcdtBΔ16::Spe | Derivative of D7SΔltxAΔcdtB with a deletion in the 16-island replaced with a loxP5-Spe-loxP5 | See above; 9,068 |
| D7SΔltxAΔcdtBΔ16 | Derivative of D7SΔltxAΔcdtBΔ16::Spe; Spe removed leaving a loxP5 | |
| D7SΔltxAΔcdtBΔ285::Spe | Derivative of D7SΔltxAΔcdtB with a deletion in the 285-island replaced with loxP1-Spe-loxP1 | See above; 6,310 |
| D7SΔltxAΔcdtBΔ285 | Derivative of D7SΔltxAΔcdtBΔ285::Spe; Spe removed leaving a loxP1 | |
| D7SΔltxAΔcdtBΔ16Δ285::Spe | Derivative of D7SΔltxAΔcdtBΔ16 with a deletion within the 285-island replaced with loxP1-Spe-loxP1 | See above; 14,155 |
| D7SΔltxAΔcdtBΔ16Δ285 | Derivative of D7SΔltxAΔcdtBΔ16Δ285::Spe; Spe cassette removed leaving a loxP1 | |
| D7SΔ285C::Spe(R) | Derivative of D7SΔ285(R) restored with a loxP4-Spe-loxP4 and the 285-island | N/A |
| D7SΔ285C(R) | Derivative of D7SΔ285C::Spe(R); Spe removed leaving a loxP4 | |
| D7SΔltxAΔcdtBΔ285C::Spe | Derivative of D7SΔltxAΔcdtBΔ285 restored with a loxP4-Spe-loxP4 and the 285-island | See above; 1,223 |
| D7SΔltxAΔcdtBΔ285C | Derivative of D7SΔltxAΔcdtBΔ285C::Spe; Spe removed leaving a loxP4 |
Table 2.
Plasmids used in this work
| Plasmid | Feature descriptions | Reference |
|---|---|---|
| pBluescript II KS | Ampr; Cloning vector | Stratagene |
| pAT/Cre | Tcr; shuttle plasmid of E. coli and A. actinomycetemcomitans; pPK1 derivative containing the cre gene | 17 |
| ploxw-Spe | Ampr,Sper; pBluescript II KS derivative containing two wildtype loxP sites (the loxPW spacer sequence: ATGTATGC) flanking a Sper gene | This work |
| plox1-Spe | Ampr,Sper; pBluescript II KS derivative containing two mutant loxP1 sites (the loxP1 spacer sequence: ATGcATGC*) flanking a Sper gene | This work |
| plox2-Spe | Ampr,Sper; pBluescript II KS derivative containing two mutant loxP2 sites (the loxP2 spacer sequence: ATGgATGC*) flanking a Sper gene | This work |
| plox3-Spe | Ampr,Sper; pBluescript II KS derivative containing two mutant loxP3 sites (the loxP3 spacer sequence: ATGTATaC*) flanking a Sper gene | This work |
| plox4-Spe | Ampr,Sper; pBluescript II KS derivative containing two mutant loxP4 sites (the loxP4 spacer sequence: AaGTATcC*) flanking a Sper gene | This work |
| plox5-Spe | Ampr,Sper; pBluescript II KS derivative containing two mutant loxP5 sites (the loxP5 spacer sequence: ATGTgTaC*) flanking a Sper gene | This work |
| plox6-Spe | Ampr,Sper; pBluescript II KS derivative containing two mutant loxP6 sites (the loxP6 spacer sequence: AgaTcTGC*) flanking a Sper gene | 17 |
the lower case letter(s) indaicated the altered base(s) located in the variant loxP spacer sequences
DNA manipulations
A. actinomycetemcomitans genomic DNA was prepared by phenol-chloroform method or GenElute Bacterial genomic Kit (Sigma, Saint Louis, MO). Plasmid DNA was isolated by QIAprep Spin Miniprep kit (Qiagen, Valencia, CA). Transformation of E coli was carried out by electroporation using a MicroPulser® (BioRad, Hercules, CA). Restriction enzymes, T4 DNA ligase and Taq DNA polymerase were purchased from New England Biolabs (Beverly, MA), and used as suggested by the manufacturer. The polymerase chain reactions (PCR) were performed as described previously15 and the PCR products were purified with QIAquick PCR purification kit and GIAquick Gel Extraction kit (Qiagen, Valencia, CA). Table 3 lists the sequences of primers used for cloning, deletion, and mutation.
Table 3.
List of Primers
| Primers | Sequences(5′→3′)a | Location and usageb |
|---|---|---|
| ltxA-UpF-Apa | ATAGGGCCCGGCATTAACTAATG | Upstream of ltxA (600424-600411); for deletion of ltxA |
| ltxA-UpR-Xho | TATCTCGAGTATTCCTCAAGCATTC | Upstream of ltxA (599669-599684); for deletion of ltxA |
| ltxA-DwF-Spe | TATACTAGTAAAGGTCGCACCGGT | Downstream of ltxA (598753-598738); for deletion of ltxA |
| ltxA-DwR-Sac | TACGAGCTCTTGAGGTGAAATTGT | Downstream of ltxA (597736-597750); for deletion of ltxA |
| ltxA-IntF | TGAACATATCGCGAATCAGC | Inside of ltxA (599431-599412); for confirmation of ltxA deletion |
| ltxA-IntR | ACGTGTAACGGCATGTTGAA | Inside of ltxA (598876-598895); for confirmation of ltxA deletion |
| cdtB-UpF-Apa | ATAGGGCCCATTGATACGCCAACGAA | Upstream of cdtB (2171088-2171072); for deletion of cdtB |
| cdtB-UpR-Xho | CACCTCGAGAGCAAGCACGTGAA | Upstream of cdtB (2170488-2170501); for deletion of cdtB |
| cdtB-DwF-Spe | TACACTAGTACCAATGCGGATACCTA | Downstream of cdtB (2170192-2170176); for deletion of cdtB |
| cdtB-DwR-Sac | AATGAGCTCTCTTCATCCAAGAATGG | Downstream of cdtB (2169234-2169250); for deletion of cdtB |
| cdtB-IntF | CGTGGTAAATGTGCGTCATG | Inside of cdtB (2170482-2170463); for confirmation of cdtB deletion |
| cdtB-IntR | TTACAGTGCATGCTTTGGCC | Inside of cdtB (2170206-2170225); for confirmation of cdtB deletion |
| 16-UpF | GGCGTCTGCTCGGTGATAA | Upstream of the 16-island (1997280-1997262); for deletion of the 16-island |
| 16-UpR-Dra | TGCACGTGGTGTTGTATGACGTAGA | Upstream of the 16-island (1996774-1996787); for deletion of the 16-island |
| 16-DwF-Dra | ATCACGTGGTGTTCCAACACGGC | Downstream of the 16-island (1988918-1988907); for deletion of the 16-island |
| 16-DwR | CCAATTCGGTGCGGACATTCC | Downstream of the 16-island (1988335-1988355); for deletion of the 16-island |
| 16-IntF | CGGCGGTCAGAAGATTGGG | Inside of the 16-island (1991452-1991434); for confirmation of the 16-island deletion |
| 16-IntR | AGTCCTGCTCACCGCCACG | Inside of the 16-island (1990908-1990926); for confirmation of the 16-island deletion |
| 285-UpF | TAAACCTACCGCCGAAGCG | Upstream of the 285-island (1056124-1056106); for deletion of the 285-island; |
| 285-UpR-Dra | CTCACGTGGTGATGGTGTTGTTA | Upstream of the 285-island (1055356-1055367); for deletion of the 285-island |
| 285-DwF-Dra | TACACGTGGTGCTGATTATTTGGGT | Downstream of the 285-island (1050256-1050243); for deletion of the 285-island |
| 285-DwR | CTGCCTTATTCCACTTCCACCC | Downstream of the 285-island (1049587-1049608); for deletion of the 285-island |
| 285-IntF | GCGCTGCAACATCATAAAGC | Inside of the 285-island (1052447-1052428); for confirmation of the 285-island deletion |
| 285-IntR | CCAGCCACAGCCATAATCAA | Inside of the 285-island (1051915-1051934); for confirmation of the 285-island deletion |
| 285s-UpF | TGGAATTCTATCCTCCGGTGTTACTGA | Upstream of the 285-island (1055865-1055839); for complementation of the 285-island |
| 285s-UpR II | TTCACGTGGTGTCAGCACCATTAAGACG | Upstream of the 285-island (1050253-1050269); for complementation of the 285-island |
| 285s-DwF II | TACACGTGGTGTTATTTGGGTGTGGGGT | Downstream of the 285-island (1050252-1050236); for complementation of the 285-island |
| 285s-DwR II | CCAATCTTGGTGCGTCATCAAGGCTAAT | Downstream of the 285-island (1049645-1049672); for complementation of the 285-island |
| acrB-UpF | TCTCGCAGAACGGGTTTATCAAACAGTAT | Upstream of acrB (1054510-1054538); for deletion of acrB |
| acrB-UpR | ATCACGTGGTGTTCGCCTAAGGATATTCA | Upstream of acrB (1055313-1055296); for deletion of acrB |
| acrB-DwF | CGCACGTGGTGTAATCTTTTTGAAAAAATACC | Downstream of acrB (1055878-1055898); for deletion of acrB |
| acrB-DwR | GATAAGTTTGTCGCGTGGAATGGTTAAGT | Downstream of acrB (1056805-1056777); for deletion of acrB |
| acrB-IntF | GCGTGGTGATGGTGTTGTTA | Inside of acrB (1055348-1055367); for confirmation of acrB deletion |
| acrB-IntR | ATCCTCCGGTGTTACTGACG | Inside of acrB (1055856-1055837); for confirmation of acrB deletion |
| p2579-UpF | GGCGTTTTTACAACCTGCAGAAGCCTTGAAA | Upstream of gene encoding protein with p-02579 (429323-429353) ; for deletion of p-cluster 02579 |
| p2579-UpR | TTCACGTGGTGTAGACGGCGTTTCTTTCTAC | Upstream of gene encoding protein with p-02579 (429918-429937); for deletion of p-cluster 02579 |
| p2579-DwF | ATCACGTGGTGAAATTCTCTCCTCTTCACGA | Downstream of gene encoding protein with p-02579 (430925-430944); for deletion of p-cluster 02579 |
| p2579-DwR | CGTTAAGCGGATAAATTCCGGCCATGATGTT | Downstream of gene encoding protein with p-02579 (431650-431620); for deletion of p-cluster 02579 |
| p2579-IntF | GGCAATTTGGCACTTTTGTT | Inside of gene encoding protein with p-02579 (430732-430713); for confirmation of p-cluster 02579 deletion |
| p2579-IntR | GAAACGCGTACCTTCGGTAA | Inside of gene encoding protein with p-02579 (430142-430161); for confirmation of p-cluster 02579 deletion |
| p2639-UpF | ATCGTGCAGGAGATTTGGACATCACCAG | Upstream of gene encoding protein with p-02639 (651694-651721); for deletion of p-cluster 02639 |
| p2639-UpR | ATCACGTGGTGGAAATTGTCCCCAACGTA | Upstream of gene encoding protein with p-02639 (652650-652633); for deletion of p-cluster 02639 |
| p2639-DwF | AACACGTGGTGTAAAGCACGCGCTGCGTC | Downstream of gene encoding protein with p-02639 (653311-653328); for deletion of p-cluster 02639 |
| p2639-DwR | CATCACCATGTCGATTTTGCCGCCGAAAT | Downstream of gene encoding protein with p-02639 (654021-653993); for deletion of p-cluster 02639 |
| p2639-IntF | GAAGTGATGGCGAACATTGA | Inside of gene encoding protein with p-02639 (652780-652799); for confirmation of p-cluster 02639 deletion |
| P2639-IntR | CCTTGGCGGTACGAATAAAA | Inside of gene encoding protein with p-02639 (653280-653261); for confirmation of p-cluster 02639 deletion |
| pulG-UpF | GCGCTGAGCAACAATAACAAACTCATCGT | Upstream of pulG (611176-611204); for deletion of pulG |
| pulG-UpR | ATCACGTGGTGTAGAGCGTTTTTCCACGT | Upstream of pulG (611960-611943); for deletion of pulG |
| pulG-DwF | ATCACGTGGTGTTGATGGGTATTTTTGGG | Downstream of pulG (612576-612593); for deletion of pulG |
| pulG-DwR | GGTGGCTATTCCGTTCAAGATTTACAACA | Downstream of pulG (613537-613509); for deletion of pulG |
| pulG-IntF | TGCCGTTCGTTTATTTTTCC | Inside of pulG (612543-612524); for confirmation of pulG deletion |
| pulG-IntR | TGACGCAAGCACTCAAAGAT | Inside of pulG (611972-611991); for confirmation of pulG deletion |
| Spe-UpF | GCAGGTCGATTTTCGTTC | Inside of Sper gene; for identification of Sper intermediate deletion mutants |
| Spe-DwR | GCCACTGCATTTCCCGCATA | Inside of Sper gene; for identification of Sper intermediate deletion mutants |
Underlined sequences are the restriction sites
The nucleotide coordinates of the primers are based on strain D7S-1
Construction of vectors containing the wildtype loxP-Spe-loxP cassette and its variants
Vectors cloned with a spectinomycin-resistance marker (Spe) flanked by two loxP sites or two of its variants were generated. As an example, for the construction of the Spe cassette with the wildtype loxP (loxPW) sites two partially complementary oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA):
5′ TCGACACCACGTGGATCCATAACTTCGTATAATGTATGCTATACGAAGTTATCTGCAGATAACTTCGTATAATGTATGCTATACGAAGTTATGTCGACACGTGGTG3′, and
3′ GATCCACCACGTGTCGACATAACTTCGTATAATGTATGCTATACGAAGTTATCTGCAGATAACTTCGTATAATGTATGCTATACGAAGTTATGGATCCACGTGGTG3′
(loxP sites were underlined, and bold letters indicated the spacer sequence of loxP. Sal I, Dra III, Bam HI, and Pst I were engineered in these two oligonucleotides). These two DNA fragments were annealed, and cloned into pBluescript II KS at the Bam HI and Sal I sites. A 1.1 kb Sper cassette released from Pst I-digested plox2-Spe plasmid12 was inserted in the Pst I site between two loxPW sites to generate a plasmid bearing a loxPW-Spe-loxPW gene cassette. All recombinant plasmids were confirmed by sequencing the PCR products with T3 and T7 primers. With the same strategy plasmids with variants of the loxP-Spe-loxP cassette were generated (see Table 2 for variant loxP spacer sequences). The variant cassettes contained a pair of mutant loxP with one or two bases altered in the loxP spacer region based on their specificity in recombination16. This will allow successive deletions with the loxP/Cre system without interference from existing loxP sites of the genome.
Site-specific gene deletion with the loxP /Cre system
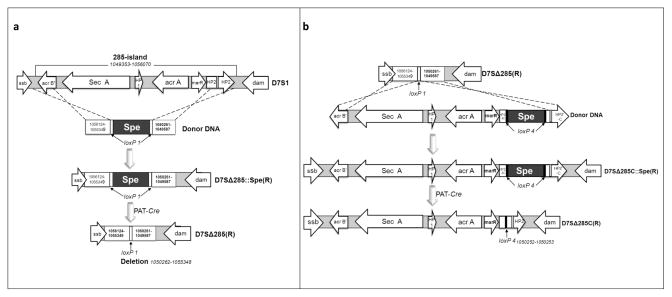
The strategy for gene deletion with the loxP/Cre system has been described previously17. Briefly, three steps were involved: (i) construction of the donor DNA with the loxP-Spe-loxP cassette flanked by homologous regions upstream and downstream of the gene to be deleted, (ii) allelic replacement of the target gene in A. actinomycetemcomitans by natural transformation, and (iii) removal of the loxP-Spe-loxP leaving a loxP scar at the deletion site (see Fig 1a for an example).
Figure 1. Deletion (a) and gene restoration (b) of the 285-island of A. actinomycetemcomitans strain D7S-1.
(a) The upstream (1056124-1055349) and downstream (1050261-1049587) regions of the 285-island (1049353-1056070) were amplified and ligated to the loxP1-Spe-loxP1 cassette in vitro. The 2.7 kb fragment was then used as donor DNA by natural transformation to generate an intermediate deletion mutant Δ285::Spe(R). Finally, the plasmid pAT/Cre was introduced and the recombination between two loxP1 sites removed the Sper marker, resulting in the markerless deletion mutant Δ285(R). (b) The upstream (1055865-1050253) and downstream (1050252-1049645) regions of the 285-island were amplified and ligated to the loxP4-Spe-loxP4 cassette in vitro. The 7.4 kb fragment was then used as donor DNA by natural transformation to generate an intermediate 285-island restored mutant Δ 285C::Spe(R). Recombination between two loxP4 sites removed the Sper marker, leaving one copy of loxP4 in the final markerless 285-island restored mutant Δ285C(R).
In this study, two approaches were used to construct the donor DNA. The first approach was by the in vitro ligation. Two pairs of primers were designed to amplify the upstream and downstream homologous DNA fragments flanking the deletion target. A Dra III site was engineered in the proximal end of the two homologous DNA fragments (Table 3). Approximately 1600 ng of PCR amplicons of the upstream and downstream homologous DNA fragments isolated by QIAquick PCR purification kit (Qiagen, Valencia, CA), were mixed with approximately 800 ng of recombinant plasmid with a loxP-Spe-loxP cassette, which had Dra III sites in each end. The mixture was digested with Dra III at 37°C for an hour, then purified by the QIAquick PCR purification kit, and ligated at 16 °C overnight. After heat inactivation of the ligation reaction, the ligation mixture was used directly for transformation. The alternative method for donor DNA preparation involved cloning. Two pairs of primers were designed to generate the upstream and downstream homologous regions (>500 bp) flanking the deletion target by PCR, and the DNA fragments were cloned sequentially into the upstream and downstream of the loxP-Spe-loxP cassette in a recombinant plasmid constructed above. The DNA fragment containing the homologous regions and the loxP-Spe-loxP cassette was then released from the vector by restriction enzyme digestion, purified and served as donor DNA. Gene transfer by natural transformation was performed as described previously18. The transformants (with the target gene replaced by the loxP-Spe-loxP cassette) were verified by PCR and designated as “intermediate” gene deletion mutants. In the final step the spectinomycin-resistance marker was removed by introducing a vector containing Cre as described previously17. Mutants of A. actinomycetemcomitans strain D7S-1 with deletions of multiple genes were constructed step-wise with the loxP/Cre system as described above.
Restoration of the deleted genes
Essentially the reversal of the process of the deletion is applied for gene restoration (see Fig 1b for an example). Two fragments (one of which includes the deleted region) were constructed by PCR amplification. The two fragments were ligated to a loxP-Spe-loxP cassette in vitro and the DNA mixture was used directly for transformation. The transformants were identified from selective media and the resistance marker spliced out leaving a loxP scar in the genome. The gene-restored mutants were then confirmed by PCR analysis and sequencing as before.
Bacterial growth assays in broth
Bacterial suspensions were prepared by the indirect suspension method19. Briefly, bacteria were collected from agar plates with a sterilized plastic loop and spread as a thin layer slightly above the solution face on the test tube wall. The bacteria were dispersed into the mTSB broth by mixing with a Vortex, and the above steps were repeated several times. The optical density of each starter bacterial suspension was determined (OD of approximately 0.25–0.3) and then diluted 1:5 with fresh broth. Aliquots of 20 μl of the bacterial suspension were transferred to each well of a 100-well Bioscreen C plate preloaded with 180 μl of prewarmed fresh media. To prevent evaporation of the liquid culture, 15 μl of mineral oils were added in each well. The plate was incubated at 37°C for 40 hours with continuous shaking, and the absorbance at 420–580 nm visible light was recorded every 30 minutes. Wells containing media served as background controls. The data from duplicate wells for each strain were averaged to represent one data point. The growth of each strain was independently tested at least three times on different dates.
To calculate the doubling time, the exponential phase of the growth was first defined as the time period between two measurements of the optical density (OD): the minimum OD plus 0.1 and the maximum OD minus 0.1. After subtraction of the background OD (OD of the media only; without bacteria), the OD was transformed into log2 (optical density). A linear regression of the data points was performed and accepted if the R2 was > 0.95, otherwise the experiments were repeated. The reciprocal of the slope gives the doubling time directly. The comparison of doubling time and maximum OD among strains was performed with SPSS 15.0 by using the Student t test or the analysis of variance (ANOVA).
Biofilm formation assay
Aliquots of 20 μl of bacterial suspension of A. actinomycetemcomitans were prepared as described above and transferred into wells preloaded with 180 μl of prewarmed media. The plate was incubated at 37°C for 48 hours in air supplemented with 5% CO2. After incubation, the media was gently removed and the wells were washed twice with 200 μl PBS, then air dried. A solution of 0.1% crystal violet was used to stain the dried wells for 10 minutes. Subsequently, the staining solution was removed, and the wells were washed twice with 200 μl PBS, then air-dried again. The stained biofilms were destained with 100% (v/v) ethanol for 5 minutes. The absorbance of the dye was quantified at 540 nm visible light using a microplate reader. Strains were tested in triplicate for each condition tested.
Results
Construction of mutants
Eight single-deletion mutants were constructed (Table 1). Each set included an “intermediate” mutant with spectinomycin resistance marker and a final markerless mutant. The deletion targets were selected to include well-studied core genes that were unlikely to affect growth and biofilm formation (ltxA and cdtB), four small accessory genes of unknown functions and two genomic islands of unknown functions. Four sets of mutants with deletion of multiple genes were constructed (Table 1). They were made to test the protocol for sequential deletions by the Cre/loxP system. The step-wise process of a quadruple gene-deletion mutant strain D7SΔltxAΔcdtBΔ16Δ285 is given here as an example. The construction began from the single gene deletion mutant D7SΔltxA with a loxPW scar. This mutant was transformed with the genomic DNA from the D7SΔcdtB::Spe (with loxP6 sites flanking the Sper marker) mutant. The transformants were identified and the resistance marker subsequently removed, leaving a loxP6 scar in the genome. The same steps were repeated to generate a triple knockout mutant D7SΔltxAΔcdtBΔ16 and the quadruple knockout mutant D7SΔltxAΔcdtB 16Δ285 (leaving a loxPW, a loxP6, a loxP5 and a loxP1scars in the genome).
Growth and biofilm formation
Table 4 provides a summary of the results of the growth assays. Most of the mutants did not show altered growth. However, it was noted that a mutant with a deletion of the 285-island appeared to grow faster than the wildtype. The restoration of the island in the deletion mutant reduced the growth rate to the level of the wildtype D7S-1. The genetic map of the 285-island and its deletion and restoration are illustrated in Figure 1a and 1b. The island is 6,660 bp, and has 6 predicted ORFs. The deleted region and the restored region are also marked. The difference in the wildtype and the mutant restored of the 285-island was the loxP4 scar within the downstream hypothetical protein HP2. The insertion of a loxP within HP2 is not expected to change the phenotype.
Table 4.
Doubling times of A. actinomycetemcomitans wildtype and mutants
| Fimbriated wildtype and derived mutants | ||
|---|---|---|
| Strain | Doubling Time | P valuea |
| D7S-1 | 4.86 ± 0.09 | - |
| D7SΔltxA | 4.79 ± 1.71 | 1.000 |
| D7SΔcdtB | 5.53 ± 0.55 | 0.840 |
| D7SΔacrB | 5.38 ± 0.21 | 0.357 |
| D7SΔpulG | 4.94 ± 0.27 | 1.000 |
| D7SΔp2579 | 5.82 ± 0.30 | 0.233 |
| D7SΔp2639 | 5.42 ± 0.31 | 0.576 |
| D7SΔ16 | 5.47 ± 0.62 | 0.230 |
| D7SΔ285(R) | 2.86 ± 0.24 | 0.004 |
| D7SΔltxAΔcdtB | 5.65 ± 0.15 | 0.054 |
| D7SΔltxAΔcdtB Δ16 | 3.74 ± 0.28 | 0.135 |
| D7SΔltxAΔcdtB Δ285 | 2.17 ± 0.15 | 0.001 |
| D7SΔltxAΔcdtB Δ16Δ285 | 2.09 ± 0.24 | 0.011 |
| Gene-restored mutants | ||
| Strain | Doubling Time | P valueb |
| D7SΔ285C(R) | 4.86 ± 0.53 | 0.678* |
| D7SΔltxAΔcdtB Δ285C | 6.58 ± 0.57 | 0.239** |
The data are the averages of three independent experiments and standard deviations are shown. Significant levels are determined by a One-way ANOVA or bthe Student t test.
compared to D7S-1,
compared to D7SΔltxAΔcdtB.
Biofilms formation was evaluated for the wildtype D7S-1 and mutants. There were no significant differences in the biofilm formation among strains. Instead, the primary determinant for biofilm formation was the fimbriation status of the test strains (data not shown).
Discussion
The long-term goal of this study was to identify a suitable genetic approach to examine the functions of strain-specific genes of A. actinomycetemcomitans. Several genetic protocols have been developed for mutagenesis and gene complementation of A. actinomycetemcomitans, including allelic exchange and homologous integration20–22, insertional mutagenesis23, 24 and transposon-mediated mutagenesis14, 25, 26. These methods involve a cloning step, which could prolong the experiments or present an obstacle if the cloned products are toxic to the E. coli host. Our approach for genetic manipulation could prepare the recombinant DNA by PCR amplification and in vitro ligation, and use it directly as donor DNA for competence-mediated gene transfer. The transformation frequencies of were approximately 10−4 to 10−5 for naturally competent A. actinomycetemcomitans12. A typical experiment generated approximately 100 to 1000 transformants with 2×107 competent bacteria. A gene deletion mutant could be generated in less than a week with minimal work.
In this study the Cre/loxP recombination system was employed to delete the antibiotic resistance marker. This approach was taken primarily for the ease of construction of mutants with deletion of multiple genes, and to avoid the possible effects of antibiotic resistance markers to the phenotypes of the bacteria. The Cre recombinase catalyzes cofactor independent recombination between two loxP sites, which consist of two 13 bp inverted repeats separated by an asymmetric 8 bp spacer region27. The Cre/loxP recombination system shows high recombination efficiency and has been widely used in eukaryotes. For prokaryotic organisms, Cre/loxP has been involved in genetic works for Escherichia coli28, 29, Lactobacillus plantarum30, and other species of Gram-negative bacteria31–33, but limited in use for A. actinomycetemcomitans17, 34.
A novel feature of our approach is the use of different loxP for construction of mutants with deletion of multiple genes. Consecutive deletion of genes using the same Cre/loxP may lead to unexpected deletions or inversions via recombination between the existing loxP of the genome and the new loxP introduced by the donor recombinant DNA, especially if the loxP sites were close to each other33. Therefore, we developed several loxP sites each with a variant spacer based on the previous study that showed incompatibility of certain loxP spacers in recombination16. We successfully generated deletion mutants of multiple genes, and did not notice any unexpected problem.
In this study we restored the deleted genes into the same locus as in the wildtype bacteria. By this process only a loxP scar was introduced into the genome in the gene-restored mutants. The loxP scar can be placed in a region that does not affect the phenotypes of the bacteria. Therefore, the restored mutants have exactly the same genetic background, and we avoid the problems with other complementation analysis that may affect gene expression due to variable copy numbers of the plasmid, different gene regulation mechanisms of the plasmid and the genome, or interference from the expression of an antibiotic resistance gene of the plasmid. Another advantage is the relative lack of size constraint for the restored genes. It is feasible and efficient to restore genes of 10–20 kb in one experiment.
The growth effect due to the mutation of 285-island was unexpected. The apparently functional ORFs in the 285-island are secA, acrA, a marR-family transcriptional regulator and a small hypothetical protein. In E. coli, SecA protein is a major component of the cellular mechanism that mediates the translocation of proteins across the plasma membrane35, and AcrA and AcrB are related to the multidrug efflux pump, known as two major components of the tripartite efflux system36, 37. The MarR (multiple antibiotic resistance regulators) family of prokaryotic transcriptional regulators includes proteins critical for control of virulence factor production, bacterial response to antibiotic and oxidative stresses, and catabolism of environmental aromatic compounds38, 39. Therefore, the function of the 285-island could be for regulation of antibiotic resistance. However, strain D7S-1 has another efflux pump homologue. The deletion of the 285-island may or may not affect its resistance to antibiotics. The E test was performed to examine the antibiotics resistance profiles of the 285-island deletion and complementation mutants, but no corresponding results were shown. More studies are needed to verify the possible modulation of growth by the 285-island and its mechanism of A. actinomycetemcomitans.
In conclusion, this study has demonstrated the feasibility of employing Cre/loxP recombination system in genetic manipulation of A. actinomycetemcomitans. The protocols are relatively easy and efficient in the construction of mutants with deletion of single or multiple genes. The approach could be used as a tool to identify functional strain-specific genes or genomic islands for further hypothesis testing.
Acknowledgments
This study was supported by NIDCR grant R01 DE12212 to CC.
References
- 1.Asikainen S, Chen C. Oral ecology and person-to-person transmission of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Periodontol 2000. 1999;20:65–81. doi: 10.1111/j.1600-0757.1999.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 2.Poulsen K, Theilade E, Lally ET, Demuth DR, Kilian M. Population structure of Actinobacillus actinomycetemcomitans: a framework for studies of disease-associated properties. Microbiology. 1994;140:2049–60. doi: 10.1099/13500872-140-8-2049. [DOI] [PubMed] [Google Scholar]
- 3.Chen C, Wang T, Chen W. Occurrence of Aggregatibacter actinomycetemcomitans serotypes in subgingival plaque from United States subjects. Mol Oral Microbiol. 2010;25:207–14. doi: 10.1111/j.2041-1014.2010.00567.x. [DOI] [PubMed] [Google Scholar]
- 4.Asikainen S, Chen C, Saarela M, Saxen L, Slots J. Clonal specificity of Actinobacillus actinomycetemcomitans in destructive periodontal disease. Clin Infect Dis. 1997;25 (Suppl 2):S227–9. doi: 10.1086/516211. [DOI] [PubMed] [Google Scholar]
- 5.Haubek D, Ennibi OK, Poulsen K, Vaeth M, Poulsen S, Kilian M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet. 2008;371:237–42. doi: 10.1016/S0140-6736(08)60135-X. [DOI] [PubMed] [Google Scholar]
- 6.DiRienzo JM, McKay TL. Identification and characterization of genetic cluster groups of Actinobacillus actinomycetemcomitans isolated from the human oral cavity. J Clin Microbiol. 1994;32:75–81. doi: 10.1128/jcm.32.1.75-81.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kittichotirat W, Bumgarner RE, Asikainen S, Chen C. Identification of the pangenome and its components in 14 distinct Aggregatibacter actinomycetemcomitans strains by comparative genomic analysis. PLoS One. 2011;6:e22420. doi: 10.1371/journal.pone.0022420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kittichotirat W, Bumgarner R, Chen C. Markedly different genome arrangements between serotype a strains and serotypes b or c strains of Aggregatibacter actinomycetemcomitans. BMC Genomics. 2010;11:489. doi: 10.1186/1471-2164-11-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sreenivasan PK, Fives-Taylor P. Isolation and characterization of deletion derivatives of pDL282, an Actinobacillus actinomycetemcomitans/Escherichia coli shuttle plasmid. Plasmid. 1994;31:207–14. doi: 10.1006/plas.1994.1022. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Chen C. Mutation analysis of the flp operon in Actinobacillus actinomycetemcomitans. Gene. 2005;351:61–71. doi: 10.1016/j.gene.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Liu A, Chen C. Genetic basis for conversion of rough-to-smooth colony morphology in Actinobacillus actinomycetemcomitans. Infect Immun. 2005;73:3749–53. doi: 10.1128/IAI.73.6.3749-3753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Shi W, Chen W, Chen C. Type IV pilus gene homologs pilABCD are required for natural transformation in Actinobacillus actinomycetemcomitans. Gene. 2003;312:249–55. doi: 10.1016/s0378-1119(03)00620-6. [DOI] [PubMed] [Google Scholar]
- 13.Brogan JM, Lally ET, Demuth DR. Construction of pYGK, an Actinobacillus actinomycetemcomitans-Escherichia coli shuttle vector. Gene. 1996;169:141–2. doi: 10.1016/0378-1119(95)00792-x. [DOI] [PubMed] [Google Scholar]
- 14.Thomson VJ, Bhattacharjee MK, Fine DH, Derbyshire KM, Figurski DH. Direct selection of IS903 transposon insertions by use of a broad-host-range vector: isolation of catalase-deficient mutants of Actinobacillus actinomycetemcomitans. J Bacteriol. 1999;181:7298–307. doi: 10.1128/jb.181.23.7298-7307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 16.Lee G, Saito I. Role of nucleotide sequences of loxP spacer region in Cre-mediated recombination. Gene. 1998;216:55–65. doi: 10.1016/s0378-1119(98)00325-4. [DOI] [PubMed] [Google Scholar]
- 17.Fujise O, Wang Y, Chen W, Chen C. Adherence of Aggregatibacter actinomycetemcomitans via serotype-specific polysaccharide antigens in lipopolysaccharides. Oral Microbiol Immunol. 2008;23:226–33. doi: 10.1111/j.1399-302X.2007.00416.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Goodman SD, Redfield RJ, Chen C. Natural transformation and DNA uptake signal sequences in Actinobacillus actinomycetemcomitans. J Bacteriol. 2002;184:3442–9. doi: 10.1128/JB.184.13.3442-3449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karched M, Paul-Satyaseela M, Asikainen S. A simple viability-maintaining method produces homogenic cell suspensions of autoaggregating wild-type Actinobacillus actinomycetemcomitans. J Microbiol Meth. 2007;68:46–51. doi: 10.1016/j.mimet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Balashova NV, Park DH, Patel JK, Figurski DH, Kachlany SC. Interaction between leukotoxin and Cu, Zn superoxide dismutase in Aggregatibacter actinomycetemcomitans. Infect Immun. 2007;75:4490–7. doi: 10.1128/IAI.00288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao H, James D, Lamont RJ, Demuth DR. Differential interaction of Aggregatibacter (Actinobacillus) actinomycetemcomitans LsrB and RbsB proteins with autoinducer 2. J Bacteriol. 2007;189:5559–65. doi: 10.1128/JB.00387-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fives-Taylor P, Meyer DH, Mintz KP, Brissette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontol 2000. 1999;20:136–67. doi: 10.1111/j.1600-0757.1999.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 23.Kolodrubetz D, Phillips LH, Ezzo PJ, Kraig E. Directed genomic integration in Actinobacillus actinomycetemcomitans: generation of defined leukotoxin-negative mutants. Infect Immun. 1995;63:2780–4. doi: 10.1128/iai.63.7.2780-2784.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guthmiller JM, Kolodrubetz D, Kraig E. Mutational analysis of the putative leukotoxin transport genes in Actinobacillus actinomycetemcomitans. Microb Pathog. 1995;18:307–21. doi: 10.1006/mpat.1995.0028. [DOI] [PubMed] [Google Scholar]
- 25.Mintz KP. Identification of an extracellular matrix protein adhesin, EmaA, which mediates the adhesion of Actinobacillus actinomycetemcomitans to collagen. Microbiology. 2004;150:2677–88. doi: 10.1099/mic.0.27110-0. [DOI] [PubMed] [Google Scholar]
- 26.Isaza MP, Duncan MS, Kaplan JB, Kachlany SC. Screen for leukotoxin mutants in Aggregatibacter actinomycetemcomitans: genes of the phosphotransferase system are required for leukotoxin biosynthesis. Infect Immun. 2008;76:3561–8. doi: 10.1128/IAI.01687-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langer SJ, Ghafoori AP, Byrd M, Leinwand L. A genetic screen identifies novel non-compatible loxP sites. Nucleic Acids Res. 2002;30:3067–77. doi: 10.1093/nar/gkf421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mlynarova L, Libantova J, Vrba L, Nap JP. The promiscuity of heterospecific lox sites increases dramatically in the presence of palindromic DNA. Gene. 2002;296:129–37. doi: 10.1016/s0378-1119(02)00841-7. [DOI] [PubMed] [Google Scholar]
- 29.Adams DE, Bliska JB, Cozzarelli NR. Cre-lox recombination in Escherichia coli cells. Mechanistic differences from the in vitro reaction. J Mol Biol. 1992;226:661–73. doi: 10.1016/0022-2836(92)90623-r. [DOI] [PubMed] [Google Scholar]
- 30.Bron PA, Grangette C, Mercenier A, de Vos WM, Kleerebezem M. Identification of Lactobacillus plantarum genes that are induced in the gastrointestinal tract of mice. J Bacteriol. 2004;186:5721–9. doi: 10.1128/JB.186.17.5721-5729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marx CJ, Lidstrom ME. Broad-host-range cre-lox system for antibiotic marker recycling in gram-negative bacteria. Biotechniques. 2002;33:1062–7. doi: 10.2144/02335rr01. [DOI] [PubMed] [Google Scholar]
- 32.Palmeros B, Wild J, Szybalski W, Le Borgne S, Hernandez-Chavez G, Gosset G, et al. A family of removable cassettes designed to obtain antibiotic-resistance-free genomic modifications of Escherichia coli and other bacteria. Gene. 2000;247:255–64. doi: 10.1016/s0378-1119(00)00075-5. [DOI] [PubMed] [Google Scholar]
- 33.Quenee L, Lamotte D, Polack B. Combined sacB-based negative selection and cre-lox antibiotic marker recycling for efficient gene deletion in pseudomonas aeruginosa. Biotechniques. 2005;38:63–7. doi: 10.2144/05381ST01. [DOI] [PubMed] [Google Scholar]
- 34.Oscarsson J, Karched M, Thay B, Chen C, Asikainen S. Proinflammatory effect in whole blood by free soluble bacterial components released from planktonic and biofilm cells. BMC Microbiol. 2008;8:206. doi: 10.1186/1471-2180-8-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chou YT, Swain JF, Gierasch LM. Functionally significant mobile regions of Escherichia coli SecA ATPase identified by NMR. J Biol Chem. 2002;277:50985–90. doi: 10.1074/jbc.M209237200. [DOI] [PubMed] [Google Scholar]
- 36.Swick MC, Morgan-Linnell SK, Carlson KM, Zechiedrich L. Expression of Multidrug Efflux Pump Genes acrAB-tolC, mdfA, and norE in Escherichia coli Clinical Isolates as a Function of Fluoroquinolone and Multidrug Resistance. Antimicrob Agents Chemother. 2011;55:921–4. doi: 10.1128/AAC.00996-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eicher T, Brandstatter L, Pos KM. Structural and functional aspects of the multidrug efflux pump AcrB. Biol Chem. 2009;390:693–9. doi: 10.1515/BC.2009.090. [DOI] [PubMed] [Google Scholar]
- 38.Perera IC, Lee YH, Wilkinson SP, Grove A. Mechanism for attenuation of DNA binding by MarR family transcriptional regulators by small molecule ligands. J Mol Biol. 2009;390:1019–29. doi: 10.1016/j.jmb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson SP, Grove A. Ligand-responsive transcriptional regulation by members of the MarR family of winged helix proteins. Curr Issues Mol Biol. 2006;8:51–62. [PubMed] [Google Scholar]