Abstract
Brain-computer interface (BCI) systems allow users to interact with their environment by bypassing muscular control to tap directly into the users’ thoughts. In the present study, we investigate the role of prior experience with yoga and meditation, examples of formalized mind-body awareness training (MBAT), in learning to use a one-dimensional sensorimotor rhythm based BCI. Thirty-six human subjects volunteered to participate in two different cohorts based on past experience with MBAT — experienced MBAT practitioners and controls. All subjects participated in three BCI experiments to achieve competency in controlling the BCI system. The MBAT cohort achieved BCI competency significantly faster than the control cohort. In addition, the MBAT cohort demonstrated enhanced ability to control the system on various measures of BCI performance and improved significantly more over time when compared to control. Our work provides insight into valuable strategies for reducing barriers to BCI fluency that limit the more widespread use of these systems.
INTRODUCTION
In recent years, research efforts have been increasingly made in the development of brain-computer interface (BCI) systems as potential therapeutic outlets for individuals suffering from a variety of neuromuscular diseases1. Diseases such as amyotrophic lateral sclerosis (ALS), spinal cord injury, brainstem stroke, and cerebral palsy have all garnered attention as potential beneficiaries of this technology. Although physically disabled, the cognitive abilities of such individuals are still intact. As such, there is a great need for these patients to communicate with and manipulate their environment in a way that bypasses muscular control2. A BCI is a system that senses and decodes the cognitive intent of the user and generates commands that control a computer or external device in the user’s environment using only signals detected from the user’s central nervous system3–6.
In an attempt to gain the ability of movement control, various forms of brain signals have been used, including electrophysiological signals acquired over the scalp (electroencephalography; EEG), over the cortical surface (electrocorticography; ECoG), and within the brain (single-neuron action potentials (single units) and local field potentials; LFPs). Invasive BCI systems have received considerable attention for accomplishing thought-based BCI control6–12. While such invasive BCI systems have made substantial progress in recent years using recording electrodes within the brain or over the brain surface, these systems are accompanied by significant risks relating to the implantation of intracranial electrodes. Parallel to the investigation of BCI control using invasive electrodes, non-invasive BCI systems have been developed using scalp-recorded EEG to decode the user’s intention from sensorimotor rhythms (SMRs)13–15, or event related potentials (ERPs)1,16–19. While ERP-based BCIs provide a higher information transfer rate and require less training, SMR-based BCIs are controlled asynchronously by the user’s intention without external stimuli1,16,17. SMRs are generated by the primary sensory and motor cortices of the brain. Utilizing a motor imagery paradigm13–15,20–28, SMR-based BCIs employ two characteristic states: event-related synchronization (ERS) and event-related desynchronization (ERD). The mu rhythm from 8 to 12 Hz and the beta rhythm from 13 to 26 Hz have been distinctly useful as control signals in SMR-based BCIs. When the brain processes sensory information or plans to execute movement, a response known as ERD occurs in which the sensorimotor rhythms decrease in amplitude on the hemisphere contralateral to the body region for imagined movement. A simultaneous increase in the amplitude of the mu rhythm on the ipsilateral hemisphere has been characterized and referred to as ERS. Teaching users to intentionally modulate these physiological phenomena is at the basis of many motor imagery-based BCI systems.
The recordings of SMRs that are produced from motor imaginations have provided users with the ability to control a computer cursor or a virtual helicopter in up to three dimensions25–27. Thus, the methods for training subjects in up to 3-dimensional (3D) control have been established and the utilization of the mu and beta rhythms as control signals has been well distinguished. However, substantial limitations still exist in the application of noninvasive BCI systems to clinical scenarios. These limitations include the lengthy training time that is required by users to achieve satisfactory performance and that, even after training, only a suboptimal proportion of BCI users ever achieve acceptable BCI performance29,30. Current noninvasive EEG-based BCI systems for up to 3D cursor control require weeks to months of training before acceptable levels of performance are attained. Even after training, some BCI users still fail to achieve adequate control of the system29,30.
Algorithm, sensor, and system development are making substantial progress within the field of BCI, but perhaps plateauing in terms of the impact on the change that these refinements may offer in the level of user control. Owing to these advances, in the last decade there have been dramatic improvements in performance measures of control accuracy, information transfer rate (ITR), and speed. However, the field of neural interface control faces a challenge to show that these gradually enhanced changes in control are translating to clinically meaningful applications. System developments have approached only a minimal clinically important difference in terms of the neuro-rehabilitation outcome measures for patients31–33. While researchers are dedicating substantial effort into the machine side of BCI utility, little effort has been focused on enhancing the users themselves. As such, there exists an important need for user-centered training techniques that focus on the refinement of the mental rehearsal practices to improve the signal produced by the user.
User-centered BCI training approaches have been pursued with more interest recently34,35. User-centered approaches emphasize early focus on the BCI users, tasks, and the environment, and the active participation of users with the goal of improving the performance of BCIs from the “brain” perspective of the brain-computer interface. Given that BCI is the interface between the “brain” and “computer”, the exploration of both aspects will be important to further improve the performance of BCIs and contribute to its translation for wide applications.
It has been suggested that mental rehearsal and concentration ability can improve the performance of an EEG-based BCI36,37. However, the level of performance depends on a variety of factors including individual differences and quality of motor imagery. Mental rehearsal refers to the repetition of a physical activity in the mind, without any physical movement of the body, and with the intention of learning and refining. The effects of mental rehearsal on motor learning are the result of practice on the central motor system. According to this logic, it seems reasonable that mental rehearsal should modulate the neuronal activity in the primary sensorimotor cortex and, as a result, change the performance of an SMR-based BCI38–40.
In both the world of public health and the popular culture at large, mind-body awareness training (MBAT), in the forms of yoga and meditative practices, has become increasingly prevalent due to an increase in awareness of the potential health benefits and improvements in concentration that this training can provide to practitioners. A growing body of evidence has supported the idea that yoga and meditation benefit both physical and mental health via down-regulation of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. In particular, several studies have been conducted that examined the impact of yoga and meditation on specific health conditions including cardiovascular disease, metabolic syndrome, diabetes, cancer, and anxiety41–44. These studies have contributed to the large body of supporting evidence attesting to the positive health benefits of yoga and meditation. Many forms of yoga practices such as Nidra, Vinyasa, and Hatha in addition to related meditative practices such as Mindfulness and Transcendental Meditation all employ particular mental techniques that produce increases and decreases in the spectral power of the alpha and theta band during the meditative state of the mind44–46. These mind-body awareness practices have demonstrated the capability of enabling the user to enhance concentration, and to induce different measurable states of consciousness and mind-body awareness during the performance of a mental task.
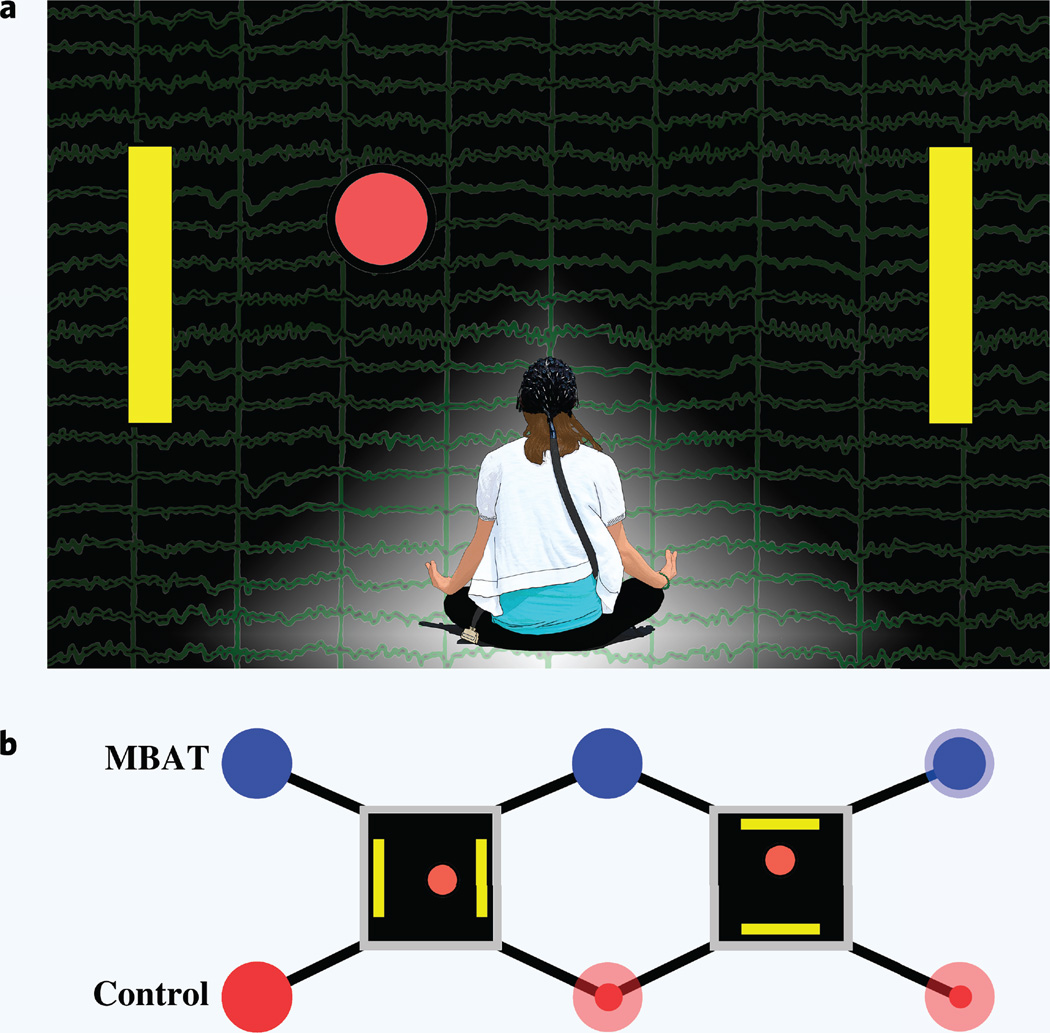
The aim of the present study is to investigate the role of experience with yoga and/or meditation, examples of formalized mind-body awareness practices, in the initial learning of a one-dimensional (1D) SMR-based BCI. Furthermore, we evaluate subject performance and the rate of subject learning using standard and modified metrics of classification accuracy, information transfer rate, and the number of hits per run, together with EEG analyses of various neural correlates of BCI control. Figure 1a displays a conceptual diagram of the present study and the potential role of MBAT in the context of an SMR-based BCI. The EEG signal that is produced from motor imaginations is depicted in the background of the figure. The schematic diagram in Fig. 1b represents the 1D cursor task that is used for initial training using the widely disseminated BCI2000 development platform.
Figure 1.
(a) A conceptual diagram of the study design and the potential role of mind body awareness training (MBAT) in the context of a sensorimotor rhythm based brain-computer interface (BCI). The EEG signal that is produced from motor imaginations is depicted in the background of the figure. The yellow target bars displayed on the left and right sides of the figure, in addition to the red ball in the middle, represent the standard left vs. right cursor task that is used for initial one-dimensional (1D) BCI training. (b) Experimental paradigms. Subjects belong to one of two cohorts — MBAT practitioners and controls. All subjects undergo the same task progression starting with a left vs. right cursor task. The subjects pass when they have completed the task with 80% accuracy (or higher) for four consecutive runs or if their accuracy averages 80% or higher over ten runs. Those who pass the left vs. right cursor task move onto the up vs. down cursor task with the same passing criteria. Subjects who pass the up vs. down cursor task are deemed proficient in 1D BCI control. Opaque dots on the figure represent the percentage of subjects (drawn to scale) who have passed each stage of the protocol. Translucent dots represent the original pool of subjects.
INNOVATION
Mind-body awareness practices and the ability to refine mental training techniques in order to focus for an extended amount of time are intuitively important skills that may help to bridge the gap between users who struggle with BCI control and those to whom it comes naturally. As such, a formal scientific investigation of MBAT practices in the context of SMR-based BCI training may identify a means to reduce the training obstacles to BCI utility. No brain-computer interface applications to date have investigated previous long-term experience with MBAT such as yoga or meditation in the context of an EEG-based brain-computer interface.
METHODS
Data acquisition and cursor control
This study was conducted according to a human subject protocol approved by the Institutional Review Board (IRB) of the University of Minnesota. Thirty-six healthy volunteers, 17 female and 19 male (ages 22 to 35 years old), participated in this study. Subjects were seated facing a computer monitor while wearing a 64-channel EEG cap, which was set up according to the international 10–20 system. The scalp-recorded EEG signals were sampled at 1000 Hz and filtered from DC–200 Hz by a Neuroscan Synamps 2 amplifier (Neuroscan Lab, VA) before they were imported into BCI2000 with no spatial filtering. The control signal was extracted as the difference between the autoregressive (AR) spectral amplitudes of electrodes C3 and C4 at 12 Hz. The magnitude of the cursor movement was determined by the normalized AR amplitude difference. At the end of each 3-minute trial, the control signal was normalized so that it had a zero mean and unit variance across a multiple trial buffer. In the BCI2000 1D cursor task, a yellow-colored, rectangular target appeared on either the left or right side of the computer screen (for the left vs. right task) or at the top or bottom of the screen (for the up vs. down task). Because SMRs were used as the control signal for this BCI, subjects were instructed to use motor imaginations of their right hand movement to move a computer cursor (red circle) to the right target, and left hand movement to move the cursor to the left target. In comparison, subjects used motor imaginations of both hands to move the cursor up, and a volitional rest to move the cursor down.
Study design
The 36 subjects fell into one of two groups. The first group included 12 subjects with at least one year of previous MBAT experience, who practiced at least 2 times per week for at least 1 hour. The specific types of MBAT practices most commonly used in this cohort were reported as Yoga Nidra and Vinyasa in addition to Reiki, Mindfulness, and Transcendental Meditation. The second group included 24 subjects with little or no MBAT experience (less than 10 total MBAT sessions), and served the purpose of representing healthy controls from the general population.
Regardless of the group that subjects were assigned to, each subject was previously naive to BCI, and participated in three, 2-hour BCI experiments over the period of 1 to 4 weeks. Each experiment consisted of ten, 3-minute trials using the standard BCI2000 cursor task47. Subjects were first introduced to and trained in the 1D left vs. right cursor task26–28. All subjects were instructed to use imaginations of either left or right hand movements to move a computer cursor to hit a target on the left or right side of a computer screen respectively. If subjects achieved accuracies of ≥ 80% over four consecutive 3-minute runs or an overall session (ten, 3-minute runs) accuracy of ≥ 80%, subjects progressed to an up vs. down control task, which consisted of imagining both hands versus a volitional rest state to control the movement of the cursor to targets located at top or bottom of a computer screen respectively. Again, if subjects achieved accuracies of ≥ 80% over four consecutive 3-minute runs or an overall session accuracy of ≥ 80% for this up vs. down task, subjects were deemed proficient in 1D BCI control. All subjects were given 3 BCI sessions, each consisting of ten, 3-minute trials each, to attempt to reach 1D BCI competency. Figure 1b illustrates the experimental design of subject progression for the MBAT and control groups.
Performance analysis
A time-to-event analysis was employed to determine the significance of passing rates between the pair of cohorts. The time-to-event analysis was 3-fold. Firstly, a Kaplan-Meier plot was created for the pair of cohorts, which visually displayed the percentages of subjects who passed the paradigm against the number of attempts. Secondly, a log rank test, calculated as a chi-square value, was constructed using the data to determine the significance of separation between the pair. Lastly, the chi-square value that was calculated from the log rank test was converted to a P value; α = 0.05 for all statistical analyses in this study.
Several metrics of subject performance were analyzed in order to formally compare the two experimental groups. These measures included percent valid correct (PVC), the average number of hits per run, information transfer rate (ITR) in bits per minute, the average scalp R2 (correlation coefficient) value with respect to the topographical locations of control electrodes C3 and C4, and the magnitude of the spectral power difference (SPD) from electrodes C3 and C4. The weighted average (± SEM) for each metric was calculated for each subject and across all the data pooled from each group.
PVC was calculated by determining the ratio of target hits to valid outcomes. Thus, invalid outcomes corresponding to aborted trials were not included in the calculation. The time required for subjects to hit the target was determined from the time during which the cursor was under cortical control. For this experimental paradigm, the time began when the cursor first appeared, and ended either when the cursor hit the correct target, the cursor hit the “incorrect” target (i.e., the subject controlled the cursor to the opposite, or incorrect side of the computer screen where the imaginary incorrect target was located), or the trial was aborted. Each 3-minute run provided the subject with 6 seconds to control the cursor to the desired target, before the trial was classified as an aborted trial.
A particularly useful measure of comparing BCIs is via their information transfer rate, either in bits/trial or bits/min. As provided in Wolpaw et al.3, bits/trial can be calculated from the following equation:
| (1) |
In this equation, B is the ITR in bit rate (bits/symbol), N is the number of possible choices (targets) and P is the probability that the desired choice will be selected, also called the classification accuracy. Typically, Bt in bits/min is used to indicate the ITR of a BCI system
| (2) |
where T (seconds/symbol) is the time needed to convey each symbol.
A valuable aspect of using this specific measure is that it combines both accuracy and speed into one number. Thus, ITR in bits per minute was calculated for each run for this protocol.
EEG data analysis
During motor imaginations of the upper extremity, a decrease (ERD) of spectral power over the contralateral hemisphere and an increase (ERS) of spectral power over the ipsilateral hemisphere occur21,22,38. Two additional measures based on the EEG data analysis — the average scalp R2 value and SPD were used to quantitatively evaluate the neural correlates of subject performance. The average R2 value is a topographical measure of the correlation between movement imagination and mu rhythm source activities15,23,24,28. Specifically, R2 was calculated as the total proportion of variance in mu rhythm amplitude between the two imagination state pairings that was due to target position, and based on single-trial source estimates. For left vs. right trials, the control signal was extracted as the difference between the autoregressive spectral amplitudes of electrode C3 subtracted from C4 at 12 Hz. For up vs. down trials, the control signal was calculated as the sum of the spectral amplitudes of electrodes C3 and C4 relative to the resting state. The magnitudes of these differences in spectral power (measured in µV2 at 12 Hz) were calculated as neural correlates of cursor control. As for previously mentioned metrics, the R2 values and SPD were calculated for each subject and across all the data pooled from each group.
Performance progress analysis
In order to quantitatively examine the process of learning and refining BCI control over time, each subject’s outcome data for each left vs. right three-minute run were first plotted as a line graph and visually inspected for trends. Next, an approximation for average rates of subject performance progress for each metric was calculated as: Average Rate = (Final Metric Value − Initial Metric Value) / Number of Runs. These values were group-averaged and an unpaired, two-way t-test was also performed to evaluate the significance between the two groups of interest. Due to this particular experimental design of subject progression, the number of completed left vs. right and up vs. down runs varied considerably between subjects. The introduced dependence of the average rate on the number of runs helps correct this disparity.
RESULTS
During the course of the study, we quantitatively examined three main components of data: the proportion of subjects that achieved the predetermined threshold of 1D BCI competency as a time-to-event analysis, how subjects performed on average in each metric over the course of the study, and subject learning over time, as analyzed in a linear regression. Multiple measures for each of those components are presented below.
Time-to-event analysis
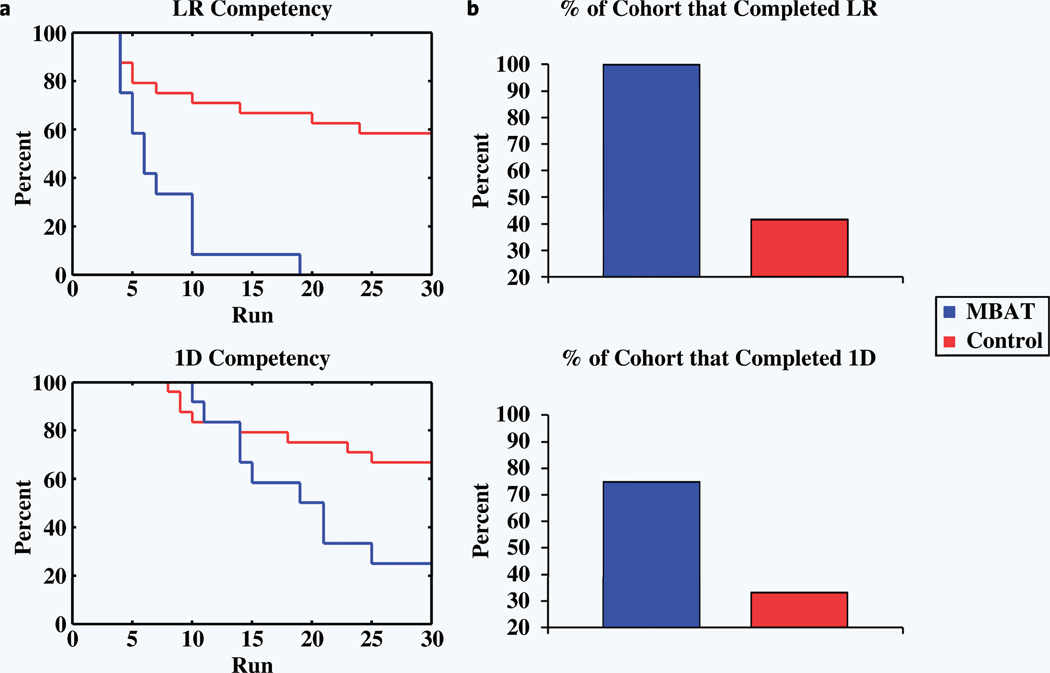
Time-to-event analysis was used to provide a quantitative representation of the overall trends of learning rates between the two cohorts. The final event was defined as passing the paradigm (either left vs. right or overall 1D). Figure 2a displays the Kaplan-Meier plots that were constructed for the two cohorts for each of the paradigms. For the left vs. right cursor task, the experienced MBAT group passed the paradigm significantly faster than the control group. The log rank test yielded a significant P value of 9.21E-5. The overall 1D paradigm experienced parallel results. The MBAT cohort passed the paradigm significantly faster than the control group (P = 0.0296). Figure 2b displays the percentage of each cohort that completed left-right and overall 1D training by the end of 30, three-minute runs.
Figure 2.
(a) Kaplan-Meier plots between pairs of cohorts. The top plot illustrates time-to-event analyses for the left-right task, while the bottom plot reports the overall 1D results. In the present paradigm, the event of interest is passing the left-right and up-down tasks to achieve 1D BCI competency. Initially, all subjects fail to pass. For both left-right and overall 1D control, the MBAT cohort showed significantly faster passing rates compared to the control group. (b) The percentage of each cohort that completed left-right (top plot) and overall 1D (bottom plot) training by the end of 30, three-minute experimental runs.
Performance analysis
On average, subjects from the MBAT group attained higher accuracies of control compared to the control group. PVC is the classification accuracy in all trials that resulted in a valid outcome (either correct or incorrect). Table 1 displays the group-weighted average left-right and overall 1D performance results for the three groups, in addition to the standard errors of the mean (SEM) within each group. For the left vs. right task, the group-weighted averages for the MBAT and control groups were 82.0% and 63.0%, respectively. For 1D cursor control, the group-weighted average accuracies for the two groups were 74.4% and 65.7%, respectively. A summary of these and all other performance metrics can be seen in Table 1.
Table 1.
A summary of the group-weighted average (± SEM) performance results during the three BCI sessions (each session including ten, 3-minute runs) during which subjects completed the experimental task. This summary includes the results for both left-right and overall 1D (left-right and up-down) results.
| Cohort | PVC (%) | Hits/Run | ITR (bits/min) | |||
|---|---|---|---|---|---|---|
| LR | 1D | LR | 1D | LR | 1D | |
| MBAT | 82.03 ± 1.70 | 74.42 ± 1.20 | 14.30 ± 0.60 | 12.78 ± 0.37 | 8.03 ± 0.72 | 5.96 ± 0.43 |
| Control | 63.01 ± 0.83 | 65.73 ± 0.80 | 8.48 ± 0.19 | 9.34 ± 0.21 | 2.64 ± 0.27 | 3.36 ± 0.22 |
A similar metric that evaluated subject performance was the average number of hits per (3-minute) run. Overall, subjects from the MBAT group achieved more hits per run than subjects from the control group. For the left vs. right task, the group-weighted averages for target acquisition rate for the MBAT and control groups were 14.3 hits/run and 8.5 hits/run, respectively. For overall 1D cursor control, including both left-right and up-down training, the group-weighted average target acquisition rates were 12.8 hits/run and 9.3 hits/run, respectively.
ITR is a standard metric of BCI control that combines both speed and accuracy into one measure. As hypothesized, subjects from the MBAT group achieved higher average ITRs than subjects from the control group, with the two experimental groups reaching 8.0 bits/min and 2.6 bits/min respectively, for the left vs. right task. The group-weighted averages for overall 1D control for the two groups were 6.0 bits/min and 3.4 bits/min, respectively.
EEG analysis
In order to quantitatively examine the neural correlates of subject control, the average R2 value and the SPD were calculated for each subject, and across all the data pooled from each group. For both left vs. right and overall 1D control, the group-weighted average neural power measures were greater for the MBAT cohort compared to the control group. For the left vs. right control task, the group-weighted average SPD results for the MBAT and control groups were 232.1 µV2 and 45.9 µV2, respectively. For overall 1D control, the group-weighted average results were 209.7 µV2 and 91.4 µV2, respectively. A summary of these neural measures of control in addition to the group-weighted average scalp R2 values for C3 and C4 can be seen in Table 2.
Table 2.
A summary of the group-weighted average (± SEM) left-right and 1D results for the quantitative analysis of various neural correlates of BCI control, including the spectral power difference of control electrodes C3 and C4 (µV2) and the average scalp R2 values at C3 and C4.
| Cohort | SPD (µV2) | Scalp R2 (C3) | Scalp R2 (C4) | |||
|---|---|---|---|---|---|---|
| LR | 1D | LR | 1D | LR | 1D | |
| MBAT | 232.13 ± 41.96 | 209.65 ± 28.15 | 0.15 ± 0.02 | 0.15 ± 0.01 | 0.18 ± 0.02 | 0.16 ± 0.01 |
| Control | 45.86 ± 9.03 | 91.42 ± 10.82 | 0.08 ± 0.00 | 0.10 ± 0.00 | 0.09 ± 0.00 | 0.09 ± 0.01 |
Performance progress analysis
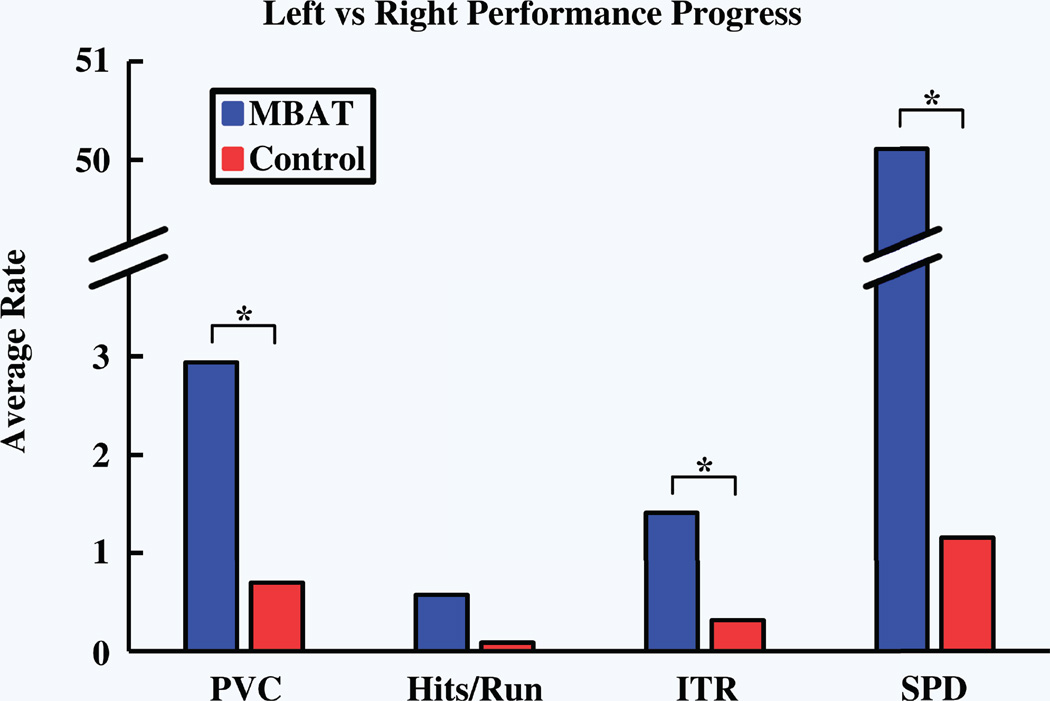
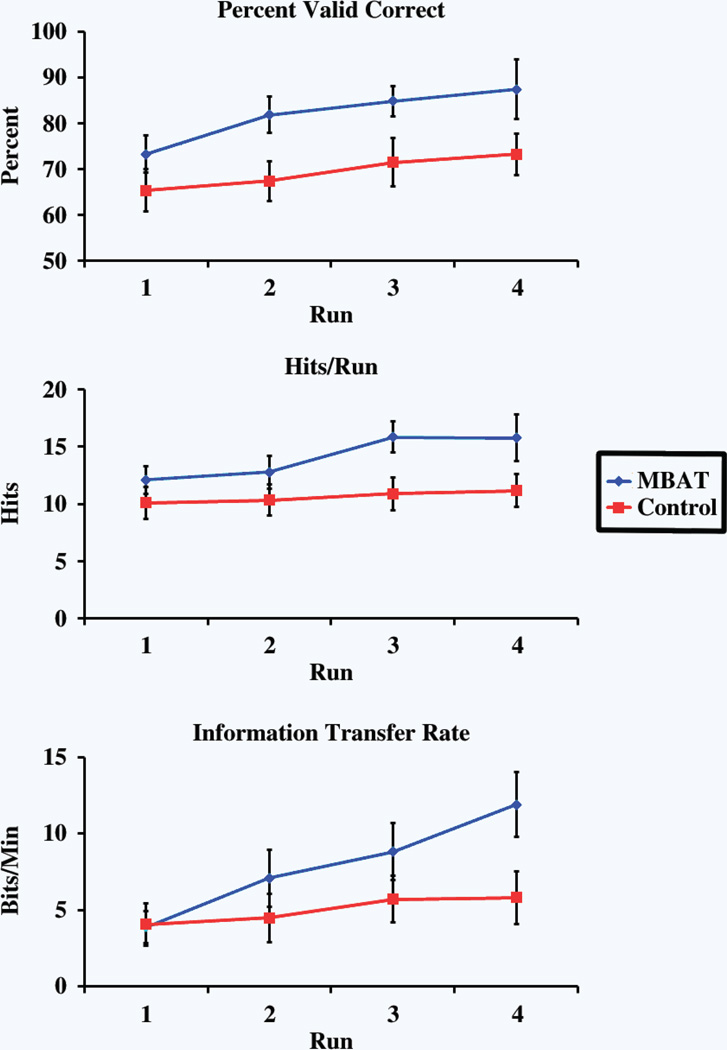
An important feature of this study was to investigate whether experience with MBAT can accelerate the early stages of learning to control a BCI. Subjects with MBAT experience demonstrated the ability to learn at a significantly greater rate than control subjects. The average rate of subject performance progress, as measured by the change in left vs. right performance outcomes as a function of number of runs, is displayed in Fig. 3. Asterisks indicate significance between the two groups evaluated. Due to the nature of the experimental design, the number of completed left vs. right runs varied appreciably between subjects. The number of trials that subjects completed was directly related to their performance ability. Subjects who passed the left vs. right paradigm quickly had relatively higher performance rates due to the smaller number of runs. Subjects from the MBAT cohort showed superior performance in all evaluated metrics in the first four runs of the left-right control task. Figure 4 displays the group-weighted average (± SEM) outcomes for the evaluated performance measures for the first four left vs. right runs of all subjects.
Figure 3.
Performance progress. Average rates were calculated as the change in performance over the total number of runs. An unpaired, two-way t-test was performed to determine the significance of progression rates between the control and practitioner groups. Asterisks indicate P-value < 0.01.
Figure 4.
Experimental performance results for the group-weighted average (± SEM) outcomes for the first four, three-minute runs of the left vs. right task. The topmost plot reports accuracy (% valid correct). The second plot reports the number of target hits per 3-minute run (hits/run) and bottom plot reports the information transfer rate (bits/min).
DISCUSSION
Establishing brain control of movement for patients suffering from various neurological disorders and the general healthy population represents a grand challenge to neuroscience and neuroengineering1,48. The goal of the present study was to test the hypothesis that experience with mind-body awareness training (MBAT), such as yoga/meditation, can improve learning to control a sensorimotor rhythm (SMR) based brain-computer interface (BCI). MBAT subjects not only outperformed control subjects in various measures of BCI control, these subjects also demonstrated the ability to learn at a significantly greater rate than control subjects. The reason for this substantially greater performance in the MBAT group may be due to the process of learning and refining particular mental techniques that provide subjects with the experience and practice of modulating their sensorimotor rhythms prior to even participating in a BCI task. Several forms of such yoga and meditative practices utilize such specific mental techniques that intentionally produce increases and decreases in the spectral power of the alpha and beta bands during training45,46. Since many of these practices do not require physical exertion, they may find utility in training physically disabled patient populations in the use of SMR-based BCIs. As such, future studies that investigate MBAT in the context of patient populations are needed to confirm this hypothesis.
The fact that learning occurred within the three experimental sessions does not necessarily signify that subjects were fully trained over this period. The main purpose of this study was to investigate the early learning stages of a BCI with prior MBAT experience. As such, all subjects completed only three, 2-hour experiments over the period of 1 to 4 weeks. By that time, 75% of the MBAT subjects passed the threshold for 1D competency compared to the passing rate of only 33.3% for the control group. The 1D BCI training times for contemporary publications in SMR-based BCI’s have demonstrated that subjects typically require weeks to even months to achieve adequate performance levels of BCI control26,28–30. Based on these findings, it is most likely that a greater proportion of the control subjects from the present study would eventually pass the 1D competency threshold had they been given more time to train. It is also important to note that all subjects were assigned to the same control signal (C3–C4 at 12 Hz) for the purposes of this particular paradigm. In the absence of completely automated control signal optimization, the process of signal optimization was an extra variable that could become difficult to separate from user learning. It was the characterization of the trajectory of learning in the absence of algorithmic or system side enhancement that was the central goal of this paradigm. Because of this, it is possible that more subjects, from both the MBAT and control cohorts, would have passed the 1D paradigm had their control signals been optimized using the wealth of methodologies developed to do so. Nevertheless, the present study indicates that subjects with MBAT experience demonstrated over 200% increase in their ability to reach 1D BCI competency by the end of the 30 experimental runs as compared with controls. This supports the notion that such mental training techniques may provide a valuable tool for substantially reducing the ubiquitous obstacle to BCI utility of lengthy training time.
The significant improvement of BCI performance in the MBAT cohort over the control cohort may be due to the improved skills of concentration in MBAT subjects. Figure 3 and Table 2 show significant differences between the MBAT and control cohorts in their ability to modulate mu rhythm as recorded in C3 and C4, two locations close to the left and right motor cortex. Yoga/meditation practices may contribute to the mental rehearsal ability of MBAT subjects, improving the ability of the brain to generate stronger synchronized responses from the neural population that is involved in mental rehearsal. This may be considered as a mental skill for human subjects, and MBAT may serve as a means of acquiring such skill for human subjects that could be translated to improved BCI performance using a motor imagery paradigm.
In contrast to similar objectives described in Mahmoudi et al.36, an important difference in the present study is that subjects in the MBAT group began BCI training with prior MBAT experience. One reason for this particular feature of the study design was to address the issue of a possible third variable of BCI learning that may have contributed to an increase in performance levels in place of mental practice and concentration skill development. Furthermore, we wanted to investigate the utility of various forms of MBAT in the early training of an SMR-based BCI. Recruiting subjects with a wide diversity of MBAT experience, ranging from Yoga Nidra and Vinyasa to Mindfulness and Transcendental Meditation, we demonstrate the importance of general mind-body awareness in learning to modulate SMRs to control a BCI. However, it is likely that some forms of MBAT are more useful than others in the specific context of SMR-based BCI control. As such, future studies are necessary to address which particular meditative techniques are optimal in EEG-based BCI training.
All of the recruited MBAT subjects began BCI training with at least one prior year of MBAT experience, practiced at least two times per week. Thus, this particular paradigm investigated relatively long-term experience with MBAT in learning to control an SMR-based BCI. In contrast, previous research explored the utility of short-term meditative training (evaluating subjects 10 and 20 days after the initial experiment) in the performance of an EEG-based BCI. Future studies that compare short-term and long-term exposure to MBAT practices are thus warranted in order to address the question of how much time is necessary for MBAT to have a significant impact on the performance of an EEG-based BCI. Another important distinction between this study and previous studies is that we used a more comprehensive method of evaluating subject performance and, in particular, learning. In contrast to the evaluation of only classification accuracies in previous studies, we quantitatively examined three main components of the data: the percentage of subjects that achieved 1D competency, the group-weighted average levels of performance of several measures of control, in addition to the rate of subject learning over time. These results extend the findings of previous research and provide a more thorough illustration of the benefits of MBAT in EEG-based BCI training.
Because a successful BCI system is dependent on the extent to which neural activity can be voluntarily controlled, it is important that user-centered training techniques are investigated in parallel to the optimization of sensing and decoding techniques of the machine. Current EEG-based BCI training practices rely on difficult and oft en frustrating methods of learning to modulate SMRs. Using abstract target bars as feedback can be a challenge to BCI users, especially for older subjects from a patient population in which the concept of motor imagery can be difficult to comprehend. Furthermore, experimenters often lack expertise in providing the mental training that is necessary to teach users how to optimally modulate their sensorimotor rhythms. MBAT offers a convenient and affordable method of training users to voluntarily control their neural activity and to improve concentration. Occurring in a comfortable and relaxing environment, MBAT avoids the intimidating context of contemporary scientific laboratory environments, while maintaining the rigorous training techniques that are crucial in developing the requisite skills for controlling an EEG-based BCI. As all BCIs are systems of communication between the human mind and a machine, ensuring optimal performance of both through system development and user training is a necessity of any successful BCI system.
It is important to note that there are different ways of improving performance of BCIs. It is known that mental activity affects the heart rate, and the heart rate changes may be observed during motor imagery. It was demonstrated that BCI performance could be improved when a hybrid BCI utilized both the EEG signal and a different type of user input such as the heart rate or a signal from an external device such as an eye tracking system35. The present study adds to what has been explored in the past — that mind-body awareness training such as yoga and meditation may offer an alternative means of improving BCI performance.
CONCLUSION
In the present study, we have evaluated the role that mind-body awareness training may play in the initial learning of a motor imagery-based brain-computer interface. To our knowledge, there are currently no other studies that have reported the effects of long-term experience with yoga or meditation in the context of sensorimotor rhythm based BCI applications. The experienced mind-body awareness training (MBAT) cohort not only passed both left vs. right and one-dimensional paradigms significantly faster than the control group but also outperformed the control group on a broad diversity of accepted measures of brain-computer interface performance. Lastly, the MBAT cohort demonstrated significantly greater improvement over time (learning) compared to the control group. Therefore, MBAT may provide an effective and widely accessible means to augment current training practices for brain-computer interface technology and in doing so, broaden the population of users for whom these devices can become meaningful.
ACKNOWLEDGEMENTS
The authors wish to thank the Your Yoga studio in Minneapolis, MN, for assistance in the recruitment of experienced MBAT subjects, and are grateful to Emal Alwis, Brad Edelman, Arman Shahriar, Xiaotong Zhang, Clara Zhang, and Keith Jamison for technical assistance and useful discussions. This work was supported in part by NSF CBET-1264782, DGE-1069104, NIH EB006433, and in part by the Institute for Engineering in Medicine of the University of Minnesota.
Footnotes
AUTHOR CONTRIBUTIONS
Conceived the concept: B.H. Designed the experiments: K.C., A.D. and B.H. Performed the experiments: K.C., A.Y. and A.D. Analyzed the data: K.C. and A.Y. Wrote the paper: K.C., A.Y., A.D. and B.H.
REFERENCES
- 1.He B, Gao S, Yuan H, Wolpaw J. Brain computer interface. In: He B, editor. Neural Engineering. 2nd Ed. Berlin: Springer; 2013. pp. 87–151. (ed.) [Google Scholar]
- 2.Kunst CB. Complex genetics of amyotrophic lateral sclerosis. Am. J. Hum. Genet. 2004;75:933–947. doi: 10.1086/426001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughn TM. Brain-computer interfaces for communication and control. Clin. Neurophysiol. 2002;133:767–791. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 4.Vallabhaneni A, Wang T, He B. Brain computer interface. In: He B, editor. Neural Engineering. Kluwer Academic/Plenum Publishers; 2005. pp. 85–122. [Google Scholar]
- 5.Yuan H, He B. Brain-computer interfaces using sensorimotor rhythms: Current state and future perspectives. IEEE Trans. Biomed. Eng. 2014;61:1425–1435. doi: 10.1109/TBME.2014.2312397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J. Neurosci. 1982;2:1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collinger JL, et al. High performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013;381:557–564. doi: 10.1016/S0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakor NV. Translating the brain-machine interface. Sci. Transl. Med. 2013;5:210ps17. doi: 10.1126/scitranslmed.3007303. [DOI] [PubMed] [Google Scholar]
- 9.Schalk G, et al. Two-dimensional movement control using electrocorticographic signals in humans. J. Neural Eng. 2008;5:75–84. doi: 10.1088/1741-2560/5/1/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochberg LR, et al. Reach and grasp by people with tetraplegia using a neutrally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy PR, Bakay RAE, Moore MM, Adams K, Goldwaithe J. Direct control of a computer from the human central nervous system. IEEE Trans. Rehabil. Eng. 2000;8:198–202. doi: 10.1109/86.847815. [DOI] [PubMed] [Google Scholar]
- 12.Musallam S, Corneil BD, Greger B, Scherberger H, Andersen RA. Cognitive control signals for neural prosthetics. Science. 2004;305:258–262. doi: 10.1126/science.1097938. [DOI] [PubMed] [Google Scholar]
- 13.Pfurtscheller G, Flotzinger D, Kalcher J. Brain-computer interface — A new communication device for handicapped persons. J. Microcomp. Appl. 1993;16:293–299. [Google Scholar]
- 14.Wolpaw JR, McFarland DJ, Neat GW, Forneris CA. An EEG-based brain-computer interface for cursor control. Electroencephalogr. Clin. Neurophysiol. 1991;78(3):252–259. doi: 10.1016/0013-4694(91)90040-b. [DOI] [PubMed] [Google Scholar]
- 15.Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc. Natl. Acad. Sci. USA. 2004;101:17849–17854. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao S, et al. Visual and auditory brain-computer interfaces. IEEE Trans. Biomed. Eng. 2014;61:1436–1447. doi: 10.1109/TBME.2014.2300164. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman T, et al. Face stimuli effectively prevent brain-computer interface inefficiency in patients with neurodegenerative disease. Clin. Neurophysiol. 2013;124:893–900. doi: 10.1016/j.clinph.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, et al. A hybrid BCI system combining P300 and SSVEP and its application to wheelchair control. IEEE Trans. Biomed. Eng. 2013;60:3156–3166. doi: 10.1109/TBME.2013.2270283. [DOI] [PubMed] [Google Scholar]
- 19.Kimura Y, et al. SSVEP-based brain-computer interfaces using FSK-modulated visual stimuli. IEEE Trans. Biomed. Eng. 2013;60:2831–2838. doi: 10.1109/TBME.2013.2265260. [DOI] [PubMed] [Google Scholar]
- 20.Samek W, et al. Transferring subspaces between subjects in brain-computer interfacing. IEEE Trans. Biomed. Eng. 2013;60:2289–2298. doi: 10.1109/TBME.2013.2253608. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Deng J, He B. Classifying EEG-based motor imagery tasks by means of time-frequency synthesized spatial patterns. Clin. Neurophysiol. 2004;115:2744–2753. doi: 10.1016/j.clinph.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Qin L, He B. A Wavelet-based time-frequency analysis approach for classification of motor imagery for brain-computer interface applications. J. Neural Eng. 2005;2:65–72. doi: 10.1088/1741-2560/2/4/001. [DOI] [PubMed] [Google Scholar]
- 23.Yuan H, Doud A, Gururajan A, He B. Cortical imaging of event-related (de) synchronization during online control of brain-computer interface using minimum-norm estimates in frequency domain. IEEE Trans. Neural Syst. Rehabil. Eng. 2008;16:425–431. doi: 10.1109/TNSRE.2008.2003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan H, Perdoni C, He B. Relationship between speed and EEG activity during imagined and executed hand movements. J. Neural Eng. 2010;7:026001. doi: 10.1088/1741-2560/7/2/026001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McFarland DJ, Sarnacki WA, Wolpaw JR. Electroencephalographic (EEG) control of three-dimensional movement. J. Neural Eng. 2010;7:036007. doi: 10.1088/1741-2560/7/3/036007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royer AS, Doud AJ, Rose ML, He B. EEG control of a virtual helicopter in 3-dimensional space using intelligent control strategies. IEEE Trans. Neural Syst. Rehabil. Eng. 2010;18:581–589. doi: 10.1109/TNSRE.2010.2077654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doud AJ, Lucas JP, Pisansky MP, He B. Continuous three-dimensional control of a virtual helicopter using a motor imagery based brain-computer interface. PLoS ONE. 2011;6:e26322. doi: 10.1371/journal.pone.0026322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaFleur K, Cassady K, Doud A, Shades K, Rogin E, He B. Quadcopter control in three-dimensional space using a noninvasive motor imagery-based brain-computer interface. J. Neural Eng. 2013;10:046003. doi: 10.1088/1741-2560/10/4/046003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradberry TJ, Gentili RJ, Contreras-Vidal JL. Fast attainment of computer cursor control with noninvasively acquired brain signals. J. Neural Eng. 2011;8:036010. doi: 10.1088/1741-2560/8/3/036010. [DOI] [PubMed] [Google Scholar]
- 30.Tkack D, Reimer J, Hatsopoulos NG. Observation-based learning for brain-machine interfaces. Curr. Opin. Neurobiol. 2008;18:589–594. doi: 10.1016/j.conb.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasad G, et al. Applying a brain-computer interface to support motor imagery practice in people with stroke for upper limb recovery: A feasibility study. J. Neuroeng. Rehabil. 2010;7:60. doi: 10.1186/1743-0003-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silvoni S, et al. Brain-computer interface in stroke: A review of progress. Clin. EEG Neurosci. 2011;42:245–252. doi: 10.1177/155005941104200410. [DOI] [PubMed] [Google Scholar]
- 33.Ang K, et al. A large clinical study on the ability of stroke patients to use an EEG-based motor imagery brain-computer interface. Clin. EEG Neurosci. 2011;42:253–258. doi: 10.1177/155005941104200411. [DOI] [PubMed] [Google Scholar]
- 34.Holz EM, et al. BCI-controlled gaming: Evaluation of usability by severely motor restricted end-users. Artif. Intelli. Med. 2013;59:111–120. doi: 10.1016/j.artmed.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Pfurtscheller G, et al. The hybrid BCI. Front. Neurosci. 2010;4:30. doi: 10.3389/fnpro.2010.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmoudi B, Erfanian A. Electro-encephalogram based brain-computer interface: Improved performance by mental practice and concentration skills. Med. Biol. Eng. Comput. 2006;44:959–969. doi: 10.1007/s11517-006-0111-8. [DOI] [PubMed] [Google Scholar]
- 37.Lakey CE, Berry DR, Sellers EW. Manipulating attention via mindfulness induction improves p300-based brain-computer interface performance. J. Neural Eng. 2011;8:025019. doi: 10.1088/1741-2560/8/2/025019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfurtscheller G, Neuper C. Motor imagery and direct brain-computer communication. Proc. IEEE. 2001;89:1123–1134. [Google Scholar]
- 39.Wolpaw JR, Ramoser H, McFarland DJ, Pfurtscheller G. EEG-based communication: Improved accuracy by response verification. IEEE Trans. Rehabil. Eng. 1998;6:326–333. doi: 10.1109/86.712231. [DOI] [PubMed] [Google Scholar]
- 40.Neuper C, et al. Motor imagery and action observation: Modulation of sensorimotor brain rhythms during mental control of a brain-computer interface. Clin. Neurophysiol. 2009;120:239–247. doi: 10.1016/j.clinph.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Ross A, Thomas S. The health benefits of yoga and exercise: A review of comparison studies. J. Altern. Complement. Med. 2010;16:3–12. doi: 10.1089/acm.2009.0044. [DOI] [PubMed] [Google Scholar]
- 42.Upadhyay AK, Balkrishna A, Upadhyay RT. Effect of pranayama (voluntary regulated yoga breathing) and yogasana (yoga postures) in diabetes mellitus (DM): A scientific review. J. Complement. Integr. Med. 5(1) article 3. [Google Scholar]
- 43.Bower JE, et al. Yoga for cancer patients and survivors. Cancer Control. 2005;12:165. doi: 10.1177/107327480501200304. [DOI] [PubMed] [Google Scholar]
- 44.Kirkwood G, et al. Yoga for anxiety: A systematic review of the research evidence. Br. J. Sports Med. 2005;39:884–891. doi: 10.1136/bjsm.2005.018069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hebert R, et al. Enhanced EEG alpha time-domain phase synchrony during transcendental meditation: Implications for cortical integration theory. Signal Process. 2005;85:2213–2232. [Google Scholar]
- 46.Aftanas LI, Golocheikine SA. Non-linear dynamic complexity of the human EEG during meditation. Neurosci. Lett. 2002;330:143–146. doi: 10.1016/s0304-3940(02)00745-0. [DOI] [PubMed] [Google Scholar]
- 47.Schalk G, et al. BCI2000: A general-purpose brain-computer interface (BCI) system. IEEE Trans. Biomed. Eng. 2004;51:1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 48.He B, et al. Grand challenges in interfacing engineering with life sciences and medicine. IEEE Trans. Biomed. Eng. 2013;60:589–598. doi: 10.1109/TBME.2013.2244886. [DOI] [PubMed] [Google Scholar]