Summary
The relatively recent discovery of persistent adult neurogenesis has led to the experimental isolation and characterization of central nervous system neural stem cell populations. Protocols for in vitro analysis and expansion of neural stem cells are crucial for understanding their properties and defining characteristics. The methods described here allow for cell and molecular analysis of individual clones of cells—neurospheres—derived from neural stem/progenitor cells. Neurospheres can be cultivated from a variety of normal, genetically altered, or pathological tissue specimens, even with protracted postmortem intervals, for studies of mechanisms underlying neurogenesis, cell fate decisions, and cell differentiation. Neurosphere-forming cells hold great promise for the development of cell and molecular therapeutics for a variety of neurological diseases.
Keywords: Neural stem cells, subependymal zone, transplantation, neurogenesis, culture
1. Introduction
Until relatively recently, it was widely held, despite isolated reports to the contrary (1), that de novo generation of neurons in the mammalian central nevous system (CNS) did not persist past perinatal development. This perception drastically changed in the last decade of the twentieth century when it was determined that a persistent germinal zone containing neural stem cells (NSCs) with the capacity to differentiate into neurons and glia existed within the CNS of adult mammals (2). It is now known that constitutive in vivo genesis of neurons occurs throughout life, and it is restricted primarily to two specific regions: the periventricular subependymal zone (SEZ), which generates neurons destined for the olfactory bulb; and the subgranular zone (SGZ) of the hippocampus, which generates neurons destined for the dentate gyrus (3,4). Although the SEZ and SGZ are the regions of adult neurogenesis, recent studies have revealed that there may be additional, latent, pools of NSCs in other regions of the brain.
In vitro, NSCs can be propagated from a variety of rodent and human tissues, including cerebral cortex, SEZ, hippocampus, and spinal cord (5–7). Clones of NSCs can be cultivated either as monolayers of substrate-anchored cells (6,8) or as suspended, spherical structures called “neurospheres” (9,10). We describe a method that our laboratory has developed for the generation and study of neurospheres (5,11), which involves cultivating single-cell suspensions in the absence of cell–cell and cell–substrate interactions. This method is based on the theory that our culture conditions will allow for the clonal expansion of cells and that they will maintain cells in a primitive ontogenetic state, because substrate attachment is necessary for differentiation to occur.
Work in our laboratory generally involves postnatal mice (1–8 days), but neurospheres can be generated from differing ages and from a variety of CNS structures in mice. Because neurosphere yield from the SEZ is much higher than other structures, it is technically easier to make a discreet isolation of the SEZ compared with embryonic animals, thereby increasing the signal-to-noise ratio, and the dissociation procedure seems to be far gentler (compared with older animals that have already undergone significant myelination), resulting in better cell survival.
We also have demonstrated that it is possible to generate neurospheres from SEZ after extensive postmortem intervals by using the same methods (12). However, neurosphere yield declines precipitously if the brain is kept at room temperature. Storing the brain at 4°C dramatically lengthens the neurogenic potential of postmortem tissue such that it is possible to cultivate neurospheres almost 1 week after death.
A major benefit of our protocol is that neurospheres are easily manipulated and lend themselves to many different analyses, both individually and collectively. The plating density that we use is low enough to allow single neurospheres to be quickly removed with a hand-held pipettor. Once isolated, a neurosphere can be used for immunocharacterization or gene analysis, or it can be passaged to generate secondary neurospheres. Populations of numerous neurospheres are also suitable for these purposes and can, in addition, be used for ultrastrucural analysis, long-term cryostorage, or transplantation (see Fig. 4 and Subheading 3. for methods). Regarding preparation of neurospheres for transplantation, it is possible to start with a variety of transgenic animals or transfected cells that contain marker genes useful for subsequent discernment of donor versus host-derived cells.
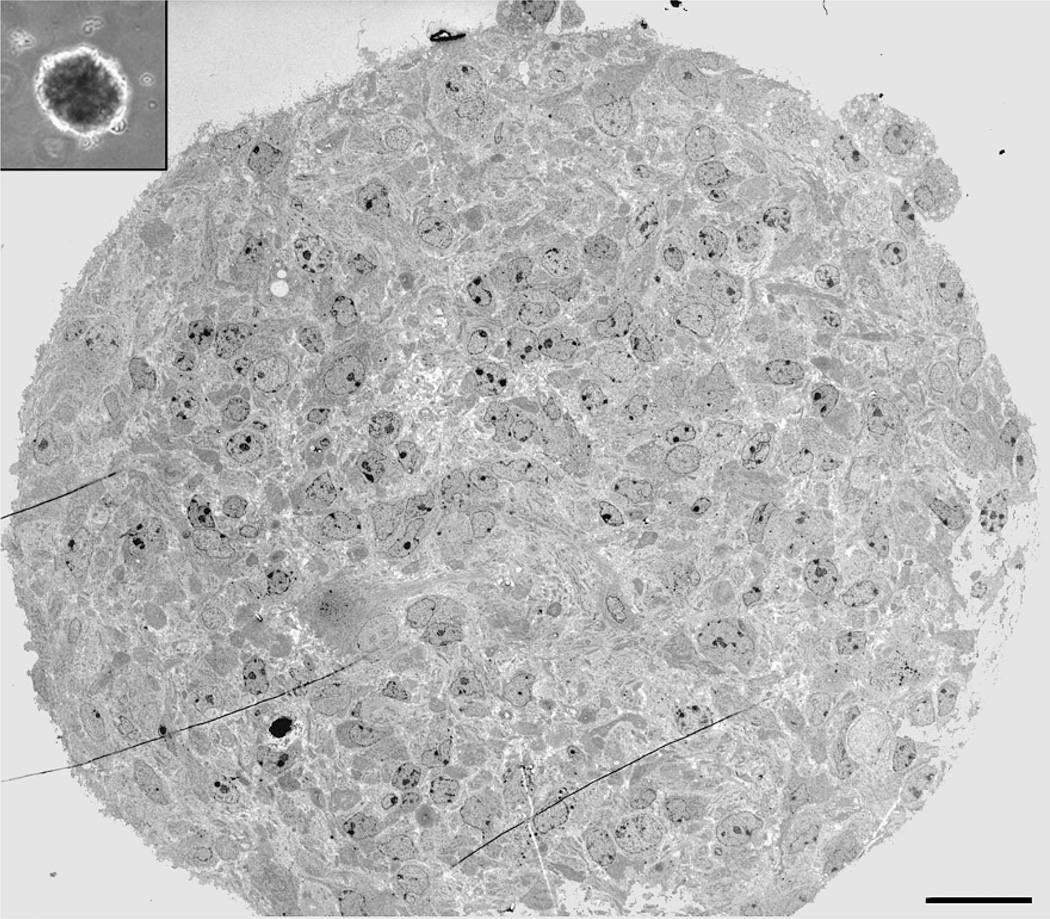
Fig. 4.
Ultrastructure of a single neurosphere. Inset contains a lower power phase micrograph of a representative neurosphere derived from surgical biopsy of adult human SEZ. Ultrastructural analysis of these neurospheres (obtained via transmission electron microscopy) reveals a diverse population of cells in different states of differentiation from a presumed stem/progenitor cell to differentiated neurons and glia (5). Bar = 30 µm.
Recent evidence has accumulated suggesting that glial cells have stem cell characteristics in vivo and that they may represent the neurosphere-forming cell in vitro. Specifically, certain astrocytes have been shown to undergo mitosis and to give rise to neuroblasts in the adult mouse SEZ (13). Furthermore, work from our laboratory has demonstrated that subpopulations of cultured mouse astrocytes (derived from a variety of CNS regions) can generate neurospheres in a regionally and temporally restricted manner (14). Astrocytes cultured from the cerebral cortex, cerebellum, and spinal cord can generate neurospheres when grown in the presence of growth factors, but only if these cultures are derived from animals younger than postnatal day 11. Astrocytes cultured from the SEZ can generate neurospheres when derived from both perinatal and adult animals. Furthermore, this cell population is amenable to SEZ transplantation and engraftment in a manner remarkably similar to neurosphere cell populations, with migratory, donor-derived progeny present in the rostral migratory stream (RMS) and olfactory bulb weeks after transplantation (see Fig. 3). The expandability of the adherent monolayer culture system is beneficial in that a significantly larger population of cells can be generated for transplantation in a relatively short period compared with the neurosphere culture system.
Fig. 3.
Characterization and use of neurospheres. A Phase microscopy of a single neurosphere attached to a coverslip for 5 days. A nearly confluent monolayer of cells has migrated away from the main mass of the neurosphere. B Immunofluorescence labeling of attached neurosphere cells reveals a population of β-III-tubulin–positive neurons. C Dissociated neurospheres derived from a GFP transgenic mouse are useful for transplantation because constitutive GFP renders the donor cells easily distinguishable from the host tissue, as seen in this fluorescent photographic montage of ×20 images of a transplant into the lateral ventricle of an adult C57BL/6 mouse 3 weeks after surgery. Bar = 100 µm in A.
2. Materials
2.1. Generation of Neurospheres from Acutely Dissociated SEZ
1× Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 (Invitrogen, Carlsbad, CA. cat. no. 12500-062).
N2 culture supplement (500× stock solution) containing pituitary extract (Sigma- Aldrich, St. Louis, MO, cat. no. P1476; 714 µl/500 ml, 10 mg total), putrescine (Sigma-Aldrich, cat. no. P5780; 8.06 mg/500 ml, 100 µM final), insulin (Sigma-Aldrich, cat. no. I5500), 2.5 mg/500 ml, 67.5 units), progesterone (Sigma-Aldrich, cat. no. P7556; 3.145 mg/ml, 0.22 mg/500 ml), transferrin (Sigma-Aldrich, cat. no. T1428; 12.5 mg/500 ml), and sodium selenite (Sigma- Aldrich, cat. no. S5261; 2.59 µg/500 ml, 30 nM final).
1× DMEM/Ham’s F-12 containing 5% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA, cat. no. S11150).
0.25% Trypsin-EDTA solution (Invitrogen, cat. no. 15405-012).
Ultralow attachment, anti-adhesive six-well plates (Corning Life Sciences, Acton, MA, cat. no. 3471).
Fire-polished Pasteur pipettes: prepare medium and narrow bore sets by briefly exposing the tip of the pipette to the flame of a Bunsen burner to narrow the lumen.
Add a cotton plug to the proximal end and autoclave before use.
15-ml Falcon tubes (TPP AG, Trasadingen, Switzerland, cat. no. TP91015).
-
Growth factor stock solution:
cultures require supplementation with 20 ng/ml epidermal growth factor (EGF; Invitrogen, cat. no. 13247-010) and 10 ng/ml basic fibroblast growth factor (bFGF) (Sigma-Aldrich, cat. no. F0291) every 2–3 days.
Because each culture well will contain approximately 2 ml of medium, we supplement with 50-µl aliquots of 40× stock (8,000 ng of EGF and 4,000 ng of bFGF in 10 ml of DMEM/Ham’s F-12).
Stock can be prepared more concentrated if desired, but we do not recommend a less concentrated stock, because correspondingly larger aliquots will quickly reduce the viscosity of the neurosphere cloning medium.
PBS or DMEM/Ham’s F-12 containing antibiotic/antimycotic (Sigma-Aldrich, cat. no. A9909).
2.2. Generation of Adherent Monolayers from Acutely Dissociated SEZ
PBS or DMEM/Ham’s F-12 containing antibiotic/antimycotic (Sigma-Aldrich, cat. no. A9909).
N2 culture supplement.
1× DMEM/Ham’s F-12 medium containing 5% FBS (Atlanta Biologicals).
0.25% Trypsin-EDTA solution (Invitrogen, cat. no. 15405-012).
15-ml Falcon tubes (TPP AG).
T-75 tissue culture flasks (TPP AG, cat. no. TP90076).
2.3. Immunolabeling
Standard small-volume pipettor with sterile tips.
12-well culture plates (TPP AG, cat. no. TP92412).
-
18-mm round coverglass (Fisher Scientific, Pittsburgh, PA, cat. no. 12-546) coated sequentially with poly-l-ornithine and laminin.
Prepare by incubating coverglass overnight at room temperature in H2O containing 10 mg/ml poly-l-ornithine (Sigma-Aldrich, cat. no. P4957).
Wash three times with H2O and incubate 8–10 h at 37°C in PBS containing 2.5 mg/ml laminin (Sigma-Aldrich, cat. no. L2020).
Wash three times with PBS.
Plates can be stored long-term in PBS at −20°C or short-term in PBS at 4°C.
Sterilize before use with PBS containing antibiotic/antimycotic and irradiate with an ultraviolet germicidal lamp.
1× DMEM/Ham’s F-12 containing 1% FBS.
Inverted phase microscope.
2.4. Ultrastructural Analysis
Embedding plastic (TAAB, Reading, UK).
Electron microscopy (EM) fixative: 0.1 M sodium cacodylate buffer (Electron Microscopy Sciences, Hatfield, PA, cat. no. 12300) containing 2% paraformaldehyde (Sigma-Aldrich, cat. no. P6148), 2% glutaraldehyde (Electron Microscopy Sciences, cat. no. 16350).
2% uranyl acetate (Electron Microscopy Sciences, cat. no. 22400) in 0.9% saline.
1% OsO4 (Electron Microscopy Sciences, cat. no. 19100) in PBS.
Small plastic microcentrifuge tubes.
Graded ethanols (50, 70, 80, 90, 100%).
2.5. Gene Analysis
Microtip sonicator.
0.6-ml tubes.
SuperScript reverse transcriptase (Invitrogen, cat. no. 18053-017), Standard reverse transcription-polymerase chain reaction (PCR) reagents, including: RNase-free water, RNase inhibitor, and RNase-H.
Solutions for sterilizing the microtip sonicator: 1 M HCl, 1 M NaOH, 1 M Tris-HCl, and double-distilled H2O (ddH2O).
2.6. Transplantation into Neonatal and Adult Mouse Brains
5-µl Hamilton syringe with attached 26-gauge needle (Hamilton, Reno, NV, cat. no. 84851).
Avertin (2.5% 2-2-2-tribromoethanol) anesthetic solution (Aldrich Chemical, Milwaukee, WI, T4 840-2) plus 1.5% tert-amyl alcohol, in H2O).
Surgical scalpel, forceps, and sutures.
Rodent stereotaxic apparatus.
3. Method
3.1. Generation of Neurospheres from Acutely Dissociated SEZ
The following protocol is the standard method our laboratory has developed to produce neurospheres from mouse and adult human brains (5,11). Any culture dish configuration can be used, but we prefer six-well plates because: they allow for multiple experimental manipulations of the same sample, they lend themselves to rapid visual screening (without the optical interference common to plates with a smaller well diameter), potential infections are contained within single wells, and infections can be removed without sacrificing the entire sample.
Decapitate mouse pup and briefly dip the head in ethanol (EtOH).
Remove the brain and place it on a clean surface suitable for cutting.
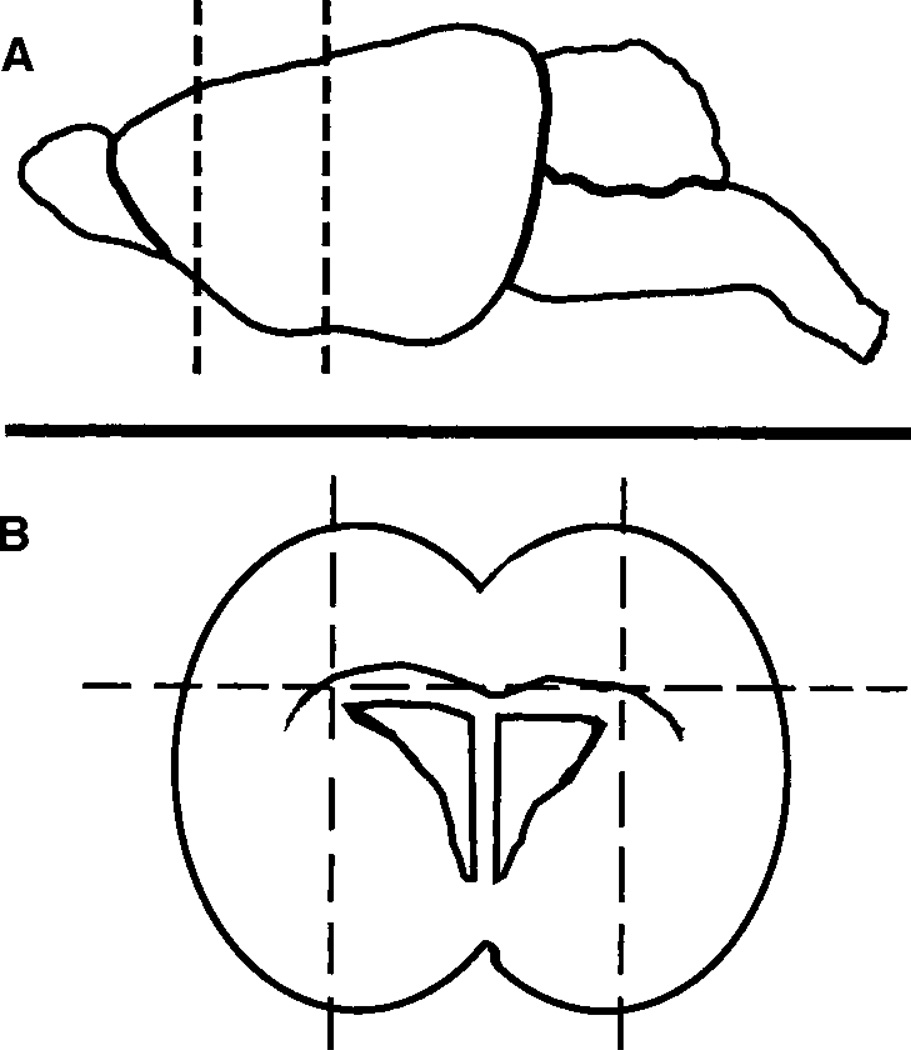
With a razor blade, make a coronal block, about 2 mm in thickness, in the area between the rhinal fissure and the hippocampus (see Fig. 1A). Lay the block flat on the cutting surface and use the razor blade to make two parasagittal cuts just lateral to the lateral ventricles, and a horizontal cut to remove the tissue above the corpus callosum (see Fig. 1B). This procedure leaves a small, rectangular chunk of tissue surrounding the lateral ventricles containing a high density of NSCs.
Wash the tissue chunk for several minutes in medium or PBS containing antibiotics/antimycotics. All subsequent work should be performed with sterile materials in a laminar flow hood.
Remove antibiotics/antimycotics and incubate tissue in trypsin-EDTA solution at 37°C for 5 min.
Gently triturate tissue through a series of descending-diameter, fire-polished Pasteur pipettes to make a single-cell suspension.
Add several volumes of DMEM/Ham’s F12 containing 10% FBS. Centrifuge to pellet cells and wash several times with fresh medium.
Count cells using a hemacytometer.
In a 15-ml Falcon tube, combine 6 ml of DMEM/Ham’s F-12 medium, 60,000 cells, and 50 µl of 40× growth stock. Add neurosphere cloning medium to bring the final volume to 12 ml.
Mix for several minutes by repeatedly inverting the tube.
Distribute 2 ml to each well of a six-well plate coated previously with antiadhesive. The final cell density will be about 1,000 cells/cm2, although the viscosity of the cloning medium makes precise volumetric measurements diffuse.
Add 50-µl aliquots of 40X growth factor stock every 2–3 days. Neurospheres will be visible under phase optics after 7–10 days. True neurospheres are characterized by near perfect spherical shape as well as very sharp, phase-bright outer edges. Importantly, individual cells should not be seen with low-power phase optics (see Note 3 and Fig. 2).
Fig. 1.
Schematic of the dissection protocol. Starting with whole brain, make two coronal cuts in the area between the rhinal fissure and the hippocampus (broken lines; A). Lay the resulting chunk on its posterior surface, and make two parasagittal cuts just lateral to the lateral ventricles, and one horizontal cut at about the level of the corpus callosum (broken lines; B). Make neurospheres by dissociating the central, rectangular piece of tissue containing the lateral ventricles.
Fig. 2.
Phase micrographs of neurospheres in suspension culture. A Sphereforming cells immediately after plating (×10). B,C After 10–14 days, neurospheres are approaching their greatest diameter, and they look like large globes with sharp, phase-contrast bright outer borders. Bar = 40 µm in B and 150 µm in C.
3.2. Generation of Adherent Monolayers from Acutely Dissociated SEZ
The following protocol describes our method of generating astrocytic monolayers that can subsequently be used to generate multipotent neurospheres. Once monolayers are established, they can be replated under neurosphere-generating conditions, as described above, where 1–10% of plated cells form neurospheres.
Decapitate mouse pup and briefly dip the head in EtOH.
Remove the brain and place it on a clean surface suitable for cutting.
Use a razor blade or microknife to isolate your CNS area or interest (e.g., SEZ; see Subheading 3.1.).
Wash tissue briefly in a 15-ml Falcon tube containing medium or PBS with antibiotics/antimycotics.
Remove antibiotics/antimycotics and incubate tissue in 5–10 ml of trypsin-EDTA solution for 37°C for 5 min.
Triturate with a 5-ml serological pipette for 2–3 min to break up the tissue into small chunks. It is not necessary to make a single-cell suspension.
Add 1–2 ml of FBS to neutralize trypsin and centrifuge cells to form a pellet.
Aspirate supernatant and wash by trituration with fresh medium. Pellet and repeat three to four times.
Resuspend in DMEM/Ham’s F-12 medium containing 10% FBS, plate in T-75 culture flasks (use one flask for each brain), and place in incubator overnight.
Remove the culture supernatant and replate into fresh T-75 flasks (discard original flasks which contain primarily microglia).
Replace medium every 2–3 days with fresh DMEM/Ham’s F-12 containing 10% FBS until astrocytic monolayers become confluent.
Remove astrocytes from flasks by aspirating culture supernatant and incubating in trypsin-EDTA for 5–10 min.
Collect cells in a Falcon tube, add serum to neutralize trypsin, and proceed with step 8 (see Subheading 3.1.).
3.3. Immunolabeling
The following protocol is our standard method for immunolabeling neurospheres (see Note 4) after they have attached to a favorable substratum and have begun to migrate and differentiate.
Place coated coverslips in 12-well plates and put a drop (50–100 µl) of medium near the center of each. Keep in a laminar flow hood.
Remove the cover from a six-well plate containing neurospheres and visualize with the inverted microscope (contamination of wells is rare, even though the plate is opened outside the hood, but wash the microscope and pipettor with EtOH first).
While looking through the microscope, guide the tip of the pipettor set for 2–5 µl to the neurosphere, and aspirate it into the tip.
Eject the neurosphere into the medium on the coverslip. Repeat as often as desired. We typically place 2–10 neurospheres on each coverslip.
Place 12-well plates in an incubator. Neurospheres should be attached firmly to the coverslip by the next day, at which time they can be fixed or left to cultivate for a longer period. If the neurospheres are to be cultured for more than 1–2 days, it is important to carefully flood the coverslip with fresh medium after attachment has taken place to prevent evaporation of the media.
Wash, fix, and process coverslips for standard immunolabeling, scanning EM, or both (see Fig. 3).
3.4. Ultrastructural Analysis
We have developed a method for generating electron micrographs of suspended neurospheres. Owing to the need for visually tracking the sample during processing, this method does not allow for the ultrastructural analysis of a single, prospectively identified neurosphere, but rather requires that a large number of neurospheres be processed together before retrospectively choosing individual examples to section and analyze.
Liquefy 2% agar by placing in water bath (85°C).
Use a transfer pipet to pool several hundred neurospheres in a 15-ml Falcon tube.
Centrifuge to pellet neurospheres. Aspirate medium and gently resuspend in EM fixative. Incubate for 30 min at room temperature.
Wash two to three times by gently pelleting and resuspending in PBS.
After a final pelleting, aspirate as much PBS as possible. Resuspend in a small volume (20–50 µl) of PBS and transfer to a plastic microcentrifuge tube. Quickly add an equal volume of melted agar to the neurospheres and mix gently (work quickly so that the agar does not solidify before being mixed with the neurospheres). Place tube at 4°C for 15 min to harden agar.
Cut off the tip of the tube with a razor blade, and use a small spatula to pry the agar plug out. Place the plug into OSO4 for 2 h at room temperature; the osmium will turn the neurospheres brown, rendering them apparent to the naked eye.
Rinse for 30 min at room temperature in H2O and then place into uranyl acetate for 1 h.
Dehydrate through graded alcohols, place in embedding plastic, and section for standard transmission EM (see Fig. 4).
3.5. Gene Analysis
Gene profiling (see Note 6) of large numbers of pooled neurospheres can be performed using standard techniques for RNA isolation. However, sometimes there may be a desire to examine transcripts present in an individual neurospheres. Because neurospheres consist of at most, several thousand cells, and because these cells are embedded in a dense extracellular matrix, RNA extraction can be tricky, and the normally low RNA yields can be lost if subjected to the additional step of RNA isolation. To address these problems, we have developed a method that combines sonication and RT-PCR without RNA isolation (15). All procedures must be performed in the same microcentrifuge tube, and the results are much better than those obtained with extraction by either the guanidine cyanide method or freeze-thawing, both of which lead to significant loss of material.
Place a single neurospheres in a 0.6-ml tube containing 10 µl of RNase-free water with 5 U of RNase inhibitor. Keep tube on ice.
Release RNA by sonicating with microtip sonicator tip (Kontes Glass, Vineland, NJ) by gently touching the surface of the water for 4–10 s. Immediately put the tube back on ice.
If working with multiple samples, sequentially wash the sonicator tip between samples in ice-cold 1 M HCl, 1 M NaOH, 1 M Tris-HCl, pH 7.5, and ddH2O.
Perform first strand cDNA synthesis by using SuperScript reverse transcriptase (Invitrogen) according to the manufacturer’s instructions.
Add 4 U of RNaseH to remove the cDNA:RNA hybrid.
This solution is now ready to use as template in standard PCR reactions optimized for each primer set.
3.6. Transplantation into Neonatal and Adult Mouse Brains
We routinely transplant dissociated neurospheres derived from transgenic (green fluorescent protein [GFP]) mouse SEZ into the brains of both adult and neonatal C57BL/6 mice in a variety of structures (SEZ, RMS, cortex, hippocampus, and cerebellum). Although the adult host model is a relevant system for the analysis of adult neurogenesis and regeneration, the neonatal model is an attractive alternative for the analysis of donor cells, because engraftment is significantly more robust in the brains of early postnatal mice (presumably because residual CNS development persists in the days after birth in the mouse).
Use a transfer pipette to pool several hundred neurospheres in a 15-ml Falcon tube.
Centrifuge to pellet neurospheres. Aspirate medium and resuspend in 2 ml of trypsin-EDTA. Incubate at 37°C for 5 min.
Neutralize trypsin by adding approximately 0.5 ml of FBS via a small bore fire-polished pipette. Using the same pipette (the FBS will have coated the inner wall of the pipette and has been found to prevent the trypsinized cells from adhering to the glass), triturate the neurospheres 20 times, or until a majority of the visible clumps are no longer visible.
Wash cells in N2 media and then centrifuge to pellet.
Determine cell number using a hemocytometer or equivalent technique, then resuspend in a volume of yielding 50,000 cells/µl DMEM/Ham’s F-12.
Recipient mice should be anesthetized (we prefer to use Avertin; see Subheading 2.6.), placed into a stereotaxic apparatus, the scalp surgically exposed, and the dura exposed at the required location. Cells (100,000; 2 µl) can be stereotaxically injected into the lateral ventricle via a 5-µl Hamilton syringe attached to a 26-gauge needle at the following coordinates: A-P, −0.2; M-L:, −1.2; and H-D, −2.5. Rostral migratory stream injections can be stereotaxically performed at the following coordinates: A-P, 3.0; M-L, 0.8; and H-D, 3.0.
Allow transplanted animals to recover and then return to general housing.
For neonatal animals, the transplant technique is somewhat cruder than for adults, and it lacks stereotaxic accuracy. Nevertheless, it is not difficult to direct cells to the large lateral ventricles. As before, load 100,000 dissociated neurosphere-derived cells into a 5-µl Hamilton syringe attached to a 28-gauge needle. Host pups can be cryoanesthetized by placing them at −20°C for 5 min, and cryoanesthesia is maintained during transplantation by placing the animal on a prechilled block. Using the bregma cranial suture as a landmark, the cells can be injected at the desired locale simply by inserting the needle into the skull (we find that the neonatal skull is soft enough for this procedure until around postnatal day 3).
Once the desired time for engraftment has been reached, sacrifice transplanted animals via trans-cardiac perfusion using 4% paraformaldehyde. After perfusion, decapitate the animal and remove the brain in its entirety, taking care to leave no pertinent CNS structures behind. Postfix brain(s) overnight in 4% paraformaldehyde then section the tissue in the desired plane and thickness by using a Vibratome or equivalent tissue sectioning equipment.
Sections can be processed for immunohistological analysis or directly mounted to glass slides for fluorescent and/or confocal microscopy (see Fig. 3).
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke grants NS37556 and HL70143 (to D.A.S.) and NS056019 (to E.D.L.).
Footnotes
We feel it is important to point out that the neurosphere protocol described in this chapter is not for strict clonal analysis. Should the goal of the experiment be to determine the clonal properties of NSCs, the researcher would be best served by plating single cells from primary neural tissue in individual microwells of a 96-well plate or similar cell sequestering protocol.
In addition to issues of clonality, the subject of neurospheres as an in vitro manifestation of NSCs and as a model for NSC biology needs to be addressed. As with other tissue-specific stem cells, NSCs are expected to demonstrate three cardinal features: clonal expansion, multilineage differentiation, and extensive self-renewal. The first two features leave little room for interpretive differences. Clonal expansion has a single definition: all cells within a neurosphere are the progeny of a single NSC founder cell. Although it is certainly possible, in highdensity cultures, to grow mixed, polyclonal spherical structures that resemble clonal neurospheres, few would disagree that at least the capacity for clonal expansion is a requisite feature of neurosphere if they truly do represent NSCs. Likewise, multilineage differentiation is fairly unambiguous. Neurospheres must be capable of generating progeny belonging to all three neural lineages, and this is generally assayed by phenotypic immunolabeling. Again, although it may be possible to experimentally manipulate the fate of neurospheres progeny by directing differentiation toward one or another lineage, the capacity for multilineage differentiation, whether realized or not, is an essential feature of true neurospheres. It is the third essential NSC feature—extensive self-renewal—that tends to be the most variable and poorly defined as it applies to neurosphere formation (16,17). What does extensive self-renewal mean? In many studies, neurospheres are dissociated and recultured to generate a number of secondary NSCs. This is clearly evidence of self-renewal, but does it constitute the extensive self-renewal that should characterize NSCs? This dilemma has yet to be conclusively resolved, but there are currently studies underway that may allow for the accurate analysis of NSC activity in vitro using the neurosphere assay (17).
- False neurospheres: Undissociated tissue pieces can, over time, begin to resemble spheres, which is why it is important to begin with single-cell suspensions. However, these pieces do not have sharply defined outer borders, and they are only rarely spherical enough to be easily confused with true neurospheres. It should be possible to eliminate these pieces from your culture by more thorough trituration with smaller diameter pipettes. Additionally, you can allow the dissociated cell suspension to sediment for several minutes, which permits the larger, undissociated chunks to settle to the bottom of the tube. The upper portion can then be transferred to a new tube, added to cloning medium, and be plated. We have observed that single, dissociated cells can clump together to form aggregates that resemble neurospheres. These cells, too, lack sharp outer edges, and it should be easy to discern individual cells within the mass using phase optics. If aggregation is a problem, try plating at a lower density.
- Infection. You may, from time to time, encounter infected wells. Use a repeating pipettor when applying growth factor aliquots, because this will minimize the number of times you need to open the growth factor stock solution. Remember to sterilize your tools and cutting surface with EtOH and to flame before each dissection. Micrococcus infections readily originate from the skin of the donor animal, so take care to thoroughly wash the head in EtOH before removing the brain.
-
Low neurosphere yield. This protocol typically yields dozens of neurospheres in each well, depending on the age of the animal. If you want to increase your yield, try making a cleaner dissection by removing more of the tissue surrounding the SEZ; the less of these other tissues (e.g., striatum, cortex), the greater the percentage of plated cells that will generate neurospheres. It is also possible to plate at a higher cell density, but beware that too high a density will increase the likelihood of forming nonclonal aggregates.During the dissociation, take care not to triturate too harshly as to lyse the cells. Determine empirically the largest bore pipette that results in a single cell suspension. Also, do not overincubate the tissue in trypsin, as this will eventually lead to cell death.Finally, it is possible to subclone primary neurospheres by dissociating and recloning them. A single dissociated neurospheres typically will give rise to 5–15 secondary neurospheres. Dissociation can be performed by collecting neurospheres in a tube containing trypsin-EDTA, and triturating with a smallbore pipette. The resulting cell suspension can then be plated in cloning medium as in Subheading 3.1.7.
- Attachment of cells to the culture dish. Occasionally, cells will attach and differentiate on the bottom of culture dishes that have been coated with anti-adhesive, possibly due to cracks or abrasions in the plastic. These attached cells are apparent under phase optics, and they can, in sufficient numbers, form a favorable substrate for the attachment and differentiation of neurospheres. If significant numbers of cells are seen attaching to the dish surface, the remaining suspended cells and neurospheres should be collected and transferred to a new plate.
Poor attachment of neurospheres to coverslip. It is not uncommon for a small percentage of neurospheres to not readily attach. Allowing more time for attachment (up to 48 h) often solves this problem. In general, a neurospheres that has not attached after 48 h will never attach. Increasing FBS to 5–10% usually improves attachment; however, higher serum levels alter differentiation. It may be worthwhile to increase serum concentration to facilitate attachment, and decrease it again after differentiation and migration occurs.
Also, very young neurospheres do not attach readily. Avoid plating neurospheres that have been in culture less than 7 days, and choose those only >50 µm in diameter.
Finally, apply and aspirate solutions (e.g., PBS, fixative) slowly and gently to avoid dislodging lightly attached neurospheres.
Contamination of six-well plates. If your plate often becomes infected during the process of removing neurospheres, be sure that you minimize your work directly over the open plate. Maintaining the pipettor at a steep angle as you approach a neurosphere will help. If possible, work in a small room that can be exposed to a germicidal lamp for several minutes before use.
Low RNA yield. This method normally yields enough RNA from a single neurosphere to serve as a template for 30–40 PCR runs by using primers for high abundance genes (e.g., housekeeping genes). If you have trouble achieving this level, you may need to adjust the sonication protocol. Sonicating for too long will increase the sample temperature, which increases RNase activity and can lead to reduced yield; too little sonication will not effectively release RNA, again leading to low yield. If you are still unable to sufficiently increase yield, or if your primer set is designed to reveal low-abundance transcripts, it may be necessary to amplify the RNA sample after sonication.
References
- 1.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla A, Lois C. Neuronal stem cells in the brain of adult vertebrates. Stem Cells. 1995;13:263–272. doi: 10.1002/stem.5530130307. [DOI] [PubMed] [Google Scholar]
- 3.Gage F, Ray J, Fisher L. Isolation, characterization, and use of stem cells from the CNS. Annu. Rev. Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 4.McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 5.Kukekov VG, Laywell ED, Suslov ON, et al. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp. Neurol. 1999;156:333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 6.Palmer TD, Markakis EA, Willhoite AR, Safar R, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the CNS. J. Neurosci. 1999;109:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheffler B, Horn M, Bluemcke I, Kukekov V, Laywell ED, Steindler DA. Marrow-mindedness: a perspective on neuropoiesis. Trends. Neurosci. 1999;22:348–357. doi: 10.1016/s0166-2236(99)01416-2. [DOI] [PubMed] [Google Scholar]
- 8.Richards LJ, Kilpatrick TJ, Bartlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc. Natl. Acad. Sci. USA. 1992;89:8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kukekov VG, Laywell ED, Thomas LB, Steindler DA. A nestin-negative precursor cell from the adult mouse brain gives rise to neurons and glia. Glia. 1997;21:399–407. doi: 10.1002/(sici)1098-1136(199712)21:4<399::aid-glia7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Laywell ED, Kukekov VG, Steindler DA. Multipotent neurospheres can be derived from forebrain subependymal zone and spinal cord of adult mice after protracted postmortem intervals. Exp. Neurol. 1999;156:430–433. doi: 10.1006/exnr.1999.7029. [DOI] [PubMed] [Google Scholar]
- 13.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 14.Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc. Natl. Acad. Sci. USA. 2000;97:3883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suslov ON, Kukekov VG, Laywell ED, Scheffler B, Steindler DA. RT-PCR amplification of mRNA from single brain neurospheres. J. Neurosci. Methods. 2000;96:57–61. doi: 10.1016/s0165-0270(99)00177-6. [DOI] [PubMed] [Google Scholar]
- 16.Seaberg RM, van der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci. 2003;26:125–131. doi: 10.1016/S0166-2236(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds BA, Rietze RL. Neural stem cells and neurospheres-reevaluating the relationship. Nat. Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]