Abstract
Purpose
3’-[F-18]Fluoro-3’-deoxythymidine (FLT) is an analog of thymidine that is being developed for imaging cellular proliferation. The goal of this study was to prove that the dose of FLT used for positron emission tomography imaging produces no significant toxicity.
Procedures
Twelve patients with gliomas with either recurrence or suspected radionecrosis were imaged with FLT. Before and at several time points after imaging, subjects underwent general physical and neurological examinations with review of systems and tests of hematologic, hepatic, renal, and several other metabolic parameters. Vital signs and electrocardiograms were monitored during and after the imaging session.
Results
There were no significant adverse effects from FLT injected at a dose of 0.07 mCi/kg (maximum of 5 mCi) at specific activities of 1.25 Ci/µmol or higher. The FLT mass administered for imaging was 0.0001% to 0.0009% of the least toxic cumulative dose administered in clinical trials of FLT as an antiretroviral agent.
Conclusions
FLT is a safe radiotracer for quantifying proliferation in the human cancer setting.
Keywords: 3’-[F-18]fluoro-3’-deoxythymidine, FLT, Fluorothymidine, Positron emission tomography (PET), Glioma, Glioblastoma, Safety, Toxicity, Proliferation
Introduction
3’-[F-18]Fluoro-3’-deoxythmidine (FLT) is a radiolabeled structural analog of the DNA constituent thymidine that is being developed for imaging cellular proliferation with positron emission tomography (PET) [1, 2]. Although FLT is not incorporated into DNA, it is trapped in the cell due to phosphorylation by thymidine kinase-1 (TK-1), via the pyrimidine nucleoside salvage pathway. TK-1 is up-regulated before and during the DNA synthesis phase of the cell cycle such that FLT has the potential to image tumor proliferation in proportion to DNA synthesis through this pathway [3]. In the normal brain, FLT is not taken up because there is little, if any, exchange across an intact blood–brain barrier, and cellular proliferation is extremely low. This gives FLT a distinct advantage for imaging brain tumors compared to 2-deoxy-2-[F-18]fluoro-d-glucose, as it should only be concentrated in proliferating tumor cells and not in normal brain. As a marker for proliferation, FLT-PET imaging may prove to be useful for assessing response of tumors to therapy and for improving the capacity of PET to differentiate between radionecrosis and recurrence after therapy.
The pharmacology of FLT is based on its action as an inhibitor of DNA synthesis [4–7]. Intracellular metabolism of FLT produces FLT phosphates, but these nucleotides inhibit endogenous DNA polymerases because they lack the 3’-hydroxyl substituent required for chain propagation. This results in premature chain termination for DNA and accounts for the prominent hematological and liver toxicity that has been reported from investigations of FLT as an antiretroviral drug in humans [8–11]. Depending on the cumulative drug exposure, significant anemia, granulocytopenia, and peripheral neuropathy were reported at doses of 0.125 mg/kg or lower every 12 h [9]. The unexpected death of two subjects from hepatic failure lead to termination of FLT treatment trials.
Numerous papers have been published on the use of FLT to image proliferation in tumors; yet, there is only one report on the safety of this tracer at the doses used for PET imaging [12]. These investigators studied 20 patients with lung cancer. Comprehensive metabolic panel, total bilirubin, complete blood, and platelet counts were collected from each patient at multiple times before and after FLT-PET. In addition, a standard neurological examination was performed for each patient before and immediately after FLT-PET. No side effects were reported by the patients. No changes were observed in the neurological status of patients. Only albumin, red blood cell count (RBC), hemoglobin (Hgb), and hematocrit (Hct) showed statistically significant decreases over time. These were attributed to IV hydration during PET imaging and to subsequent blood loss at surgery performed after the PET session.
The intent of the present investigation was to confirm the safety of FLT administered in diagnostic radiotracer amounts and to explore preliminarily its capacity to distinguish radionecrosis in gliomas from recurrent disease (Spence et al., in preparation). A comparison between the FLT dose administered for imaging and the doses used in clinical trials of FLT as an antiretroviral drug will be presented.
Materials and Methods
Patients
Twelve adult patients were included in this safety analysis (male, ten; female, two; age range, 36, 64): two glioblastoma multiforme, one gliosarcoma, four anaplastic astrocytoma, two anaplastic oligodendroglioma, and three grade 2 oligodendroglioma (Table 1). All tumors were graded by the World Health Organization (WHO) grading scheme. The weight range of the patients was 65 to 118 kg.
Table 1.
Age, sex, pathology, and location of tumor
| Number | Age (Sex) | Pathology (location)1 |
|---|---|---|
| 1 | 62 M | GM (left temporal) |
| 2 | 44 M | Ogo 2 (right temporal) |
| 3 | 47 M | Ogo 2 (right frontal) |
| 4 | 54 M | GM (left thalamus) |
| 5 | 50 M | AA (right frontal) |
| 6 | 54 M | GS (right frontal) |
| 7 | 47 F | AA (left frontal) |
| 8 | 37 F | AA (right frontal) |
| 9 | 40 M | AA (left frontal) |
| 10 | 62 M | AO (right temporal) |
| 11 | 36 M | AO (right temporal) |
| 12 | 50 M | Ogo 2 (left parietal) |
GM Glioblastoma multiforme, GS gliosarcoma, AA anaplastic astrocytoma, Ogo oligodendroglioma, AO anaplastic oligodendroglioma
These studies were conducted under an investigational new drug (IND) sponsored by the National Cancer Institute. The studies were approved by the University of Washington Human Subjects Committee, Fred Hutchinson Cancer Research Center IRB, and the University of Washington Radiation Safety Committee. Each patient signed informed consent.
Patient Eligibility
Enrollment criteria required all subjects to have a glioma treated previously with radiotherapy with or without chemotherapy. All 12 patients had conventional external beam radiotherapy with doses ranging from 55.8 to 70 Gy. Two patients had radiosurgery, and one had brachytherapy. Eleven patients had chemotherapy with temozolomide and/or a nitrosourea-containing regimen. Patients could not enroll if there were clinically significant signs of uncal herniation, such as acute pupillary enlargement, rapidly developing motor changes (over hours), or rapidly decreasing level of consciousness.
Female patients were required to be postmenopausal for a minimum of 1 year or be surgically sterile or on a reliable method of birth control for a minimum of 1 month before the PET scans. Negative pregnancy test was required for reproductively capable women.
The following laboratory tests were required within 14 days before the PET studies: liver enzymes [aspartate aminotransferase (AST), alanine aminotransferase (SGPT), alkaline phosphatase (ALK Phos), gamma glutamyl transferase (GGT), and lactate dehydrogenase (LDH)], bilirubin (direct and total), total protein, albumin, haptoglobin, amylase, serum electrolytes, calcium, phosphate, complete blood count with platelet and absolute neutrophil counts (ANC), prothrombin time, partial thromboplastin time, blood urea nitrogen (BUN), creatinine, and urinalysis. These were required to be within normal limits for age with the following exceptions. The GGT could be no greater than five times the institutional upper limit of normal (ULN). The other liver enzymes (SGOT, SGPT, ALK Phos, and LDH) could be no greater than two times the institutional ULN. The white blood cell count (WBC), ANC, and platelets were required to be greater than 3.0, 1.5, and 75,000/µl, respectively, and hemoglobin greater than 10 g/dl. All of these tests were repeated at approximately 3 h, 24 h, and 1 month after the FLT-PET scan.
All patients were required to undergo a physical and complete neurological examination by a board-certified neurologist at four time points: on the day of FLT scan, just before the FLT scan, immediately after the FLT scan, 24 h after, and 1 month later. This included assessment of higher integrative functions, cranial nerves, and motor, reflex, sensory, and coordination functions. All were required to submit to a review of systems after the FLT scan, 24 h later, and at 1 month after exposure to FLT. This included inquiries about abdominal, chest, or other pain, fever, injection site reaction, flushing, nausea, diarrhea, vomiting, dyspnea, rash, itching, hives, sweating, cyanosis, visual disturbance, numbness of fingers or toes, muscle weakness, or burning sensations.
FLT Synthesis
The 3’-deoxy-3’-[18F]fluorothymidine used in this study was prepared locally by the PET Radiochemistry Group at the University of Washington where this radiopharmaceutical was first developed. The precursors for the radiosynthesis included 18F prepared at the University of Washington RDS Eclipse cyclotron from proton irradiation of [18O]water and an organic precursor, 5’-O-benzoyl-2,3’-anhydrothymidine, supplied by ABX through NCI/CIP exclusively for this radiosynthesis. The other reagents used in the synthesis were potassium carbonate (99.99%), anhydrous dimethylsulfoxide, anhydrous acetonitrile, and kryptofix [2.2.2] (a phase transfer agent) all obtained from Sigma Aldrich and USP absolute ethanol, USP sodium phosphates for dilution, USP sterile water for injection, and USP saline for injection. The final FLT product contained <175 endotoxin units per dose (endotoxin levels were always below detectable limits), <5,000 ppm each of acetone and dimethyl sulfoxide and <400 ppm of acetonitrile, <50 µg/ml Kryptofix [2.2.2.], and <6.1 µg of FLT. The mass and purity of the FLT were measured using high-performance liquid chromatography (HPLC) with mass spectrometry and 254 or 266 nm UV absorbance and radiation detection. The HPLC system used was Aquasil C18 [2.1 × 150 mm (5 µm)] column, eluted with 14% methanol/86% water (v/v). Silica Gel TLC was also used to measure radioactive purity. The radiopharmaceutical product was a clear and colorless liquid that was stored at room temperature in a sterile serum vial. The average product at the end of synthesis had a specific activity of 133,000 GBq/mmol at injection (range, 46,000 to 370,000 GBq/mmol) and could be used for several patients. The FLT had an expiration time of 8 h after sterile filtration or when the GBq/mmol or MBq/ml fell below the specified limits, 7,400 and 18.5, respectively.
Patient Monitoring During Imaging
Vital signs were monitored and recorded closely during the FLT imaging procedures at baseline, during infusion at 5, 10, 15, 20, 30, 60, 90, and 120 min, and then approximately 3 and 24 h after the infusion. These included blood pressure, pulse, temperature, respiratory rate, and oxygen saturation. The electrocardiogram was recorded continuously starting before the infusion and running through the whole imaging procedure.
FLT Imaging Protocol
This followed exactly the procedure reported by Muzi et al. [13]. Briefly, FLT doses were calculated based on patient weight (2.69 Mbq/kg, 0.07 mCi/kg) with a maximum dose of 185 Mbq (5 mCi). The lowest specific activity allowed for injection was 6.1 µg per 5 mCi, which is 0.24 Ci/µmol. Table 2 shows the specific activity, millicuries injected and micrograms injected per patient. FLT was administered IV over 1 min using an infusion pump. Dynamic PET acquisition was carried out for 90–120 min. A radial artery line was inserted to sample arterial blood for determination of radioactivity and plasma metabolite analysis. Arterial samples were obtained at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 5, 6, 7, 8, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, and 90 ± 120 min using an automated blood sampler [14]. Three patients had hand-drawn arterial samples at the following time points: 1, 2, 3, 5, 10, 15, 20, 30, 45, 60, 90, and 120 min. All subjects received 500 ml of normal saline intravenously over the course of the PET scans. The blood volume removed by blood sampling and venipuncture for lab tests after FLT imaging was approximately 160 cc. The amount of heparinized saline injected to prevent clotting of the arterial line was 130 cc.
Table 2.
For each subject the details of the FLT injected for imaging
| Patient no. | Specific activity Ci/mmol at injection |
mCi injected | µg FLT in dose at time of injection |
µg FLT/kg in dose at time of injection |
AUC12 ng.h/ml |
|---|---|---|---|---|---|
| 1 | 2,630 | 4.59 | 0.43 | 0.0039 | 0.012 |
| 2 | 2,390 | 4.76 | 0.49 | 0.0057 | 0.021 |
| 3 | 2,590 | 4.16 | 0.41 | 0.0048 | 0.009 |
| 4 | 2,900 | 5.19 | 0.49 | 0.0060 | 0.018 |
| 5 | 4,320 | 4.69 | 0.27 | 0.0036 | 0.007 |
| 6 | 1,250 | 4.72 | 0.99 | 0.0145 | 0.035 |
| 7 | 2,100 | 4.84 | 0.57 | 0.0078 | 0.030 |
| 8 | 5,150 | 4.51 | 0.22 | 0.0030 | 0.013 |
| 9 | 10,000 | 4.88 | 0.13 | 0.0016 | 0.004 |
| 10 | 2,190 | 4.88 | 0.59 | 0.0067 | 0.026 |
| 11 | 4,830 | 4.9 | 0.27 | 0.0023 | 0.009 |
| 12 | 2,780 | 4.79 | 0.43 | 0.0066 | 0.008 |
| Average | 3,594 | 4.74 | 0.44 | 0.0056 | 0.016 |
| SD | 2,330 | 0.25 | 0.22 | 0.0034 | 0.010 |
| Range | 1,250–10,000 | 4.16–5.19 | 0.13–0.99 | 0.0016–0.0145 | 0.004–0.035 |
The PET studies were performed on a GE Advance PET tomograph (GE Medical Systems, Waukesha, WI, USA) providing 35 image planes over a 15-cm axial field of view with a 4.25-mm slice spacing [15]. Images were acquired in 3D mode with the following dynamic sequence: 10 × 10 s, 4 × 20 s, 3 × 40 s, 3 × 1 min, 5 × 2 min, 4 × 3 min, and 12 × 5 min or 18 × 5 min time frames for a total duration of 90 or 120 min. After correction for scatter and random coincidences, images were reconstructed by the method of 3D re-projection using 4.5-mm radial and 6-mm axial smoothing filters (Hanning) resulting in approximately isotropic image resolution of about 6 mm [16].
Blood Sampling and Metabolite Analysis
For each arterial blood sample, 0.2 ml plasma was assayed for radioactivity using a Cobra gamma counter (Packard Corp, Meriden, CT, USA). Since FLT has negligible serum protein binding [17], all of the activity associated with FLT in blood was assumed to be available for tissue uptake. An aliquot (0.4 ml) of the plasma from eight arterial samples (5, 10, 15, 20, 30, 45, 60, and 90 min) was assayed for the relative amount of FLT and FLT-glucuronide as previously described [18]. The fraction of total activity present as FLT in each blood sample was fitted to a mono-exponential curve to provide a continuous function describing the fraction of the total plasma activity associated with FLT versus metabolites [18].
In the report of Flexner et al., the FLT dosing regimens were reported as the area under the blood concentration curve over 12 h (AUC12), as this was the time interval at which the patients received the drug [9]. To compare the data from our 12 patients to those of the Flexner report, we estimated the AUC12 from the arterial blood samples collected during the 90 or 120 min of imaging. The contribution to the blood radioactivity from the FLT glucuronide metabolite was subtracted. From these data, we generated curve fits of the time activity curves out to 12 h according to the method reported by Graham [19] and then calculated the AUC12 of these curve fits.
PET Image Analysis
This is described in a separate report.
Statistics
Summary statistics (mean, standard deviation) were calculated. Laboratory test values were evaluated with the one-way analysis of variance test for repeat sampling with Dunett’s post-hoc comparison to the pre-study value. The statistical software package used was Statistical Package for the Social Sciences (SPSS Inc.). Significance was p < 0.05 or less.
Results
Vital Signs and Electrocardiogram During the FLT-PET Procedure
Vital signs were monitored and recorded closely during the FLT imaging procedures at baseline, during infusion, then at multiple intervals, and then approximately 3 and 24 h after the infusion. Subject 4 experienced an asymptomatic rise in blood pressure from 120/78 to 163/74 2 h after the injection of FLT. This was attributable to discomfort from the head immobilization device, positioning, and needing to hold still for the long procedure. Otherwise, there were no significant changes in blood pressure, pulse, temperature, respiratory rate, oxygen saturation, or electrocardiogram in any subject during any of the imaging procedures.
Review of Systems
The review of systems reports at the end of the imaging session, 1 day later and 1 month after the FLT-PET revealed no symptoms in any subjects at all attributable to the study.
Neurological Examinations
Over the intervals tested, there were no changes of consequence on any of the neurological tests performed. The occasional single-point deviation on the mental status examination, for better or worse, was not considered significant, as practice effects or fatigue could account for these. Specifically, no symptoms or signs of peripheral neuropathy occurred in any patient at any time.
Laboratory Investigations of Liver, Renal, Hematological, and Metabolic Functions
Multiple laboratory tests listed in “Materials and Methods” were collected before, right after, 1 day after, and 1 month after the FLT-PET studies. Table 3 summarizes the mean and standard deviation for each test over time. Statistically significant decreases at 3 h post-FLT administration were observed in potassium, carbon dioxide, total protein, and albumin (all p < 0.05). All returned toward baseline at subsequent time points. Scattered individual values were observed to be above or below normal range for individual parameters, but the only pattern observed was that many were lower at 3 h post-FLT administration. The changes in these tests can, with confidence, be attributed to the withdrawal of blood from arterial blood sampling plus the effect of dilution from intravenous saline and the heparinized saline injected to keep the arterial catheter from clotting during the procedure.
Table 3.
The results of all the laboratory examinations at all the time points tested
| Analyte | Time relative to [18F]FLTFLT administration |
|||
|---|---|---|---|---|
| Pre | 3 h post | 1 day post | 1 month post | |
| Amylase | ||||
| N | 12 | 12 | 10 | 12 |
| Mean | 71.0 | 68.5 | 77.8 | 75.0 |
| SD | 26.7 | 27.6 | 33.9 | 32.2 |
| Minimum | 23 | 26 | 29 | 27 |
| Maximum | 111 | 112 | 129 | 133 |
| Na+ | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 140.9 | 138.4 | 139.3 | 140.7 |
| SD | 3 | 5 | 3 | 3.3 |
| Minimum | 135 | 125 | 131 | 136 |
| Maximum | 145 | 142 | 143 | 148 |
| K+ | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 4.2 | 3.9* | 4.2 | 4.1 |
| SD | 0.5 | 0.2 | 0.3 | 0.3 |
| Minimum | 3.5 | 3.4 | 3.8 | 3.6 |
| Maximum | 5.3 | 4.2 | 4.8 | 4.5 |
| Cl− | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 106 | 106 | 104 | 105.8 |
| SD | 3.75 | 4.57 | 3.59 | 3.8 |
| Minimum | 100 | 94 | 98 | 99 |
| Maximum | 112 | 111 | 110 | 110 |
| CO2 total | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 27.6 | 24.6* | 26.9 | 27.00 |
| SD | 2.8 | 2.3 | 2.1 | 2.49 |
| Minimum | 22 | 20 | 24 | 22 |
| Maximum | 32 | 28 | 30 | 30 |
| Glucose | ||||
| N | 13 | 12 | 11 | 12 |
| Mean | 119.6 | 98.7 | 125.2 | 114.3 |
| SD | 46 | 33 | 73 | 68.9 |
| Minimum | 66.0 | 70.0 | 77.0 | 75.0 |
| Maximum | 247 | 194 | 339 | 330.0 |
| BUN | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 12.58 | 10.58 | 11.00 | 13.33 |
| SD | 3.90 | 4.01 | 3.03 | 4.62 |
| Minimum | 5.0 | 6.0 | 7.0 | 7.0 |
| Maximum | 19.0 | 18.0 | 17.0 | 20.0 |
| Creatinine | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 1 | 1 | 1 | 1.01 |
| SD | 0.2 | 0.2 | 0.2 | 0.3 |
| Minimum | 0.5 | 0.5 | 0.4 | 0.5 |
| Maximum | 1.4 | 1.1 | 1.4 | 1.4 |
| Protein total | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 6.43 | 5.66* | 6.14 | 6.35 |
| SD | 0.38 | 0.37 | 0.57 | 0.73 |
| Minimum | 5.9 | 5.0 | 5.4 | 5.00 |
| Maximum | 7.1 | 6.2 | 7.1 | 7.80 |
| Albumin | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 4.11 | 3.66* | 4.01 | 4.03 |
| SD | 0.27 | 0.18 | 0.51 | 0.50 |
| Minimum | 3.7 | 3.4 | 3.2 | 2.6 |
| Maximum | 4.5 | 3.9 | 4.6 | 4.5 |
| Bilirubin total | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 0.70 | 0.79 | 0.79 | 0.67 |
| SD | 0.17 | 0.24 | 0.17 | 0.14 |
| Minimum | 0.3 | 0.5 | 0.5 | 0.4 |
| Maximum | 0.9 | 1.5 | 1.0 | 0.9 |
| Ca++ | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 9.34 | 9.01 | 9.38 | 9.25 |
| SD | 0.18 | 0.29 | 0.39 | 0.52 |
| Minimum | 8.9 | 8.6 | 8.7 | 8.1 |
| Maximum | 9.5 | 9.4 | 9.9 | 10.1 |
| AST | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 26.3 | 22.5 | 23.7 | 26.4 |
| SD | 5.7 | 4.4 | 5.0 | 5.0 |
| Minimum | 21 | 16 | 16 | 21 |
| Maximum | 40 | 29 | 31 | 38 |
| Alk Phos | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 88.8 | 78.5 | 84.3 | 87.0 |
| SD | 23.8 | 26.4 | 27.9 | 29.0 |
| Minimum | 55 | 50 | 52 | 48 |
| Maximum | 125 | 122 | 127 | 133 |
| GPT | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 38.58 | 30.17 | 32.73 | 32.1 |
| SD | 15.32 | 9.58 | 11.20 | 8.04 |
| Minimum | 19.0 | 15.0 | 16.0 | 17.0 |
| Maximum | 64.0 | 42.0 | 48.0 | 43.00 |
| GGT | ||||
| N | 11 | 12 | 11 | 11 |
| Mean | 45.4 | 42.3 | 47.2 | 45.5 |
| SD | 20.2 | 22.4 | 23.3 | 25.0 |
| Minimum | 16 | 14 | 18 | 18 |
| Maximum | 89 | 90 | 90 | 92 |
| LDH | ||||
| N | 11 | 11 | 10 | 11 |
| Mean | 217.1 | 151.8 | 174.9 | 240.9 |
| SD | 102.9 | 41.9 | 57.9 | 196.6 |
| Minimum | 125 | 98 | 115 | 120 |
| Maximum | 437 | 247 | 279 | 790 |
| Phosphate | ||||
| N | 9 | 12 | 10 | 10 |
| Mean | 3 | 3 | 3 | 3.09 |
| SD | 0.5 | 0.6 | 0.6 | 0.4 |
| Minimum | 2 | 2 | 2 | 2.2 |
| Maximum | 3.90 | 3.80 | 4.00 | 3.5 |
| Prothrombin | ||||
| N | 11 | 11 | 11 | 10 |
| Mean | 12.83 | 13.59 | 13.12 | 12.91 |
| SD | 1.35 | 0.52 | 0.94 | 1.34 |
| Minimum | 10.1 | 12.8 | 11.4 | 9.4 |
| Maximum | 14.3 | 14.3 | 14.8 | 13.9 |
| INR | ||||
| N | 12 | 11 | 11 | 11 |
| Mean | 1.03 | 1.05 | 1.01 | 1.02 |
| SD | 0.05 | 0.05 | 0.09 | 0.07 |
| Minimum | 1.0 | 1.0 | 0.9 | 0.9 |
| Maximum | 1.1 | 1.1 | 1.2 | 1.1 |
| PTT | ||||
| N | 12 | 11 | 11 | 11 |
| Mean | 27.2 | 29.4 | 30.9 | 26.6 |
| SD | 3.3 | 5.7 | 15.8 | 2.9 |
| Minimum | 23 | 21 | 21 | 21 |
| Maximum | 33 | 43 | 78 | 31 |
| WBC | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 6.09 | 5.75 | 6.96 | 5.73 |
| SD | 1.82 | 1.40 | 3.98 | 2.04 |
| Minimum | 3.9 | 3.9 | 4.3 | 2.7 |
| Maximum | 9.5 | 8.2 | 17.7 | 10.3 |
| RBC | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 4.59 | 4.37 | 4.50 | 4.47 |
| SD | 0.44 | 0.39 | 0.38 | 0.50 |
| Minimum | 4.0 | 3.8 | 3.8 | 3.3 |
| Maximum | 5.6 | 5.0 | 4.9 | 5.3 |
| Hgb | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 14.33 | 13.36 | 13.92 | 13.97 |
| SD | 1.24 | 0.94 | 1.07 | 1.53 |
| Minimum | 12.0 | 12.1 | 11.5 | 10.7 |
| Maximum | 16.1 | 15.0 | 15.3 | 16.6 |
| Hct | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 42.0 | 39.9 | 41.1 | 41.2 |
| SD | 3.5 | 3.4 | 3.0 | 4.3 |
| Minimum | 36 | 35 | 35 | 31 |
| Maximum | 49 | 46 | 45 | 47.3 |
| Platelets | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 234.4 | 226.2 | 220.9 | 222.3 |
| SD | 51.7 | 42.7 | 47.8 | 50.6 |
| Minimum | 170 | 153 | 168 | 172 |
| Maximum | 346 | 307 | 333 | 319 |
| ANC | ||||
| N | 12 | 12 | 11 | 12 |
| Mean | 4.09 | 3.79 | 5.02 | 3.81 |
| SD | 1.35 | 1.15 | 3.34 | 1.38 |
| Minimum | 2.3 | 2.5 | 2.4 | 2.1 |
| Maximum | 6.8 | 6.7 | 13.4 | 6.6 |
| Haptoglobin | ||||
| N | 11 | 12 | 11 | 11 |
| Mean | 119.9 | 113.6 | 125.0 | 139.6 |
| SD | 38.5 | 41.1 | 43.5 | 68.3 |
| Minimum | 54 | 46 | 54 | 36 |
| Maximum | 202 | 183 | 193 | 265 |
p < 0.05
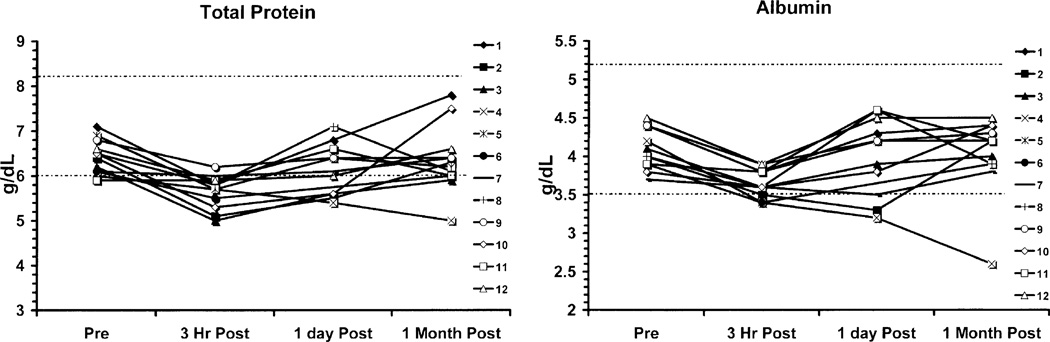
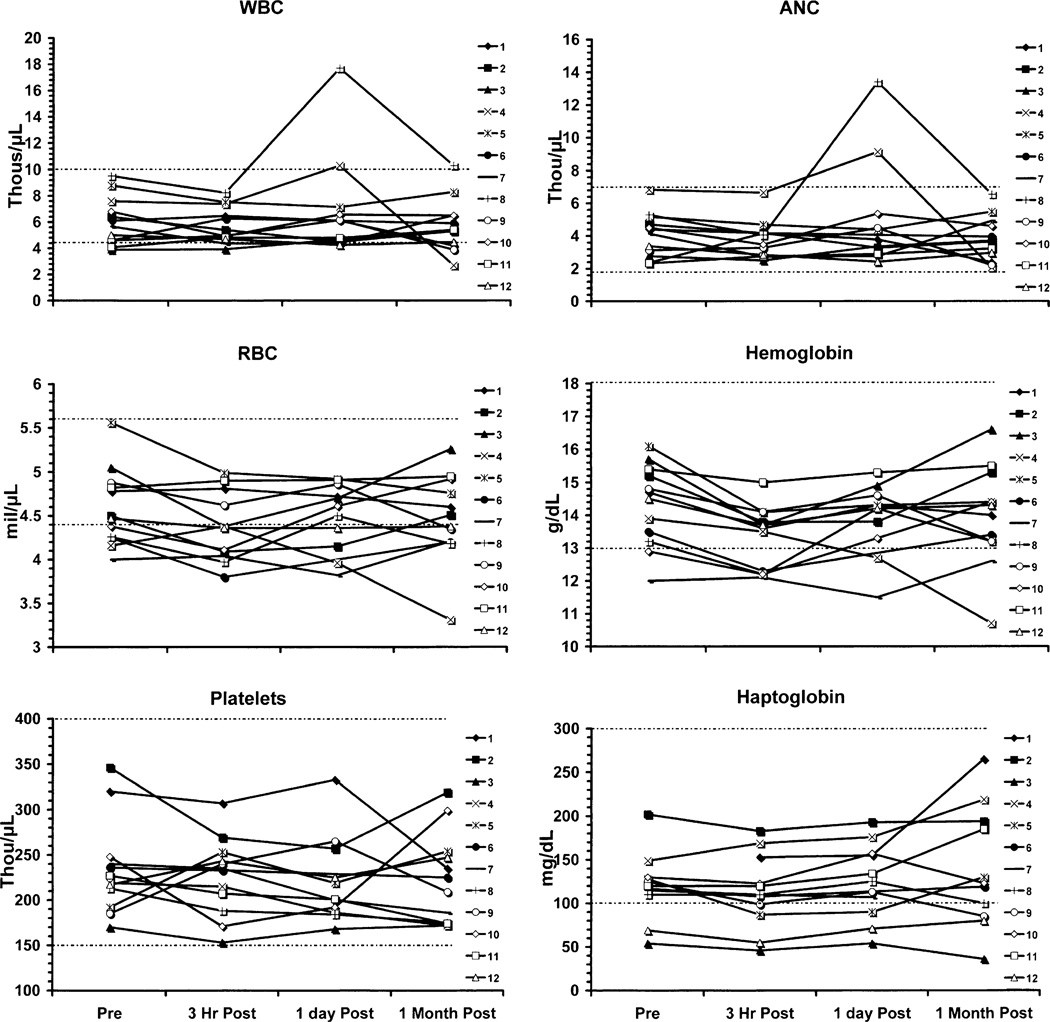
Values for individual patients with time for the parameters that showed statistically significant decreases are shown in Figs. 1 and 2. For total protein and albumin, the majority (10/12) of the patients were slightly below normal range at 3 h as was the mean value, but at 1 month, the majority were within normal range (10/12). Patient number 4 was an exception in that he went on to receive treatment with bevacizumab and irinotecan. His course was complicated by postural hypotension and epistaxis requiring many non-protein containing fluids orally and intravenously, poor oral intake of nutrients and fluids, and diarrhea. These complications of his chemotherapy explain the failure of his albumin and protein levels to recover and explain reductions in his RBC, Hct, Hgb, and ANC.
Fig. 1.
Graphs of total protein and albumin for each subject at the four time points of data collection. Subject 4 showed declines in both parameters. Complications of bevacizumab and irinotecan treatment explain these declines.
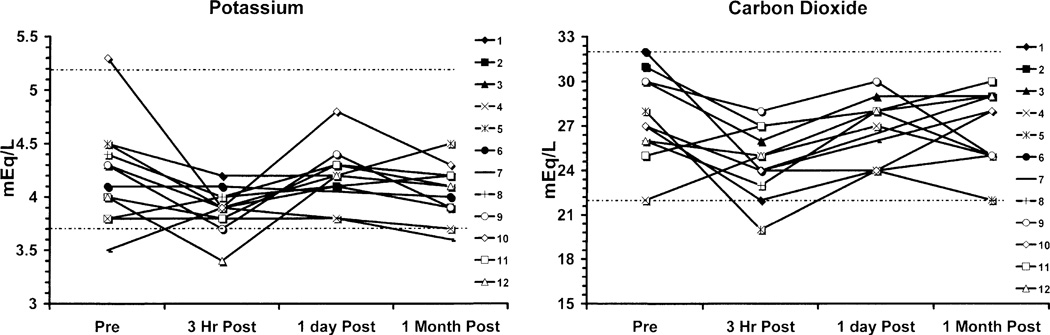
Fig. 2.
Graphs of potassium and CO2 levels show declines at 3 h post-FLT injection, all likely due to hydration and blood sampling effects.
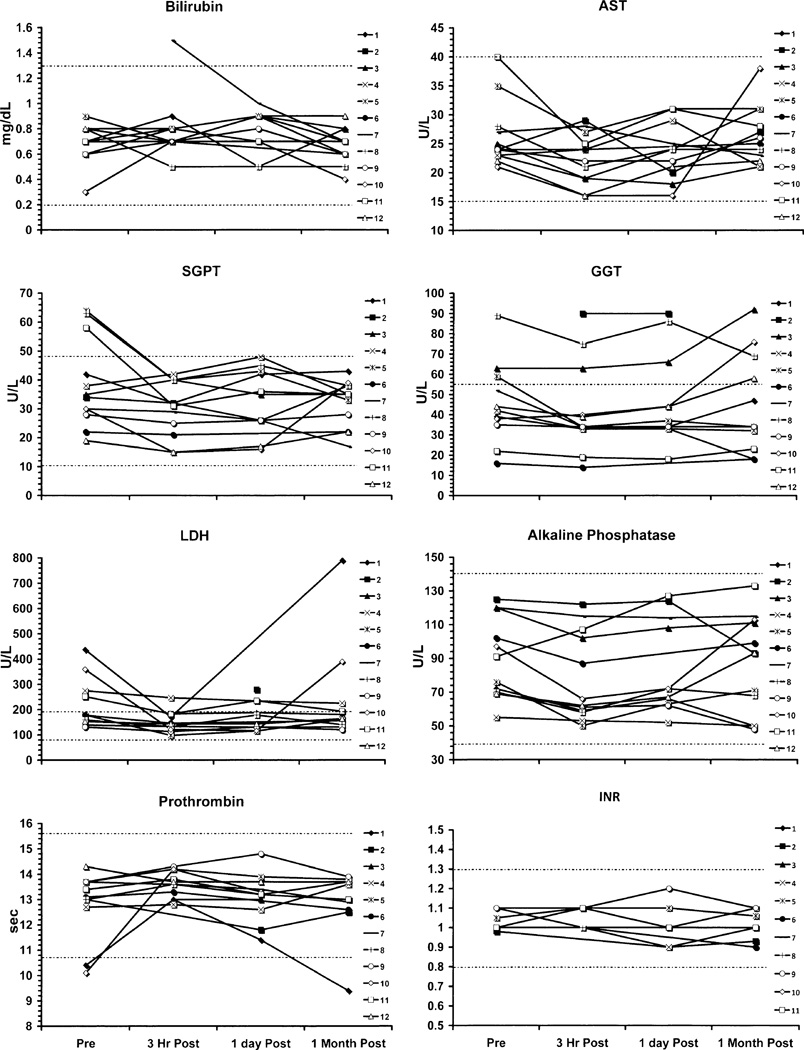
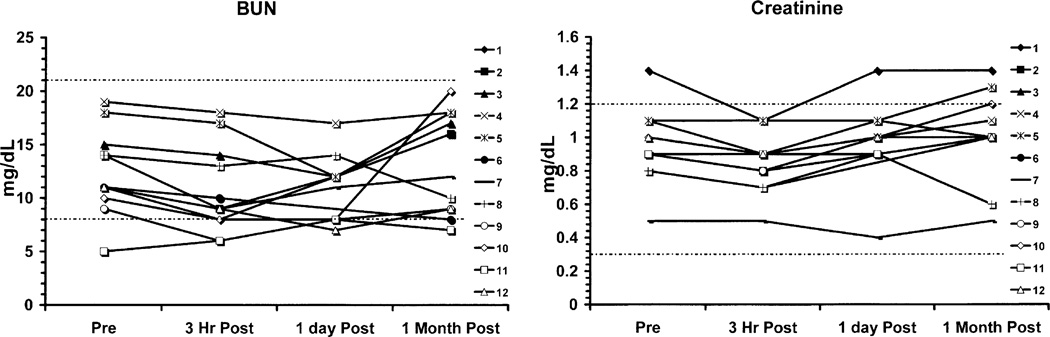
There were minor deviations in the potassium and carbon dioxide measurements that were statistically significant, but these likely related to dietary, hydration, or lack of replacement effects (Fig. 2). Hepatic function (Fig. 3) otherwise did not show any patterns of changes related to drug administration, and there were no statistically significant changes in total bilirubin, AST, SGPT, GGT, LDH, ALK Phos, prothrombin time, or INR. Similarly, renal function (Fig. 4) showed no patterns of change in BUN or creatinine. Selected hematology parameters are shown in Fig. 5, and no patterns of change were observed. Haptoglobin levels did not reveal any evidence of hemolysis Fig. 5. There were no significant changes recorded in the urinalyses.
Fig. 3.
Graphs of liver function tests show no significant impact from exposure to FLT.
Fig. 4.
Graphs of BUN and creatinine show no significant changes at any time point after FLT.
Fig. 5.
The hematological measurements show no significant declines in WBC, ANC, RBC, Hgb, or platelets except in patient 4 where the declines were due to bevacizumab and irinotecan. Haptoglobin levels show no evidence of hemolysis.
The AUC12 values calculated from the arterial blood samples extended out to 12 h are shown in Table 2. The range was 0.004 to 0.035 ng.h/ml with a mean of 0.016 and SD of 0.010 ng.h/ml.
These correspond to 0.008% to 0.07% of the lowest and least toxic single dose clinical trial AUC12 of 50 ng.h/ml reported by Flexner et al. [9]. This single injection also corresponds to a nearly 105- to 106-fold lower cumulative dose than in Flexner’s twice-daily FLT therapeutic trial.
Discussion
The chief intent of the present investigation was to confirm the safety of FLT administered in radiotracer amounts for PET studies of tumor proliferation. The safety of any PET imaging agent, particularly one that has been used as a therapeutic and been shown to have toxicity, is important to document as part of the development of the agent for clinical use. This involved an assessment of potential symptoms and signs in subjects exposed to FLT and analysis of an extensive survey of laboratory investigations of hepatic, renal, hematologic, and metabolic functions. This also necessitated a comparison of the FLT dose administered for a single imaging session to the doses previously used in clinical trials of FLT as an antiretroviral drug, wherein significant hematological, hepatic, and neurologic toxicity were encountered [9].
In the initial phases of testing FLT for antiretroviral treatment, HIV-positive patients were treated in an open-label concentration-control trial over 16 weeks with 0.125 mg/kg orally every 12 h with a target AUC12 of 300 ng.h/ml [9]. The mean cumulative drug exposure (AUC12) was 417 ng. h/ml. Six of ten subjects experienced grade 3 hematological toxicity. At 300 ng.h/ml, grade 2 or greater anemia (fall in hemoglobin to <9.4 g/dl) developed within 4 weeks in 9 of 12 subjects that completed at least 4 weeks of therapy. Subsequently, 48 patients were randomized into three groups with different FLT concentration target levels to define better the pharmacokinetics, anti-viral activity, and toxicity. The AUC12 targets for the groups were 50, 100, or 200 ng. h/ml for a maximum of 16 weeks on trial. At 200 ng.h/ml, grade 2 or greater anemia developed in three of 14 subjects, whereas at 100 ng.h/ml, only one of 15 developed this complication and none did at 50 ng.h/ml. Grades 3 and 4 neutropenia occurred in four of 14 subjects and one of 14 subjects at 200 ng.h/ml, respectively, but none occurred at the lower AUC12 levels. The neutropenia and anemia reversed with discontinuation of FLT. At all doses, peripheral neuropathy (worsening vibration sense scores) occurred within a median time to onset of 40 days (one, two, five, and two subjects at 300, 200, 100, and 50 ng.h/ml, respectively). After 12 weeks, one subject in the group at AUC12 of 200 ng.h/ml died from acute hepatic failure. This complication plus a death from hepatic failure in a similar European trial lead to closure of the trial [9]. All surviving subjects were followed closely for 4 weeks after stopping FLT, and none had evidence of clinically significant liver disease or other adverse effects. Overall, 25 of the 44 subjects that received at least two doses of FLT completed the 16 week study without any significant adverse effects. These literature data in sum demonstrated that the lowest toxic dose of 50 ng.h/ml for a 12-h oral dosing schedule produced peripheral neuropathy at a median of 40 days in two of 15 subjects and no hematological or hepatic toxicity.
Our study included 12 patients with gliomas who underwent FLT-PET imaging when conventional magnetic resonance imaging and clinical evaluations raised the question of recurrence versus radionecrosis. These subjects were exposed to no more than 0.035 ng.h/ml, a value typical for diagnostic radiopharmaceuticals.
The monitoring of vital signs and electrocardiogram (EKG) during the imaging sessions demonstrated no significant effects. The review of systems questions administered immediately after the FLT-PET session, 24 h later, and 1 month later revealed no symptoms whatsoever to suggest any adverse reaction to the FLT injection. The neurological evaluations at all time points did not reveal any evidence of neuropathy or other toxicity from FLT.
Multiple laboratory tests were collected before, right after, 1 day after, and 1 month after the FLT-PET studies. These encompassed liver, renal, and hematological function tests and amylase, glucose, serum electrolytes, calcium, and phosphate. There were no significant changes in the renal function tests. All liver function tests except the protein and albumin showed no significant changes from the FLT exposure. The protein and albumin changes can, with confidence, be attributed to the withdrawal of blood from arterial blood sampling plus the effect of dilution from intravenous saline and the heparinized saline injected to keep the arterial catheter from clotting during the procedure. The changes in patient 4 were due to complications of his subsequent treatment with bevacizumab and irinotecan. The minor deviations in the potassium and carbon dioxide measurements were secondary to dietary, hydration, or lack of replacement effects, not to FLT.
It is important to point out that all but one of the subjects in this series had been exposed to cytotoxic chemotherapeutic agents before FLT-PET, many to agents that have the capacity to deplete bone marrow stem cells. Such subjects would be expected to be more sensitive to marrow toxic agents such as FLT than chemotherapy-naive subjects. Even in this study’s patient sampling, therefore, it is remarkable that there was no significant hematological toxicity demonstrated.
It is also important to point out that there were additional laboratory investigations in the present study not included in the report of Turcotte et al. [12]. These included GGT, LDH, total protein, prothrombin time, partial thromboplastin time, ANC, haptoglobin (all patients), CO2 total, amylase, Ca++, phosphate, and urinalysis.
From above, it follows that the safety of a single dose of FLT administered to patients for PET imaging needs to be compared to the least toxic regimen used in the clinical trials of Flexner et al. [9]. This was the 12-h schedule at 50 ng.h/ml at which there was no hematological or hepatic toxicity and two of 15 subjects developed mild peripheral neuropathy at a median of 40 days on treatment (80 doses) [9]. The dose per kilogram to yield an AUC12 of 50 ng.h/ml in that study can be estimated from the 0.125 mg/kg dose administered that yielded an AUC12 of 417 ng.h/ml. From this, for an AUC12 of 50 ng.h/ml, the dose per 12 h would be 0.015 mg/kg such that the cumulative dose was on the order of 80-fold higher or 1.2 mg/kg.
In the 12 subjects of the present study, the AUC12 values estimated from assaying arterial blood samples, millicuries of FLT injected, and specific activities ranged from 0.004 to 0.035 ng.h/ml with a mean of 0.016. These estimates were corrected to eliminate the FLT glucuronide contribution to plasma radioactivity. The radiopharmaceutical mass levels correspond to 0.008% to 0.07% of the least toxic clinical trial single dose level for AUC12 of 50 ng.h/ml of the Flexner trial [9]. Assuming 40 day’s exposure and a 12 hour dosing schedule as in the trial of Flexner et al. these radiopharmaceutical mass levels correspond to 0.0001% to 0.0009% of the mass levels in that trial.
The dose of FLT in microgram per kilogram in our patients was 0.0016 to 0.0145 (average 0.0056). This amounts to 0.00011 to 0.00097 of the single dose from the Flexner report and 1.3 × 10−6 to 1.2 × 10−5 of the cumulative dose of that report. Normalizing this to a single radiotracer dose of 5 mCi for a 70-kg adult at the lowest acceptable specific activity of 0.24 Ci/µmol, the FLT exposure would be 0.073 µg/kg, 0.00006 of the least toxic cumulative clinical trial dose. However, the subjects of this trial received less FLT than would be in 0.24 Ci/µmol. The lowest specific activity we injected was 1.25 Ci/µmol, about five times greater than 0.24 Ci/µmol.
In the report of Turcotte et al. [12], the entire blood radioactivity was assumed to be FLT. This was incorporated into their estimates of the AUC12 that were subsequently compared the AUC12 data of Flexner et al. The contribution to the blood radioactivity from FLT glucuronide was not subtracted from the total to yield a more accurate estimate of the FLT blood concentration. Our results show that performing this subtraction yields a lower estimate of the AUC12 FLT exposure (mean, 32%; range, 21% to 45%).
Conclusion
The results of this study show no evidence of toxicity from FLT injected as a single radiotracer imaging dose of 0.07 mCi/kg (maximum, 5 mCi) at specific activities of 1.25 Ci/µmol or higher. The neurological evaluations and laboratory investigations before and after imaging and the monitoring of vital signs and EKG’s during the imaging sessions provided no evidence of any adverse events whatsoever. The FLT dose administered for imaging from this study was on the order of 0.0001% to 0.0009% of the least toxic cumulative dose administered in clinical trials of FLT as a therapeutic agent [9]. Altogether, the evidence from this report and the prior one of Turcotte et al. [12] argue that FLT is a safe radiotracer and support the continued testing and development of FLT for quantifying proliferation in the human cancer setting. The presented data also support the assumption that FLT-PET is probably safe when administered over several imaging time points to assess changes in proliferation in response to treatment interventions.
Acknowledgments
Lalitha K. Shankar, M.D., Ph.D., and Paula M. Jacobs, Ph.D. are gratefully acknowledged for their indispensable help with the contracts and the statistical analyses. This study was supported by National Cancer Institute Contract N01-CM-37008, Subcontract NCI (CIP/SAIC) BOA 24XS036 and S10 RR17229.
References
- 1.Grierson JR, Shields AF. Radiosynthesis of 3’-deoxy-3’-[(18)F]fluorothymidine: [(18)F]FLT for imaging of cellular proliferation in vivo. Nucl Med Biol. 2000;27:143–156. doi: 10.1016/s0969-8051(99)00104-3. [DOI] [PubMed] [Google Scholar]
- 2.Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 3.Grierson JR, Schwartz JL, Muzi M, Jordan R, Krohn KA. Metabolism of 3’-deoxy-3’-[F-18]fluorothymidine in proliferating A549 cells: validations for positron emission tomography. Nucl Med Biol. 2004;31:829–837. doi: 10.1016/j.nucmedbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Langen P, Etzold G, Hintsche R, Kowollik G. 3’-Deoxy-3’-fluorothymidine, a new selective inhibitor of DNA-synthesis. Acta Biol Med Ger. 1969;23:759–766. [PubMed] [Google Scholar]
- 5.Langen P, Graetz H. Cell death resulting from inhibition by 3’-deoxy-3’-fluorothymidine, cytosine-arabinoside, 5-fluoro-2’-deoxyuridine, and hydroxyurea of DNA synthesis in cultured Ehrich ascites carcinoma cells. Studia Biophys. 1972;31/32:359–366. [Google Scholar]
- 6.Langen P, Kowollik G, Etzold G, Venner H, Reinert H. The phosphorylation of 3’-deoxy-3’-fluorothymidine and its incorporation into DNA in a cellfree system from tumor cells. Acta Biol Med Ger. 1972;29:483–494. [PubMed] [Google Scholar]
- 7.Matthes E, Lehmann C, Scholz D, Rosenthal HA, Langen P. Phosphorylation, anti-HIV activity and cytotoxicity of 3’-fluorothymidine. Biochem Biophys Res Commun. 1988;153:825–831. doi: 10.1016/s0006-291x(88)81170-7. [DOI] [PubMed] [Google Scholar]
- 8.Faraj A, Fowler DA, Bridges EG, Sommadossi JP. Effects of 2’,3’-dideoxynucleosides on proliferation and differentiation of human pluripotent progenitors in liquid culture and their effects on mitochon-drial DNA synthesis. Antimicrob Agents Chemother. 1994;38:924–930. doi: 10.1128/aac.38.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flexner C, van der Horst C, Jacobson MA, et al. Relationship between plasma concentrations of 3’-deoxy-3’-fluorothymidine (alovudine) and antiretroviral activity in two concentration-controlled trials. J Infect Dis. 1994;170:1394–1403. doi: 10.1093/infdis/170.6.1394. [DOI] [PubMed] [Google Scholar]
- 10.Matthes E, Lehmann C, Scholz D, et al. Inhibition of HIV-associated reverse transcriptase by sugar-modified derivatives of thymidine 5’-triphosphate in comparison to cellular DNA polymerases alpha and beta. Biochem Biophys Res Commun. 1987;148:78–85. doi: 10.1016/0006-291x(87)91078-3. [DOI] [PubMed] [Google Scholar]
- 11.Sundseth R, Joyner SS, Moore JT, Dornsife RE, Dev IK. The anti-human immunodeficiency virus agent 3’-fluorothymidine induces DNA damage and apoptosis in human lymphoblastoid cells. Antimicrob Agents Chemother. 1996;40:331–335. doi: 10.1128/aac.40.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turcotte E, Wiens LW, Grierson JR, Peterson LM, Wener MH, Vesselle H. Toxicology evaluation of radiotracer doses of 3’-deoxy-3’-[18F]fluorothymidine (18F–FLT) for human PET imaging: laboratory analysis of serial blood samples and comparison to previously investigated therapeutic FLT doses. BMC Nucl Med. 2007;7:3. doi: 10.1186/1471-2385-7-3. Electronic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muzi M, Spence AM, O’Sullivan F, et al. Kinetic analysis of 3’-deoxy-3’-18F–fluorothymidine in patients with gliomas. J Nucl Med. 2006;47:1612–1621. [PubMed] [Google Scholar]
- 14.Graham MM, Lewellen BL. High-speed automated discrete blood sampling for positron emission tomography. J Nucl Med. 1993;34:1357–1360. [PubMed] [Google Scholar]
- 15.Lewellen TK, Kohlmyer SG, Miyaoka RS, Kaplan MS, Stearns CW, Schubert SF. Investigation of the performance of the General Electric ADVANCE positron emission tomograph in 3D mode. IEEE Trans Nucl Sci. 1996;43:2199–2206. [Google Scholar]
- 16.Kinahan PE, Townsend DW, Beyer T, Sashin D. Attenuation correction for a combined 3D PET/CT scanner. Med Phys. 1998;25:2046–2053. doi: 10.1118/1.598392. [DOI] [PubMed] [Google Scholar]
- 17.Lundgren B, Bottiger D, Ljungdahl-Stahle E, et al. Antiviral effects of 3’-fluorothymidine and 3’-azidothymidine in cynomolgus monkeys infected with simian immunodeficiency virus. J Acquir Immune Defic Syndr. 1991;4:489–498. [PubMed] [Google Scholar]
- 18.Muzi M, Mankoff DA, Grierson JR, Wells JM, Vesselle H, Krohn KA. Kinetic modeling of 3’-deoxy-3’-fluorothymidine in somatic tumors: mathematical studies. J Nucl Med. 2005;46:371–380. [PubMed] [Google Scholar]
- 19.Graham MM. Physiologic smoothing of blood time-activity curves for PET data analysis. J Nucl Med. 1997;38:1161–1168. [PubMed] [Google Scholar]