Abstract
Objective
Although the theoretical risk of elevated temperatures during endoscopic ear surgery has been reported previously, neither temperature change nor heat distribution associated with the endoscope has been quantified. In this study, we measure temperature changes during rigid middle ear endoscopy in a human temporal bone model and investigate whether suction can act as a significant cooling mechanism.
Study Design
Human temporal bone model of endoscopic middle ear surgery.
Methods
Fresh human temporal bones were maintained at body temperature (~36°C). Temperature fluctuations were measured as a function of 1) distance between the tip of a 3mm 0° Hopkins rod and round window membrane, and 2) intensity of the light source. Infrared imaging determined the thermal gradient. For suction, a #20 French was utilized.
Results
We found: 1) an endoscope maximally powered by a xenon or LED light source resulted in a rapid temperature elevation up to 46°C within 0.5–1mm from the tip of the endoscope within 30–124 seconds; 2) elevated temperatures occurred up to 8mm from the endoscope tip; and 3) temperature decreased rapidly within 20–88 seconds of turning off the light source or applying suction.
Conclusion
Our findings have direct implications for avoiding excessive temperature elevation in endoscopic ear surgery. We recommend: 1) using submaximal light intensity, 2) frequent repositioning of the endoscope, and 3) removing the endoscope to allow tissue cooling. Use of suction provides rapid cooling of the middle ear space and may be incorporated in the design of new instrumentation for prolonged dissection.
Keywords: endoscope, middle ear surgery, tympanoplasty
Introduction
Endoscopic middle ear surgery is gaining popularity in the otolaryngology community. One of the important benefits of an endoscope compared to the microscope is the wide-field view of the middle ear afforded by the location of the light source at the tip of the instrument and the availability of angled lenses. Furthermore, middle ear procedures that employ a rigid endoscope for viewing reduce the need to drill for enhanced exposure to the operative field. In contrast, the traditional otologic operating microscopes typically require larger portals (e.g. post-auricular or endaural approaches) to enable adequate passage of light for intraoperative viewing and follow-up surveillance in clinic.1,2 As the clinical indications for this new technique are currently evolving in the literature,3 the use of rigid endoscopes to perform ear surgery (rather than just to visualize view the middle ear) is increasing and primed to expand as refined instrumentation and operative approaches become available.
Essential equipment for endoscopic middle ear surgery includes a light source and rigid endoscope coupled to a high-definition (HD) camera and video monitor. Currently, the Hopkins rod-lens system is a popular choice among otolaryngologists and provides endoscopes of varying diameters, lengths, and angles of view. The rigid endoscope diameters commonly used for ear surgery are 2.7 mm, 3 mm, and 4 mm. As the diameter expands, the image quality improves as more light is transmitted to the operative field. However, the wider diameter endoscopes decrease the working space available for additional instruments. Typical endoscope shaft lengths in middle ear surgery are 11 cm, 14 cm, and 18 cm. The angles of view most commonly employed are by 0° and 30° angled endoscopes. In addition to endoscope parameter choices, light of varying brightness and quality is offered by three different types of light sources: halogen, xenon, and LED.1
One potential drawback to the use of endoscopes in middle ear surgery is the elevated temperatures caused by transmission of heat from the light source to the endoscope. Previous reports suggest a theoretical risk of elevated temperatures with endoscopy; however, few attempts have been made to quantify or localize temperature elevations.4 During traditional middle ear surgery with an otologic operating microscope, irrigation is frequently utilized to cool tissue and bone as well as remove debris and blood. In contrast, during endoscopic middle ear surgery, a dry field is preferable for improved viewing and operative efficiency. Indeed, irrigation in endoscopic ear surgery is often not necessary as bleeding is typically minimal following elevation of the tympanomeatal flap.
Over the past two decades, as endoscopy has gained in popularity for sinus and skull base surgery and is now the principle instrument for viewing therein, research studies have attempted to address temperature fluctuations in this domain.5,6 However, these findings are not directly applicable to the diagnostic or therapeutic use of endoscopy in the ear: In contrast to the nasal cavity’s relatively large surface area, extensively vascularized tissue, and specialized function to facilitate airflow, the middle ear space is small, bony, and less vascular. In theory, these properties of the middle ear increase the potential for significant temperature elevation and damage to adjacent critical structures. Most importantly, neurosensory cells in the cochlea (and vestibular end organs) are sensitive to trauma from various forms of environmental changes,7,8 and therefore local temperature increases for extended time periods near the cochlea should be minimized.
As the application of endoscopes in middle ear surgery is still in its infancy, few data describe temperature changes within the unique anatomy of the temporal bone. Nearly two decades ago, Bottrill et al. designed a human temporal bone and animal model to examine the role of temperature elevation in endoscope-induced vertigo in clinic patients.9 This group found that a variety of endoscopes in the middle ear results in a warm caloric effect and maximum temperature difference of ~8° C for a 3 mm Hopkins rod in the lateral semicircular canal in their temporal bone model. Although their work importantly set the stage for future studies, Bottrill et al. performed their temporal bones studies at room temperature, which does not recapitulate the physiologic temperature of the middle ear. Further, there is no description of the cooling of tissue after removal of the endoscope. Other studies by Tomazic et al. and MacKeith et al. also describe temperature changes due to varying light sources and endoscopes, and potential use of suction as a cooling mechanism.5,6 These investigators, however, tested endoscopes in room air. It is, thus, difficult to apply their findings to the temporal bone, given its complex architecture and thermal properties resulting in variable temperature distribution. Given the resultant difficulty in generalizing their data, these studies are not directly applicable to a surgical model.
In our study, we characterize and quantify temperature changes associated with the use of rigid endoscopy in an unfixed human temporal bone model. We recreate similar thermal characteristics in a live human by heating the surrounding tissue of the middle ear to maintain a constant body temperature. We hypothesize that the use of endoscopes results in substantial temperature rise in the middle ear space, given the thermal properties of the surrounding tissue. In addition, we hypothesize that using suction near the tip of the endoscope will create a cooling effect that will mitigate temperature elevation. We aim to provide data that will be readily applicable to current surgical techniques and enable the continued development of safe endoscopic procedures and surgical equipment.
Methods and Materials
Endoscopes, Light Sources, and Temperature Measurements
Rigid endoscopes of 3 mm diameter, 14 cm length, and 0° angled scope was used (Karl Storz Endoskope, Tuttlingen, Germany). Two types of light sources were tested: a Xenon 300 and a Power LED 175 (Karl Storz Endoskope). Temperatures were measured with thermocouple sensors (HH147U data logger and SPHT-K-6 sensor, OMEGA, United States) that can measure 0.1° C increments and ±0.1% reading + 0.7° C. This model allows simultaneous temperature recordings of four different locations at one-second increments. The infrared (IR) camera used (FLIR ThermaCAM SC300, FLIR Systems, Inc, United States) measures between −20° C to +120° C with accuracy of ±2%.
Human Temporal Bone Model of Endoscopic Ear Surgery Set-Up
A temporal bone removed within 24 hours post-mortem in the manner described by Nadol (1996), then frozen until utilized, represented the soft tissue and bony architecture in endoscopic middle ear surgery. For proper placement of thermocouple sensors, the mastoid cavity was drilled and facial recess exposed, allowing for direct viewing of the round window. The experiment was conducted in a thermostatically controlled 36° C sound chamber, and a homeothermic blanket (Harvard Apparatus, United States) maintained the temporal bone at 34–37° C. In living tissue, energy in the form of heat is constantly being circulated to maintain body temperature, making it potentially difficult for additional heat from an endoscope to escape the middle ear. By maintaining the temporal bone at constant body temperature, we attempted to simulate the thermal properties of the living ear.
The temporal bone was fixed in place and the tympanic membrane was elevated. Using a stereotaxic micro-manipulator, a thermocouple sensor was positioned at the round window membrane (Fig. 1). Using a second manipulator, the endoscope was advanced into the external auditory canal (EAC) until the tip was near the level of the thermocouple sensor adjacent to the round window. After the thermocouple sensor and endoscope were positioned, the temperature data logger hardware was initiated. Temperatures were recorded at all thermocouple sensor positions for a baseline temperature measurement of 15–30 seconds, a 6 minute period with the LED or xenon light source turned on at 100% or 50% power, and a subsequent 3 minute cooling period after turning the light source off.
Figure 1. Middle Ear Endoscopy and Human Temporal Bone Set-Up.
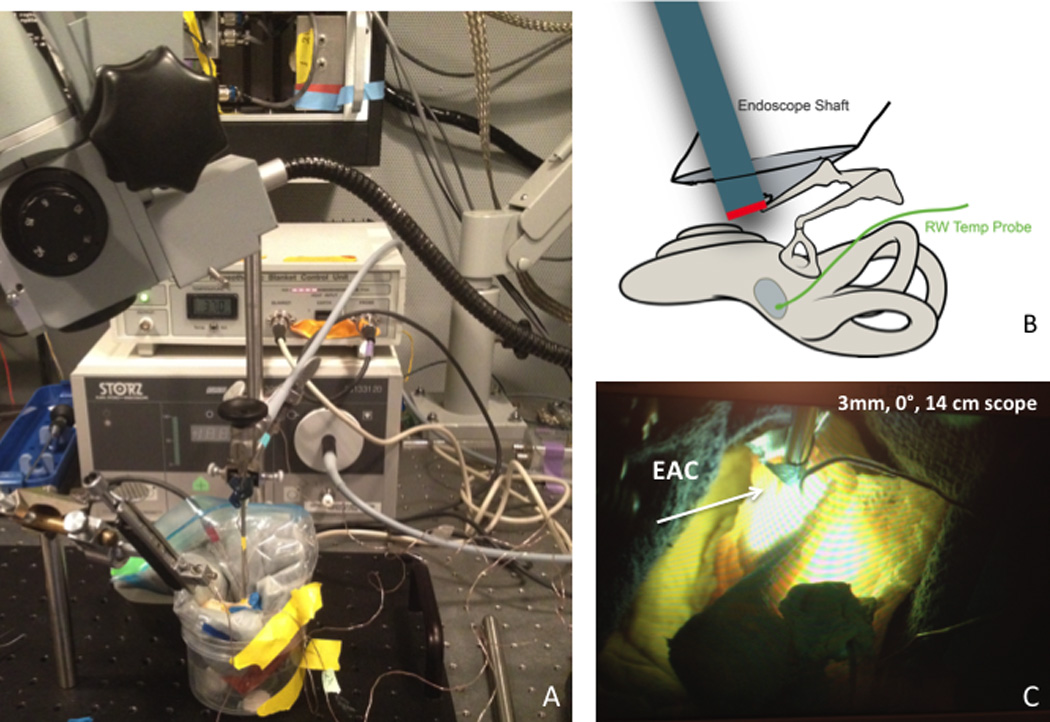
A: Fresh human temporal bone placed in homeothermic blanket with temperature sensors in place. Experiment was performed in small sound booth chamber capable of reaching 35° C. B: Illustration demonstrating placement of endoscope relative to round window (RW) with temperature sensor placed adjacent to RW. C: Fresh human temporal bone with endoscope in place and light source turned on. Light is visible both through EAC and facial recess.
After allowing for proper cooling, the endoscope tip was then incrementally withdrawn from the EAC in 2 mm steps via the stereotactic manipulator, and temperature recordings with the power source turned on were repeated at each location as described above. Temperature measurements were specifically made at 2 mm, 4 mm, 6 mm, and 8 mm distal to the round-window thermocouple sensor (Fig. 2). In addition to temperature measurements at the round window, continuous temperature recordings were made in soft tissue of the sigmoid sinus to ensure that there were no unexpected temperature fluctuations during the experiment.
Figure 2. Endoscopeic View of Middle Ear and Tempreature Sensor.

A: View of EAC from a 3 mm, 14 cm scope. B: View of round window (RW) from facial recess. Part of RW membrane is visible. C: View from endoscope as endoscope approaches RW niche. D: View of temperature sensor adjacent to RW.
For the suction component of the experiment, a #20 French suction was placed next to the endoscope tip. Using a similar set-up, the IR camera recorded gross temperature measurements within the temporal bone, specifically at the level of the tip of the endoscope and along the shaft.
Data Analysis
Temperature recordings were uploaded to a computer via the Temp Monitor_S2 for the HH147U Thermometer (OMEGA, United States). IR data was analyzed with the Researcher 2.10 Pro (FLIR Systems, Inc, United States). Statistical analyses were conducted with Microsoft Excel (Microsoft, United States). Temperature values are displayed in degrees Celsius (°C), and time is given in seconds.
Results
Temperature at the Round Window in the Temporal Bone is affected by the Proximity of the Endoscope Tip
Temperature elevation at the round window increases with proximity of the endoscope tip with both xenon and LED light sources (Fig. 3 and Fig. 4, respectively). The greatest temperature elevation occurs with the xenon light source (100% power) closest to the round window. The temperature sensor reached a maximum temperature of 46.9° C. Figure 3 demonstrates the temperature elevations observed after the xenon light source was turned on. The range of time to 75% of the maximum temperature was 30–124 seconds. There was also a similarly rapid decline in temperature to within 25% of baseline temperature after the light source was turned off (range 22–88 seconds).
Figure 3. Temperature Elevation Is Directly Proportional to Proximity of the Endoscope to the Round Window Sensor with Xenon Light Source.

A-E: Rapid temperature elevation after light source is turned on within 30–124 seconds. Temperature plateaus quickly. After light source is turned off, there is a rapid decline in temperature within 22–88 seconds.
Figure 4. Temperature Elevation Is Directly Proportional to Proximity of the Endoscope to the Round Window Sensor with LED Light Source.
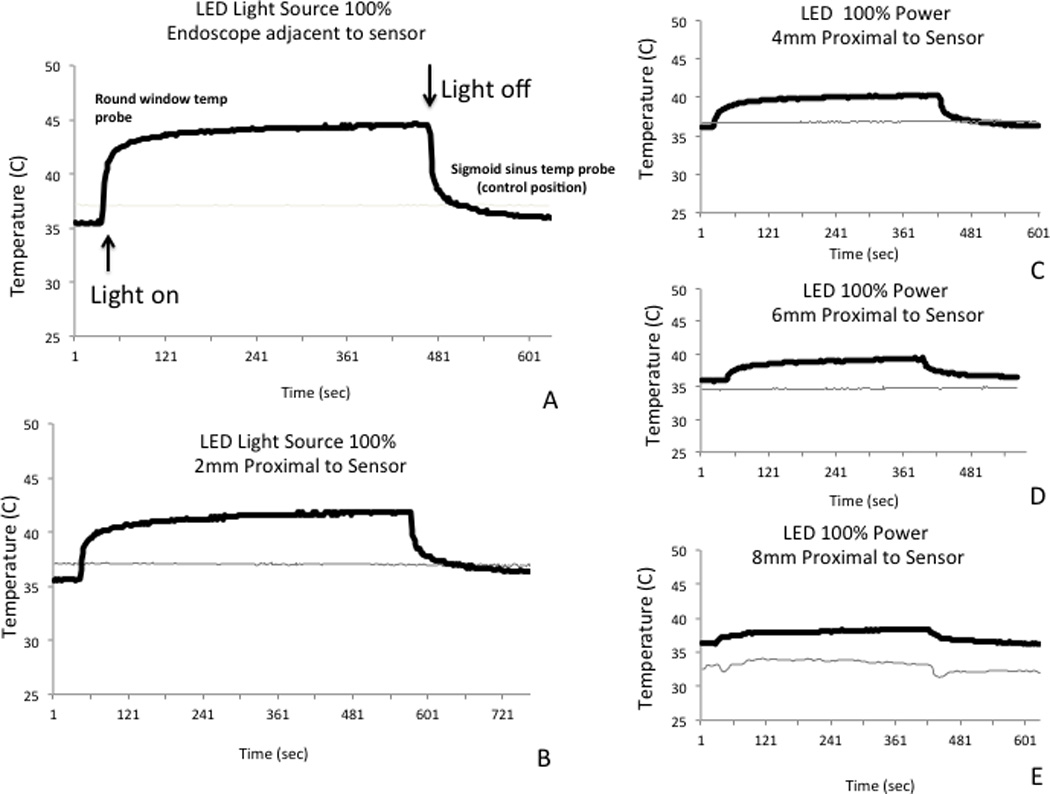
A-E: Similar to xenon light source, rapid temperature elevation after light source is turned on. Temperature plateaus quickly. After light source is turned off, there is a rapid decline in temperature.
Figure 4 demonstrates the similar though less pronounced findings with the LED light source. The maximum temperature elevation observed with the LED light source was to 44.6° C at the round window. Decreasing power level to 50% diminished temperature elevations with both xenon and LED light sources (Fig. 5).
Figure 5. Maximum Temperature by Power Setting with Xenon or LED Light Source.

The xenon light source results in higher temperatures compared to the LED light source at matched power settings.
Infrared Camera Demonstrates Elevated Temperatures Near the Tip of the Endoscope
To further confirm the temperature profile near the tip of the endoscope, we used an infrared camera system. We found that the greatest temperature rise was localized within millimeters to the tip of the endoscope, which was consistent with the thermocouple experiments (Fig. 6C). Similar to our previous experiments, we exposed the temporal bone middle ear space to an endoscope with an LED light source and identified a distinct area of elevated temperature near the tip of the endoscope with the infrared camera (Fig. 6D). The area resolution of the infrared camera was not detailed enough to clearly identify specific pinpoint locations such as the round window, but the region near the tip of the endoscope encompassing the region in which the round window resides had a similar temperature profile as was recorded with the thermocouple sensor.
Figure 6. Infrared Camera Demonstrates Elevated Temperatures Near the Endoscope Tip.

A: Representative infrared camera picture of rigid endoscope that demonstrates higher temperatures at the viewport and the connection to light source. B: Set-up for human temporal bone. C: Endoscope shaft showing elevated temperatures close to the endoscope tip with the highest temperatures found within 4 mm from the tip. D: View of human temporal bone with light source turned on and elevated temperatures identified near the endoscope tip.
Suction Results in Robust Cooling of Middle Ear Space
We repeated the previous experiments while applying a #20 French suction near the tip of the endoscope using either the xenon or LED light sources. Similar to previous experiments, we saw a rapid rise in temperature after turning on the light sources. After suction was initiated, we identified a significant temperature drop of ~11° C (from 42.5° C; Fig. 7A) while the xenon light source remained on. This drop occurred precipitously within 20 seconds, resulting in a temperature below physiologic baseline. Suction was also applied while the LED light source was turned on and resulted in a similar, rapid decline in temperature (Fig. 7B).
Figure 7. Suction Results in Robust Cooling of the Middle Ear Space.

In a human temporal bone heated to body temperature, light sources were turned on (A: xenon; B: LED), and the expected rapid rise in temperature was demonstrated. Suction was positioned next to the endoscope tip, which resulted in rapid temperature decreases to below baseline temperature.
Discussion
In our human temporal bone model, we observed that an endoscope powered by a xenon or LED light source resulted in a rapid temperature elevation at the tip of the endoscope that reached up to 44–46° C (from ~36° C physiologic baseline) within 30–124 seconds, depending on the proximity of the endoscope tip to the round window. Further, significantly elevated temperatures occurred at distances within 8 mm from the endoscope tip. Additionally, temperature decreased significantly after suction was applied or light sources were turned off.
Our findings have direct implications for endoscopic otologic operations. First, we found that the elevated temperatures in the middle ear due to the endoscope occur up to 8 mm from the tip. Second, our findings affirm the advised surgical technique of periodic removal of the endoscope to allow tissue cooling, as this is likely an effective method given the observed rapid decrease in temperature to physiologic baseline when the light sources were turned off. Although usage of the LED light source resulted in lower temperatures compared to the xenon light source, the relative decrease observed was minor. Thus, LED use alone does not provide a solution to the issue of elevated temperatures. Third, the power settings for all light sources should be as low as possible without compromising visibility, as routinely recommended by manufacturers. Fourth, there is interest among otolaryngologists in developing an endoscope holder for otologic surgery. Any designs for an endoscope holder and the related operative approaches should take into account our findings of elevated temperatures in the middle ear cavity due to a stationary endoscope.
In our study, we found that suction applied near the endoscope tip resulted in a rapid and substantial drop in temperature. Suction removes heat by convection and is particularly effective in a small, enclosed space such as the middle ear cavity. In our experiments, the degree of the temperature decrease caused by suction eclipsed the elevation in temperature caused by the endoscope, suggesting suction may serve as a potential solution to elevated temperatures within the middle ear due to endoscopy. Although irrigation is often used in otologic surgery as a means of cooling, in endoscopic procedures, irrigation may be less than desirable as it can obscure the operative field. Thus, suction may offer an advantage by providing necessary cooling and maintaining a dry operative field for prolonged, unobscured viewing of the middle ear space. Future otologic instrument designs, including those for rigid endoscopes, may incorporate the use of suction for both removal of debris and cooling purposes.
Our primary aim was to investigate the extent of temperature elevation near the round window in the middle ear space during endoscopy within the constraints of the three-dimensional architecture of the human temporal bone. We further sought to evaluate the temperature at the round window with the endoscope tip at varying proximity. We designed our model of endoscopic middle ear surgery to simulate transcanal-based otologic cases, in which the endoscope may advance beyond the annulus, as would occur in a transcanal tympanoplasty. Our experimental paradigm was devised to investigate a “worst-case scenario” simulation in which the round window was continuously exposed to the heat from the endoscope tip and the light source was placed on highest settings. This location was chosen for its close proximity to the sensitive neurosensory cells in the inner ear.
Our study also raises questions regarding the functional consequences of elevated temperatures. The obvious question is “How hot is too hot?” Previous studies document functional consequences of elevated temperature in the auditory system. Hyperthermia exacerbates the effects of noise exposure on the ear: Noise-induced reduction of the chinchilla cochlear response is more pronounced at higher body temperatures of up to 39° C,7 and hyperthermic C57BL/6J mice (at 40° C) exposed to noise demonstrate greater threshold elevations in the cochlear nerve envelope response than do euthermic mice.10 Hyperthermia also exacerbates aminoglycoside ototoxicity in preweanling mice (20.4 dB threshold elevation compared to 9.3 dB euthermic mice).11 Elevation of temperature from 18° C to 39° C alters the latency, duration, and amplitude of the cochlear microphonic and neural components measured at the round window in response to acoustic clicks in the hamster cochlea.12 Tuning characteristics of gecko single auditory nerve fibers are temperature dependent as well: The frequency-threshold tuning curve of a fiber shifts to higher frequencies as the otic capsule and inner ear temperatures increase from 19° C to 30° C.13 Human subjects in heated climatic chambers, experiencing hyperthermia up to an average of 38.4° C, demonstrate significant inhibition of transient evoked otoacoustic emissions, suggesting outer hair cell micromechanical activity is also sensitive to body temperature.8 Thus, in both mammalian and nonmammalian species alike, auditory physiology and pathophysiology appear temperature dependent as elevated temperatures induce a range of functional consequences.
The absolute temperatures estimated by our data should be considered cautiously as various factors could augment or dampen temperature changes in vivo. Further, as evident by our data, temperature recordings are exquisitely sensitive to temperature sensor positioning. We do not expect our absolute values of temperature to describe exact conditions of inner ear temperature fluctuations but instead offer a guide and preliminary quantification. Our cadaveric tissue is not perfused, and blood flow might decrease the rise in temperature as circulating fluid may carry away heat. Bottrill et al.’s work with a canine model demonstrated that perfusion limits temperature elevation.9 With possible implications for temperature changes, our model temporal bone is without a pinna, and the facial recess is exposed. In comparison to the relatively closed space of an unaltered temporal bone, our model may be prone to more rapid lowering of temperature due to greater surface area exposure.
We believe our endoscopic middle ear surgery model offers a reasonable initial approach to examine thermal properties during middle ear endoscopy and identifies a potential solution to increased temperature in the use of suction. Future studies will seek to examine the functional consequences of elevated temperatures in a live animal model.
Conclusion
In conclusion, we demonstrate that the use of the endoscope in the middle ear results in significant temperature elevations at the round window. This effect is localized around the tip of the endoscope when powered by a xenon or LED light source. Our study finds that removal of the light source results in rapid cooling. Further, we provide data that show suction is an effective means to decrease the temperature during endoscopic viewing of the middle ear. Our data may be instructive in guiding endoscopic ear surgery procedures and in developing new endoscopic otologic equipment.
Acknowledgments
We would like to thank Dr. Dennis Poe, Associate Professor of Otology and Laryngology of Harvard Medical School / Boston Children’s Hospital for his thoughtful review of our manuscript. We would also like to thank Jeananne Phillips of the Joseph B. Nadol, Jr., M.D. Otolaryngology Surgical Training Laboratory at the Massachusetts Eye and Ear Infirmary for her continued support of surgical equipment and expertise necessary to complete this project. We would like to acknowledge Diane Jones for procuring the human temporal bones.
Works Cited
- 1.Badr-El-Dine M, James AL, Panetti G, Marchioni D, Presutti L, Nogueira JF. Instrumentation and technologies in endoscopic ear surgery. Otolaryngologic clinics of North America. 2013;46:211–225. doi: 10.1016/j.otc.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 2.James AL. Endoscopic middle ear surgery in children. Otolaryngologic clinics of North America. 2013;46:233–244. doi: 10.1016/j.otc.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Tarabichi M. Transcanal Endoscopic Management of Cholesteatoma. Otology & Neurotology. 2010:580–588. doi: 10.1097/MAO.0b013e3181db72f8. [DOI] [PubMed] [Google Scholar]
- 4.Wackym PA, King WA, Meyer GA, Dennis S. Poe M. Endoscopy in neuro-otologic surgery. Otolaryngol Clin N Am. 2002:297–323. doi: 10.1016/s0030-6665(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 5.Tomazic PV, Hammer GP, Gerstenberger C, Koele W, Stammberger H. Heat development at nasal endoscopes' tips: danger of tissue damage? A laboratory study. The Laryngoscope. 2012;122:1670–1673. doi: 10.1002/lary.23339. [DOI] [PubMed] [Google Scholar]
- 6.MacKeith SA, Frampton S, Pothier DD. Thermal properties of operative endoscopes used in otorhinolaryngology. The Journal of laryngology and otology. 2008;122:711–714. doi: 10.1017/S0022215107000734. [DOI] [PubMed] [Google Scholar]
- 7.Drescher DG. Noise-Induced Reduction of Inner-Ear Microphonic Response: Dependence on Body Temperature. Science, New Series. 1974;185:273–274. doi: 10.1126/science.185.4147.273. [DOI] [PubMed] [Google Scholar]
- 8.Ferber-Viart C, Savourey G, Garcia C, Duclaux R, Bittel J, Collet L. Influences of hyperthermia on cochlear micromechanical properties in humans. Hearing Research. 1995;91:202–207. doi: 10.1016/0378-5955(95)00193-x. [DOI] [PubMed] [Google Scholar]
- 9.Bottrill I, Perrault DF, Poe D. In Vitro and In Vivo Determination of the Thermal Effect of Middle Ear Endsocopy. The Laryngoscope. 1996:213–216. doi: 10.1097/00005537-199602000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Henry KR. Hyperthermia exacerbates and hypothermia protects from noise-induced threshold elevation of the cochlear nerve envelope response in the C57BL/6J mouse. Hearing Research. 2003;179:88–96. doi: 10.1016/s0378-5955(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 11.Henry KR, Guess MB, Chole RA. Hyperthermia increases aminoglycoside ototoxicity. Acta Otolaryngol. 1983;95:323–327. doi: 10.3109/00016488309130949. [DOI] [PubMed] [Google Scholar]
- 12.Kahana L, Rosenblith WA, Gallambos R. Effect of Temperature Change on Round-Window Response in the Hamster. The American Journal of Physiology. 1950;163:213–223. doi: 10.1152/ajplegacy.1950.163.2.213. [DOI] [PubMed] [Google Scholar]
- 13.Eatock R, Manley G. Auditory nerve fibre activity in the Tokay gecko: II. Temperature effect on tuning. J Comp Physiol. 1981;142:219–226. [Google Scholar]


