Abstract
Background
Ukraine has the highest HIV burden of any European country with much of the current HIV epidemic concentrated among people who inject drugs (PWIDs) and their sexual partners. Opiate substitution therapy (OST) is limited in Ukraine and expansion of OST is urgently needed to help stem the tide of the HIV epidemic.
Methods
We accessed publicly available data in Ukraine in order to explore geographic variability with respect to prevalence of HIV, PWIDs and OST programmes.
Results
The regions of Ukraine with the largest number of opioid dependent persons (the south and eastern portions of the country) correspond to the regions with the highest HIV prevalence and HIV incidence. The number of opioid PWIDs per 100,000 population as well as the number of all OST treatment slots per 100,000 varied significantly across the three HIV prevalence categories. Overall, the proportion of individuals receiving either methadone maintenance therapy (MMT) or buprenorphine maintenance therapy (BMT) was quite low: average across categories: 7.3% and 0.4%, respectively. Additionally, less than half of OST patients receiving MMT or BMT were HIV positive patients.
Conclusion
There is significant geographic variability in both numbers of HIV positive individuals and numbers of PWIDs across Ukraine, however, there may be a more concentrated epidemic among PWIDs in many regions of the country. Scale up of addiction treatment for PWID, especially OST, can have a significant impact on preventing injection related morbidity, such as HIV and HCV infection. Ukraine can learn from the mistakes other nations have made in denying critical treatment opportunities to PWID.
Keywords: Ukraine, People who inject drugs (PWID), HIV, Opiate substitution therapy (OST)
Introduction
Ukraine, with a population of 45 million, has the highest HIV burden of any European country; recent data estimates the national HIV prevalence at 0.5% (Degenhardt et al., 2014). According to sentinel surveillance data, the HIV epidemic in Ukraine is still concentrated in the most-at-risk groups, with people who inject drugs (PWIDs) and their sexual partners comprising one of the leading risk groups. This is in part attributable to a general lack of access to substance use and HIV treatment among PWID in much of the country. Among the nearly 310,000 estimated PWIDs in Ukraine (Ministry of Health of Ukraine, 2012), fewer than 3% of PWIDs receive methadone or buprenorphine treatment under national programme funded by the Global Fund (Wolfe, Carrieri, & Shepard, 2010). The vulnerability of this population, coupled with their general lack of access to medical care, has resulted in nearly a quarter of PWID in Ukraine being infected with HIV (Ministry of Health of Ukraine, 2012).
It is estimated that only about a quarter of HIV positive PWIDss in Ukraine are receiving ART while PWIDs comprise more than 60% of all HIV infections in the country (Wolfe et al., 2010). Barriers to accessing HIV/AIDS services, including substance use treatment, among PWID populations in Ukraine include stigmatization of both HIV/AIDS and drug use, as well as widespread discriminatory practices among government and community based service providers towards HIV positive PWIDs (Spicer et al., 2011). Widespread stigma, discrimination and harassment have contributed to the limited proportion of HIV positive PWIDs on antiretroviral therapy (ART) (Mimiaga et al., 2010). Booth and colleagues have reported that HIV positive PWID are more likely than non-PWID to report an adversarial relationship with law enforcement (Booth et al., 2013) which may further impede access to both addiction and HIV treatment. An additional impediment to accessing HIV care among some populations of HIV positive PWID is a lack of knowledge regarding HIV treatment related services (Spicer et al., 2011). Finally, it is important to note that health services in Ukraine are primarily designed as a rigid, hospital-centred vertical system with multiple parallel clinics that provide specialized care but have very little coordination between them. Ukraine has a history of “modularization” of the health care system so that HIV care and treatment for substance use by “narcologists” are all done in separate places and by separate specialties. This has translated into very little or no integration among and between clinical specialties and subspecialties. Thus, integrated care for HIV positive PWIDs is a significant challenge.
While multiple attempts to more broadly introduce opiate substitution therapy in Ukraine have occurred over the last decade, these attempts have been significantly hampered by the perception by policy makers that opiate substitution treatment (OST) medications are themselves dangerous or ineffective (Golovanevskaya, Vlasenko, & Saucier, 2012). Adverse attitudes of both government agencies and law enforcement towards PWIDs, and OST programmes, in Ukraine have severely limited OST capacity expansion and overall treatment access (Golovanevskaya et al., 2012; Wu & Clark, 2013). This is despite evidence from several systematic reviews suggesting that: PWID who receive OST have less than half the risk of HIV infection compared with PWID not on OST (MacArthur et al., 2012); OST is associated with greater adherence to ART (Malta, Magnanini, Strathdee, & Bastos, 2010); OST is associated with reductions in illicit opioid use, injecting behaviour, and sharing of injection equipment (Gowing, Farrell, Bornemann, Sullivan, & Ali, 2011); and OST is associated with a reduction by more than half in all-cause mortality among PWID (Degenhardt et al., 2011). These international findings have also been confirmed in studies conducted specifically in Ukraine (Bachireddy et al., 2013; Lawrinson et al., 2008; Schaub, Chtenguelov, Subata, Weiler, & Uchtenhagen, 2010; Schaub, Subata, Chtenguelov, Weiler, & Uchtenhagen, 2009). Additionally, modelling data suggesting that expanding harm reduction services, including OST, and access to ART are the most cost-effective methods of controlling the HIV epidemic in Ukraine (Alistar, Owens, & Brandeau, 2011).
Advocacy among substance using populations, as well as their physicians and families, for expanded access to opiate substitution therapy has increased in the past decade. For example, Golovanevskaya and colleagues describe how NGOs, patient groups and physician advocates have all been working to create broader access to OST in a variety of settings, including easier allowances for take-home dosing and continuity of treatment during inpatient hospitalizations (Golovanevskaya et al., 2012). And there has been an easing of policing of OST sites, initially promoted to decrease medication diversion, in recent years (Humphreys, 2013). Yet, restrictive policies, such as drug user registries and overreliance on detoxification rather than treatment, remain problematic (Bojko, Dvoriak, & Altice, 2013).
Data presented by Degenhardt et al. provide estimates for number of OST clients (7503), number of OST clients per 100 PWID (Wolfe et al., 2010) and the number of OST sites (133) in Ukraine (Ministry of Health of Ukraine, 2012). Here we build upon these estimates and present national data for the numbers of both methadone and buprenorphine clients, including those who are HIV positive, as well as present information about regional trends and increases in the number of OST treatment slots. We also explore geographic variability with respect to prevalence of HIV, PWIDs and OST programmes using national level data through November of 2013.
Methods
We accessed numerous publicly available data sets in Ukraine in order to better understand national estimates of HIV prevalence and substance use treatment among PWID. Data sources are described below. Most data are through November 2013 unless otherwise specified.
Data sources
Ukraine Centers for Disease Prevention and Control (UCDC)
Data are routinely compiled by the Ukrainian CDC through combining data from special medical forms from all AIDS Centers, OST sites, Narcology Centers, TB and General hospitals in the country. Data are updated monthly with regard to regional HIV prevalence, regional HIV incidence and regional AIDS incidence. Data are also routinely collected by Ukrainian CDC from all medical facilities which have an integrated HIV treatment and OST site. These data are site-level and collected monthly. They include numbers of individuals on OST, including average dosing, new admissions, discharges, etc. Data are publicly available through the official web-site of the UCDC: http://ucdc.gov.ua/uk/.
Ukrainian monitoring centre for alcohol and drugs
Data are routinely collected by narcological dispensaries at the regional level and then consolidated in one statistical form. Data include officially diagnosed and registered cases of opioid dependency per 100,000 population. Data were obtained through an official data request to the Centre and represents official numbers on the prevalence of dependence from opioids.
International HIV/AIDS Alliance in Ukraine
The Alliance conducts a bi-annual survey among most-at-risk groups. HIV prevalence is measured among PWID in all regional capital cities in Ukraine. The survey is conducted every 2 years. Survey data, narcological registry data and mathematical computation methods are used to estimate the numbers of PWID in the region. For example, a capture–recapture method is used for estimating the PWID population size in the region. All estimated region-level population sizes were discussed with key regional stakeholders and approved by regional coordination councils.
National Statistics Committee of Ukraine
The Centre produces routine data on population size, for both oblasts and regional capitals. Data are publicly available through official web-site: http://www.ukrstat.gov.ua/.
Statistical analyses
First, we regionalized all 27 oblasts in Ukraine into five regions (West, Central, North, East and South, see Table 1) in order to graphically depict the HIV prevalence, HIV incidence and PWID prevalence in each geographic region. Next, we stratified the data described above by HIV prevalence: less than 150 HIV cases per 100,000 population; 150–300 HIV cases per 100,000 population; and greater than 300 HIV cases per 100,000 population.
Table 1.
Oblasts included into five regions.
| Region | Oblasts included |
|---|---|
| West | Volyn oblast, Zakarpattia oblast, Ivano-Frankivsk oblast, Lviv oblast, Rivne oblast, Ternopil oblast, Chernivtsi oblast |
| Central | Vinnytsia oblast, Kirovohrad oblast, Poltava oblast, Khmelnytskyi oblast, Cherkasy oblast |
| North | Zhytomyr oblast, City of Kyiv, Kyiv oblast, Sumy oblast, Chernihiv oblast |
| East | Dnipropetrovsk oblast, Donetsk oblast, Luhansk oblast, Kharkiv oblast |
| South | Zaporizhia oblast, Mykolaiv oblast, Odesa oblast, City of Sevastopol, AR of Crimea, Kherson oblast |
Oblasts were categorized for analysis purposes as being of low, medium or high HIV prevalence per 100,000 head of population and prevalence of PWID per 100,000 of population. The designations of low, medium and high were determined based on the range of values seen across oblasts. As these data were not normally distributed, we elected to use, and report, median values of prevalence and proportion of PWID estimates. Median values are not as influenced by extreme data points, i.e. outliers, as compared with mean values. Maps of HIV and PWID prevalence were constructed using freely available blank outline maps.
We tested for differences (one-way ANOVA) between these three HIV prevalence areas with respect to the proportion of PWID living in each area as well as OST treatment indicators, such as the number of individuals receiving specific types of OST. We also graphically display the absolute increases in OST availability in each area over a five-year time period (2008–2012).
Results
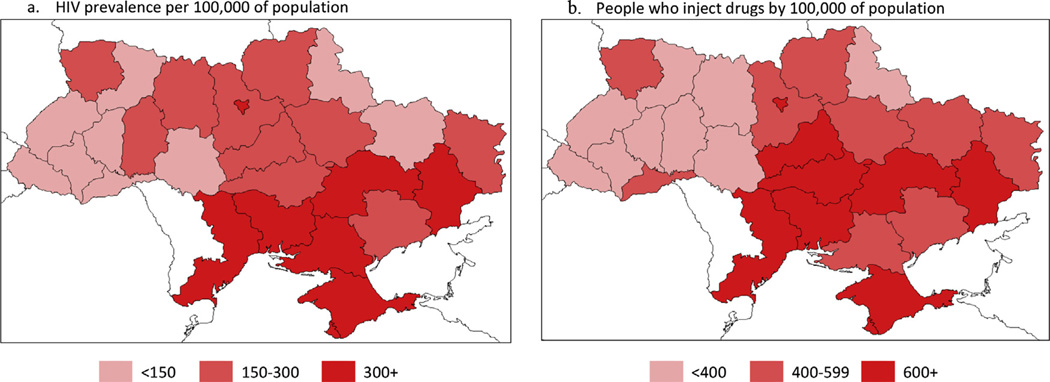
Ukraine is divided into 24 oblasts and three special administrative regions. At the time of the writing of this manuscript, Crimea was still a part of Ukraine. Fig. 1 shows registered number of HIV positive individuals and estimated number of PWID per 100,000 population in each of Ukraine’s oblasts and administrative regions. As depicted by the darker red colour in the maps in Fig. 1, six of the seven oblasts with the highest prevalence of HIV (AR of Crimea, Dnipropetrovsk, Donetsk, Mykolaiv, Odesa, oblasts and City of Kyiv) − >300 HIV positive person per 1000,000 population, were also among the oblasts with the greatest number of PWID per 100,000 population (>600/100,000 population). However, two oblasts (Kirovohrad oblast and Cherkasy oblast were among those with the highest PWID concentrations (>600/100,000) but had intermediate HIV prevalence (150–299/100,000). Additionally, HIV prevalence was intermediate in the oblasts of Khmelnytskyi and Zhytomyr, despite low prevalence of PWID.
Fig. 1.
HIV prevalence (a) per 100,000 of population (2013) and (b) people who inject drugs by 100,000 of population (2013).
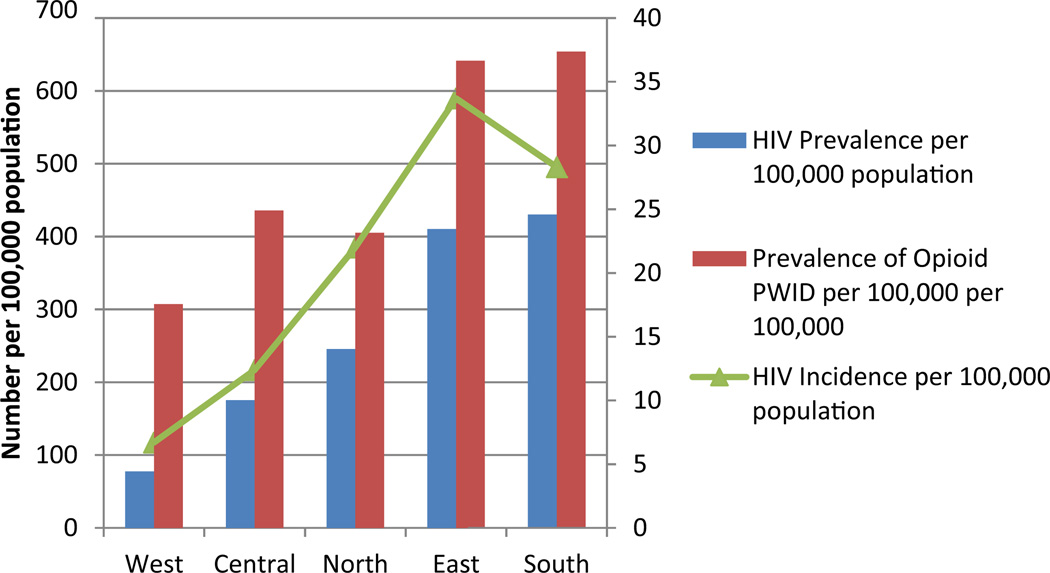
In order to more fully explore the geographic overlap between HIV and opioid use specifically, we sought to regionalize estimates for HIV prevalence, HIV incidence and prevalence of opioid dependence into five regions (West, Central, North East and South) as shown in Table 1. Fig. 2 graphically depicts these estimates (expressed by total number per 100,000 population) by region. Similar to the maps in Fig. 1, the regions of the country with the largest number of opioid dependent persons (the south and eastern portions of the country) correspond to the regions with the highest HIV prevalence and HIV incidence.
Fig. 2.
Regional rates of HIV and opioid use, Ukraine (2013).
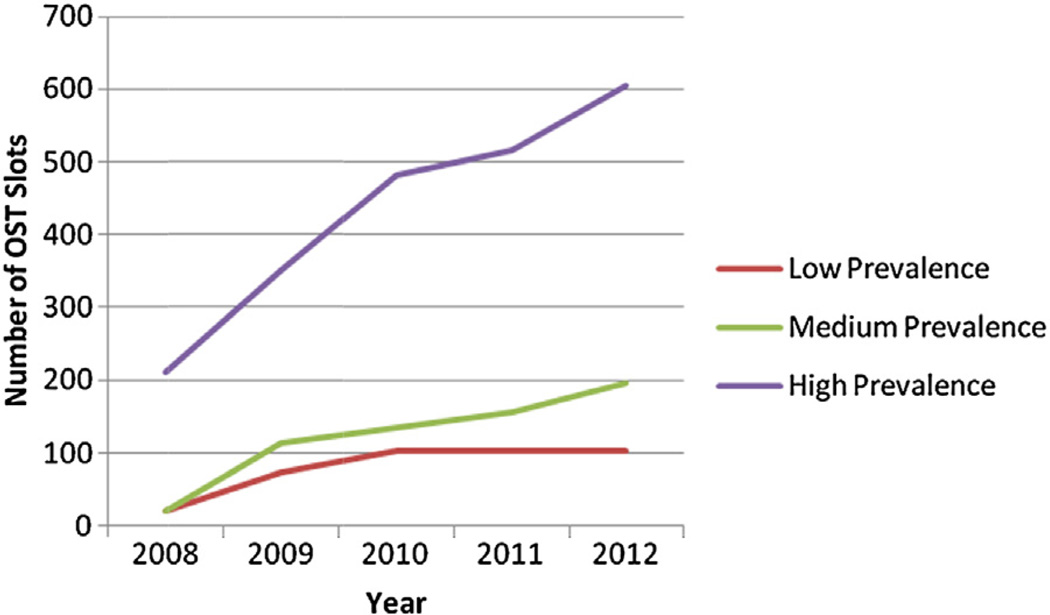
We then used the HIV prevalence in Fig. 1, (low: <150/100,000; medium: 150–299/100,000, high: >300/100,000) to explore changes in OST treatment availability, as defined by total number of global fund to fight AIDS, tuberculosis and malaria (GF) funded and state subsidized treatment slots, between 2008 and 2012. While Fig. 3 shows modest increases in the number of OST treatment slots during this time, in areas defined as having a high HIV prevalence (>300/100,000), treatment slots more than tripled to a total of more than 600 (Fig. 3).
Fig. 3.
OST treatment slots in low, medium and high HIV prevalence regions in Ukraine.
Finally, we examined differences in OST treatment type, e.g. methadone or buprenorphine, across HIV prevalence categories (low, medium and high as previously defined, Table 2). The median number of opioid PWIDs per 100,000 population as well as the median number of all OST treatment slots per 100,000 varied significantly across the three HIV prevalence categories. Similarly, number of each type of OST, methadone maintenance (MMT) and buprenorphine maintenance (BMT), also significantly differed across categories (Table 2). However, the total proportion of individuals receiving either MMT or BMT as a function of the total number of estimated opioid users in each HIV prevalence category was quite low (average across categories: 7.3% and 0.4%, respectively). Overall, for both MMT and BMT, the median age for patients was approximately 35 and the majority of individuals receiving treatment were male. Finally, with respect to the proportion of HIV positive patients receiving any OST, less than half of OST patients receiving MMT or BMT were HIV positive patients across prevalence categories (Table 2).
Table 2.
Opioid substitution treatment stratified by HIV prevalence categories (2013).
| Variable | Low HIV prevalence (less than 150 per 100,000 population) |
Medium HIV prevalence (150–300 per 100,000 population) |
High HIV prevalence (greater than 300 per 100,000) |
P-Value (one-way ANOVA) |
|---|---|---|---|---|
| Median estimated number of opioid PWIDs per 100,000 population | 307.3 | 431 | 799.4 | <0.001 |
| Median number of OST slots per 100,000 population | 141 | 210 | 647 | <0.001 |
| Median number of MMT patients (% of total median number of PWIDs) | 104 (2.8) | 170 (2.5) | 533 (2.9) | 0.001 |
| Median number of BMT patients (% of total median number of PWIDs) | 20 (0.5) | 18 (0.3) | 52 (0.3) | 0.03 |
| Median number of female MMT patients (% of total median number of MMT patients) | 23 (22.1) | 34 (20.0) | 106 (19.9) | 0.004 |
| Median number of female BMT patients (% of total median number of BMT patients) | 4 (20.0) | 3 (16.7) | 11 (21.1) | 0.04 |
| Median number of HIV positive MMT patients (% of total median number of MMT patients) | 32 (30.7) | 64 (37.6) | 211 (39.6) | 0.001 |
| Median number of HIV positive BMT patients (% of total median number of BMT patients) | 7 (30.4) | 8 (44.4) | 30 (57.7) | 0.01 |
| Median average age for MMT patients | 35.6 | 35.3 | 34.8 | 0.78 |
| Median average age for BMT patients | 35.1 | 34 | 39.4 | 0.001 |
| Median average dose of MMT | 72.5 | 83 | 81.5 | 0.10 |
| Median average dose of BMT | 11 | 11.2 | 12.2 | 0.81 |
Discussion
Here we report national treatment data for Ukraine in specific geographic regions. The geographic variability in both numbers of HIV positive individuals and numbers of PWIDs suggests that in some oblasts there appears to be a more generalized HIV epidemic among both PWID and the general population while in other areas there may be a more concentrated epidemic among PWIDs. In part this may be attributable to differences in socioeconomic, cultural and religious contexts as well as to drug trafficking routes across Ukraine. This observation is consistent with recent data suggesting that the HIV epidemic in Ukraine is becoming more generalized with populations of PWID, commercial sex workers and men who have sex with men (MSM) serving as potential “bridge populations” to the general Ukrainian (Barnett, Whiteside, Khodakevich, Kruglov, & Steshenko, 2000; Beyrer et al., 2010; Booth, Kwiatkowski, Brewster, Sinitsyna, & Dvoryak, 2006; Burruano & Kruglov, 2009; Goodwin, Kozlova, Nizharadze, & Polyakova, 2004; Hamers & Downs, 2003).
Although the number of OST slots has been increasing, particularly in areas with high HIV prevalence, overall coverage of OST in Ukraine remains quite low. Between 3% and 4% of PWID are currently receiving OST (methadone (2.5–2.9%), buprenorphine (0.3–0.5%)). Thus, there appears to be a general lack of committed resources from the national government, either through allocation of in-country or international funding, to meeting the growing need for OST among the already sizable population of PWID in Ukraine. Even in the regions of the country with the greatest prevalence of both PWID (>600 per 100,000) and HIV (>300 per 100,000), the total number of individuals receiving OST is unacceptably low. Bringing treatment to scale while maintaining quality of care is likely to be challenging given the limited capacity to provide OST in country, but will be cost-effective by bringing substantial reductions in HIV prevalence, particularly if provided in combination with high coverage of needle and syringe programmes and HIV antiretroviral therapy (Alistar et al., 2011; Strathdee et al., 2010). Among the small proportion of the opioid dependent population accessing OST, median methadone and buprenorphine doses were within the therapeutic ranges recommended by the World Health Organization (Guidelines, 2009). Additional work is urgently needed to move from a more punitive approach to drug use to a more holistic, integrated model of care for PWID, particularly those PWID with HIV and/or other infectious diseases (Wu & Clark, 2013). In particular, the discrimination that many PWID encounter from both law enforcement and government agencies (Booth et al., 2013; Spicer et al., 2011) needs to be addressed through more structured education through the existing systems of training and education for medical and legal professionals. Furthermore, data from Alistar et al. (2011) suggest that investment in harm reduction measures, including expansion of OST, needs to be effectively communicated to policy makers in Ukraine such that they better understand the dynamics between untreated addiction and HIV among PWID and the national HIV epidemic.
Integration of care
Importantly, numerous policies in Ukraine and elsewhere continue to impede access to treatment services, both HIV care and drug treatment, for PWID. These include, but are not limited to, expanding access to high quality OST, integrating HIV, OST and other medical services for PWID, and providing new leadership to move away from criminalization of drug use (Ministry of Health of Ukraine, 2012). The existing infrastructure in Ukraine, with numerous types of clinical specialties, i.e. TB, HIV, Narcology, etc., is often is implicated in providing fractured care However, the numerous clinical settings this system supports may provide a unique opportunity to provide better integration of HIV treatment and buprenorphine (Bruce, Dvoryak, Sylla, & Altice, 2007), provided the treatment siloes are dismantled. Integration of HIV and substance use treatment among PWID is particularly important as several countries have had significant successes in expanded access to ART among PWID and in decreasing new HIV infections through expanded access to syringe exchange programmes and OST (Ministry of Health of Ukraine, 2012). Bachireddy and colleagues found that integration of healthcare services for nearly 300 HIV positive PWID in Ukraine improved access to ART, while also improving substance use related outcomes such as more appropriate OST dosing based on WHO guidelines (Bachireddy et al., 2013). Importantly, this study found that OST alone was sufficient in improving the health related quality of life for HIV positive PWID (Bachireddy et al., 2013). Additionally, integration of methadone at inpatient TB facilities in Ukraine has also demonstrated favourable outcomes with respect to TB treatment retention and adherence (Morozova, Dvoryak, & Altice, 2013).
Buprenorphine as an opportunity for better integration of medical care and addiction treatment?
The availability of buprenorphine in community-based settings expands the treatment options for opioid addicted individuals and has the potential to increase the overall number of individuals in drug treatment. Buprenorphine has a longer duration of action than methadone (24–72 h), stable serum levels, and allows for the option of either daily or thrice weekly dosing. Buprenorphine has been used in a diverse array of clinical settings (Alford et al., 2007; Colameco, Armando, & Trotz, 2005; Fiellin et al., 2008; Mintzer et al., 2007). Given the emphasis on specialized clinics to provide treatment for specific illnesses in Ukraine, an opportunity exists to provide collaborative care with different types of clinical specialists. This approach has demonstrated some effectiveness in urban settings (Alford et al., 2007). Bachireddy et al. (2013) found that clinical settings in Ukraine that had better integration of OST and medical care were associated with greater quality of healthcare indicators. This supports the idea that integration is possible and leads to better care outcomes. However, additional support for addiction training programmes for clinicians in Ukraine is crucial. Additionally, while training on appropriate prescribing of buprenorphine among clinicians is an important first step, data suggest that training alone is insufficient in facilitating clinicians prescribing buprenorphine in their clinical settings. Clinics also need to focus on provision of ancillary services such as mental health care and social support (Hutchinson, Catlin, Andrilla, Baldwin, & Rosenblatt, 2014).
Limitations
It is important to consider some limitations when interpreting our findings. Data were administrative in nature and therefore may be subject to errors in reporting. Also, there may be geographic differences in data reporting for both estimates of PWID per 100,000 and HIV prevalence across oblasts. Additionally, it is important to note that the use of central tendency measures, such as median values, do not always portray the variability, or skew, of the data. We acknowledge that some oblasts in Ukraine are experiencing much larger HIV epidemics than others and that the number of PWIDs varies substantially across oblasts. However, we felt that median values provide more accurate and precise countrywide estimates of HIV prevalence and PWID per 100,000 as median values are not as heavily influenced by outlying data points.
Political unrest: implications for treatment
In February 2014, the administrative region of Crimea was annexed by Russia. The implications of this for access to OST are likely to be significant. Russian law forbids OST and Russian media has reported on plans to close OST programmes in the region (Hutchinson et al., 2014); there are more than 800 patients currently on OST in Crimea. Importation of methadone and buprenorphine into Crimea has been prevented, and with supplies dwindling, treatment providers have begun to reduce patient dosages (Holt, 2014; Klepikov, 2014). There are concerns that the withdrawal of treatment will lead to increases in high-risk injecting drug use, with concomitant HIV transmission. Of particular concern with the current conflict in Ukraine is that HIV/AIDS prevention gains, including expansion of OST programmes and efforts to better integrate HIV and OST treatment that have been achieved during the past decade are at risk. It is crucial to have the continued support of the Ukrainian government in order to continue expansion of HIV prevention and OST programmes in the country. The current conflict may divert critical resources away from HIV prevention and treatment efforts such that additional progress is impeded. In other parts of the world, political and social instability have served as driving forces for increased HIV transmission (Premkumar & Tebandeke, 2011). To avoid treatment gaps, the Alliance, with support from the International Renaissance Foundation, has put a system in place where OST patients from Crimea can continue to receive their OST in other Ukrainian cities. Furthermore, scale up of addiction treatment for PWID, especially OST, can have significant impact on preventing injection related morbidity, such as HIV and HCV infection (MacArthur et al., 2012). Ukraine can learn from the mistakes other nations have made in denying critical treatment opportunities to PWID. These mistakes have cost lives and have resulted in disastrous social consequences, including transmission of infectious diseases, such as HIV and viral hepatitis, mass incarceration and family and economic disruption, for many thousands of people.
Acknowledgements
This work was supported by grants from: Elena Pinchuk Anti-AIDS Foundation and National Institute of Allergy and Infectious Diseases (P30-AI042853).
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
References
- Alford DP, LaBelle CT, Richardson JM, O’Connell JJ, Hohl CA, Cheng DM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. Journal of General Internal Medicine. 2007;22(2):171–176. doi: 10.1007/s11606-006-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: A modeling analysis for Ukraine. PLoS Medicine. 2011;8(3):e1000423. doi: 10.1371/journal.pmed.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachireddy C, Soule MC, Izenberg JM, Dvoryak S, Dumchev K, Altice FL. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug and Alcohol Dependence. 2013;134:106–114. doi: 10.1016/j.drugalcdep.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett T, Whiteside A, Khodakevich L, Kruglov Y, Steshenko V. The HIV/AIDS epidemic in Ukraine: Its potential social and economic impact. Social Science and Medicine. 2000;51(9):1387–1403. doi: 10.1016/s0277-9536(00)00104-0. [DOI] [PubMed] [Google Scholar]
- Beyrer C, Baral SD, Walker D, Wirtz AL, Johns B, Sifakis F. The expanding epidemics of HIV type 1 among men who have sex with men in low- and middle-income countries: Diversity and consistency. Epidemiologic Reviews. 2010 Apr;32(1):137–151. doi: 10.1093/epirev/mxq011. [DOI] [PubMed] [Google Scholar]
- Bojko MJ, Dvoriak S, Altice FL. At the crossroads: HIV prevention and treatment for people who inject drugs in Ukraine. Addiction. 2013 Oct;108(10):1697–1699. doi: 10.1111/add.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth RE, Dvoryak S, Sung-Joon M, Brewster JT, Wendt WW, Corsi KF, et al. Law enforcement practices associated with HIV infection among injection drug users in Odessa, Ukraine. AIDS and Behavior. 2013:1–11. doi: 10.1007/s10461-013-0500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth RE, Kwiatkowski CF, Brewster JT, Sinitsyna L, Dvoryak S. Predictors of HIV sero-status among drug injectors at three Ukraine sites. AIDS. 2006;20:2217–2223. doi: 10.1097/QAD.0b013e328010e019. [DOI] [PubMed] [Google Scholar]
- Bruce RD, Dvoryak S, Sylla L, Altice FL. HIV treatment access and scale-up for delivery of opiate substitution therapy with buprenorphine for IDUs in Ukraine – Programme description and policy implications. International Journal of Drug Policy. 2007;18:326–328. doi: 10.1016/j.drugpo.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burruano L, Kruglov Y. HIV/AIDS epidemic in Eastern Europe: Recent developments in the Russian Federation and Ukraine among women. Gender Medicine. 2009 Apr;6(1):277–289. doi: 10.1016/j.genm.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Colameco S, Armando J, Trotz C. Opiate dependence treatment with buprenorphine: One year’s experience in a family practice residency setting. Journal of Addictive Diseases. 2005;24(2):25–32. doi: 10.1300/J069v24n02_03. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, et al. Mortality among regular or dependent users of heroin and other opioids: A systematic review and meta-analysis of cohort studies. Addiction. 2011 Jan;106(1):32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Mathers BM, Wirtz A, Wolfe D, Kamarulzaman A, Carrieri MP, et al. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. International Journal of Drug Policy. 2014;25:53–60. doi: 10.1016/j.drugpo.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Moore BA, Sullivan LE, Becker WC, Pantalon MV, Chawarski MC, et al. Long-term treatment with buprenorphine/naloxone in primary care: Results at 2–5 years. American Journal on Addictions. 2008;17(2):116–120. doi: 10.1080/10550490701860971. [DOI] [PubMed] [Google Scholar]
- Golovanevskaya M, Vlasenko L, Saucier R. In control?: Ukrainian opiate substitution treatment patients strive for a voice in their treatment. Substance Use & Misuse. 2012;47:511–521. doi: 10.3109/10826084.2012.644117. [DOI] [PubMed] [Google Scholar]
- Goodwin R, Kozlova A, Nizharadze G, Polyakova G. High-risk behaviors and beliefs and knowledge about HIV transmission among school and shelter children in Eastern Europe. Sexually Transmitted Diseases. 2004 Nov;31(11):670–675. doi: 10.1097/01.olq.0000143092.56513.32. [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell M, Bornemann R, Sullivan L, Ali R. Substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database of Systematic Reviews. 2011 CD004145. [Google Scholar]
- Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. Geneva: World Health Organization; 2009. [Accessed 8.01.14]. Available from: http://www.who.int/hiv/pub/idu/opioid. [PubMed] [Google Scholar]
- Hamers FF, Downs AM. HIV in central and eastern Europe. Lancet. 2003;361:1035–1044. doi: 10.1016/S0140-6736(03)12831-0. [DOI] [PubMed] [Google Scholar]
- Holt E. Fears over future of opioid substitution therapy in Crimea. Lancet. 2014;383:1113. doi: 10.1016/s0140-6736(14)60234-8. [DOI] [PubMed] [Google Scholar]
- Humphreys G. Opioid treatment in Ukraine risks losing momentum. Bulletin of the World Health Organization. 2013 Feb;91(2):87–88. doi: 10.2471/BLT.13.020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E, Catlin M, Andrilla CH, Baldwin LM, Rosenblatt RA. Barriers to primary care physicians prescribing buprenorphine. Annals of Family Medicine. 2014 Mar-Apr;12(2):128–133. doi: 10.1370/afm.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepikov A. Annexation of Crimea: What will happen to 14,000 drug users? The Huffington Post. 2014 [Google Scholar]
- Lawrinson P, Ali R, Buavirat A, Chiamwongpaet S, Dvoryak S, Habrat B, et al. Key findings from the WHO collaborative study on substitution therapy for opioid dependence and HIV/AIDS. Addiction. 2008;103(9):1484–1492. doi: 10.1111/j.1360-0443.2008.02249.x. [DOI] [PubMed] [Google Scholar]
- MacArthur G, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, et al. Evidence for the effectiveness of opioid substitution treatment in relation to HIV transmission in people who inject drugs: A systematic review and meta-analysis. British Medical Journal. 2012;345:e5945. doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malta M, Magnanini MM, Strathdee SA, Bastos FI. Adherence to antiretro-viral therapy among HIV-infected drug users: A meta-analysis. AIDS and Behavior. 2010;14:731–747. doi: 10.1007/s10461-008-9489-7. [DOI] [PubMed] [Google Scholar]
- Mimiaga MJ, Safren SA, Dvoryak S, Reisner SL, Needle R, Woody G. We fear the police, and the police fear us: Structural and individual barriers and facilitators to HIV medication adherence among injection drug users in Kiev, Ukraine. AIDS Care. 2010;22:1305–1313. doi: 10.1080/09540121003758515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health of Ukraine. Bulletin 37: HIV-infection in Ukraine. Kiev: Ministry of Health of Ukraine; 2012. [Google Scholar]
- Mintzer IL, Eisenberg M, Terra M, MacVane C, Himmelstein DU, Woolhandler S, et al. Treating opioid addiction with buprenorphine-naloxone in community-based primary care settings. Annals of Family Medicine. 2007;5(2):146–150. doi: 10.1370/afm.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova O, Dvoryak S, Altice FL. Methadone treatment improves tuberculosis treatment among hospitalized opioid dependent patients in Ukraine. International Journal of Drug Policy. 2013;24:e91–e98. doi: 10.1016/j.drugpo.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar R, Tebandeke A. Political and socio-economic instability: Does it have a role in the HIV/AIDS epidemic in sub-Saharan Africa? Sahara Journal. 2011;8(2):65–73. doi: 10.1080/17290376.2011.9724987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M, Chtenguelov V, Subata E, Weiler G, Uchtenhagen A. Feasibility of buprenorphine and methadone maintenance programmes among users of home-made opioids in Ukraine. International Journal of Drug Policy. 2010;21(3):229–233. doi: 10.1016/j.drugpo.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Schaub M, Subata E, Chtenguelov V, Weiler G, Uchtenhagen A. Feasibility of buprenorphine maintenance therapy programs in the Ukraine: First promising treatment outcomes. European Addiction Research. 2009;15(3):157–162. doi: 10.1159/000217586. [DOI] [PubMed] [Google Scholar]
- Spicer N, Bogdan D, Brugha R, Harmer A, Murzalieva G, Semigina T. ‘It’s risky to walk in the city with syringes’: Understanding access to HIV/AIDS services for injecting drug users in the former Soviet Union countries of Ukraine and Kyrgyzstan. Globalization and Health (N.Y.) 2011;7:22. doi: 10.1186/1744-8603-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Hallett TB, Bobrova N, Rhodes T, Booth R, Abdool R, et al. IV and risk environment for injecting drug users: The past, present and future. Lancet. 2010;376:268–284. doi: 10.1016/S0140-6736(10)60743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drugusers with HIV infection: A review of barriers and ways forward. Lancet. 2010;376:355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- Wu Z, Clark N. Scaling up opioid dependence treatment in low- and middle-income settings. Bulletin of the World Health Organization. 2013;91:82–82A. doi: 10.2471/BLT.12.110783. [DOI] [PMC free article] [PubMed] [Google Scholar]