Abstract
Different clinical variants of probable Alzheimer’s disease (AD) share underlying plaques and tangles but show distinct atrophy patterns. We included 52 posterior cortical atrophy (PCA), 29 logopenic variant primary progressive aphasia (lvPPA), 53 early-onset (EOAD) and 42 late-onset AD (LOAD) patients, selected for abnormal CSF-Aβ42, with CSF and MRI data available. Bootstrapping revealed no differences in the prevalence of abnormal CSF total-tau and phosphorylated-tau between probable AD variants (range total-tau: 84.9–92.3%, phosphorylated-tau: 79.2–93.1%, p>0.05). Voxel-wise linear regressions showed various relationships between lower CSF-Aβ42 and syndrome-specific atrophy, involving precuneus, posterior cingulate, and medial temporal lobe (MTL) in EOAD, occipital cortex and middle temporal gyrus in PCA; anterior cingulate, insular cortex and precentral gyrus (left>right) in lvPPA; and MTL, thalamus, and temporal pole in LOAD (all at p<0.001 uncorrected). In contrast, CSF-tau was not related to gray matter atrophy in any group. Our findings suggest that lower CSF-Aβ42 – and not increased total-tau and phosphorylated-tau – relates to reduced gray matter volumes, mostly in regions that are typically atrophied in distinct clinical variants of probable AD.
Keywords: Alzheimer’s disease, cerebrospinal fluid, magnetic resonance imaging, amyloid, tau, atrophy
1. INTRODUCTION
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that can present with a variety of clinical manifestations. Patients with late-onset AD (LOAD, defined as age-at-onset ≥65 years in most studies) typically present with memory deficits, while early-onset AD (EOAD, <65 years) patients show more impaired attention, language, visuo-spatial abilities and executive functions (Koedam, et al., 2010, Koss, et al., 1996, Smits, et al., 2012, Stopford, et al., 2008). AD can also present with focal non-amnestic syndromes, including posterior cortical atrophy (PCA) affects occipital, parietal and occipitotemporal cortices and is clinically characterized by predominant visuo-spatial and visuo-perceptive deficits (Benson, et al., 1988, Crutch, et al., 2012). Logopenic variant primary progressive aphasia (lvPPA) is associated with brain atrophy in the language-dominant left hemisphere resulting in a progressive language disorder (Gorno-Tempini, et al., 2008, Mesulam, et al., 2008). PCA and lvPPA usually manifest at a young age and are caused by AD pathology (i.e. amyloid-beta (Aβ) plaques and neurofibrillary tangles) in the majority of patients (Galton, et al., 2000, Mesulam, et al., 2014, Renner, et al., 2004). Due to their atypical, non-amnestic presentation, they may pose clinicians with great diagnostic dilemmas. In the past decades, several pathophysiological and neurodegenerative biomarkers, including Aβ42, total-tau (t-tau) and phosphorylated-tau (p-tau) concentrations in cerebrospinal fluid (CSF) (Blennow, et al., 2010, Schoonenboom, et al., 2012), have been developed to support the clinical diagnosis of AD.
Previous studies on CSF biomarkers in distinct clinical variants of probable AD have shown that CSF Aβ42 levels are reduced (reflecting greater amyloid burden) independent of phenotype (Baumann, et al., 2010, Bouwman, et al., 2009, Coppi, et al., 2014, de Souza, et al., 2011a, de Souza, et al., 2011b, Magnin, et al., 2014, Santangelo, et al., 2014, Seguin, et al., 2011, Teng, et al., 2014). In contrast, for CSF t-tau and p-tau results have shown discrepancies between studies. Some studies have found comparable t-tau and p-tau levels between amnestic AD and PCA (Baumann, et al., 2010, Coppi, et al., 2014, de Souza, et al., 2011a, de Souza, et al., 2011b, Seguin, et al., 2011), amnestic AD and lvPPA (Santangelo, et al., 2014, Teng, et al., 2014), and between EOAD and LOAD (Bouwman, et al., 2009). One study found lower CSF t-tau and p-tau levels in PCA than in lvPPA and amnestic AD (Teng, et al., 2014) and another study found higher CSF t-tau and p-tau levels in lvPPA compared to amnestic AD (Magnin, et al., 2014). In general, sample sizes of these studies have been small, included only two or three different clinical variants of probable AD and did not always select for patients with abnormal CSF biomarker profiles.
CSF t-tau and p-tau are believed to reflect axonal neurodegeneration and tangle pathology (Blennow, et al., 2010), and have been associated with greater rates of atrophy in AD patients (Hampel, et al., 2005, Henneman, et al., 2009). CSF tau is thus associated with neurodegeneration in AD, but in other conditions that involve massive neurodegeneration, such as frontotemporal dementia, CSF tau is not elevated consistently (Blennow, et al., 2010, Scherling, et al., 2014, Schoonenboom, et al., 2012). One possible explanation is that the likelihood of tau spill-in into CSF is related to the site of neurodegeneration and its proximity to the ventricular space (e.g. medial temporal lobes versus neocortex (Murray et al., 2011)). To the best of our knowledge, no study has investigated the relationships between CSF biomarkers and patterns of brain atrophy at a voxelwise level across multiple probable AD variants. In this study, we aimed to determine the prevalence of abnormal CSF biomarkers and their relationships to brain atrophy in LOAD, EOAD, PCA and lvPPA patients. We hypothesized that 1) t-tau and p-tau CSF biomarkers would be comparable across probable AD variants, and 2) t-tau and p-tau, and not Aβ42, would be associated with syndrome-specific patterns of brain atrophy.
2. METHODS
2.1 Participants
A total of 176 AD patients were included from the Amsterdam Dementia Cohort (van der Flier, et al., 2014). All patients underwent standard dementia screening that included a medical history and physical examination, a structured caregiver interview, brain MRI and neuropsychological testing. Clinical diagnosis was established by consensus in a multidisciplinary team. All patients fulfilled National Institute on Ageing-Alzheimer’s Association (NIA-AA) criteria for probable AD (McKhann, et al., 2011) or mild cognitive impairment (MCI) due to AD (Albert, et al., 2011), with at least intermediate likelihood due to reduced Aβ42 levels in CSF (see below). Patients with PCA and lvPPA additionally met specific diagnostic criteria for PCA (Mendez, et al., 2002, Tang-Wai, et al., 2004) or lvPPA (Gorno-Tempini, et al., 2011). We included all PCA and lvPPA patients in the VUMC database with available CSF and MRI data. Next, AD patients that did not meet criteria for PCA or lvPPA were categorized as EOAD (defined as <65 years at time of diagnosis) or LOAD (defined as (≥65 years at time of diagnosis). 53 EOAD patients were selected from a larger pool based on matching criteria (i.e. age, sex, disease severity (CDR and MMSE) and MRI scanner type) to the PCA and lvPPA patients. Similar matching criteria – except for age – were applied to select 42 LOAD patients. LOAD patients had predominantly memory presentations, while EOAD patients were also memory-predominant but showed more diffuse cortical symptoms in addition to their memory deficits. Informed consent was obtained from all subjects or their assigned surrogate decision-makers, and the VUMC institutional review board for human research approved the study.
2.2 Cerebrospinal Fluid Biochemical Analysis
CSF was obtained by lumbar puncture during dementia screening using a 25-gauge needle and collected in 10-mL polypropylene tubes (Sarstedt, Nümbrecht, Germany). Part of the CSF was used for routine analysis including leukocyte count, erythrocyte count, glucose concentration, and total protein concentration. Within two hours, the remaining CSF was centrifuged at 1800g for 10 minutes at 4°C, transferred to new polypropylene tubes, and stored at −20°C until biomarker analysis (within 2 months). Aβ42, total tau (t-tau), and phosphorylated tau (p-tau) were measured with commercially available ELISAs (Innotest β-amyloid(1–42), Innotest hTAU-Ag and Innotest Phosphotau(181P), respectively; Innogenetics, Ghent, Belgium) on a routine basis as described before (Mulder, et al., 2010). Only patients with a high likelihood of harboring amyloid pathology were included since we aimed to study patients with underlying AD pathology and a small proportion of PCA and lvPPA clinical syndromes are caused by non-AD pathologies (Crutch, et al., 2012). We used a threshold of CSF Aβ42 <640 ng/L that showed excellent correspondence with global [11C]PIB PET binding in VUMC patients (Zwan, et al., 2014). This threshold is similar to a recent independent PET-CSF study (cut-off: <647 ng/L), in which there was also excellent agreement between amyloid PET positivity and reduced CSF Aβ42 (Palmqvist, et al., 2014).
2.3 Structural MRI Image Acquisition
Structural MRI scans were performed on a 1T (Magnetom Impact, Siemens, n=21; 8 EOAD, 7 PCA, 3 lvPPA and 3 LOAD), 1.5T (Sonata, Siemens, n=34; 10 EOAD, 10 PCA, 7 lvPPA and 7 LOAD) or 3T (SignaHDxt, GE Healtcare, n=121; 35 EOAD, 35 PCA, 19 lvPPA and 32 LOAD) unit. Acquisition parameters have been published previously (Moller, et al., 2013, Ossenkoppele, et al., 2012, Sluimer, et al., 2008). The proportion of patients scanned on each scanner was balanced across the groups and all statistical models included scanner type as nuisance variable. MRI acquisition and lumbar puncture were performed on the same day in the majority of patients (151/176, median=0, range: 0–6 months).
2.4 Structural MRI Imaging Data Processing and Analysis
MRI data were segmented using the New Segment toolbox implemented in the Statistical Parametric Mapping (SPM) 8 software (Wellcome Trust Centre for Neuroimaging, Institute of Neurology at University College London). DARTEL was used to generate a study-specific template by aligning the gray matter (GM) images non-linearly to a common space (Ashburner, 2007). Native GM and white matter (GM) images were spatially normalized to the DARTEL template using individual flow fields (modulation was applied to preserve the total amount of signal). Images were smoothed using a 8mm full width at half maximum (FWHM) isotropic Gaussian kernel. Images were inspected visually after each step in the processing pipeline and the final smoothed-modulated-warped GM images were checked for sample homogeneity using the VBM8 toolbox to identify potential outliers. Finally, we created implicit masks thresholded at GM volumes between 0.05–0.3 (at 0.05 steps), visually assessed how each of these masks overlaid on the study specific gray matter template, and finally selected an implicit mask thresholded at 0.1 as the best fit. This implicit mask was used for statistical analysis and provided the optimal balance between noise reduction and preservation of actual GM.
2.5 Statistical Analysis
Differences between groups for baseline characteristics were assessed using ANOVA with post hoc Bonferroni tests for continuous variables, X2 tests for dichotomous data and Kruskal-Wallis tests for ordinal data. Further statistical analysis was divided into three steps. First, we investigated whether the prevalence of abnormal CSF biomarkers (t-tau and p-tau) differed among probable AD variants. Aβ42 and tau/Aβ42 ratios were not tested as CSF Aβ42 was used as an inclusion criterion. We used a priori cutoffs (t-tau >375 ng/L and p-tau >52 ng/L) defined previously for clinical practice at VU University Medical Center Amsterdam (Mulder, et al., 2010). For each biomarker and pair of probable AD variants, we calculated a 95% confidence interval (CI) by non-parametric bootstrapping for the difference in frequency of abnormal CSF biomarkers between the two diagnoses, using R version 3.0.2 (R Foundation for Statistical Computing, 2012). If the 95% CI did include zero, we concluded that the prevalence of abnormal CSF biomarkers did not differ for the probable AD variants. Note that we did not perform this comparison for CSF Aβ42, since subjects with normal CSF Aβ42 levels were already excluded. Second, a combined CSF-MRI analysis was performed using SPM8 software, where we tested for associations between CSF biomarkers and brain atrophy using voxel-based morphometry. We applied 3 linear regression models within each probable AD variant, using one CSF biomarker at the time (Aβ42, t-tau, or p-tau as continuous variables) as independent variable and whole-brain gray matter volumes as dependent variable. The models further included age, sex, total intracranial volume and scanner type as nuisance variables. The statistical threshold was set at p<0.001, uncorrected for multiple comparisons. Third, for each probable AD variant we masked the cluster with the greatest T-value derived by the previous analysis, and calculated the mean gray matter probability within this cluster using the Marsbar ROI toolbox implemented in SPM8. We then performed linear regressions between CSF Aβ42 levels and the mean GM probability within each cluster, and calculated both unadjusted bivariate and adjusted (for age, sex, TIV and MRI scanner type) multivariate standardized correlation coefficients (expressed as standardized β) using R version 3.0.2.
3. RESULTS
3.1 Subjects
Demographic and clinical characteristics of 52 PCA, 29 lvPPA, 53 EOAD and 42 LOAD patients are presented in Table-1. On average, patients showed mild disease severity (mean MMSE: 22.0±4.1, mean CDR: 0.8±0.2), and LOAD and lvPPA patients older than PCA and EOAD patients. There were no other significant differences between the groups. Specifically, mean Aβ42, t-tau and p-tau levels in CSF did not differ across groups.
TABLE 1.
Demographic and clinical characteristics according to AD phenotype
| EOAD | PCA | lvPPA | LOAD | |
|---|---|---|---|---|
| N | 53 | 52 | 29 | 42 |
| Age | 61.5±4.4 | 62.6±7.6 | 67.0±7.7a | 75.8±3.0b |
| Sex (% male) | 55.2 | 58.5 | 59.5 | 51.9 |
| Education* | 5.3±1.0 | 5.0±1.1 | 4.7±1.5 | 4.9±1.3 |
| MMSE | 21.5±5.4 | 22.2±3.4 | 21.8±4.3 | 22.1±3.9 |
| CDR | 0.7±0.3 | 0.8±0.2 | 0.9±0.2 | 0.8±0.2 |
| TIV (L) | 1.56±0.13 | 1.57±0.14 | 1.61±0.14 | 1.57±0.14 |
| CSF Aβ42(ng/L)** | 480±90 | 459±98 | 469±117 | 433±103 |
| CSF Total tau (ng/L) | 695±358 | 728±309 | 788±376 | 785±336 |
| % CSF total tau >375 | 84.9 | 92.3 | 85.7 | 90.5 |
| CSF Phospho tau (ng/L) | 92±40 | 93±38 | 96±34 | 97±39 |
| % CSF phospho tau >52 | 79.2 | 90.4 | 93.1 | 90.5 |
Abbreviations: PCA = Posterior cortical atrophy; lvPPA = Logopenic variant primary progressive aphasia; EOAD = Early-onset Alzheimer’s disease; LOAD = Late-onset Alzheimer’s disease; MMSE = Mini-mental state examination; CDR = Clinical dementia rating scale; TIV = Total intracranial volume.
Data are presented as mean ± SD unless otherwise stated. Differences between groups were analyzed using ANOVA with post-hoc Bonferroni tests (age, MMSE, CDR, TIV, CSF), X2 tests (sex), Kruskal-Wallis with post-hoc Mann-Whitney U-tests (education) and bootstrapping (% of suprathreshold CSF concentrations).
using Verhage’s classification (scale 1–7(Verhage, 1964)),
Only patients with CSF Aβ42 <640 were included,
lvPPA > PCA & EOAD, p<0.01;
LOAD > all other groups, p<0.001.
3.2 Prevalence of Abnormal CSF T-tau and P-tau Biomarkers
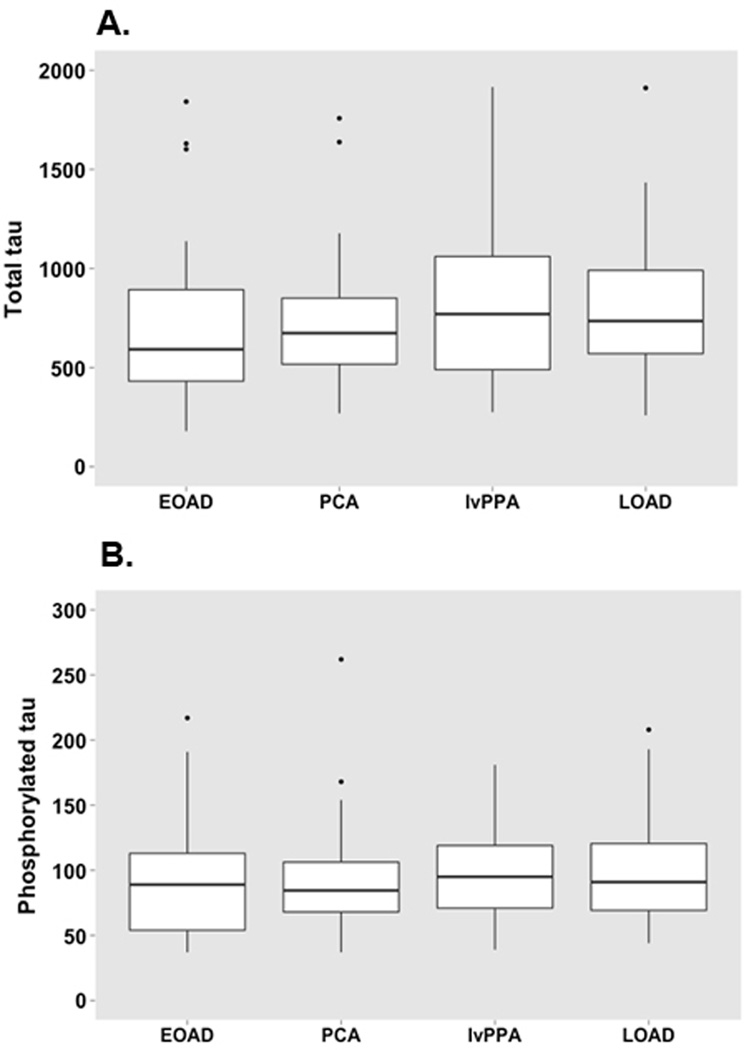
Next, we tested whether the prevalence of abnormal CSF t-tau and p-tau biomarkers (as defined by the a priori thresholds) varied between different variants of probable AD. In general, the CSF biomarker levels were comparable across the AD variants (Table-1). The only exception was that p-tau concentrations in the EOAD group were less often abnormal, although the differences were not statistically significant. Figure 1 represents boxplots of t-tau and p-tau for each probable AD variant.
FIGURE 1.
Boxplots for CSF total tau (A) and phosphorylated tau (B) levels (ng/L) for each AD variant. ANOVA with post-hoc Bonferroni tests revealed no differences between groups.
3.3 Associations Between CSF biomarkers and Cerebral Atrophy
3.3.1 CSF Aβ42
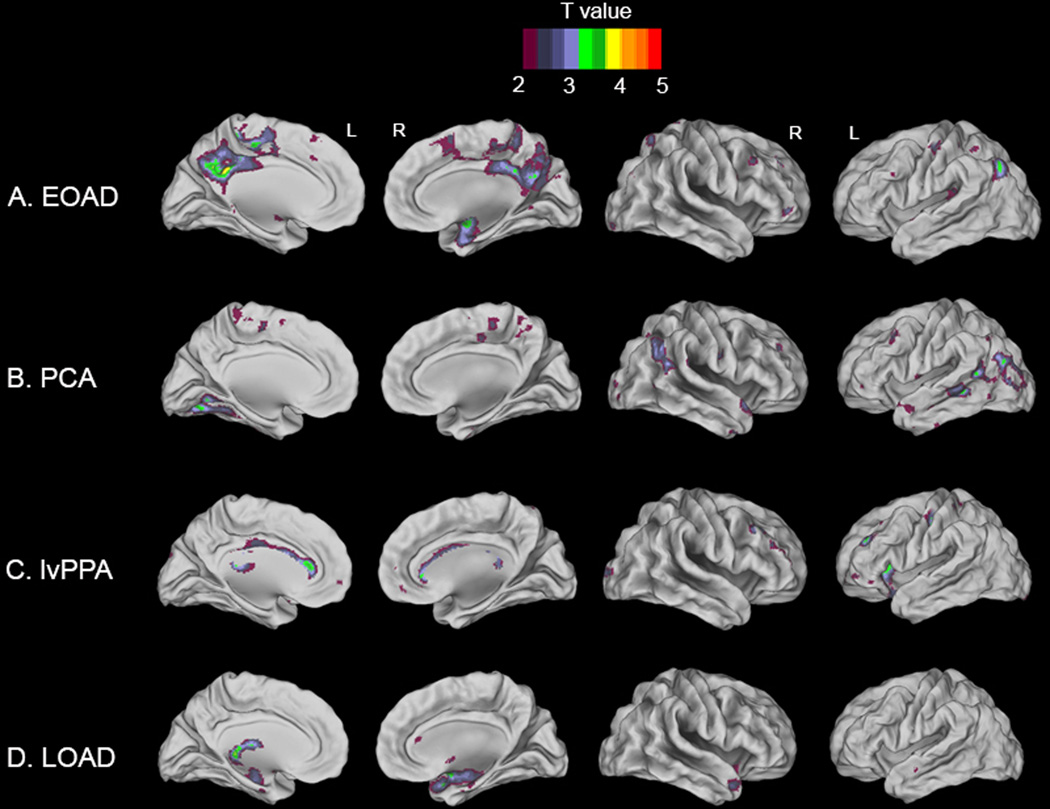
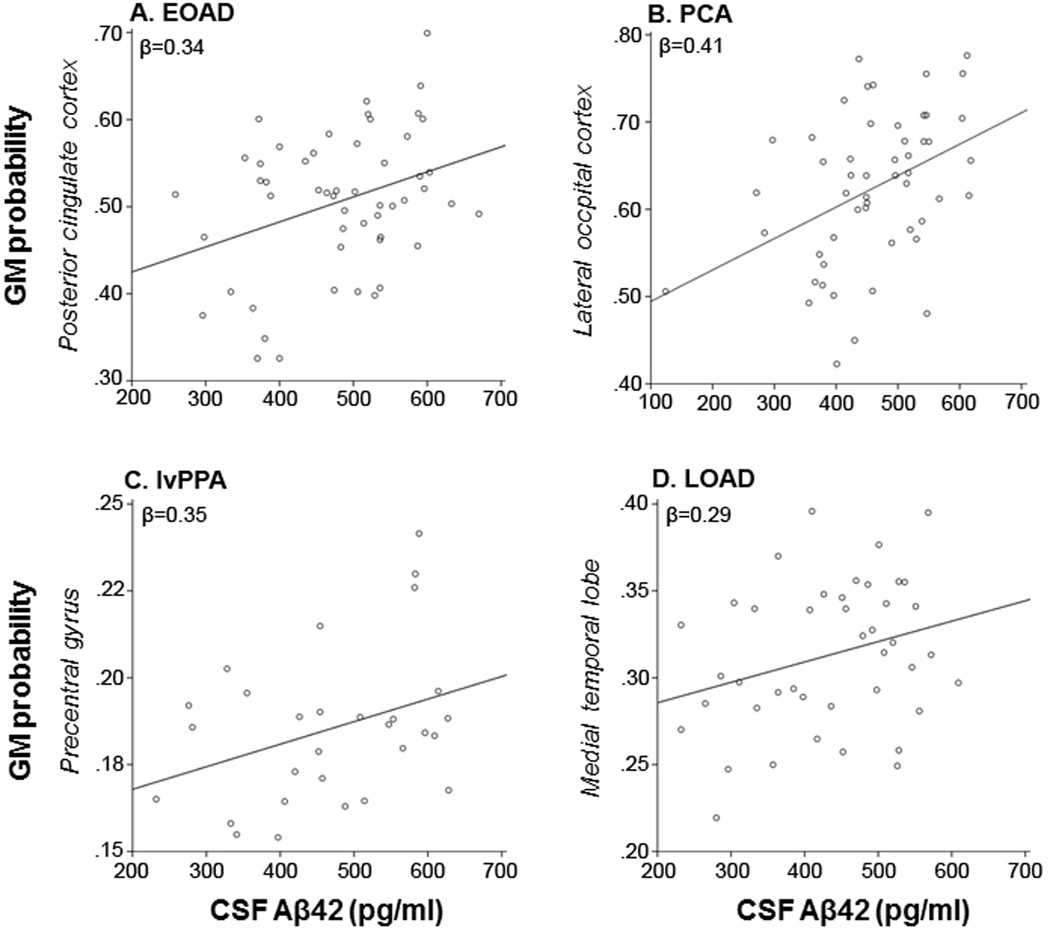
Voxelwise linear regression models with adjustment for age, sex, TIV and scanner type showed several relationships between reduced Aβ42 levels in CSF and reduced gray matter volumes on MRI, mostly in regions that are typically atrophied in the distinct clinical of probable AD. In EOAD, lower Aβ42 was associated with significant clusters in bilateral precuneus, posterior cingulate cortex and right hippocampus and amygdala (Figure-2A). PCA patients showed significant associations between decreased Aβ42 and reduced gray matter volumes in left fusiform and lingual gyri, bilateral lateral occipital cortex and left middle temporal gyrus (Figure-2B). In lvPPA, there was a positive relationship between CSF Aβ42 and gray matter volumes in the anterior cingulate cortex (left more than right), left insular cortex and left precentral gyrus (Figure-2C). Finally, in LOAD patients lower Aβ42 related to reduced gray matter volumes in left thalamus, and right hippocampus, amygdala and temporal pole (Figure-2D). None of those clusters survived family-wise error correction, and analyses with all AD probable variants together did not yield any significant clusters at p<0.001, uncorrected. Adjusting for disease severity by (additionally) entering CDR as a nuisance variable did essentially not change the results (data not shown). Figure-3 illustrates for each AD variant the relationship between CSF Aβ42 and mean gray matter probability within the cluster showing the highest T-values in the previous analysis. Linear regression models yielded the following standardized correlation coefficients ± standard error: β=0.34±0.08 (unadjusted bivariate analysis) and β=0.61±0.07 (adjusted (for age, sex, TIV and MRI scanner type) multivariate analysis) in posterior cingulate for EOAD, β=0.41±0.08 and β=0.85±0.05 in lateral occipital cortex for PCA, β=0.35±0.02 and β=0.77±0.02 in precentral gyrus for lvPPA, and β=0.29±0.04 and β=0.84±0.02 in medial temporal lobe for LOAD.
FIGURE 2.
Voxelwise linear regression analyses between CSF Aβ42 and gray matter volume on MRI in early-onset AD (A), posterior cortical atrophy (B), logopenic variant primary progressive aphasia (C) and late-onset AD (D). T-maps are superimposed on a brain template implemented in Caret software, ranging from 2 to 5 for visualization purposes (T values of 3.27 for EOAD, 3.28 for PCA, 3.48 for lvPPA and 3.33 for LOAD correspond to the p<0.001 threshold; In the text only the significant regions have been reported).
FIGURE 3.
Scatterplots for each probable AD variant of CSF Aβ42 and gray matter probability in the cluster with the greatest T-value: Posterior cingulate cortex for EOAD (A), lateral occipital cortex for PCA (B), precentral gyrus for lvPPA (C), and medial temporal lobe for LOAD (D).
3.3.2 CSF t-tau and p-tau
Linear regressions with t-tau and p-tau as independent variables did not reveal significant relationships with cerebral atrophy in any of the clinical variants of probable AD at p<0.001, uncorrected.
4. DISCUSSION
In the present study we investigated the prevalence of abnormal t-tau and p-tau concentrations in CSF of patients with LOAD, EOAD, PCA and lvPPA, and the relationships between CSF Aβ42, t-tau and p-tau and cerebral atrophy patterns within these probable AD variants. We found that overall CSF biomarker levels were comparable across probable AD variants and CSF t-tau and p-tau showed similar sensitivity in clinical phenotypes of Alzheimer’s disease according to a priori defined thresholds. Voxel-based morphometry showed relationships between lower Aβ42 concentrations and reduced gray matter volume, mostly in regions that correspond to the symptomatology of the distinct probable AD variants. We conclude that CSF t-tau and p-tau biomarkers do not distinguish various clinical phenotypes of AD. Contrary to our expectations, in this sample of mild AD patients with biomarker evidence of amyloid pathology, lower CSF Aβ42 levels - and not t-tau and p-tau - were associated with brain atrophy in patterns typically observed in distinct clinical phenotypes. We speculate that lower CSF Aβ42, even within the pathological range, represents a more advanced neurobiological disease stage associated with greater syndrome-specific neurodegeneration.
A remarkable and unexpected finding of this study was that within probable AD variants, CSF Aβ42 - and not t-tau or p-tau – related to brain atrophy in syndrome-specific patterns described in previous MRI studies on EOAD, PCA, LOAD and to a lesser extent lvPPA (Frisoni, et al., 2007, Lehmann, et al., 2012, Migliaccio, et al., 2009, Moller, et al., 2013, Ridgway, et al., 2012, Rogalski, et al., 2014). We know from neuropathological and amyloid PET studies that Aβ deposits in a relatively diffuse and symmetric fashion throughout the neocortex (Braak and Braak, 1991, Klunk, et al., 2004), even in focal syndromes such as PCA and lvPPA (Lehmann, et al., 2013, Rosenbloom, et al., 2011). Furthermore, Aβ pathology shows only modest associations with the extent of neurodegeneration, particularly in the very earliest and latest stages of AD (Bateman, et al., 2012, Benzinger, et al., 2013, Ossenkoppele, et al., 2012). Consequently, it has been proposed that Aβ pathology is not a driver of the disease but exerts its effects by facilitating other pathogenic processes (especially spread of tau pathology) that lead to neuronal dysfunction and brain atrophy (Jack and Holtzman, 2013). Several biomarker studies have suggested that Aβ continues to accumulate over time in the MCI and early stage of AD dementia (Ossenkoppele, et al., 2011, Rinne, et al., 2010, Villain, et al., 2012, Villemagne, et al., 2011). This suggests that even within the pathological range, there is still considerable variability in early disease stages. Reduced CSF Aβ42 concentrations in the current study may thus be reflective of a more advanced neurobiological disease stage, explaining why lower Aβ42 correlated with greater brain atrophy. The fact that lower Aβ42 was associated with syndrome-specific atrophy patterns further supports this notion. It is conceivable that these relationships are best captured within early clinical stages, as exemplified by patients in the present study, when Aβ deposition and brain atrophy may show dynamic changes at the same time (Jack and Holtzman, 2013).
Animal and post-mortem studies have consistently linked tau pathology to neurodegeneration and disease severity (Nelson, et al., 2012, Spires-Jones and Hyman, 2014). Furthermore, the regional distribution of tau mirrors clinical symptoms and neurodegenerative patterns in various probable AD variants (Johnson, et al., 1999, Mesulam, et al., 2014, Ossenkoppele, et al., 2014, Renner, et al., 2004, Tang-Wai, et al., 2004). Contrary to our hypothesis, however, increased t-tau and p-tau concentrations did not correlate with gray matter reductions in any of the clinical variants. A potential explanation is that CSF t-tau and p-tau may reflect processes that are not specific to AD. For instance, CSF t-tau concentrations increase rapidly in the face of acute lesions associated with Creutzfeld-Jacob disease (Skillback, et al., 2014), stroke (Hesse, et al., 2001) or traumatic brain injury (Ost, et al., 2006). Neurofibrillary tangle pathology is typically absent in those conditions, suggesting that CSF t-tau indicates axonal degeneration in general. CSF p-tau levels, on the other hand, have been proposed to reflect tangle load since CSF p-tau is increased primarily in AD and not in most non-AD dementias (Blennow, et al., 2010). However, the relationship between CSF p-tau and tangle burden has been non-existent or weak in neuropathological studies (Buerger, et al., 2006, Engelborghs, et al., 2007). The aforementioned studies suggest that more rapid cell death could result in increased release of tau proteins in CSF. CSF tau concentrations may therefore be a derivative of the rate of cerebral atrophy, rather than reflecting the current state of the brain. This is in line with previous biomarker studies showing that CSF tau is a better predictor for disease progression than for baseline brain atrophy or cognition (Kester, et al., 2009, Tosun, et al., 2011, van der Vlies, et al., 2009).
In line with previous studies (Baumann, et al., 2010, Bouwman, et al., 2009, Coppi, et al., 2014, de Souza, et al., 2011a, de Souza, et al., 2011b, Magnin, et al., 2014, Santangelo, et al., 2014, Seguin, et al., 2011, Teng, et al., 2014), we found that CSF Aβ42 concentrations were similar across clinical variants of probable AD, although we narrowed the dynamic range by including only patients within the pathological range for Aβ. Discrepant findings for t-tau and p-tau in probable AD variants have been reported previously (Baumann, et al., 2010, Bouwman, et al., 2009, Coppi, et al., 2014, de Souza, et al., 2011a, de Souza, et al., 2011b, Magnin, et al., 2014, Santangelo, et al., 2014, Seguin, et al., 2011, Teng, et al., 2014), but in this relatively large study we observed comparable CSF levels in all probable AD variants and the prevalence of abnormal t-tau and p-tau concentrations was essentially the same across AD phenotypes. This is an important finding as CSF biomarkers have been incorporated in research criteria for AD and are increasingly used in clinical practice (Duits, et al., 2014, Mattsson, et al., 2012). Currently there exist two different biomarker informed sets of criteria for AD: the NIA-AA criteria (McKhann, et al., 2011) and the International Working Group-2 (IWG-2) criteria (Dubois, et al., 2014). Both NIA-AA and IWG-2 discuss different subtypes of AD, but neither proposes differential usage of CSF biomarkers to diagnose AD based on the clinical phenotype. While IWG-2 requires the presence of reduced CSF Aβ42 in combination with increased t-tau or p-tau (or a positive amyloid PET scan or the presence of an autosomal dominant mutation) for a diagnosis of AD dementia, NIA-AA proposes that the likelihood of underlying AD pathology contributing to the clinical presentation can be high (when both CSF Aβ42 and tau are positive) or intermediate (when either CSF Aβ42 or tau is positive). Our observation that CSF t-tau and p-tau are equally useful in all clinical phenotypes is thus compatible with both NIA-AA and IWG-2.
The strength of this study is the relatively large sample size, encompassing various clinical variants of probable AD. There are also some limitations. First, CSF samples were analyzed continuously in clinical practice rather than in one single run. This may introduce variability in biomarker levels, both due to factors related to analytical procedures and to the analytical kits used for measurements (Mattsson, et al., 2013). Because of this, the VUMC neurochemistry laboratory has implemented an elaborate quality control system in order to minimize this variability and acts as reference laboratory for the Netherlands. Second, we did not examine diagnostic sensitivity for CSF Aβ42 concentrations or tau/Aβ42 ratios, since we excluded patients with negative Aβ42 markers to reduce the possibility of clinical misdiagnosis in EOAD and LOAD (Ossenkoppele, et al., 2013, Sanchez-Juan, et al., 2014) and to rule out the small proportion of PCA and lvPPA clinical syndromes that are caused by non-Aβ pathologies (Crutch, et al., 2012). This approach seems justified, as previous studies did not detect differences in CSF Aβ42 between AD variants (Baumann, et al., 2010, Bouwman, et al., 2009, Coppi, et al., 2014, de Souza, et al., 2011a, de Souza, et al., 2011b, Magnin, et al., 2014, Santangelo, et al., 2014, Seguin, et al., 2011, Teng, et al., 2014), so we could focus on the less established diagnostic performance of t-tau and p-tau. Third, we used three different MRI scanner types. We adjusted for this by entering scanner type as a covariate in all imaging regression analyses. Fourth, although the clinical diagnosis of AD was supported by CSF Aβ42 biomarkers, the presence of non-AD pathologies can not be excluded as autopsy data are not available. CSF Aβ42 may be specific to amyloid pathology, it is not specific to AD as (comorbid) amyloid pathology can be present in other neurodegenerative diseases. Finally, our voxelwise regressions were performed using a liberal threshold (p<0.001 uncorrected) and did not survive conservative family-wise error correction for multiple comparisons. This may have induced a false positive finding in the lvPPA group, showing a less plausible atrophy pattern compared to PCA, EOAD and LOAD. On the other hand, the lvPPA patients showed - in line with the literature (Ridgway, et al., 2012, Rogalski, et al., 2014) – left hemispheric predominant atrophy, and the most severely affected regions (i.e. left insula and anterior cingulate) were also identified in a longitudinal study (Rohrer, et al., 2013).
5. CONCLUSIONS
In this group of probable AD patients who were are all likely to harbor cerebral amyloid pathology as determined by reduced CSF Aβ42 levels, we found that the prevalence of abnormal CSF t-tau and p-tau biomarkers was comparable across all clinical probable AD variants. We also found that CSF Aβ42, but not CSF t-tau or p-tau, was related to regional atrophy in patterns largely corresponding to symptomatology in the different syndromes. This suggests that decreased CSF Aβ42, even after crossing the cut-off for amyloid-positivity, represents a more advanced neurobiological disease stage associated with greater syndrome-specific neurodegeneration in distinct variants of probable AD.
ACKNOWLEDGEMENTS
This research was supported by a Marie Curie FP7 International Outgoing Fellowship [628812] (to R.O.), the donors of [Alzheimer’s Disease Research], a program of BrightFocus Foundation (to R.O.), National Institute on Aging grant [R01-AG045611] (to G.D.R.) and the John Douglas French Alzheimer’s Foundation (to G.D.R). Research of the VUMC Alzheimer Center is part of the Neurodegeneration research program of the Neuroscience Campus Amsterdam. The VUMC Alzheimer Center is supported by Alzheimer Nederland and Stichting VUMC funds. The clinical database structure was developed with funding from Stichting Dioraphte. Aegon NV sponsored a three-month visiting professorship of Dr. Rabinovici to VUMC Alzheimer Center, which was partly dedicated to work on the present project.
REFERENCES
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC Dominantly Inherited Alzheimer, N. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. New Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann TP, Duyar H, Sollberger M, Kuhle J, Regeniter A, Gomez-Mancilla B, Schmidtke K, Monsch AU. CSF-tau and CSF-Abeta (1–42) in posterior cortical atrophy. Dement geriatr cog dis. 2010;29:530–533. doi: 10.1159/000314679. [DOI] [PubMed] [Google Scholar]
- Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch neurol. 1988;45:789–793. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- Benzinger TL, Blazey T, Jack CR, Jr, Koeppe RA, Su Y, Xiong C, Raichle ME, Snyder AZ, Ances BM, Bateman RJ, Cairns NJ, Fagan AM, Goate A, Marcus DS, Aisen PS, Christensen JJ, Ercole L, Hornbeck RC, Farrar AM, Aldea P, Jasielec MS, Owen CJ, Xie X, Mayeux R, Brickman A, McDade E, Klunk W, Mathis CA, Ringman J, Thompson PM, Ghetti B, Saykin AJ, Sperling RA, Johnson KA, Salloway S, Correia S, Schofield PR, Masters CL, Rowe C, Villemagne VL, Martins R, Ourselin S, Rossor MN, Fox NC, Cash DM, Weiner MW, Holtzman DM, Buckles VD, Moulder K, Morris JC. Regional variability of imaging biomarkers in autosomal dominant Alzheimer's disease. Proc Nat Aca Sci. 2013;110:E4502–E4509. doi: 10.1073/pnas.1317918110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- Bouwman FH, Schoonenboom NS, Verwey NA, van Elk EJ, Kok A, Blankenstein MA, Scheltens P, van der Flier WM. CSF biomarker levels in early and late onset Alzheimer's disease. Neurobiol aging. 2009;30:1895–1901. doi: 10.1016/j.neurobiolaging.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buerger K, Ewers M, Pirttila T, Zinkowski R, Alafuzoff I, Teipel SJ, DeBernardis J, Kerkman D, McCulloch C, Soininen H, Hampel H. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- Coppi E, Ferrari L, Santangelo R, Caso F, Pinto P, Passerini G, Comi G, Magnani G. Further evidence about the crucial role of CSF biomarkers in diagnosis of posterior cortical atrophy. Neurol sci. 2014;35:785–787. doi: 10.1007/s10072-014-1644-5. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC. Posterior cortical atrophy. Lancet neurol. 2012;11:170–178. doi: 10.1016/S1474-4422(11)70289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza LC, Corlier F, Habert MO, Uspenskaya O, Maroy R, Lamari F, Chupin M, Lehericy S, Colliot O, Hahn-Barma V, Samri D, Dubois B, Bottlaender M, Sarazin M. Similar amyloid-beta burden in posterior cortical atrophy and Alzheimer's disease. Brain. 2011a;134:2036–2043. doi: 10.1093/brain/awr130. [DOI] [PubMed] [Google Scholar]
- de Souza LC, Lamari F, Belliard S, Jardel C, Houillier C, De Paz R, Dubois B, Sarazin M. Cerebrospinal fluid biomarkers in the differential diagnosis of Alzheimer's disease from other cortical dementias. J neurol neurosurg psychiatry. 2011b;82:240–246. doi: 10.1136/jnnp.2010.207183. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert MO, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Duits FH, Prins ND, Lemstra AW, Pijnenburg YA, Bouwman FH, Teunissen CE, Scheltens P, van der Flier WM. Diagnostic impact of CSF biomarkers for Alzheimer's disease in a tertiary memory clinic. Alzheimers dement. 2014 doi: 10.1016/j.jalz.2014.05.1753. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Engelborghs S, Sleegers K, Cras P, Brouwers N, Serneels S, De Leenheir E, Martin JJ, Vanmechelen E, Van Broeckhoven C, De Deyn PP. No association of CSF biomarkers with APOEepsilon4, plaque and tangle burden in definite Alzheimer's disease. Brain. 2007;130:2320–2326. doi: 10.1093/brain/awm136. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Pievani M, Testa C, Sabattoli F, Bresciani L, Bonetti M, Beltramello A, Hayashi KM, Toga AW, Thompson PM. The topography of grey matter involvement in early and late onset Alzheimer's disease. Brain. 2007;130:720–730. doi: 10.1093/brain/awl377. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123:484–498. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, Perani D, Garibotto V, Cappa SF, Miller BL. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Burger K, Pruessner JC, Zinkowski R, DeBernardis J, Kerkman D, Leinsinger G, Evans AC, Davies P, Moller HJ, Teipel SJ. Correlation of cerebrospinal fluid levels of tau protein phosphorylated at threonine 231 with rates of hippocampal atrophy in Alzheimer disease. Arch neurol. 2005;62:770–773. doi: 10.1001/archneur.62.5.770. [DOI] [PubMed] [Google Scholar]
- Henneman WJ, Vrenken H, Barnes J, Sluimer IC, Verwey NA, Blankenstein MA, Klein M, Fox NC, Scheltens P, Barkhof F, van der Flier WM. Baseline CSF p-tau levels independently predict progression of hippocampal atrophy in Alzheimer disease. Neurology. 2009;73:935–940. doi: 10.1212/WNL.0b013e3181b879ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse C, Rosengren L, Andreasen N, Davidsson P, Vanderstichele H, Vanmechelen E, Blennow K. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci letters. 2001;297:187–190. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Holtzman DM. Biomarker modeling of Alzheimer's disease. Neuron. 2013;80:1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch neurol. 1999;56:1233–1239. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- Kester MI, van der Vlies AE, Blankenstein MA, Pijnenburg YA, van Elk EJ, Scheltens P, van der Flier WM. CSF biomarkers predict rate of cognitive decline in Alzheimer disease. Neurology. 2009;73:1353–1358. doi: 10.1212/WNL.0b013e3181bd8271. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Koedam EL, Lauffer V, van der Vlies AE, van der Flier WM, Scheltens P, Pijnenburg YA. Early-versus late-onset Alzheimer's disease: more than age alone. J Alzheimer dis. 2010;19:1401–1408. doi: 10.3233/JAD-2010-1337. [DOI] [PubMed] [Google Scholar]
- Koss E, Edland S, Fillenbaum G, Mohs R, Clark C, Galasko D, Morris JC. Clinical and neuropsychological differences between patients with earlier and later onset of Alzheimer's disease: A CERAD analysis, Part XII. Neurology. 1996;46:136–141. doi: 10.1212/wnl.46.1.136. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Barnes J, Ridgway GR, Ryan NS, Warrington EK, Crutch SJ, Fox NC. Global gray matter changes in posterior cortical atrophy: a serial imaging study. Alzheimers dement. 2012;8:502–512. doi: 10.1016/j.jalz.2011.09.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Ghosh PM, Madison C, Laforce R, Jr, Corbetta-Rastelli C, Weiner MW, Greicius MD, Seeley WW, Gorno-Tempini ML, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer's disease. Brain. 2013;136:844–858. doi: 10.1093/brain/aws327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnin E, Paquet C, Formaglio M, Croisile B, Chamard L, Miguet-Alfonsi C, Tio G, Dumurgier J, Roullet-Solignac I, Sauvee M, Thomas-Anterion C, Vighetto A, Hugon J, Vandel P. Increased cerebrospinal fluid tau levels in logopenic variant of Alzheimer's disease. J Alzheimers dis. 2014;39:611–616. doi: 10.3233/JAD-131382. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Andreasson U, Persson S, Carrillo MC, Collins S, Chalbot S, Cutler N, Dufour-Rainfray D, Fagan AM, Heegaard NH, Robin Hsiung GY, Hyman B, Iqbal K, Lachno DR, Lleo A, Lewczuk P, Molinuevo JL, Parchi P, Regeniter A, Rissman R, Rosenmann H, Sancesario G, Schroder J, Shaw LM, Teunissen CE, Trojanowski JQ, Vanderstichele H, Vandijck M, Verbeek MM, Zetterberg H, Blennow K, Kaser SA Alzheimer's Association, Q.C.P.W.G. CSF biomarker variability in the Alzheimer's Association quality control program. Alzheimers dement. 2013;9:251–261. doi: 10.1016/j.jalz.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Rosen E, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek MM, Olde Rikkert M, Tsolaki M, Mulugeta E, Aarsland D, Visser PJ, Schroder J, Marcusson J, de Leon M, Hampel H, Scheltens P, Wallin A, Eriksdotter-Jonhagen M, Minthon L, Winblad B, Blennow K, Zetterberg H. Age and diagnostic performance of Alzheimer disease CSF biomarkers. Neurology. 2012;78:468–476. doi: 10.1212/WNL.0b013e3182477eed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer's disease. Dement geriatr cog dis. 2002;14(1):33–40. doi: 10.1159/000058331. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, Weintraub S, Bigio EH. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Annals of neurology. 2008;63(6):709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain : a journal of neurology. 2014;137(Pt 4):1176–1192. doi: 10.1093/brain/awu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio R, Agosta F, Rascovsky K, Karydas A, Bonasera S, Rabinovici GD, Miller BL, Gorno-Tempini ML. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology. 2009;73(19):1571–1578. doi: 10.1212/WNL.0b013e3181c0d427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller C, Vrenken H, Jiskoot L, Versteeg A, Barkhof F, Scheltens P, van der Flier WM. Different patterns of gray matter atrophy in early- and late-onset Alzheimer's disease. Neurobiology of aging. 2013;34(8):2014–2022. doi: 10.1016/j.neurobiolaging.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Mulder C, Verwey NA, van der Flier WM, Bouwman FH, Kok A, van Elk EJ, Scheltens P, Blankenstein MA. Amyloid-beta(1–42), total tau, and phosphorylated tau as cerebrospinal fluid biomarkers for the diagnosis of Alzheimer disease. Clinical chemistry. 2010;56(2):248–2453. doi: 10.1373/clinchem.2009.130518. [DOI] [PubMed] [Google Scholar]
- Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet neurol. 2011;10:785–796. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kovari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. Journal of neuropathology and experimental neurology. 2012;71(5):362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Prins ND, Pijnenburg YA, Lemstra AW, van der Flier WM, Adriaanse SF, Windhorst AD, Handels RL, Wolfs CA, Aalten P, Verhey FR, Verbeek MM, van Buchem MA, Hoekstra OS, Lammertsma AA, Scheltens P, van Berckel BN. Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2013;9(4):414–421. doi: 10.1016/j.jalz.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, Schonhaut DR, Baker SL, O'Neil JP, Janabi M, Ghosh PM, Santos M, Miller ZA, Bettcher BM, Gorno-Tempini ML, Miller BL, Jagust WJ, Rabinovici GD. Tau, Amyloid and Hypometabolism in a Patient with Posterior Cortical Atrophy. Annals of neurology. 2014 doi: 10.1002/ana.24321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Tolboom N, Foster-Dingley JC, Adriaanse SF, Boellaard R, Yaqub M, Windhorst AD, Barkhof F, Lammertsma AA, Scheltens P, van der Flier WM, van Berckel BN. Longitudinal imaging of Alzheimer pathology using [11C]PIB, [18F]FDDNP and [18F]FDG PET. European journal of nuclear medicine and molecular imaging. 2012;39(6):990–1000. doi: 10.1007/s00259-012-2102-3. [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, van Berckel BN, Prins ND. Amyloid imaging in prodromal Alzheimer's disease. Alzheimer's research & therapy. 2011;3(5):26. doi: 10.1186/alzrt88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ost M, Nylen K, Csajbok L, Ohrfelt AO, Tullberg M, Wikkelso C, Nellgard P, Rosengren L, Blennow K, Nellgard B. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology. 2006;67(9):1600–1604. doi: 10.1212/01.wnl.0000242732.06714.0f. [DOI] [PubMed] [Google Scholar]
- Palmqvist S, Zetterberg H, Blennow K, Vestberg S, Andreasson U, Brooks DJ, Owenius R, Hagerstrom D, Wollmer P, Minthon L, Hansson O. Accuracy of Brain Amyloid Detection in Clinical Practice Using Cerebrospinal Fluid beta-Amyloid 42: A Cross-Validation Study Against Amyloid Positron Emission Tomography. JAMA neurology. 2014 doi: 10.1001/jamaneurol.2014.1358. [DOI] [PubMed] [Google Scholar]
- Renner JA, Burns JM, Hou CE, McKeel DW, Jr, Storandt M, Morris JC. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology. 2004;63(7):1175–1180. doi: 10.1212/01.wnl.0000140290.80962.bf. [DOI] [PubMed] [Google Scholar]
- Ridgway GR, Lehmann M, Barnes J, Rohrer JD, Warren JD, Crutch SJ, Fox NC. Early-onset Alzheimer disease clinical variants: multivariate analyses of cortical thickness. Neurology. 2012;79(1):80–84. doi: 10.1212/WNL.0b013e31825dce28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, Rodriguez Martinez de Liano S, Liu E, Koller M, Gregg KM, Schenk D, Black R, Grundman M. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet neurology. 2010;9(4):363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Martersteck A, Rademaker A, Wieneke C, Weintraub S, Mesulam MM. Asymmetry of cortical decline in subtypes of primary progressive aphasia. Neurology. 2014 doi: 10.1212/WNL.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Caso F, Mahoney C, Henry M, Rosen HJ, Rabinovici G, Rossor MN, Miller B, Warren JD, Fox NC, Ridgway GR, Gorno-Tempini ML. Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain and language. 2013;127(2):121–126. doi: 10.1016/j.bandl.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MH, Alkalay A, Agarwal N, Baker SL, O'Neil JP, Janabi M, Yen IV, Growdon M, Jang J, Madison C, Mormino EC, Rosen HJ, Gorno-Tempini ML, Weiner MW, Miller BL, Jagust WJ, Rabinovici GD. Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution. Neurology. 2011;76(21):1789–1796. doi: 10.1212/WNL.0b013e31821cccad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Juan P, Ghosh PM, Hagen J, Gesierich B, Henry M, Grinberg LT, O'Neil JP, Janabi M, Huang EJ, Trojanowski JQ, Vinters HV, Gorno-Tempini M, Seeley WW, Boxer AL, Rosen HJ, Kramer JH, Miller BL, Jagust WJ, Rabinovici GD. Practical utility of amyloid and FDG-PET in an academic dementia center. Neurology. 2014;82(3):230–238. doi: 10.1212/WNL.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo R, Coppi E, Ferrari L, Bernasconi MP, Pinto P, Passerini G, Comi G, Magnani G. Cerebrospinal Fluid Biomarkers Can Play a Pivotal Role in the Diagnostic Work Up of Primary Progressive Aphasia. Journal of Alzheimer's disease : JAD. 2014 doi: 10.3233/JAD-141122. [DOI] [PubMed] [Google Scholar]
- Scherling CS, Hall T, Berisha F, Klepac K, Karydas A, Coppola G, Kramer JH, Rabinovici G, Ahlijanian M, Miller BL, Seeley W, Grinberg LT, Rosen H, Meredith J, Jr, Boxer AL. Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Annals of neurology. 2014;75(1):116–126. doi: 10.1002/ana.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonenboom NS, Reesink FE, Verwey NA, Kester MI, Teunissen CE, van de Ven PM, Pijnenburg YA, Blankenstein MA, Rozemuller AJ, Scheltens P, van der Flier WM. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78(1):47–54. doi: 10.1212/WNL.0b013e31823ed0f0. [DOI] [PubMed] [Google Scholar]
- Seguin J, Formaglio M, Perret-Liaudet A, Quadrio I, Tholance Y, Rouaud O, Thomas-Anterion C, Croisile B, Mollion H, Moreaud O, Salzmann M, Dorey A, Bataillard M, Coste MH, Vighetto A, Krolak-Salmon P. CSF biomarkers in posterior cortical atrophy. Neurology. 2011;76(21):1782–1788. doi: 10.1212/WNL.0b013e31821ccc98. [DOI] [PubMed] [Google Scholar]
- Skillback T, Rosen C, Asztely F, Mattsson N, Blennow K, Zetterberg H. Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: results from the Swedish Mortality Registry. JAMA neurology. 2014;71(4):476–483. doi: 10.1001/jamaneurol.2013.6455. [DOI] [PubMed] [Google Scholar]
- Sluimer JD, van der Flier WM, Karas GB, Fox NC, Scheltens P, Barkhof F, Vrenken H. Whole-brain atrophy rate and cognitive decline: longitudinal MR study of memory clinic patients. Radiology. 2008;248(2):590–598. doi: 10.1148/radiol.2482070938. [DOI] [PubMed] [Google Scholar]
- Smits LL, Pijnenburg YA, Koedam EL, van der Vlies AE, Reuling IE, Koene T, Teunissen CE, Scheltens P, van der Flier WM. Early onset Alzheimer's disease is associated with a distinct neuropsychological profile. Journal of Alzheimer's disease : JAD. 2012;30(1):101–108. doi: 10.3233/JAD-2012-111934. [DOI] [PubMed] [Google Scholar]
- Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer's disease. Neuron. 2014;82(4):756–771. doi: 10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopford CL, Snowden JS, Thompson JC, Neary D. Variability in cognitive presentation of Alzheimer's disease. Cortex; a journal devoted to the study of the nervous system and behavior. 2008;44(2):185–195. doi: 10.1016/j.cortex.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, Caselli RJ, Knopman DS, Petersen RC. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168–1174. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- Teng E, Yamasaki TR, Tran M, Hsiao JJ, Sultzer DL, Mendez MF. Cerebrospinal fluid biomarkers in clinical subtypes of early-onset Alzheimer's disease. Dementia and geriatric cognitive disorders. 2014;37(5–6):307–314. doi: 10.1159/000355555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D, Schuff N, Shaw LM, Trojanowski JQ, Weiner MW Alzheimer's Disease NeuroImaging, I. Relationship between CSF biomarkers of Alzheimer's disease and rates of regional cortical thinning in ADNI data. Journal of Alzheimer's disease : JAD 26 Suppl. 2011;3:77–90. doi: 10.3233/JAD-2011-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier WM, Pijnenburg YA, Prins N, Lemstra AW, Bouwman FH, Teunissen CE, van Berckel BN, Stam CJ, Barkhof F, Visser PJ, van Egmond E, Scheltens P. Optimizing patient care and research: the Amsterdam Dementia Cohort. Journal of Alzheimer's disease : JAD. 2014;41(1):313–327. doi: 10.3233/JAD-132306. [DOI] [PubMed] [Google Scholar]
- van der Vlies AE, Verwey NA, Bouwman FH, Blankenstein MA, Klein M, Scheltens P, van der Flier WM. CSF biomarkers in relationship to cognitive profiles in Alzheimer disease. Neurology. 2009;72(12):1056–1061. doi: 10.1212/01.wnl.0000345014.48839.71. [DOI] [PubMed] [Google Scholar]
- Verhage F. Intelligentie en leeftijd: onderzoek bij Nederlanders van twaalf tot zevenenzeventig jaar [Intelligence and age: Study with Dutch people aged 12 to 77] Assen. 1964 [Google Scholar]
- Villain N, Chetelat G, Grassiot B, Bourgeat P, Jones G, Ellis KA, Ames D, Martins RN, Eustache F, Salvado O, Masters CL, Rowe CC, Villemagne VL, Group AR. Regional dynamics of amyloid-beta deposition in healthy elderly, mild cognitive impairment and Alzheimer's disease: a voxelwise PiB-PET longitudinal study. Brain : a journal of neurology. 2012;135(Pt 7):2126–2139. doi: 10.1093/brain/aws125. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, Ackermann U, Jones G, Szoeke C, Salvado O, Martins R, O'Keefe G, Mathis CA, Klunk WE, Ames D, Masters CL, Rowe CC. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Annals of neurology. 2011;69(1):181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwan M, van Harten A, Ossenkoppele R, Bouwman F, Teunissen C, Adriaanse S, Lammertsma A, Scheltens P, van Berckel B, van der Flier W. Concordance Between Cerebrospinal Fluid Biomarkers and [11C]PIB PET in a Memory Clinic Cohort. Journal of Alzheimer's disease : JAD. 2014 doi: 10.3233/JAD-132561. [DOI] [PubMed] [Google Scholar]