Abstract
Traffic and power generation are the main sources of urban air pollution. The idea that outdoor air pollution can cause exacerbations of pre-existing asthma is supported by an evidence base that has been accumulating for several decades, with several studies suggesting a contribution to new-onset asthma as well. In this Series paper, we discuss the effects of particulate matter (PM), gaseous pollutants (ozone, nitrogen dioxide, and sulphur dioxide), and mixed traffic-related air pollution. We focus on clinical studies, both epidemiological and experimental, published in the previous 5 years. From a mechanistic perspective, air pollutants probably cause oxidative injury to the airways, leading to inflammation, remodelling, and increased risk of sensitisation. Although several pollutants have been linked to new-onset asthma, the strength of the evidence is variable. We also discuss clinical implications, policy issues, and research gaps relevant to air pollution and asthma.
Introduction
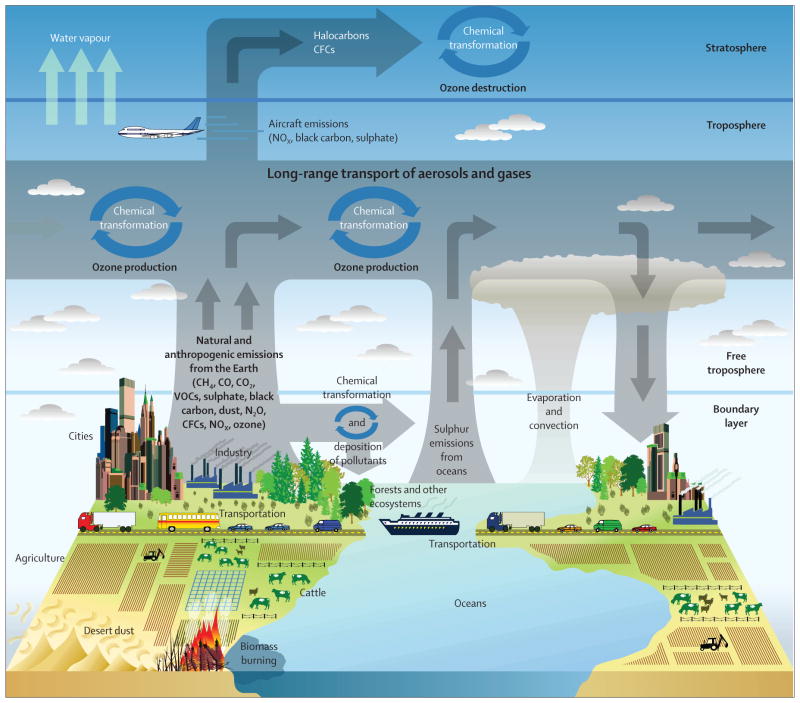
Outdoor air pollution contributed more than 3% of the annual disability-adjusted life years lost in the 2010 Global Burden of Disease comparative risk assessment, a notable increase since the previous estimate was made in 2000.1 Previous assessments of global disease burden attributed to air pollution were restricted to urban areas or by coarse spatial resolution of concentration estimates.2 In a study of ten European cities, 14% of the cases of incident asthma in children and 15% of all exacerbations of childhood asthma were attributed to exposure to pollutants related to road traffic.3 Urbanisation is an important contributor to asthma and this contribution might be partly attributed to increased outdoor air pollution (figure 1).4–6 Because many urban centres in the developing world are undergoing rapid population growth accompanied by increased outdoor air pollution, the global burden of asthma is likely to increase. In this context, it is notable that the populations of China, India, and Southeast Asia are equal to the rest of the world combined.
Figure 1. Sources, transport, transformation, and fate of atmospheric pollutants.
Reproduced from the US Climate Change Science Program. CFC=chlorofluorocarbon. CH4=methane. CO=carbon monoxide. CO2=carbon dioxide. N2O=nitrous oxide. NOx=nitrogen oxides.
In view of the burden of asthma attributed to outdoor air pollution, a better understanding of why asthmatic individuals are susceptible to this exposure should enable the design of effective preventive strategies. The idea that air pollution can cause exacerbations of pre-existing asthma is supported by an evidence base that has been accumulating for several decades,7–10 but evidence has emerged that suggests air pollution might cause new-onset asthma as well.11–21 Not all studies support a causal link between air pollution and asthma, and a recent meta-analysis22 of cross-sectional studies that compared communities with different levels of pollution showed no effect of long-term exposure to pollution on asthma prevalence. Although outdoor air pollution almost always occurs as a mixture, air quality is regulated by most jurisdictions in terms of its individual components. Such regulation has meant that experimental studies of humans and animals have been focused on individual pollutants. Because epidemiological studies inherently involve exposure to mixtures of pollutants, substantial efforts are usually made to try to identify the individual effects of pollutants, which often obscures the health effect of the mixture as a whole.
With increasing attention to traffic-related air pollution (TRAP) as the exposure variable of interest, a shift has occurred away from a focus on individual components of the pollution mixture. In this Series paper, we will attempt to discuss the effects of several gaseous pollutants (ozone, nitrogen dioxide, and sulphur dioxide), the independent effects of various forms of PM, and then focus on the effects of TRAP as a mixture. We concentrate on studies published in the past 5 years that report results relevant to both exacerbation and onset of asthma.
We focus primarily, although not exclusively, on epidemiological and experimental clinical studies. Controlled exposure studies in human beings are restricted by small sample size and an inability to study the potentially most susceptible subgroups (eg, children and adults with severe asthma) and the effects of chronic exposure. Epidemiological studies are restricted by imprecise methods of both exposure and asthma outcome assessment and often inadequate data about potentially confounding variables.
Although the potential effect of indoor air pollution on asthma is an important concern, especially in developing countries where much domestic cooking is done with solid fuels, it is outside the scope of this review.
Mechanisms
Why are individuals with asthma so affected by exposure to air pollution? At high concentrations, such as those noted in megacities in India and China, air pollutants might have direct irritant and inflammatory effects on airway neuroreceptors and epithelium, but such levels of exposure rarely occur in North America or Europe. At the lower concentrations that are more typical in high-income countries, other mechanisms are probably in operation. Specific pollutants can induce airway inflammation (eg, ozone, nitrogen dioxide, and PM <2·5 μm in diameter [PM2·5])23–28 and airway hyper-responsiveness (ozone and nitrogen dioxide),23,29 two characteristic features of asthma. In addition, oxidative stress (a feature of severe asthma) has been associated with pollutant exposures (ozone, nitrogen dioxide, and PM2·5).30–32 Therefore, exposure to these pollutants is unsurprisingly associated with exacerbations and possibly even the onset of asthma. The mechanisms by which pollutants induce these effects are not completely clear.
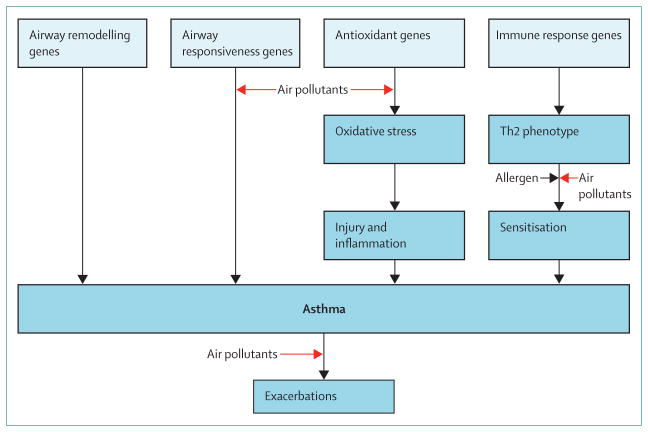
A framework for how air pollution might contribute to the development and exacerbation of asthma proposed by the UK’s Committee on the Medical Effects of Air Pollutants identified four main mechanisms: oxidative stress and damage, airway remodelling, inflammatory pathways and immunological responses, and enhancement of respiratory sensitisation to aeroallergens (figure 2).33 Variation in the genes that regulate these mechanisms could confer increased susceptibility to development of new-onset asthma or exacerbations of existing disease with exposure to air pollution.
Figure 2.
Mechanistic framework for air pollution effects in asthma
Because the pollutants of interest, including TRAP, can cause oxidative stress, the ability of antioxidant defences to handle the increased load of reactive oxygen species generated in the lungs after exposure is an important determinant of risk for subsequent adverse effects. Specific polymorphisms in antioxidant enzyme genes, such as glutathione S-transferase genes, GSTM1 and GSTP1, can modify risk of asthmatic responses to pollutants34,35 and these variants (GSTM1 null and GSTP1 Ile105Val) might also interact with a tumour necrosis factor (TNF) promoter variant (G-308A) that affects expression of TNF and hence the early inflammatory response.36 Additionally, neonatal rats are more prone to oxidative stress from PM exposure at least in part due to relative deficiency of nuclear factor-like 2 (Nrf2).37 Proinflammatory effects of oxidative stress are mediated by the redox-sensitive MAP kinase and nuclear factor-κB cascades that are responsible for the expression of cytokines, chemokines, and adhesion molecules, and reduced antioxidant capacity in the airways can result in altered expression after pollutant exposure.38
Other pathways through which oxidising pollutants might affect severity of asthma involve control of immune responses. TRAP, specifically ambient polycyclic aromatic hydrocarbons and diesel-exhaust particles, affect regulatory T cell (Treg) function through an epigenetic mechanism.39,40 Hypermethylation of CpG islands in Foxp3 associated with chronic exposure to polycyclic aromatic hydrocarbons39 or diesel-exhaust particles40 leads to suppression of Treg function and increased asthma severity as assessed by symptoms and lung function. Hypermethylation of interferon γ in effector T cells, contributing to a shift towards a Th2 response, has also been associated with exposure to air pollution.41
Studies in animals and in vitro42,43 suggest that exposure to PM results in allergic inflammation with Th2 and Th17 phenotypic differentiation, with a specific role for environmentally persistent free radicals and polycyclic aromatic hydrocarbon fractions of PM in this differentiation. In addition, exposure to diesel-exhaust particles is associated with increased serum interleukin 17 and increased symptoms in children with allergic asthma; a parallel study44 that used a murine model of allergic airway inflammation showed that combined exposure to diesel-exhaust particles and antigen from a house dust mite induced a mixed Th2/Th17 response.
A potential enhancing effect of pollutant exposure on responses to inhaled allergen has been studied in both animals and man, with evidence for such an effect on lung function and inflammatory responses to ozone, nitrogen dioxide, sulphur dioxide, and diesel-exhaust particles.45–48 Several mechanisms through which air pollutants could enhance sensitisation to aeroallergens have been proposed and include increased deposition of allergen in the airways due to carriage by particles, increased epithelial permeability due to oxidative injury, increased antigenicity of proteins from chemical modification, and a direct adjuvant effect (including for diesel-exhaust particles in human beings).49
In summary, air pollutants might cause oxidative injury to the airways that leads to inflammation and remodelling, which in a genetically predisposed individual could result in clinical asthma. One predisposing factor might be atopy, and air pollutants could increase the risk of sensitisation and the responses to inhaled allergen in individuals with asthma.
Particulate matter
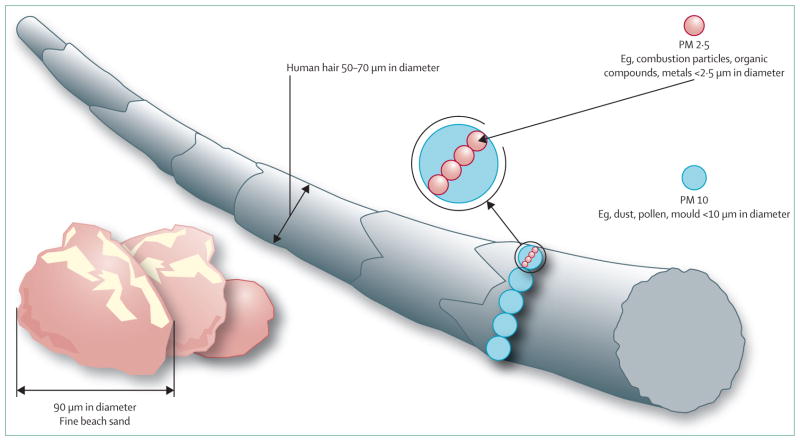
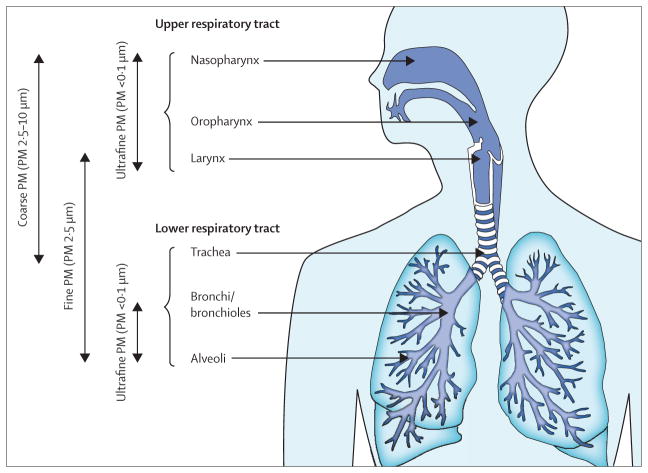
Ambient PM is a ubiquitous atmospheric aerosol with both anthropogenic and natural sources that has been associated with various health effects.50 PM is categorised on the basis of its aerodynamic diameter, with implications for its typical site of deposition when inhaled (figure 3). Coarse PM, with an aerodynamic diameter of 2·5–10 μm, deposits mainly in the head and large conducting airways. Fine PM or PM2·5 deposits throughout the respiratory tract, particularly in small airways and alveoli. Ultrafine PM (<0·1 μm) deposits in the alveoli. PM10 includes the coarse, fine, and ultrafine fractions (figure 4). The composition and size distribution of PM varies according to the source, whether it is natural or anthropogenic, and whether it is derived from combustion or not.50 Transition metals, polycyclic aromatic hydrocarbons, and environmentally persistent free radicals are constituents of PM of special interest because of their potential to cause oxidative stress and many of the phenotypic changes associated with asthma. Additionally, PM frequently contains various immunogenic substances, such as fungal spores and pollen, which have been independently associated with exacerbation of asthma symptoms.51,52
Figure 3. Particulate matter size.
Image modified with permission from the US Environmental Protection Agency.PM=particulate matter.
Figure 4.
Compartmental deposition of particulate matter
Experimental exposure to PM results in oxidative stress, airway hyper-responsiveness, and airway remodelling, either alone or in combination with allergic sensitisation.53
Short-term exposure to ambient PM2·5 and PM of diameter 2·5–10 μm in prospective cohorts of asthmatic children and adults has been associated with asthma symptoms, especially in children with allergic sensitisation.54,55 Long-term exposure to PM is associated with poorly controlled asthma and decrements in lung function in children and adults.30,56 Several studies in children and adults have shown associations between short-term and long-term exposure to PM2·5 or PM10 and increased health-care use; these associations are generally partially attenuated but persistent after adjustment for co-pollutants.57–63
Some evidence suggests PM is a cause of incident asthma (aside from the literature on TRAP). Independent associations between exposure to PM10 in utero and during infancy with asthma diagnosed by a doctor were identified in a nested case-control study within a large birth cohort.18 Although several studies have identified associations between asthma prevalence and exposure to outdoor PM,11,64,65 this finding has not always been consistent.22 Furthermore, PM is frequently strongly correlated with ozone, nitrogen oxides, and sulphur oxides, serving to confound these associations. In summary, substantial evidence supports the idea that ambient levels of PM exacerbate existing asthma, particularly by contributing to oxidative stress and allergic inflammation, and some evidence exists in support of PM as a cause of new cases of asthma.
Gases
In view of the central role of oxidative stress in asthma morbidity associated with air pollutants, oxidising gases continue to be an area of substantial research. Ground-level ozone is formed by photochemical reactions between sunlight and pollutant precursors, such as nitrogen oxides and volatile organic compounds, especially in warm conditions and peaks in summer temperatures.66 Nitrogen oxides, including nitrogen dioxide, are formed primarily by the reaction of ozone with nitric oxide emitted during fossil fuel combustion; as a result, steep concentration gradients of nitrogen oxides exist near sites of nitrogen oxide emission (eg, roadways) and relative depletion of ozone occurs in these sites.67 Indoor exposure to nitrogen dioxide derives from appliances that burn natural gas. The main sources of sulphur oxides in the developed world are primary emissions during energy production or industrial processes. These gaseous pollutants vary substantially in their environmental persistence and oxidising capacity; whereas ozone is a potent oxidising agent, nitrogen dioxide is a relatively weak oxidant and much debate surrounds whether it is a cause of asthma morbidity or just a marker of TRAP. Because sulphur dioxide is a reductant, it probably causes asthma symptoms through a different mechanism.
Responses to controlled exposure of short-term ozone and sulphur dioxide at relevant concentrations have been studied extensively. Ozone exposure results in airway inflammation, airway hyper-responsiveness, and decrements in lung function in healthy and asthmatic adults,23 whereas sulphur dioxide causes more prominent bronchoconstriction, especially in asthmatic individuals (table 1).68 Controlled exposure to nitrogen dioxide seems to have a mild airway inflammatory effect at high levels of exposure that are unlikely to be encountered in non-experimental settings, but induces little lung inflammation at common ambient concentrations. A 1992 meta-analysis69 identified an increase in airway hyper-responsiveness with relevant concentrations of nitrogen dioxide, but a 2009 review70,71 showed this effect to be small, inconsistent, and of unclear clinical significance. Consequently, focus has been placed on the adjuvant role nitrogen dioxide might have in combination with exposure to copollutants or allergens.
Table 1.
Acute effects of short-term exposures to pollutant gases in asthmatic adults
| Ozone | Nitrogen dioxide | Sulphur dioxide | |
|---|---|---|---|
| Bronchoconstriction | +/− | − | + |
| Decreased FEV1 and FVC | + | − | − |
| Increased airway responsiveness | + | + | − |
| Airway inflammation | + | + | − |
| Enhanced responses to inhaled allergen | + | + | + |
FEV1=forced expiratory volume in 1 s. FVC=forced vital capacity.
By contrast with the inconsistent experimental data on nitrogen dioxide, the body of observational data supporting its role in the exacerbation of asthma and asthma incidence continues to grow in breadth and consistency. Studies of asthmatic children and adults in the past 5 years have identified associations between nitrogen dioxide and symptoms of asthma,54,72 reduced response to bronchodilators,73 decrements in lung function,30 and exacerbation of asthma.57,60,62 Notable, several studies have identified an increase in asthma incidence or prevalence associated with exposure to nitrogen dioxide.11–15 Biological plausibility for nitrogen dioxide as a cause of asthma is supported by experimental data from animals74 and controlled exposures of healthy and asthmatic adults,75,76 showing enhanced pulmonary neutrophilic inflammation and the promotion of a Th2/Th17 phenotype, although not all studies of asthmatic patients showed such an effect of nitrogen dioxide.77 Despite weaknesses in toxicological data, consistent results from observational studies over a broad range of exposures and in diverse populations suggest that nitrogen dioxide is associated with significant morbidity in asthmatic individuals and might be a cause of incident asthma.
Although short-term exposure to ozone has been well documented as a cause of asthma exacerbation in adults and children,58,78 whether long-term exposure can lead to new-onset asthma is somewhat less clear. Studies of adult-onset asthma have identified an increased risk associated with ozone exposure, although this effect was restricted to male individuals.79,80 In children, ozone has been associated with incident allergic sensitisation, a known risk factor for subsequent asthma, and prevalence of wheeze and asthma as diagnosed by a doctor.65,81 Studies of asthma incidence in children have identified an association with ozone, although the risk might be confined to heavily exposed, physically active children.15,82 Taken together, the available evidence suggests that ozone might be a cause of new-onset asthma in some subgroups of children.
Although exposure to sulphur dioxide has been greatly reduced in the developed world through the use of scrubbing equipment in coal-fired power plants and energy sources other than coal combustion, it is a problem in developing countries. A study11 from China noted an association with sulphur dioxide and both asthma prevalence and current symptoms among children, especially in those with atopy.
TRAP
TRAP is a complex mixture of PM derived from combustion (including elemental or black carbon) and non-combustion sources (eg, road dust, tyre wear, and brake wear) and primary gaseous emissions including nitrogen oxides. These primary emissions lead to the generation of secondary pollutants such as ozone, nitrates, and organic aerosol. Intense experimental and epidemiological research has greatly advanced our understanding of the role of TRAP in asthma and disease mechanisms underlying this association, facilitated in part by advances in modelling that allow improved spatial resolution of exposure than can be provided by central site air quality monitors. Concentrations of many of the constituent pollutants in TRAP diminish quickly with distance from roadways. A 2010 review10 suggested that distances within 300–500 m of roadways were the most relevant for effects on human health. In large North American cities, 30–45% of people live within this distance of a major roadway, and the burden of near-roadway exposure seems to be even higher in many European cities.3
Diesel-exhaust particles, in isolation, have inconsistent effects in animals and in vitro.44,83–85 However, exposure to such pollutants concomitant with or after sensitisation to a variety of allergens, results in oxidative stress, airway hyper-responsiveness, enhanced neutrophilic and eosinophilic airway inflammation, and a switch to Th2/Th17 phenotypes. Mice exposed prenatally to diesel-exhaust particles develop more substantial postnatal manifestations of allergic asthma after allergen sensitisation and upregulation of genes involved in oxidative stress responses and polycyclic aromatic hydrocarbon metabolism.86,87 Controlled human exposure of asthmatic individuals enhances non-specific airway hyper-responsiveness, but not airway inflammation. By contrast, healthy individuals develop airway inflammation without airway hyper-responsiveness.88–90
Epidemiological studies of TRAP relying on various exposure metrics have identified increases in respiratory symptoms,6,56 changes in lung function,91,92 and healthcare use93–95 in children and adults. Notably, the phase 3 ISAAC study, representing over 500 000 children and adolescents across five continents, identified a dose-response association between symptoms of asthma (ever asthma, current wheeze, and severe asthma symptoms) and self-reported exposure to truck traffic.6 Effects of short-term ambient exposure to PM2·5, nitrogen oxides, and carbon monoxide were increased by exposure to higher than median modelled traffic exposure, showing the strength of considering both regional air pollution and long-term TRAP exposure in studies of health effects.95 A study72 of two communities in Southern California estimated that reductions in traffic-related nitrogen dioxide and ozone to background levels would reduce bronchitic episodes in asthmatics by 36–70%. The London Low Emission Zone provides an opportunity to study the impact of reduced TRAP on asthma morbidity.96 Collectively, these data suggest that TRAP exposure, especially in urban areas, has a tremendous effect on disease morbidity in individuals with asthma.
A growing body of evidence supports the notion that TRAP exposure is also responsible for cases of incident asthma. Several recent prospective studies of children with never-asthma at enrolment have identified an increased incidence associated with greater TRAP exposure.15–18 A future source of information on early exposures related to asthma incidence will be the ENRIECO project in Europe, a collaboration of multiple birth cohort studies.97 Adults with never-asthma at baseline have an increased risk of incident asthma associated with TRAP.19,20 In two studies,3,98 asthma prevalence in ten European cities at least partially attributable to near-road TRAP was 14% and total cases of incident asthma in Los Angeles County attributable to traffic proximity was 8%. In view of this growing body of evidence supporting TRAP’s role in exacerbation of underlying asthma and, probably, development of new cases of asthma, we suggest additional focus be placed on assessment of the effect of strategies aimed at reduction of these exposures.
Risk modifiers
Young children with asthma have long been regarded as a group who are very susceptible to adverse effects from air pollution because of their developing lungs, immature metabolic pathways, high ventilation rates per bodyweight, and increased time exercising outdoors.99,100 Even exposures in utero might affect postnatal risk of asthma and asthma exacerbations.100,101 Low birthweight, which might be associated with narrow airways during early childhood, is a risk factor for symptoms of asthma related to air pollution.102
Asthma exacerbations are more prevalent and severe in young boys than in girls,103 and this disparity has been attributed, at least in part, to relatively narrow airways in early life. No consistent difference in air pollution effects on asthma has been evident between boys and girls, although some studies have reported differential responses based on sex.54,104 In adults, active asthma is more prevalent among women than men,103 but again no consistent sex-based difference in risk has been noted for exacerbations related to air pollution. Elderly individuals with asthma are likely to be at increased risk for adverse effects related to air pollution, but scant literature is available to support this point.
Evidence to support differences in susceptibility to air pollution on the basis of ethnicity in asthma is also restricted, but the incidence of asthma was associated with exposure to air pollution in African–American and Latino children recruited from several US cities and Puerto Rico.12 Effects of ethnicity might be confounded with those effects associated with low socioeconomic status. Asthmatic children of such families seem to have greater exposure to outdoor air pollution and greater susceptibility to the effects of pollutants than do children from families of high socioeconomic status.105–107 The factors that might contribute to this susceptibility include neighbourhood characteristics (eg, increased levels of crime, less green space, or poor food access), stress,108 and diet.
Dietary factors can play a part in susceptibility to pollutant effects independent of socioeconomic status. The body of evidence on the protective effects of a diet high in fruits and vegetables and of antioxidant vitamin supplements is sufficient to support an important role for oxidative stress in the pathways by which outdoor air pollution adversely affects asthma.109–111 Obesity might also increase susceptibility to the adverse effects of air pollution.112–114
Because secondhand tobacco smoke is a mixture that includes gases and respirable PM and is known to adversely affect asthma, it would be expected to be a modifier of the effects of air pollution. Some evidence is available to support this expectation.101,115 What is clear is that polymorphisms of genes (GSTM1, GSTP1, and TNF) that have been associated with susceptibility to adverse asthma outcomes from exposure to air pollutants have also been associated with increased risks from exposure to secondhand smoke.116–118
Clinical implications
One strategy to reduce exacerbations of asthma related to air pollution is for local governments to issue smog alerts on days when ozone or PM2·5 levels are forecast to be high (table 2). Individuals with asthma and other pre-existing cardiopulmonary disorders are urged to stay indoors on such days. Clinicians should encourage patients with asthma (and advise parents of children with asthma), to avoid unnecessary outdoor activity, especially if it entails vigorous exercise that increases minute ventilation and thus the total inhaled dose, on days when air pollution levels are high. Avoidance of outdoor activity on poor air quality days can be added to a patient’s asthma management plan. For such an avoidance strategy to be effective, however, jurisdictions ought to publicise air quality monitoring data widely and on a daily basis. Although data on the effectiveness of smog alerts are scarce, some evidence suggests that people do avoid going outdoors when alerts are issued in Southern California (especially people with known susceptibility).88–90,119
Table 2.
UK Daily Air Quality Index
| Value | Health message for at-risk individuals | |
|---|---|---|
| Low | 1–3 | Enjoy your usual outdoor activities. |
| Moderate | 4–6 | Adults and children with lung problems and adults with heart problems who have symptoms should consider reducing strenuous physical activity, particularly outdoors. |
| High | 7–9 | Adults and children with lung problems and adults with heart problems should reduce strenuous physical exertion, particularly outdoors, and particularly if they have symptoms. People with asthma might find they need to use their reliever inhaler more often. Older people should also reduce physical exertion. |
| Very high | 10 | Adults and children with lung problems, adults with heart problems, and older people should avoid strenuous physical activity. People with asthma might find they need to use their reliever inhaler more often. |
UK Daily Air Quality Index is based on concentrations of nitrogen dioxide, sulphur dioxide, ozone, PM2·5, and PM10. Similar air quality indices exist for many other countries, differing in the pollutants considered and recommendations offered. PM2·5=particulate matter with a diameter of less than 2·5 μm. PM10=particulate matter with a diameter of less than 10 μm.
Patients with asthma should ideally live at least 300 m from major roadways, especially those with heavy truck traffic. TRAP can exacerbate asthma,10,120 but concentrations of motor vehicle emissions such as ultrafine PM and black carbon particles decrease substantially by 300 m.121 In-vehicle exposure during commuting with open windows can also be very high.122
Treatment of exacerbations of asthma related to air pollution need not differ from usual clinical practice. In addition to avoidance of exposure on high pollution days, exacerbations can be prevented by maintenance inhaled corticosteroid therapy as several studies suggest this approach decreases adverse responses to pollutant exposures.123,124 Patients with severe asthma might be the most likely to have respiratory symptoms with exposure to air pollutants, despite treatment with steroids.125 Because of the potential interactive effects of air pollutants with allergens or secondhand smoke, avoidance of these exposures is prudent, especially during high pollution days, which may be concurrent with exposure to seasonal aeroallergens.
Policy issues
Motor vehicle emissions and power plants are the main sources of both primary pollutants (eg, nitrogen oxides, fine PM, and ultrafine PM) and secondary pollutants (eg, ozone, nitrates, sulphates, and organic aerosol) in developed countries. Although improved pollution controls have reduced emissions of pollutants, growing evidence of adverse health effects at levels less than present US, Canadian, and European Union standards suggests that cleaner vehicles and energy production that do not rely on combustion of fossil fuels will be necessary to achieve good air quality. Climate change is projected to increase ozone concentrations in many locations because of increased sunshine and ambient temperature.126 Because mitigation of climate change and depletion of the world’s oil reserves also require reduced reliance on fossil fuels, policy initiatives to incentivise the development of alternatively powered vehicles and renewable electricity production might be advisable.127,128
Efforts to reduce greenhouse gas emissions would have a major co-benefit in terms of lowering of primary and secondary emissions of air pollution. Thus, governments should coordinate their climate change mitigation and clean-air regulation efforts as California is trying to do.129 Governments could prioritise mitigation measures that increase human health benefits from clean air, for example by focusing on carbon sources with the most toxic PM emissions such as coal and diesel combustion.130 Elimination of coal burning, rather than regulations requiring electrostatic precipitators or scrubbers on power plant smokestacks, would maximise reduction of carbon dioxide and PM2·5 emissions. Increased production of power from non-fossil fuels is key to reduction of reliance on coal combustion.
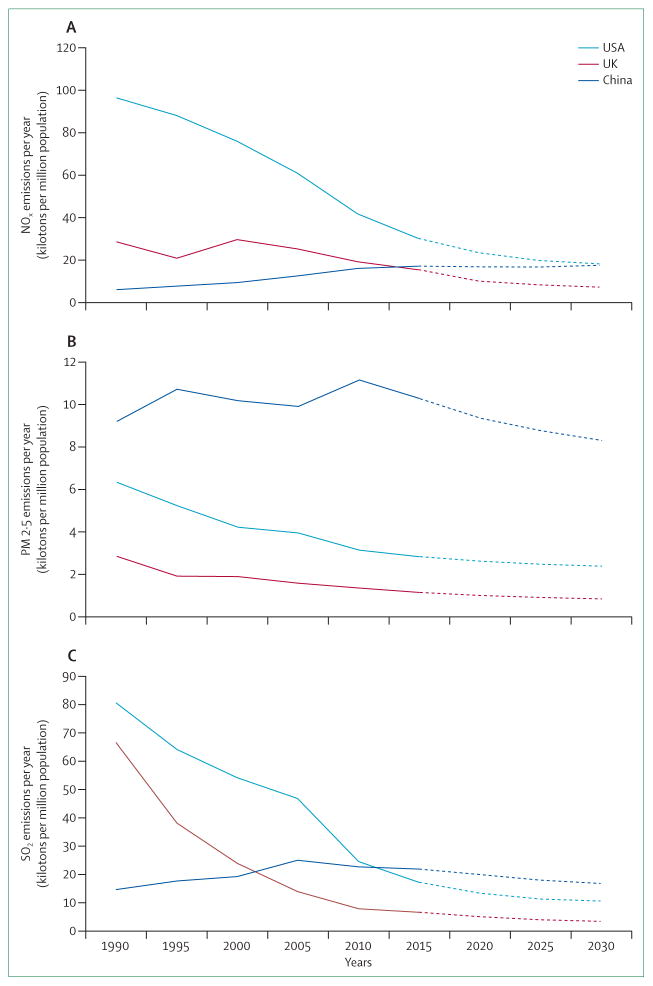
Control of air pollution in developing countries is usually complicated by efforts to accelerate economic growth, the costs of both alternative energy production and control measures, and household air pollution from the inefficient burning of solid fuels (eg, wood, dung, crop waste, and coal). Cities in countries with rapid economic and population growth, such as China and India, have some of the worst air quality in the world.131 Both countries burn a lot of coal and the number of motor vehicles on their roads is increasing rapidly. Therefore, China’s commitment of substantial resources into alternative power production is encouraging, as is India’s recognition of the need to reduce domestic cooking with biomass fuels. However, present efforts are insufficient to address both the climate change and public health imperatives of rapidly increasing fossil fuel combustion for power and transportation needs (figure 5). One concept that offers reduced reliance on coal and provision of power in the less developed rural areas of developing countries is decentralised or distributed generation, referring to electricity production at or near the point of use.133
Figure 5. National emissions of key air pollutants for USA, UK, and China with projections to 2030.
(A) Nitrogen oxides. (B) Particulate matter with a diameter <2·5 μm. (C) Sulphur dioxide. Data from the GAINS Database.132
Smart growth strategies are also necessary to reduce vehicle travel in the megacities of developing countries and in the developed world.134 Government investment in efficient public transportation systems and support of high-density, energy-efficient housing along transit system corridors will reap long-term economic and public health benefits. The paradox of urban intensification is a major concern. At the neighborhood level, improvements to public transportation will usually be insufficient to counteract the traffic congestion effect of increasing population density. This problem leaves policy makers with a poor choice: intensify and accept the local consequences or allow urban sprawl and accept the wider consequences. To address this paradox, some cities are implementing intensification accompanied by parking restrictions, closing roads to traffic, congestion pricing, and car-free zones.135
Research gaps
Although much research on air pollution and asthma has been done in the past 5 years, major gaps in our knowledge remain. Perhaps the most critical gap is in our understanding of the mechanisms by which exposure to air pollutants contributes to the onset of asthma, especially in non-atopic children and adults. Gene–environment interaction is an obvious focus of future research. The biological basis for the interactive effects of air pollution and psychological stress is another especially intriguing area in which our understanding is low.
In view of how important air pollution is as a trigger of exacerbations, improved mechanistic understanding of how these exacerbations occur is needed so that strategies to prevent them can be developed. Although exposure to PM2·5 and TRAP is associated with increased risk of asthma exacerbations, which components of the particulate mix are the most responsible is not known (eg, fine vs ultrafine particles). Nitrogen dioxide, a relatively mild oxidant at ambient concentrations, is repeatedly noted in epidemiological studies as the pollutant most associated with effects on asthma, but the reason is unclear. Is nitrogen dioxide merely a good marker of exposure to TRAP or is it involved in a biological pathway leading to asthma exacerbation? Polycyclic aromatic hydrocarbons are another class of pollutants about which more information is needed. Several recent papers have suggested that these compounds play a part in both allergy and asthma in children,39,101,136 and the epigenetic pathway in which they play a role makes these ubiquitous products of combustion a prime target for future research.
In addition to the specific chemical characteristics of air pollutants, another key question is whether short-term peak exposures (eg, 1 h) versus time-weighted averages over longer time periods (eg, 1–2 days) are associated with increased risk of adverse asthma outcomes. Some evidence points to peak exposures being more important than long-term exposure,137 although more data are needed to address this question. Furthermore, what lag time exists between exposure to air pollutants and clinical effect? Many studies show a strong effect on the day after high-level pollutant exposure, rather than on the day of exposure. Finally, although exposure science has made great strides over the years, assessment of individual-level exposure is presently primarily based on either distance from air quality monitoring stations or estimates from models. Methods for personal monitoring might allow individuals to assess their own exposure.
One of the most challenging tasks for air pollution research has been how to address the fact that people are almost always exposed to a mixture of pollutants. Effective strategies to address this confounding are needed for both epidemiological and toxicological studies. Cumulative impacts of pollutant exposures in concert with other environmental stressors (eg, stress, poor diet, or tobacco smoke) is another challenging concept for which innovative strategies are needed. The relative contributions of pollutant exposures in indoor microenvironments—home, school, workplace, transit—is also understudied, but new technologies are in development that might allow assessment of exposure in these locations.138
The adverse effects of exposure to air pollutants might vary over the life course such that future research should be directed toward differential effects at different stages of development. Even prenatal exposures to pollutants might affect risk of disease later in life, including asthma.100,101
Climate change will lead to increases in ambient concentrations of pollutants, ozone (directly), and nitrogen dioxide and PM2·5 (secondary to increased energy production required for air conditioning). Increased exposures to aeroallergens are also projected because of lengthened growing seasons and increased production of pollen.139 Thus, improved understanding of pollutant exposure-allergy interactions will be needed to address the expected increased risk of asthma exacerbations from the confluence of these effects of climate change.
Conclusions
A substantial body of research on the effects of air pollution on asthma has been published in the past 5 years, adding to the body of knowledge that has accumulated over several decades. Presently, short-term exposures to ozone, nitrogen dioxide, sulphur dioxide, PM2·5, and TRAP is thought to increase the risk of exacerbations of asthma symptoms. Increasing amounts of evidence also suggest that long-term exposures to air pollution, especially TRAP and its surrogate, nitrogen dioxide, can contribute to new-onset asthma in both children and adults. Much more about the mechanisms that are involved with exacerbations induced by pollution and onset of asthma needs to be understood, but oxidative stress and immune dysregulation are probably both involved. Young children with asthma, especially those growing up in economically disadvantaged neighbourhoods, are at increased risk of adverse effects from exposures to air pollution. Unravelling which components of the traffic pollution mixture are responsible for asthma exacerbations and onset is a substantial challenge. Improved air quality to prevent exacerbations and new cases of asthma will require strong governmental efforts to move economies in both developed and developing countries away from combustion of fossil fuels for transportation and energy production; this approach is also needed to mitigate climate change.
Search strategy and selection criteria.
We searched Pubmed from Jan 1, 2009, to Feb 28, 2014, with the search terms “Asthma” and any of the following specific terms: “Air Pollution”, “Particulate Matter”, “PM2·5”, “PM10”, “Ozone”, “O3”, “Sulfur Dioxide”, “Sulfur Oxides”, “SO2”, “SOx”, “Nitrogen Dioxide”, “Nitrogen Oxides”, “NO2”, “NOx”, “Traffic”, “Diesel”, “Elemental Carbon”, or “Black Carbon”. We searched the reference lists of recent reports and review articles produced with this search strategy to include relevant publications older than 5 years. Additionally, we included frequently referenced older publications with a high impact based on our knowledge of the subject area, particularly when more recent publications on the same subject were not available.
Footnotes
Contributors
MG did the literature search and had primary writing responsibility for the sections on particulate matter, gases, and traffic-related air pollution. JRB reviewed the search results with MG and had primary responsibility for writing the introduction and the sections on mechanisms, risk modifiers, clinical implications, policy issues, and research gaps. Both authors reviewed and contributed to the editing of the whole report.
Declaration of interests
JRB is the Physician Member of the California Air Resources Board, the state agency with regulatory responsibility for air quality and climate change mitigation policies. MG declares that he has no competing interests.
References
- 1.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brauer M, Amann M, Burnett RT, et al. Exposure assessment for estimation of the global burden of disease attributable to outdoor air pollution. Environ Sci Technol. 2012;46:652–60. doi: 10.1021/es2025752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez L, Declercq C, Iniguez C, et al. Chronic burden of near-roadway traffic pollution in 10 European cities (APHEKOM network) Eur Respir J. 2013;42:594–605. doi: 10.1183/09031936.00031112. [DOI] [PubMed] [Google Scholar]
- 4.Wong GWK, Chow CM. Childhood asthma epidemiology: insights from comparative studies of rural and urban populations. Pediatr Pulmonol. 2008;43:107–16. doi: 10.1002/ppul.20755. [DOI] [PubMed] [Google Scholar]
- 5.Robinson CL, Baumann LM, Romero K, et al. Effect of urbanisation on asthma, allergy and airways inflammation in a developing country setting. Thorax. 2011;66:1051–57. doi: 10.1136/thx.2011.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunekreef B, Stewart AW, Anderson HR, Lai CKW, Strachan DP, Pearce N. Self-reported truck traffic on the street of residence and symptoms of asthma and allergic disease: a global relationship in ISAAC phase 3. Environ Health Perspect. 2009;117:1791–98. doi: 10.1289/ehp.0800467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman MS, Powell KE, Hutwagner L, Graham LM, Teague WG. Impact of changes in transportation and commuting behaviors during the 1996 Summer Olympic Games in Atlanta on air quality and childhood asthma. JAMA. 2001;285:897–905. doi: 10.1001/jama.285.7.897. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor GT, Neas L, Vaughn B, et al. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J Allergy Clin Immunol. 2008;121:1133–39. doi: 10.1016/j.jaci.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect. 2010;118:449–57. doi: 10.1289/ehp.0900844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Health Effects Institute. Traffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects. Boston, MA: Health Effects Institute; 2010. Panel on the Health Effects of Traffic-Related Air Pollution. [Google Scholar]
- 11.Dong G-H, Chen T, Liu M-M, et al. Gender differences and effect of air pollution on asthma in children with and without allergic predisposition: northeast Chinese children health study. PLoS One. 2011;6:e22470. doi: 10.1371/journal.pone.0022470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishimura KK, Galanter JM, Roth LA, et al. Early-life air pollution and asthma risk in minority children. The GALA II and SAGE II studies. Am J Respir Crit Care Med. 2013;188:309–18. doi: 10.1164/rccm.201302-0264OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacquemin B, Sunyer J, Forsberg B, et al. Home outdoor NO2 and new-onset of self-reported asthma in adults. Epidemiology. 2009;20:119–26. doi: 10.1097/EDE.0b013e3181886e76. [DOI] [PubMed] [Google Scholar]
- 14.Jerrett M, Shankardass K, Berhane K, et al. Traffic-related air pollution and asthma onset in children: a prospective cohort study with individual exposure measurement. Environ Health Perspect. 2008;116:1433–38. doi: 10.1289/ehp.10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McConnell R, Islam T, Shankardass K, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. 2010;118:1021–26. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou C, Baiz N, Zhang T, Banerjee S, Annesi-Maesano I. Modifiable exposures to air pollutants related to asthma phenotypes in the first year of life in children of the EDEN mother-child cohort study. BMC Public Health. 2013;13:506. doi: 10.1186/1471-2458-13-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlsten C, Dybuncio A, Becker A, Chan-Yeung M, Brauer M. Traffic-related air pollution and incident asthma in a high-risk birth cohort. Occup Environ Med. 2011;68:291–95. doi: 10.1136/oem.2010.055152. [DOI] [PubMed] [Google Scholar]
- 18.Clark NA, Demers PA, Karr CJ, et al. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. 2010;118:284–90. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunzli N, Bridevaux P-O, Liu L-JS, et al. Traffic-related air pollution correlates with adult-onset asthma among never-smokers. Thorax. 2009;64:664–70. doi: 10.1136/thx.2008.110031. [DOI] [PubMed] [Google Scholar]
- 20.Modig L, Toren K, Janson C, Jarvholm B, Forsberg B. Vehicle exhaust outside the home and onset of asthma among adults. Eur Respir J. 2009;33:1261–67. doi: 10.1183/09031936.00101108. [DOI] [PubMed] [Google Scholar]
- 21.Anderson HR, Favarato G, Atkinson RW. Long-term exposure to air pollution and the incidence of asthma: meta-analysis of cohort studies. Air Qual Atmos Health. 2013;6:47–56. [Google Scholar]
- 22.Anderson HR, Favarato G, Atkinson RW. Long-term exposure to outdoor air pollution and the prevalence of asthma: meta-analysis of multi-community prevalence studies. Air Qual Atmos Health. 2013;6:57–68. [Google Scholar]
- 23.Seltzer J, Bigby BG, Stulbarg M, et al. O3-induced change in bronchial reactivity to methacholine and airway inflammation in humans. J Appl Physiol. 1986;60:1321–26. doi: 10.1152/jappl.1986.60.4.1321. [DOI] [PubMed] [Google Scholar]
- 24.Aris RM, Christian D, Hearne PQ, Kerr K, Finkbeiner WE, Balmes JR. Ozone-induced airway inflammation in human subjects as determined by airway lavage and biopsy. Am Rev Respir Dis. 1993;148:1363–72. doi: 10.1164/ajrccm/148.5.1363. [DOI] [PubMed] [Google Scholar]
- 25.Solomon C, Christian DL, Chen LL, et al. Effect of serial-day exposure to nitrogen dioxide on airway and blood leukocytes and lymphocyte subsets. Eur Respir J. 2000;15:922–28. doi: 10.1034/j.1399-3003.2000.15e19.x. [DOI] [PubMed] [Google Scholar]
- 26.Frampton MW, Boscia J, Roberts NJJ, et al. Nitrogen dioxide exposure: effects on airway and blood cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L155–65. doi: 10.1152/ajplung.2002.282.1.L155. [DOI] [PubMed] [Google Scholar]
- 27.Dales R, Wheeler A, Mahmud M, et al. The influence of living near roadways on spirometry and exhaled nitric oxide in elementary schoolchildren. Environ Health Perspect. 2008;116:1423–27. doi: 10.1289/ehp.10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delfino RJ, Staimer N, Gillen D, et al. Personal and ambient air pollution is associated with increased exhaled nitric oxide in children with asthma. Environ Health Perspect. 2006;114:1736–43. doi: 10.1289/ehp.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poynter ME, Persinger RL, Irvin CG, et al. Nitrogen dioxide enhances allergic airway inflammation and hyperresponsiveness in the mouse. Am J Physiol Lung Cell Mol Physiol. 2006;290:L144–52. doi: 10.1152/ajplung.00131.2005. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Poon R, Chen L, et al. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009;117:668–74. doi: 10.1289/ehp11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel MM, Chillrud SN, Deepti KC, Ross JM, Kinney PL. Traffic-related air pollutants and exhaled markers of airway inflammation and oxidative stress in New York City adolescents. Environ Res. 2013;121:71–78. doi: 10.1016/j.envres.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neophytou AM, Hart JE, Cavallari JM, et al. Traffic-related exposures and biomarkers of systemic inflammation, endothelial activation and oxidative stress: a panel study in the US trucking industry. Environ Health. 2013;12:105. doi: 10.1186/1476-069X-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gowers AM, Cullinan P, Ayres JG, et al. Does outdoor air pollution induce new cases of asthma? Biological plausibility and evidence; a review. Respirology. 2012;17:887–98. doi: 10.1111/j.1440-1843.2012.02195.x. [DOI] [PubMed] [Google Scholar]
- 34.Gilliland FD, Li Y-F, Saxon A, Diaz-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomised, placebo-controlled crossover study. Lancet. 2004;363:119–25. doi: 10.1016/S0140-6736(03)15262-2. [DOI] [PubMed] [Google Scholar]
- 35.Islam T, Berhane K, McConnell R, et al. Glutathione-S-transferase (GST) P1, GSTM1, exercise, ozone and asthma incidence in school children. Thorax. 2009;64:197–202. doi: 10.1136/thx.2008.099366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y-F, Gauderman WJ, Avol E, Dubeau L, Gilliland FD. Associations of tumor necrosis factor G-308A with childhood asthma and wheezing. Am J Respir Crit Care Med. 2006;173:970–76. doi: 10.1164/rccm.200508-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan JKW, Charrier JG, Kodani SD, et al. Combustion-derived flame generated ultrafine soot generates reactive oxygen species and activates Nrf2 antioxidants differently in neonatal and adult rat lungs. Part Fibre Toxicol. 2013;10:34. doi: 10.1186/1743-8977-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med. 2008;44:1689–99. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nadeau K, McDonald-Hyman C, Noth EM, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126:845–52. doi: 10.1016/j.jaci.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Brunst KJ, Leung Y-K, Ryan PH, et al. Forkhead box protein 3 (FOXP3) hypermethylation is associated with diesel exhaust exposure and risk for childhood asthma. J Allergy Clin Immunol. 2013;131:592–94. doi: 10.1016/j.jaci.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohli A, Garcia MA, Miller RL, et al. Secondhand smoke in combination with ambient air pollution exposure is associated with increasedx CpG methylation and decreased expression of IFN-gamma in T effector cells and Foxp3 in T regulatory cells in children. Clin Epigenetics. 2012;4:17. doi: 10.1186/1868-7083-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang P, Thevenot P, Saravia J, Ahlert T, Cormier SA. Radical-containing particles activate dendritic cells and enhance Th17 inflammation in a mouse model of asthma. Am J Respir Cell Mol Biol. 2011;45:977–83. doi: 10.1165/rcmb.2011-0001OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Voorhis M, Knopp S, Julliard W, et al. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS One. 2013;8:e82545. doi: 10.1371/journal.pone.0082545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandt EB, Kovacic MB, Lee GB, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132:1194–1204. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kehrl HR, Peden DB, Ball B, Folinsbee LJ, Horstman D. Increased specific airway reactivity of persons with mild allergic asthma after 7.6 hours of exposure to 0. 16 ppm ozone. J Allergy Clin Immunol. 1999;104:1198–204. doi: 10.1016/s0091-6749(99)70013-8. [DOI] [PubMed] [Google Scholar]
- 46.Tunnicliffe WS, Burge PS, Ayres JG. Effect of domestic concentrations of nitrogen dioxide on airway responses to inhaled allergen in asthmatic patients. Lancet. 1994;344:1733–36. doi: 10.1016/s0140-6736(94)92886-x. [DOI] [PubMed] [Google Scholar]
- 47.Devalia JL, Rusznak C, Herdman MJ, Trigg CJ, Tarraf H, Davies RJ. Effect of nitrogen dioxide and sulphur dioxide on airway response of mild asthmatic patients to allergen inhalation. Lancet. 1994;344:1668–71. doi: 10.1016/s0140-6736(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 48.Nel AE, Diaz-Sanchez D, Ng D, Hiura T, Saxon A. Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system. J Allergy Clin Immunol. 1998;102:539–54. doi: 10.1016/s0091-6749(98)70269-6. [DOI] [PubMed] [Google Scholar]
- 49.Diaz-Sanchez D, Garcia MP, Wang M, Jyrala M, Saxon A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J Allergy Clin Immunol. 1999;104:1183–88. doi: 10.1016/s0091-6749(99)70011-4. [DOI] [PubMed] [Google Scholar]
- 50.Kelly FJ, Fussell JC. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos Environ. 2012;60:504–26. [Google Scholar]
- 51.Delfino RJ, Zeiger RS, Seltzer JM, et al. The effect of outdoor fungal spore concentrations on daily asthma severity. Environ Health Perspect. 1997;105:622–35. doi: 10.1289/ehp.97105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ostro B, Lipsett M, Mann J, Braxton-Owens H, White M. Air pollution and exacerbation of asthma in African-American children in Los Angeles. Epidemiology. 2001;12:200–08. doi: 10.1097/00001648-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Stanek LW, Brown JS, Stanek J, Gift J, Costa DL. Air pollution toxicology—a brief review of the role of the science in shaping the current understanding of air pollution health risks. Toxicol Sci. 2011;120 (suppl 1):S8–27. doi: 10.1093/toxsci/kfq367. [DOI] [PubMed] [Google Scholar]
- 54.Mann JK, Balmes JR, Bruckner TA, et al. Short-term effects of air pollution on wheeze in asthmatic children in Fresno, California. Environ Health Perspect. 2010;118:1497–502. doi: 10.1289/ehp.0901292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meng Y-Y, Rull RP, Wilhelm M, Lombardi C, Balmes J, Ritz B. Outdoor air pollution and uncontrolled asthma in the San Joaquin Valley, California. J Epidemiol Community Health. 2010;64:142–47. doi: 10.1136/jech.2009.083576. [DOI] [PubMed] [Google Scholar]
- 56.Jacquemin B, Kauffmann F, Pin I, et al. Air pollution and asthma control in the Epidemiological study on the Genetics and Environment of Asthma. J Epidemiol Community Health. 2012;66:796–802. doi: 10.1136/jech.2010.130229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iskandar A, Andersen ZJ, Bonnelykke K, Ellermann T, Andersen KK, Bisgaard H. Coarse and fine particles but not ultrafine particles in urban air trigger hospital admission for asthma in children. Thorax. 2012;67:252–57. doi: 10.1136/thoraxjnl-2011-200324. [DOI] [PubMed] [Google Scholar]
- 58.Delamater PL, Finley AO, Banerjee S. An analysis of asthma hospitalizations, air pollution, and weather conditions in Los Angeles County, California. Sci Total Environ. 2012;425:110–18. doi: 10.1016/j.scitotenv.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malig BJ, Green S, Basu R, Broadwin R. Coarse particles and respiratory emergency department visits in California. Am J Epidemiol. 2013;178:58–69. doi: 10.1093/aje/kws451. [DOI] [PubMed] [Google Scholar]
- 60.Samoli E, Nastos PT, Paliatsos AG, Katsouyanni K, Priftis KN. Acute effects of air pollution on pediatric asthma exacerbation: evidence of association and effect modification. Environ Res. 2011;111:418–24. doi: 10.1016/j.envres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 61.Silverman RA, Ito K. Age-related association of fine particles and ozone with severe acute asthma in New York City. J Allergy Clin Immunol. 2010;125:367–73. doi: 10.1016/j.jaci.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 62.Son J-Y, Lee J-T, Park YH, Bell ML. Short-term effects of air pollution on hospital admissions in Korea. Epidemiology. 2013;24:545–54. doi: 10.1097/EDE.0b013e3182953244. [DOI] [PubMed] [Google Scholar]
- 63.Strickland MJ, Darrow LA, Mulholland JA, et al. Implications of different approaches for characterizing ambient air pollutant concentrations within the urban airshed for time-series studies and health benefits analyses. Environ Health. 2011;10:36. doi: 10.1186/1476-069X-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penard-Morand C, Raherison C, Charpin D, et al. Long-term exposure to close-proximity air pollution and asthma and allergies in urban children. Eur Respir J. 2010;36:33–40. doi: 10.1183/09031936.00116109. [DOI] [PubMed] [Google Scholar]
- 65.Akinbami LJ, Lynch CD, Parker JD, Woodruff TJ. The association between childhood asthma prevalence and monitored air pollutants in metropolitan areas, United States, 2001–2004. Environ Res. 2010;110:294–301. doi: 10.1016/j.envres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 66.US EPA, ORD, National Center for Environmental Assessment, NCEA-RTP. [accessed April 17, 2014];Integrated science assessment for ozone and related photochemical oxidants. http://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=247492.
- 67.Division UEONRTP. [accessed April 17, 2014];Integrated science assessment for oxides of nitrogen—health criteria. http://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=259167.
- 68.Johns DO, Linn WS. A review of controlled human SO(2) exposure studies contributing to the US EPA integrated science assessment for sulfur oxides. Inhal Toxicol. 2011;23:33–43. doi: 10.3109/08958378.2010.539290. [DOI] [PubMed] [Google Scholar]
- 69.Folinsbee LJ. Does nitrogen dioxide exposure increase airways responsiveness? Toxicol Ind Health. 1992;8:273–83. [PubMed] [Google Scholar]
- 70.Goodman JE, Chandalia JK, Thakali S, Seeley M. Meta-analysis of nitrogen dioxide exposure and airway hyper-responsiveness in asthmatics. Crit Rev Toxicol. 2009;39:719–42. doi: 10.3109/10408440903283641. [DOI] [PubMed] [Google Scholar]
- 71.Hesterberg TW, Bunn WB, McClellan RO, Hamade AK, Long CM, Valberg PA. Critical review of the human data on short-term nitrogen dioxide (NO2) exposures: evidence for NO2 no-effect levels. Crit Rev Toxicol. 2009;39:743–81. doi: 10.3109/10408440903294945. [DOI] [PubMed] [Google Scholar]
- 72.Perez L, Kunzli N, Avol E, et al. Global goods movement and the local burden of childhood asthma in southern California. Am J Public Health. 2009;99 (Suppl 3):S622–28. doi: 10.2105/AJPH.2008.154955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hernandez-Cadena L, Holguin F, Barraza-Villarreal A, Del Rio-Navarro BE, Sienra-Monge JJ, Romieu I. Increased levels of outdoor air pollutants are associated with reduced bronchodilation in children with asthma. CHEST. 2009;136:1529–36. doi: 10.1378/chest.08-1463. [DOI] [PubMed] [Google Scholar]
- 74.Martin RA, Ather JL, Lundblad LKA, et al. Interleukin-1 receptor and caspase-1 are required for the Th17 response in nitrogen dioxide-promoted allergic airway disease. Am J Respir Cell Mol Biol. 2013;48:655–64. doi: 10.1165/rcmb.2012-0423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barck C, Sandstrom T, Lundahl J, et al. Ambient level of NO2 augments the inflammatory response to inhaled allergen in asthmatics. Respir Med. 2002;96:907–17. doi: 10.1053/rmed.2002.1374. [DOI] [PubMed] [Google Scholar]
- 76.Pathmanathan S, Krishna MT, Blomberg A, et al. Repeated daily exposure to 2 ppm nitrogen dioxide upregulates the expression of IL-5, IL-10, IL-13, and ICAM-1 in the bronchial epithelium of healthy human airways. Occup Environ Med. 2003;60:892–96. doi: 10.1136/oem.60.11.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Witten A, Solomon C, Abbritti E, et al. Effects of nitrogen dioxide on allergic airway responses in subjects with asthma. J Occup Environ Med. 2005;47:1250–59. doi: 10.1097/01.jom.0000177081.62204.8d. [DOI] [PubMed] [Google Scholar]
- 78.Strickland MJ, Darrow LA, Klein M, et al. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med. 2010;182:307–16. doi: 10.1164/rccm.200908-1201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greer JR, Abbey DE, Burchette RJ. Asthma related to occupational and ambient air pollutants in nonsmokers. J Occup Med. 1993;35:909–15. doi: 10.1097/00043764-199309000-00014. [DOI] [PubMed] [Google Scholar]
- 80.McDonnell WF, Abbey DE, Nishino N, Lebowitz MD. Long-term ambient ozone concentration and the incidence of asthma in nonsmoking adults: the AHSMOG Study. Environ Res. 1999;80:110–21. doi: 10.1006/enrs.1998.3894. [DOI] [PubMed] [Google Scholar]
- 81.Kim B-J, Kwon J-W, Seo J-H, et al. Association of ozone exposure with asthma, allergic rhinitis, and allergic sensitization. Ann Allergy Asthma Immunol. 2011;107:214–19. doi: 10.1016/j.anai.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 82.McConnell R, Berhane K, Gilliland F, et al. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359:386–91. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- 83.Acciani TH, Brandt EB, Khurana Hershey GK, Le Cras TD. Diesel exhaust particle exposure increases severity of allergic asthma in young mice. Clin Exp Allergy. 2013;43:1406–18. doi: 10.1111/cea.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee GB, Brandt EB, Xiao C, et al. Diesel exhaust particles induce cysteine oxidation and s-glutathionylation in house dust mite induced murine asthma. PLoS One. 2013;8:e60632. doi: 10.1371/journal.pone.0060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takahashi G, Tanaka H, Wakahara K, et al. Effect of diesel exhaust particles on house dust mite-induced airway eosinophilic inflammation and remodeling in mice. J Pharmacol Sci. 2010;112:192–202. doi: 10.1254/jphs.09276fp. [DOI] [PubMed] [Google Scholar]
- 86.Manners S, Alam R, Schwartz DA, Gorska MM. A mouse model links asthma susceptibility to prenatal exposure to diesel exhaust. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang P, You D, Saravia J, Shen H, Cormier S. Maternal exposure to combustion generated PM inhibits pulmonary Th1 maturation and concomitantly enhances postnatal asthma development in off spring. Part Fibre Toxicol. 2013;10:29. doi: 10.1186/1743-8977-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nordenhall C, Pourazar J, Ledin MC, Levin JO, Sandstrom T, Adelroth E. Diesel exhaust enhances airway responsiveness in asthmatic subjects. Eur Respir J. 2001;17:909–15. doi: 10.1183/09031936.01.17509090. [DOI] [PubMed] [Google Scholar]
- 89.Behndig AF, Larsson N, Brown JL, et al. Proinflammatory doses of diesel exhaust in healthy subjects fail to elicit equivalent or augmented airway inflammation in subjects with asthma. Thorax. 2011;66:12–19. doi: 10.1136/thx.2010.140053. [DOI] [PubMed] [Google Scholar]
- 90.Stenfors N, Nordenhall C, Salvi SS, et al. Different airway inflammatory responses in asthmatic and healthy humans exposed to diesel. Eur Respir J. 2004;23:82–86. doi: 10.1183/09031936.03.00004603. [DOI] [PubMed] [Google Scholar]
- 91.Rosenlund M, Forastiere F, Porta D, De Sario M, Badaloni C, Perucci CA. Traffic-related air pollution in relation to respiratory symptoms, allergic sensitisation and lung function in schoolchildren. Thorax. 2009;64:573–80. doi: 10.1136/thx.2007.094953. [DOI] [PubMed] [Google Scholar]
- 92.Balmes JR, Earnest G, Katz PP, et al. Exposure to traffic: lung function and health status in adults with asthma. J Allergy Clin Immunol. 2009;123:626–31. doi: 10.1016/j.jaci.2008.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Delfino RJ, Chang J, Wu J, et al. Repeated hospital encounters for asthma in children and exposure to traffic-related air pollution near the home. Ann Allergy Asthma Immunol. 2009;102:138–44. doi: 10.1016/S1081-1206(10)60244-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cook AG, deVos AJBM, Pereira G, Jardine A, Weinstein P. Use of a total traffic count metric to investigate the impact of roadways on asthma severity: a case-control study. Environ Health. 2011;10:52. doi: 10.1186/1476-069X-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Delfino RJ, Wu J, Tjoa T, Gullesserian SK, Nickerson B, Gillen DL. Asthma morbidity and ambient air pollution: effect modification by residential traffic-related air pollution. Epidemiology. 2014;25:48–57. doi: 10.1097/EDE.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 96.Kelly F, Armstrong B, Atkinson R, et al. The London low emission zone baseline study. Res Rep Health Eff Inst. 2011;163:3–79. [PubMed] [Google Scholar]
- 97.Vrijheid M, Casas M, Bergstrom A, et al. European birth cohorts for environmental health research. Environ Health Perspect. 2012;120:29–37. doi: 10.1289/ehp.1103823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perez L, Lurmann F, Wilson J, et al. Near-roadway pollution and childhood asthma: implications for developing ‘win-win’ compact urban development and clean vehicle strategies. Environ Health Perspect. 2012;120:1619–26. doi: 10.1289/ehp.1104785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bateson TF, Schwartz J. Children’s response to air pollutants. J Toxicol Environ Health A. 2008;71:238–43. doi: 10.1080/15287390701598234. [DOI] [PubMed] [Google Scholar]
- 100.Miller MD, Marty MA. Impact of environmental chemicals on lung development. Environ Health Perspect. 2010;118:1155–64. doi: 10.1289/ehp.0901856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rosa MJ, Jung KH, Perzanowski MS, et al. Prenatal exposure to polycyclic aromatic hydrocarbons, environmental tobacco smoke and asthma. Respir Med. 2011;105:869–76. doi: 10.1016/j.rmed.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mortimer KM, Tager IB, Dockery DW, Neas LM, Redline S. The effect of ozone on inner-city children with asthma: identification of susceptible subgroups. Am J Respir Crit Care Med. 2000;162:1838–45. doi: 10.1164/ajrccm.162.5.9908113. [DOI] [PubMed] [Google Scholar]
- 103.Johnston NW, Sears MR. Asthma exacerbations.1: epidemiology. Thorax. 2006;61:722–28. doi: 10.1136/thx.2005.045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oosterlee A, Drijver M, Lebret E, Brunekreef B. Chronic respiratory symptoms in children and adults living along streets with high traffic density. Occup Environ Med. 1996;53:241–47. doi: 10.1136/oem.53.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sternthal MJ, Coull BA, Chiu Y-HM, Cohen S, Wright RJ. Associations among maternal childhood socioeconomic status, cord blood IgE levels, and repeated wheeze in urban children. J Allergy Clin Immunol. 2011;128:337–45. doi: 10.1016/j.jaci.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gehring U, Pattenden S, Slachtova H, et al. Parental education and children‘s respiratory and allergic symptoms in the Pollution and the Young (PATY) study. Eur Respir J. 2006;27:95–107. doi: 10.1183/09031936.06.00017205. [DOI] [PubMed] [Google Scholar]
- 107.Wilhelm M, Qian L, Ritz B. Outdoor air pollution, family and neighborhood environment, and asthma in LA FANS children. Health Place. 2009;15:25–36. doi: 10.1016/j.healthplace.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci USA. 2009;106:12406–11. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nagel G, Weinmayr G, Kleiner A, Garcia-Marcos L, Strachan DP. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) Phase Two. Thorax. 2010;65:516–22. doi: 10.1136/thx.2009.128256. [DOI] [PubMed] [Google Scholar]
- 110.Fitzpatrick AM, Teague WG, Holguin F, Yeh M, Brown LAS. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J Allergy Clin Immunol. 2009;123:146–48. doi: 10.1016/j.jaci.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barraza-Villarreal A, Sunyer J, Hernandez-Cadena L, et al. Air pollution, airway inflammation, and lung function in a cohort study of Mexico City schoolchildren. Environ Health Perspect. 2008;116:832–38. doi: 10.1289/ehp.10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dong GH, Qian Z, Liu M-M, et al. Obesity enhanced respiratory health effects of ambient air pollution in Chinese children: the Seven Northeastern Cities study. Int J Obes (Lond) 2013;37:94–100. doi: 10.1038/ijo.2012.125. [DOI] [PubMed] [Google Scholar]
- 113.Schikowski T, Schaffner E, Meier F, et al. Improved air quality and attenuated lung function decline: modification by obesity in the SAPALDIA cohort. Environ Health Perspect. 2013;121:1034. doi: 10.1289/ehp.1206145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moon K-Y, Park M-K, Leikauf GD, Park C-S, Jang A-S. Diesel exhaust particle-induced airway responses are augmented in obese rats. Int J Toxicol. 2014;33:21–28. doi: 10.1177/1091581813518355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rabinovitch N, Silveira L, Gelfand EW, Strand M. The response of children with asthma to ambient particulate is modified by tobacco smoke exposure. Am J Respir Crit Care Med. 2011;184:1350–57. doi: 10.1164/rccm.201010-1706OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rogers AJ, Brasch-Andersen C, Ionita-Laza I, et al. The interaction of glutathione S-transferase M1-null variants with tobacco smoke exposure and the development of childhood asthma. Clin Exp Allergy. 2009;39:1721–29. doi: 10.1111/j.1365-2222.2009.03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schultz EN, Devadason SG, Khoo S-K, et al. The role of GSTP1 polymorphisms and tobacco smoke exposure in children with acute asthma. J Asthma. 2010;47:1049–56. doi: 10.1080/02770903.2010.508856. [DOI] [PubMed] [Google Scholar]
- 118.Wu H, Romieu I, Sienra-Monge JJ, et al. Parental smoking modifies the relation between genetic variation in tumor necrosis factor-alpha (TNF) and childhood asthma. Environ Health Perspect. 2007;115:616–22. doi: 10.1289/ehp.9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Neidell M. Air quality warnings and outdoor activities: evidence from Southern California using a regression discontinuity design. J Epidemiol Community Health. 2010;64:921–26. doi: 10.1136/jech.2008.081489. [DOI] [PubMed] [Google Scholar]
- 120.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007;357:2348–58. doi: 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- 121.Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002;52:1032–42. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
- 122.Zhu Y, Eiguren-Fernandez A, Hinds WC, Miguel AH. In-cabin commuter exposure to ultrafine particles on Los Angeles freeways. Environ Sci Technol. 2007;41:2138–45. doi: 10.1021/es0618797. [DOI] [PubMed] [Google Scholar]
- 123.Delfino RJ, Zeiger RS, Seltzer JM, Street DH, McLaren CE. Association of asthma symptoms with peak particulate air pollution and effect modification by anti-inflammatory medication use. Environ Health Perspect. 2002;110:A607–17. doi: 10.1289/ehp.021100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bartoli ML, Vagaggini B, Malagrino L, et al. Baseline airway inflammation may be a determinant of the response to ozone exposure in asthmatic patients. Inhal Toxicol. 2013;25:127–33. doi: 10.3109/08958378.2013.763313. [DOI] [PubMed] [Google Scholar]
- 125.Lewis TC, Robins TG, Mentz GB, et al. Air pollution and respiratory symptoms among children with asthma: vulnerability by corticosteroid use and residence area. Sci Total Environ. 2013;448:48–55. doi: 10.1016/j.scitotenv.2012.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bloomer BJ, Stehr JW, Piety CA, Salawitch RJ, Dickerson RR. Observed relationships of ozone air pollution with temperature and emissions. Geophys Res Lett. 2009:36. [Google Scholar]
- 127.Balmes JR. California’s cap-and-trade program. In: Pinkerton KE, Rom WN, editors. Global climate change and public health. New York: Springer; 2014. pp. 383–91. [Google Scholar]
- 128.Delucchi MA, Yang C, Burke AF, et al. An assessment of electric vehicles: technology, infrastructure requirements, greenhouse-gas emissions, petroleum use, material use, lifetime cost, consumer acceptance and policy initiatives. Philos Trans A Math Phys Eng Sci. 2014;372:20120325. doi: 10.1098/rsta.2012.0325. [DOI] [PubMed] [Google Scholar]
- 129.West JJ, Smith SJ, Silva RA, et al. Co-benefits of mitigating global greenhouse gas emissions for future air quality and human health. Nat Clim Chang. 2013;3:885–89. doi: 10.1038/NCLIMATE2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thurston GD. Mitigation policy: Health co-benefits. Nat Clim Chang. 2013;3:863–64. [Google Scholar]
- 131.(Barely) living in smog: China and air pollution. Lancet. 2014;383:845. doi: 10.1016/S0140-6736(14)60427-X. [DOI] [PubMed] [Google Scholar]
- 132.International Institute for Applied Systems Analysis (IIASA) [accessed March 10, 2014];Greenhouse Gas and Air Pollution Interactions and Synergies (GAINS) Database. 2012 http://gains.iiasa.ac.at/models/
- 133.Kaundinya DP, Balachandra P, Ravindranath NH. Grid-connected versus stand-alone energy systems for decentralized power—a review of literature. Renew Sustain Energy Rev. 2009;13:2041–50. [Google Scholar]
- 134.Salon D, Sperling D, Meier A, Murphy S, Gorham R, Barrett J. City carbon budgets: A proposal to align incentives for climate-friendly communities. Energy Policy. 2010;38:2032–41. [Google Scholar]
- 135.Melia S, Parkhurst G, Barton H. The paradox of intensification. Transport Policy. 2011;18:46–52. [Google Scholar]
- 136.Gale SL, Noth EM, Mann J, Balmes J, Hammond SK, Tager IB. Polycyclic aromatic hydrocarbon exposure and wheeze in a cohort of children with asthma in Fresno, CA. J Expo Sci Environ Epidemiol. 2012;22:386–92. doi: 10.1038/jes.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Delfino RJ, Staimer N, Tjoa T, et al. Personal and ambient air pollution exposures and lung function decrements in children with asthma. Environ Health Perspect. 2008;116:550–58. doi: 10.1289/ehp.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Adams C, Riggs P, Volckens J. Development of a method for personal, spatiotemporal exposure assessment. J Environ Monitoring. 2009;11:1331–39. doi: 10.1039/b903841h. [DOI] [PubMed] [Google Scholar]
- 139.Rice MB, Thurston GD, Balmes JR, Pinkerton KE. Climate change. A global threat to cardiopulmonary health. Am J Respir Crit Care Med. 2014;189:512–19. doi: 10.1164/rccm.201310-1924PP. [DOI] [PMC free article] [PubMed] [Google Scholar]