Abstract
Cardiac magnetic resonance imaging (CMR) has made major inroads in the new millenium in the diagnosis and assessment of prognosis for patients with cardiomyopathies. Imaging of left and right ventricular structure and function and tissue characterization with late gadolinium enhancement (LGE) as well as T1 and T2 mapping enable accurate diagnosis of the underlying etiology. In the setting of coronary artery disease, either transmurality of LGE or contractile reserve in response to dobutamine can assess the likelihood of recovery of function after revascularization. The presence of scar reduces the likelihood of response to medical therapy and to cardiac resynchronization therapy in heart failure. The presence and extent of LGE relate to overall cardiovascular outcome in cardiomyopathies. An emerging major role for CMR in cardiomyopathies is to identify myocardial scar for diagnostic and prognostic purposes.
Keywords: Cardiac magnetic resonance imaging, Cardiomyopathies, Coronary artery disease, Late gadolinium enhancement, Ischemic cardiomyopathy, Nonischemic cardiomyopathy, Infiltrative cardiomyopathy
CMR TECHNIQUES USED IN THE EVALUATION OF CARDIOMYOPATHIES
CMR is considered the gold standard for measurement of left and right ventricular (LV, RV) structure and function. Cine imaging is used to cover the LV in short axis from apex to base, essentially creating a 3-dimensional volumetric structure for analysis. In the 1990’s, r gradient echo cine imaging was the standard but was replaced at the turn of the millennium by steady state free precession cine imaging with to its higher contrast-to-noise between the dark myocardium and bright blood pool (1). Multiple studies have demonstrated the accuracy and reproducibility of CMR for measuring left ventricular (LV) volumes, ejection fraction (EF), and regional function (2). Measurements made with this 3-dimensional (3D) data set do not require geometric assumptions and are therefore less prone to error than 2D methods such as 2D echocardiography in ventricles deformed by myocardial infarction (MI) or cardiomyopathies. Interstudy and interscan reproducibility is high, allowing for reduced sample sizes in clinical trials in heart failure (3–4). Techniques such as myocardial tissue tagging (5) or cine displacement encoded stimulated echo imaging (6) allow more detailed analysis of regional myocardial motion and deformation than cine imaging and have been applied to studies in myocardial infarction (7) and hypertrophic cardiomyopathy (8).
Late gadolinium enhancement (LGE) refers to regions of scar, necrosis, and/or inflammation discriminated from normal tissue by prolonged retention of gadolinium-based contrast agents. Since the mid-1980s, investigators have appreciated T1 shortening (increased enhancement) in regions of infarction following gadolinium administration (9–11). However, these early imaging techniques were limited by long acquisition times, artifacts, and insufficient contrast between normal and abnormal regions. With faster approaches and implementation of an inversion recovery pulse sequence with inversion time set to null normal myocardium increases the signal difference between normal and infarcted segments by 500–1000% (12). Using this approach, Kim et al. demonstrated that the spatial extent of LGE seen on CMR closely mirrored the distribution of myocyte necrosis in the early period following infarction and that of collagenous scar seen at 8 weeks(R=0.97, P<0.001) (13), while in regions of the heart subjected to reversible injury, the retention of contrast did not occur (14) (Figure 1). LGE accurately delineates infarction as defined by histology at various time points following injury (15). LGE is not unique to infarct scar and can demonstrate any cause of fibrosis or infiltration in cardiomyopathies.
Figure 1.
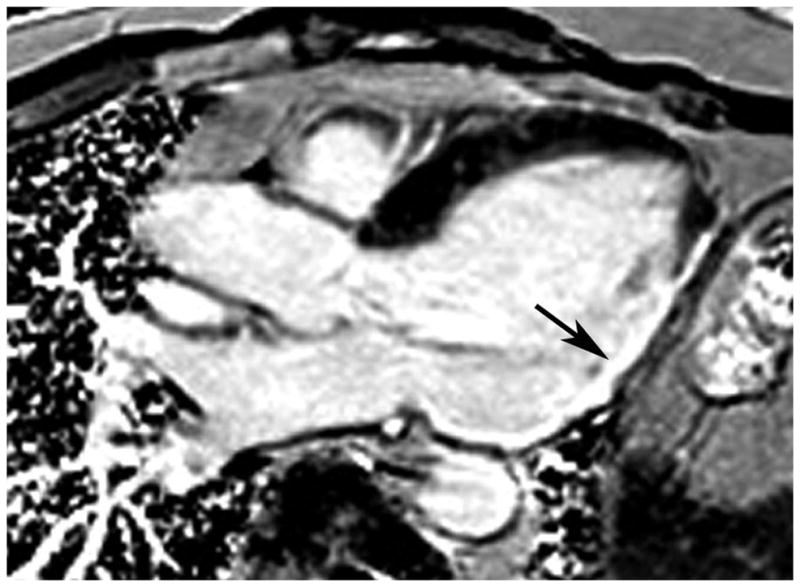
3-chamber long axis phase sensitive inversion recovery LGE image in a patient with heart failure and coronary artery disease. There is transmural LGE in the inferolateral wall (arrow).
T2-weighted (W) imaging can be used to demonstrate myocardial edema and/or injury. An understanding of the time course of edema is a key to the accurate interpretation of T2-W imaging. Abdel-Aty et al. noted in a canine model of MI that the onset of edema on CMR first became apparent 28±4 minutes after experimental coronary artery occlusion and well before the appearance of LGE or troponin elevation, signifying that CMR can visualize edema before the onset of irreversible myocardial injury (16) (Figure 2). Microsphere analysis in a canine model of reperfused acute MI suggested that T2-W imaging performed 2 days post-MI can be used to delineate the area at risk (17). Elevated T2 can be seen in any cause of myocardial injury such as acute myocarditis (18). Older T2-W imaging approaches are prone to artifacts and more recently, CMR protocols have used T2 mapping to overcome these limitations (19)
Figure 2.
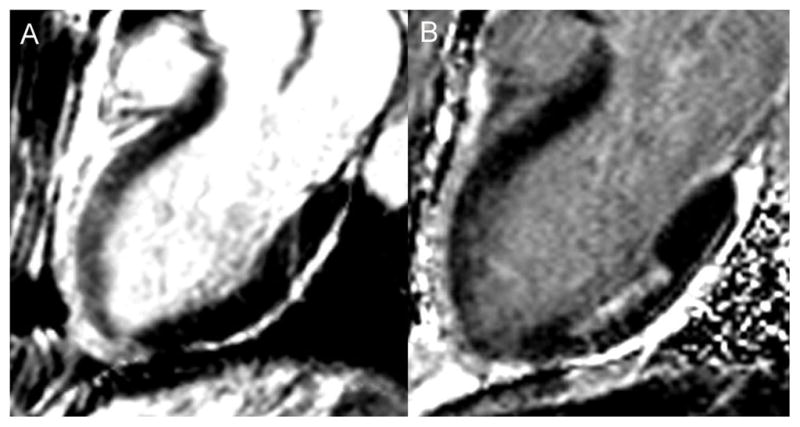
A – 3-chamber long axis bright blood T2-W image in a patient with a spontaneous LAD dissection demonstrating bright signal in the anterior wall consistent with edema. B – 3-chamber long axis phase sensitive inversion recovery late gadolinium enhanced image in the same patient showing absence of LGE in the anterior wall, but evidence of a prior 50% transmural infarct in the mid inferior wall. Together these images demonstrate evidence of myocardial stunning without infarction in the anterior wall.
T1 of the myocardium can be measured by mapping with a modified Look-Locker inversion recovery (MOLLI) sequence (20) and shorter versions of this approach, termed shMOLLI (21). Native or pre-contrast T1 can be measured before contrast infusion and then post-contrast T1 measures can be used to calculate extracellular volume fraction (ECV) in the myocardium (22). These techniques are being applied to help in the differential diagnosis of cardiomyopathies as some have characteristically high native T1 as well as high extracellular volume fraction. Native T1 is especially useful in patients with concomitant renal disease as patients with stage 4 or 5 chronic kidney disease are not candidates to receive gadolinium due to concerns with nephrogenic systemic fibrosis (NSF).
ESTABLISHING ETIOLOGY OF CARDIOMYOPATHY
The first step in the evaluation of the patient with new onset heart failure (HF) is to evaluate the underlying etiology and, importantly, to exclude ischemic heart disease as a potentially reversible cause. The presence of LGE in a coronary distribution can support the diagnosis of underlying coronary artery disease (CAD) but its absence does not rule it out, as patients with extensive hibernating myocardium may have no LGE (23) (Figure 3). In a study by Soriano et al of 71 patients with new onset HF and systolic dysfunction, the sensitivity of the infarct pattern of LGE for ischemic cardiomyopathy as defined by the presence of obstructive CAD was 81% whereas the specificity was 91% (23). Patients without obstructive CAD may have evidence of LGE in an infarct pattern due to thrombotic occlusion of a nonobstructive artery, embolization, or spontaneous coronary dissection and thereby may be misclassified. This finding was noted in 13% of 63 patients with the diagnosis of dilated cardiomyopathy with chronic HF (24). Computed tomographic coronary angiography may be an excellent way of noninvasively ruling CAD in or out in the setting of new or recent onset HF as shown in a study using the combination of x-ray coronary angiography and LGE by CMR as a gold standard for establishing the underlying etiology (25).
Figure 3.
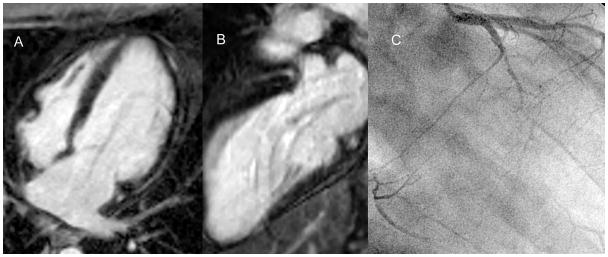
A - 4-chamber long axis phase sensitive inversion recovery LGE image in a patient with heart failure and EF 20% which shows no LGE. B - 2-chamber long axis phase sensitive inversion recovery LGE image in the same patient which shows no LGE. C – Right anterior oblique x-ray angiogram in this patient which demonstrates 3-vessel CAD with an occluded left circumflex, an occluded right coronary that fills through collaterals, and significant LAD disease. Thus, this patient demonstrates severe LV dysfunction in the setting of 3-vessel CAD without LGE signifying extensive hibernating myocardium.
ISCHEMIC CARDIOMYOPATHY
In addition to identifying coronary artery disease as the underlying etiology, CMR can define appropriate therapy as well. Response to beta-blockade can be predicted by the presence and extent of LGE. Bello et al demonstrated an inverse relationship between the extent of LGE and the likelihood of contractile improvement with 6 months of beta blockade (26). Fifty six % of segments with no scar demonstrated improved function, whereas only 3% of segments with >75% transmural scar demonstrated improvement (26).
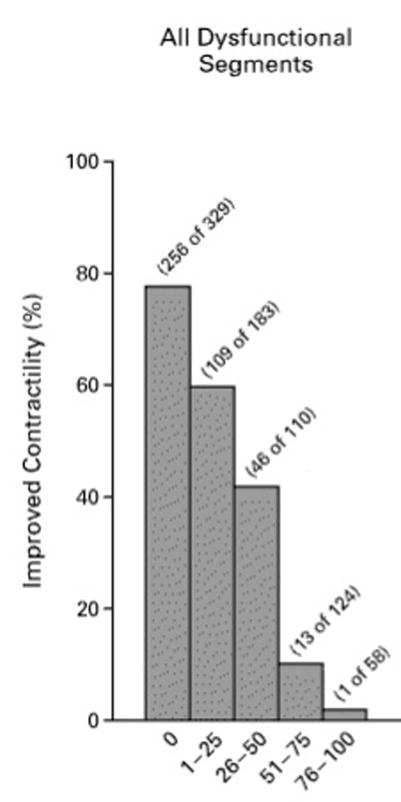
CMR plays an important role in the assessment of myocardial viability when choosing patients for revascularization. LV wall thickness on rest cine CMR imaging of less than 5.5mm at end-diastole is generally considered a marker of nonviable myocardium (27). One study, however, demonstrates that in a minority of patients with wall thinning without substantial LGE burden (4% of 1055 patients with CAD), contractile recovery can occur after revascularization (28). Non-transmural infarcts typically don’t result in severe end-diastolic wall thinning. The extent of LGE is a useful marker of likelihood of functional recovery with revascularization. In a landmark study, Kim et al studied 50 patients with LV dysfunction who were evaluated with contrast enhanced CMR prior to surgical or percutaneous revascularization (29). LGE was noted in 80% of the patients. There was an inverse relationship between the transmural extent of LGE and functional recovery with revascularization (Figure 4). Segments with >50% transmural LGE were nonviable, whereas segments with no LGE had an approximately 80% likelihood of functional recovery. Similar findings were noted in a study of 52 patients undergoing coronary artery bypass surgery (CABG) which demonstrated that 36% of segments developed new areas of LGE after surgery (30).
Figure 4.
Inverse relationship between transmurality of LGE on the x-axis and likelihood of improved function after revascularization. Adapted from (29) Kim et al, New England Journal of Medicine; 2000;43:1445–53.
Using cine imaging in long and short axis views of the LV after administration of low dose dobutamine (10 μg/kg/min) can assess contractile reserve and predicts areas of functional recovery with revascularization (27)(31). Bove et al studied patients before and after CABG and divided segments into no LGE, 1–25%, 26–50% or greater than 50% transmural LGE (31). Dysfunctional segments were defined as having wall thickening less than 27% (2 S.D < normal myocardium). After revascularization with CABG the improvement in wall thickening in segments with 1–50% transmural enhancement was 22±4% for dobutamine-responsive segments, significantly higher than that for dobutamine non-responsive segments (9±4%). These findings suggested that contractile reserve was more predictive of functional recovery than infarct transmurality in infarcts with 1–50% transmural LGE. In a complementary study, 29 patients with ischemic cardiomyopathy and severely reduced EF underwent both LGE and low-dose dobutamine cine imaging (32). Based on the area under the receiver operator characteristics (ROC) curve, contractile reserve with low-dose dobutamine was superior to the presence of LGE in predicting recovery of LV function, in particular when myocardial segments have <75% transmural LGE. A recent meta-analysis of 24 studies involving 698 patients suggested that although LGE provides the highest sensitivity and negative predictive value (95% and 90%, respectively) for predicting functional recovery after revascularization, low dose dobutamine cine imaging offered the highest specificity and positive predictive value (91% and 93%, respectively) (33).
Cardiac resynchronization therapy (CRT) with biventricular pacing has emerged as an important part of the therapeutic armamentarium in heart failure. Several studies have demonstrated that scar in the posterolateral wall is associated with a lower rate of response to CRT since the LV lead is usually placed in the lateral cardiac veins and may not appropriately capture scarred myocardium. Bleeker et al demonstrated in the 14 of 50 patients with >50% transmural posterolateral scar, only 2 (14%) responded to CRT whereas 81% of the remainder responded (34). Another study of 23 patient showed that %LV scar>15% had a sensitivity of 85% and specificity of 90% for CRT response (35). Bilchick et al studied 75 patients and demonstrated that the absence of posterolateral scar was an important predictor of CRT response in addition to dyssynchrony markers by CMR (36).
The presence and amount of LGE is prognostically important in the setting of CAD. Kwong et al studied 195 patients and found that the presence of any LGE was associated with a 8.3 hazard ratio for major adverse cardiac event (MACE) and a 10.9 hazard ratio for cardiac mortality (37). In another study of 857 patients followed for 4.4 years on average, a scar index based on LGE was an independent predictor of all-cause mortality or cardiac transplantation, in addition to other well-known risk factors including ejection fraction and age (38).
NONISCHEMIC CARDIOMYOPATHIES
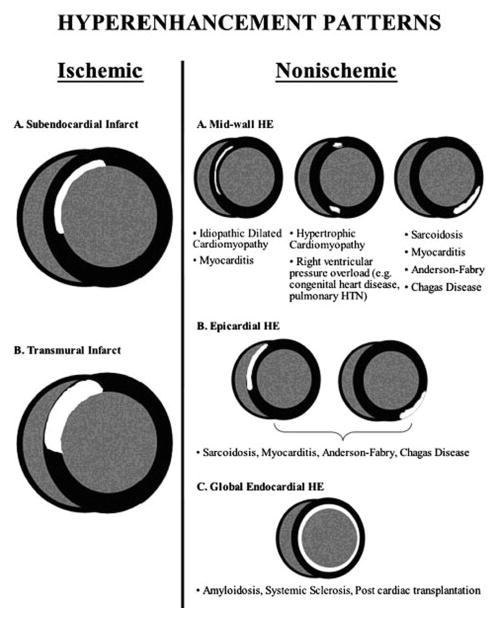
Tissue characterization by CMR is quite useful to differentiate etiology of nonischemic cardiomyopathies (Figure 5). LGE can differentiate infiltrative cardiomyopathies (39) and T1 mapping can identify certain hypertrophic and infiltrative cardiomyopathies that have characteristically high native T1 values (40). The finding of LGE has prognostic implications for nonischemic cardiomyopathies, as well as for ischemic cardiomyopathies. In a recent meta-analysis of 9 studies involving 1488 patients with nonischemic cardiomyopathy followed on average for 30 months, the presence of LGE was noted in 38% of patients and was associated with an odds ratio of 3.3 for mortality and 5.3 for sudden cardiac death (SCD) or aborted SCD (41).
Figure 5.
Patterns of late gadolinium enhancement seen in ischemic and non-ischemic cardiomyopathies. From Edelman RR, et al., eds. Clinical Magnetic Resonance Imaging, 3rd ed. New York: Elsevier Press; 2005 (75).
Dilated Cardiomyopathy
The majority patients with HF without obstructive CAD have no evidence of LGE (24). A minority of patients (13%) has LGE in an infarct pattern and may be misclassified as nonischemic (24). Approximately ¼ of patients with dilated cardiomyopathy (DCM) will have evidence of midwall fibrosis (24) (Figure 6). This likely represents the chronic healing phase of myocarditis. In a study of 472 patients with dilated cardiomyopathy followed for 5.3 years, the 142 patients with midwall fibrosis had a hazard ratio of 3.0 for all-cause mortality and 5.2 for a composite end point of SCD and aborted SCD (42). A study of 65 patients with dilated nonischemic cardiomyopathy with EF≤35% showed that the presence of LGE was associated with an 8-fold increase in heart failure, appropriate ICD firing, and cardiac death (43). The presence of midwall fibrosis is also an independent predictor of mortality and morbidity of patients with DCM undergoing cardiac resynchronization therapy (44). In fact, in this study of 97 patients with DCM and 161 patients with ischemic cardiomyopathy, DCM patients with midwall fibrosis had a similar outcome to those with ischemic disease (44). Thus, as with ischemic cardiomyopathy, the presence of fibrosis/scar is a marker of adverse outcome and worse response to device therapy.
Figure 6.
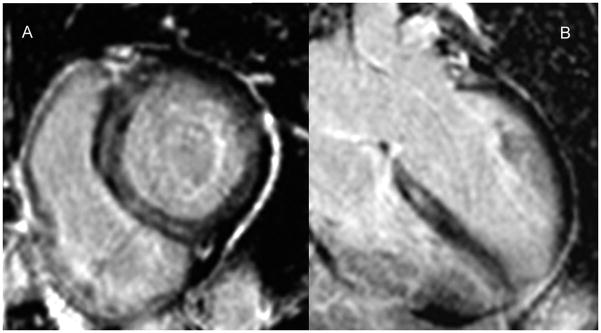
A – Basal short axis phase sensitive inversion recovery LGE image in a patient with heart failure, global LV systolic dysfunction, and EF 25%. There is a midwall stripe of LGE in the septum. B - 4-chamber long axis phase sensitive inversion recovery LGE image in the same patient again showing the mid septal stripe of LGE.
Myocarditis
A typical patient with acute myocarditis presents with chest pain, troponin elevation, and normal coronary arteries. The finding of LGE in the midwall and subepicardium of the LV is characteristic of viral myocarditis and has been validated against histology (45). Older studies suggested that early post-contrast T1-weighted enhancement of the myocardium could be a marker of inflammation in myocarditis (46). In addition, T2-W imaging demonstrating myocardial edema can be a diagnostic sign. The Lake Louise consortium in 2009 suggested that positivity of 2 of 3 techniques (early enhancement ratio, LGE, and/or increased T2 signal) may be an ideal diagnostic approach (47).
However, the early enhancement ratio has lost favor due to lack of reproducibility and T2-W imaging has been replaced by more quantitative T2 mapping. In addition, T1 mapping, both native and with contrast for measurement of extracellular volume (ECV) is also being used to make the diagnosis. Ferreira et al studied 50 patients with suspected acute myocarditis and 45 age-matched controls and showed that patients had higher T2 signal intensity ratios and native myocardial T1 than controls (48). In fact, a T1 cutoff of 990 msec at 1.5T demonstrated a sensitivity, specificity, and diagnostic accuracy of 90%, 91%, and 91% respectively. A combination of these techniques may be the best way to accurately identify acute myocarditis. In one recent study of 104 patients and 21 controls, a stepwise approach using LGE and myocardial ECV>27% demonstrated an overall accuracy of 90% (49). Another study of 61 patients with acute and 67 with chronic myocarditis showed that for acute myocarditis, diagnostic accuracy of native T1 was 99% as compared to 86% for LGE alone and 72% for increased T2-W signal (50). In chronic myocarditis, LGE alone performed better than T1 mapping (94% vs. 84% accuracy), but the combination of the 2 techniques performed even better with a 98% overall accuracy.
Hypertrophic Cardiomyopathy (HCM)
Approximately 2/3 of patients with HCM may have LGE with a characteristic pattern of patchy involvement, particularly at the right ventricular septal insertion sites and in those walls with the greatest hypertrophy. A number of studies have examined the relationship between the presence of LGE and outcome in HCM. Two recent studies, although underpowered, showed that LGE was associated with an increased risk of cardiovascular events. Bruder et al followed 243 HCM patients for 3 years for all-cause and cardiac mortality (51). LGE was seen in 67% and demonstrated an odds ratio (OR) of 5.5 for all-cause mortality and 8.0 for cardiac mortality. O’Hanlon et al followed 217 patients for 3 years for a composite endpoint including CV death, unplanned CV admission, sustained ventricular tachycardia or ventricular fibrillation, or appropriate ICD discharge (52). Sixty-three % had LGE, which was associated with an OR of 3.4 for the primary endpoint. A meta-analysis of 1063 patients pooled from 4 studies including these latter 2, followed for an average of 3.1 years, demonstrated that the presence of LGE had an odds ratio of 2.9 for cardiac death, 5.7 for heart failure death, and 4.5 for all-cause mortality, but only a trend for SCD (53). These studies treated LGE in a binary fashion, but it is likely that the extent of LGE would add discriminatory value.
A recent study of 1293 patients followed for 3.3 years showed that LGE ≥15% of LV mass was associated with a 2-fold increase in SCD event risk (54). In addition to the amount, the location and/or pattern of LGE may be more predictive of adverse outcome than the presence of LGE alone. The ongoing NIH-funded natural history study “HCMR-Novel Predictors of Prognosis in Hypertrophic Cardiomyopathy” (NCT01915615) using CMR, genetics, and biomarker evaluation of 2750 patients with HCM is likely to offer further insight in this regard.
Amyloidosis
In addition to the classic findings of thick LV walls, valves, and interatrial septum and presence of a pericardial effusion in cardiac amyloidosis, the existence of amyloid protein in the myocardial interstitium is associated with characteristic patterns of LGE due to abnormal gadolinium kinetics in the infiltrated myocardium (55). Validation against endomyocardial biopsy was performed in 33 patients with diastolic dysfunction and features concerning for amyloid (56) and the pattern of circumferential subendocardial LGE had a sensitivity of 80% and specificity of 94% (Figure 7). When the volume of distribution of gadolinium is quantified using serial measures of T1 in the myocardium after gadolinium contrast infusion, it is markedly increased compared to normal controls (57). In addition, the “native” T1 of myocardium is significantly longer in amyloidosis than in controls (57) and longer than in patients with LVH due to aortic stenosis (58).
Figure 7.
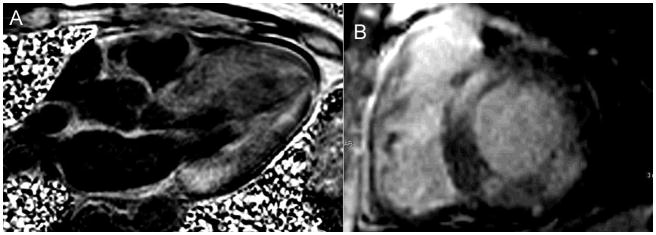
A - 4-chamber long axis phase sensitive inversion recovery LGE image in a patient with heart failure and EF 30% demonstrating diffuse subendocardial LGE and signal nulling in the blood pool, all characteristic of amyloidosis, most likely the ATTR variety. B- Basal short axis phase sensitive inversion recovery LGE image in a different patient with heart failure, global LV systolic dysfunction, and EF 25%. In this patient, the LGE is patchy and in a noncoronary distribution, more consistent with AL amyloidosis which was subsequently diagnosed on fat pad biopsy.
CMR is increasingly used to discriminate light chain (AL) amyloidosis from the transthyretin (ATTR) form of the disease. Fontana et al studied native T1 mapping in 85 ATTR patients and 79 AL patient compared with 52 normal subjects and 46 patients with HCM (59). AL disease demonstrated the highest native T1, followed by ATTR, although the area under the receiver operating curve was similar for both forms of amyloidosis vs. HCM (0.84). The potential utility of native T1 is critically important as many amyloid patients have concomitant renal dysfunction and are not candidates for gadolinium due to NSF concerns. Another study compared LGE findings in 46 patients with biopsy-proven AL and 51 with ATTR amyloidosis (60). LGE was much more extensive in ATTR with 90% demonstrating transmural LGE compared to only 37% in AL. These investigators developed an LGE scoring system that differentiated the 2 types with 87% sensitivity and 96% specificity.
Sarcoidosis
CMR can readily identify characteristic features of cardiac sarcoidosis including biventricular dilation and dysfunction. There is a variable pattern of LGE seen with cardiac sarcoid with a classic pattern of midwall or epicardial LGE, but it can also present with subendocardial or transmural enhancement in almost any distribution. One study of CMR in 58 patients with biopsy proven pulmonary sarcoid found 19 patients with evidence of LGE, mostly in the basal and lateral myocardium, a higher prevalence than identified by standard Japanese Ministry of Health (JMH) guidelines (61). Another study of 81 patients with extracardiac sarcoidosis followed on average for 21 months demonstrated a 2-fold higher rate of cardiac involvement by CMR than by JMH guidelines (62). Patients with LGE had a 9-fold higher incidence of adverse events compared to those without. In addition, T2 mapping may have a role in delineating activity of sarcoidosis. In a study of 28 patients, regions of LGE demonstrated decreased T2 which may reflect an inactive phase of the disease (63).
Arrhythmogenic Right Ventricular Cardiomyopathy
CMR is the gold standard method for the diagnosis of arrhythmogenic right ventricular cardiomyopathy. Diagnostic findings in this disease include RV dilatation and global or regional dysfunction including focal RV systolic bulging or aneurysm and are carefully defined to meet Task Force Criteria (64), In certain cases, LGE of the RV free wall can be identified (65), although this can be difficult to identify due to the thin RV wall in this disorder.
Non-compaction Cardiomyopathy
Non-compaction cardiomyopathy is defined on CMR as a noncompacted to compacted myocardial ratio of 2.3 at end-diastole (66). This ratio should be measured at a distance from the true apex. Newer definitions for the CMR diagnosis are frequently entertained (67), although the 2.3 noncompacted to compacted ratio remains the standard at present. Additional CMR findings in this disorder may include LV systolic dysfunction and LV thrombus.
Other Non-ischemic Cardiomyopathies
CMR is also able to diagnose a variety of other non-ischemic cardiomyopathies, including takotsubo cardiomyopathy demonstrating myocardial edema without LGE (68), iron-overload cardiomyopathy (using T2* mapping) (69), Anderson-Fabry disease with characteristic findings of basal lateral LGE (70), and Chagas disease with varied LGE findings (71).
LIMITATIONS OF CMR IN CARDIOMYOPATHIES
There are several limitations to the use of CMR in cardiomyopathies. For one, many patients with HF and reduced EF receive ICD’s and some with more severe HF refractory to medical therapy receive CRT devices. Both ICD’s and CRT devices are presently contraindicated for performing CMR. This limits the use of CMR in more advanced HF patients. However, there is growing evidence of safety of many of these devices under stringent conditions in the MR scanner (72). In addition, with the growth in implantation of MR-conditional pacemaker systems, there is recognition of the ability to do cardiac imaging safely in these patients. (73) Another limitation as discussed above is that patients with stage 4 or 5 chronic kidney disease cannot receive gadolinium due to concerns regarding the potential of causing nephrogenic systemic fibrosis in this population (74). Patients with active HF who are unable to lie flat are also not candidates for CMR.
CONCLUSION
CMR is an extremely useful imaging technique in cardiomyopathies. CMR is a gold standard method for measuring cardiac chamber size and function. With tissue characterization with LGE as well as with T1 and T2 mapping, the underlying etiology of heart failure can be readily established. In addition, the presence and extent of LGE in cardiomyopathies is associated with adverse cardiovascular outcomes and poor response to standard medical and interventional therapies. Thus, CMR has a major role to play in determining diagnosis and assessing prognosis in cardiomyopathies.
Acknowledgments
Dr. Kramer is supported in part by R01 HL075792 and U01HL117006-01A1
Footnotes
Disclosures – Dr. Kramer receives equipment support from Siemens Healthcare and has consulted for Bristol-Meyers Squibb, Merck, Myokardia, and Heart Metabolomics.
Reference List
- 1.Miller S, Simonetti OP, Carr J, Kramer U, Finn JP. MR imaging of the heart with cine true fast imaging with steady-state precession: influence of spatial and temporal resolutions on left ventricular functional parameters. Radiology. 2002;223:263–269. doi: 10.1148/radiol.2231010235. [DOI] [PubMed] [Google Scholar]
- 2.Isbell DC, Kramer CM. Cardiovascular magnetic resonance: structure, function, perfusion, and viability. J Nucl Cardiol. 2005;12:324–336. doi: 10.1016/j.nuclcard.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Grothues F, Smith GC, Moon JCC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 4.Bellenger NG, Davies LC, Francis JM, Coats AJS, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2:271–278. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]
- 5.Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging: a new method for noninvasive assessment of myocardial motion. Radiology. 1988;169:59–63. doi: 10.1148/radiology.169.1.3420283. [DOI] [PubMed] [Google Scholar]
- 6.Kim D, Gilson WD, Kramer CM, Epstein FH. Myocardial tissue tracking with two-dimensional cine displacement-encoded MR imaging: development and initial evaluation. Radiology. 2004;230:862–871. doi: 10.1148/radiol.2303021213. [DOI] [PubMed] [Google Scholar]
- 7.Kramer CM, Rogers WJ, Theobald TM, Power TP, Petruolo S, Reichek N. Remote noninfarcted region dysfunction soon after first anterior myocardial infarction. A magnetic resonance tagging study. Circulation. 1996;94:660–666. doi: 10.1161/01.cir.94.4.660. [DOI] [PubMed] [Google Scholar]
- 8.Kramer CM, Reichek N, Ferrari VA, Theobald T, Dawson J, Axel L. Regional heterogeneity of function in hypertrophic cardiomyopathy. Circulation. 1994;90:186–194. doi: 10.1161/01.cir.90.1.186. [DOI] [PubMed] [Google Scholar]
- 9.Rehr RB, Peshock RM, Malloy CR, et al. Improved in vivo magnetic resonance imaging of acute myocardial infarction after intravenous paramagnetic contrast agent administration. Am J Cardiol. 1986;57:864–868. doi: 10.1016/0002-9149(86)90628-4. [DOI] [PubMed] [Google Scholar]
- 10.Peshock RM, Malloy CR, Buja LM, Nunnally RL, Parkey RW, Willerson JT. Magnetic resonance imaging of acute myocardial infarction: gadolinium diethylenetriamine pentaacetic acid as a marker of reperfusion. Circulation. 1986;74:1434–1440. doi: 10.1161/01.cir.74.6.1434. [DOI] [PubMed] [Google Scholar]
- 11.de Roos A, Doornbos J, van der Wall EE, Van Voorthuisen AE. MR imaging of acute myocardial infarction: value of Gd-DTPA. AJR Am J Roentgenol. 1988;150:531–534. doi: 10.2214/ajr.150.3.531. [DOI] [PubMed] [Google Scholar]
- 12.Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 13.Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 14.Rehwald WG, Fieno DS, Chen EL, Kim RJ, Judd RM. Myocardial magnetic resonance imaging contrast agent concentrations after reversible and irreversible ischemic injury. Circulation. 2002;105:224–229. doi: 10.1161/hc0202.102016. [DOI] [PubMed] [Google Scholar]
- 15.Fieno DS, Kim RJ, Chen EL, Lomasney JW, Klocke FJ, Judd RM. Contrast-enhanced magnetic resonance imaging of myocardium at risk - Distinction between reversible and irreversible injury throughout infarct healing. J Am Coll Cardiol. 2000;36:1985–1991. doi: 10.1016/s0735-1097(00)00958-x. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG. Edema as a very early marker for acute myocardial ischemia: a cardiovascular magnetic resonance study. J Am Coll Cardiol. 2009;53:1194–1201. doi: 10.1016/j.jacc.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 17.Aletras AH, Tilak GS, Natanzon A, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging - Histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Aty H, Boye P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis - Comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 19.Giri S, Chung YC, Merchant A, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11 doi: 10.1186/1532-429X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messroghli DR, Walters K, Plein S, et al. Myocardial T1 mapping: Application to patients with acute and chronic myocardial infarction. Magn Reson Med. 2007;58:34–40. doi: 10.1002/mrm.21272. [DOI] [PubMed] [Google Scholar]
- 21.Piechnik S, Ferreira V, Dall’Armellina E, et al. Shortened modified look-locker inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12:69. doi: 10.1186/1532-429X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon J, Messroghli D, Kellman P, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soriano CJ, Ridocci F, Estornell J, Jimenez J, Martinez V, De Velasco JA. Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J Am Coll Cardiol. 2005;45:743–748. doi: 10.1016/j.jacc.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 24.McCrohon JA, Moon JC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 25.le Polain de Waroux J-Bt, Pouleur AC, Goffinet C, Pasquet A, Vanoverschelde JL, Gerber BL. Combined coronary and late-enhanced multidetector-computed tomography for delineation of the etiology of left ventricular dysfunction: comparison with coronary angiography and contrast-enhanced cardiac magnetic resonance imaging. Eur Heart J. 2008;29:2544–2551. doi: 10.1093/eurheartj/ehn381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bello D, Shah DJ, Farah GM, et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation. 2003;108:1945–1953. doi: 10.1161/01.CIR.0000095029.57483.60. [DOI] [PubMed] [Google Scholar]
- 27.Baer FM, Both E, Schneider CA, Theissen P, Schicha H, Sechtem U. Comparison of low-dose dobutamine-gradient echo magnetic resonance imaging and positron emission tomography 18F-fluorodeoxyglucose in patients with chronic coronary artery disease. Circulation. 1995;91:1006–1015. doi: 10.1161/01.cir.91.4.1006. [DOI] [PubMed] [Google Scholar]
- 28.Shah DJ, Kim HW, James O. Prevalence of regional myocardial thinning and relationship with myocardial scarring in patients with coronary artery disease. JAMA. 2013;309:909–918. doi: 10.1001/jama.2013.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 30.Selvanayagam JB, Kardos A, Francis JM, et al. Value of delayed-enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation. 2004;110:1535–1541. doi: 10.1161/01.CIR.0000142045.22628.74. [DOI] [PubMed] [Google Scholar]
- 31.Bove CM, DiMaria JM, Voros S, Conaway MR, Kramer CM. Dobutamine response and myocardial infarct transmurality: functional improvement after coronary artery bypass grafting--initial experience. Radiology. 2006;240:835–841. doi: 10.1148/radiol.2403051150. [DOI] [PubMed] [Google Scholar]
- 32.Wellnhofer E, Olariu A, Klein C, et al. Magnetic resonance low-dose dobutamine test is superior to scar quantification for the prediction of functional recovery. Circulation. 2004;109:2172–2174. doi: 10.1161/01.CIR.0000128862.34201.74. [DOI] [PubMed] [Google Scholar]
- 33.Romero J, Xue X, Gonzalez W, Garcia MJ. CMR imaging assessing viability in patients with chronic ventricular dysfunction due to coronary artery disease: a meta-analysis of prospective trials. JACC Cardiovasc Imaging. 2012;5:494–508. doi: 10.1016/j.jcmg.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Bleeker GB, Kaandorp TAM, Lamb HJ, et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113:969–976. doi: 10.1161/CIRCULATIONAHA.105.543678. [DOI] [PubMed] [Google Scholar]
- 35.White JA, Yee R, Yuan X, et al. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol. 2006;48:1953–1960. doi: 10.1016/j.jacc.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 36.Bilchick KC, Kuruvilla S, Hamirani YS, et al. Impact of mechanical activation, scar, and electrical timing on cardiac resynchronization therapy response and clinical outcomes. J Am Coll Cardiol. 2014;63:1657–1666. doi: 10.1016/j.jacc.2014.02.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 38.Cheong BYC, Muthupillai R, Wilson JM, et al. Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009;120:2069–2076. doi: 10.1161/CIRCULATIONAHA.109.852517. [DOI] [PubMed] [Google Scholar]
- 39.Karamitsos TD, Francis JM, Neubauer S. The current and emerging role of cardiovascular magnetic resonance in the diagnosis of nonischemic cardiomyopathies. Prog Cardiovasc Dis. 2011;54:253–265. doi: 10.1016/j.pcad.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Dass S, Suttie JJ, Piechnik SK, et al. Myocardial tissue characterization using magnetic resonance noncontrast T1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging. 2012;5:726–733. doi: 10.1161/CIRCIMAGING.112.976738. [DOI] [PubMed] [Google Scholar]
- 41.Kuruvilla S, Adenaw N, Katwal AB, Lipinski MJ, Kramer CM, Salerno M. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2014;7:250–258. doi: 10.1161/CIRCIMAGING.113.001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gulati A, Jabbour A, Ismail TF. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 43.Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leyva F, Taylor RJ, Foley PWX, et al. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J Am Coll Cardiol. 2012;60:1659–1667. doi: 10.1016/j.jacc.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 45.Mahrholdt H, Goedecke C, Wagner A, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 46.Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media–enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802–1809. doi: 10.1161/01.cir.97.18.1802. [DOI] [PubMed] [Google Scholar]
- 47.Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferreira VM, Piechnik SK, Dall’Armellina E, et al. T1 mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging. 2013;6:1048–1058. doi: 10.1016/j.jcmg.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Radunski UK, Lund GK, Stehning C, et al. CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imaging. 2014;7:667–675. doi: 10.1016/j.jcmg.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Hinojar Baydes R, Foote L, Ucar EA, et al. Native T1 in discrimination of acute and convalescent stage of inpatients with clinical diagnosis of myocarditis: a proposed new diagnostic algorithm using cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2015;8 doi: 10.1016/j.jcmg.2014.07.016. in press. [DOI] [PubMed] [Google Scholar]
- 51.Bruder O, Wagner A, Jensen CJ, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875–887. doi: 10.1016/j.jacc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 52.O’Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–874. doi: 10.1016/j.jacc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Green JJ, Berger J, Kramer CM, Salerno M. Prognostic value of cardiac magnetic resonance late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5:370–377. doi: 10.1016/j.jcmg.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 54.Chan RH, Maron BJ, Olivotto I, et al. Prognostic Value of Quantitative Contrast-Enhanced Cardiovascular Magnetic Resonance for the Evaluation of Sudden Death Risk in Patients With Hypertrophic Cardiomyopathy. Circulation. 2014;130:484–495. doi: 10.1161/CIRCULATIONAHA.113.007094. [DOI] [PubMed] [Google Scholar]
- 55.Maceira AM, Joshi J, Prasad SK, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–193. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 56.Vogelsberg H, Mahrholdt H, Deluigi CC, et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008;51:1022–1030. doi: 10.1016/j.jacc.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 57.Brooks J, Kramer CM, Salerno M. Markedly increased volume of distribution of gadolinium in cardiac amyloidosis demonstrated by T1 mapping. J Magn Reson Imaging. 2013;38:1591–1595. doi: 10.1002/jmri.24078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karamitsos TD, Piechnik SK, Banypersad SM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6:488–497. doi: 10.1016/j.jcmg.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 59.Fontana M, Banypersad SM, Treibel TA, et al. Native myocardial T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7:157–165. doi: 10.1016/j.jcmg.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 60.Dungu JN, Valencia O, Pinney JH, et al. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7:133–142. doi: 10.1016/j.jcmg.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 61.Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45:1683–1690. doi: 10.1016/j.jacc.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 62.Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y, Safka K, Graham JJ, et al. Correlation of late gadolinium enhancement MRI and quantitative T2 measurement in cardiac sarcoidosis. J Magn Reson Imaging. 2014;39:609–616. doi: 10.1002/jmri.24196. [DOI] [PubMed] [Google Scholar]
- 64.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tandri H, Saranathan M, Rodriguez ER, et al. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol. 2005;45:98–103. doi: 10.1016/j.jacc.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 66.Petersen SE, Selvanayagam JB, Wiesmann F, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46:101–105. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 67.Stacey RB, Andersen MM, St Clair M, Hundley WG, Thohan V. Comparison of systolic and diastolic criteria for isolated LV noncompaction in CMR. JACC Cardiovasc Imaging. 2013;6:931–940. doi: 10.1016/j.jcmg.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 68.Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P. CLinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286. doi: 10.1001/jama.2011.992. [DOI] [PubMed] [Google Scholar]
- 69.Kirk P, Roughton M, Porter JB, et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation. 2009;120:1961–1968. doi: 10.1161/CIRCULATIONAHA.109.874487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moon JCC, Sachdev B, Elkington AG, et al. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Eur Heart J. 2003;24:2151–2155. doi: 10.1016/j.ehj.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 71.Rochitte CE, Oliveira PF, Andrade JM, et al. Myocardial delayed enhancement by magnetic resonance imaging in patients with chagas disease: a marker of disease severity. J Am Coll Cardiol. 2005;46:1553–1558. doi: 10.1016/j.jacc.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 72.Nazarian S, Hansford R, Roguin A, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med. 2011;155:415–424. doi: 10.7326/0003-4819-155-7-201110040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wollmann C, Thudt K, Kaiser B, Salomonowitz E, Mayr H, Globits S. Safe performance of magnetic resonance of the heart in patients with magnetic resonance conditional pacemaker systems: the safety issue of the ESTIMATE study. J Cardiovasc Magn Reson. 2014;16:30. doi: 10.1186/1532-429X-16-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaewlai R, Abujudeh H. Nephrogenic systemic fibrosis. AJR. 2012;199:W17–W23. doi: 10.2214/AJR.11.8144. [DOI] [PubMed] [Google Scholar]
- 75.Edelman RR, Hesselink JR, Zlatkin MB, Crues JV., III . Clinical Magnetic Resonance Imaging. New York, NY: Elsevier; 2005. [Google Scholar]