Abstract
Background
Epoetin therapy used to treat anemia among ESRD patients has cost Medicare ~$40 billion. Since January 2011, epoetin has been reimbursed via a new bundled prospective payment system (PPS). Our aim is to determine changes in epoetin dosing and hematocrit levels in response to PPS by different types of dialysis providers.
Methods
Data from the USRDS were used to identify 187,591 and 206,163 Medicare-eligible ESRD patients receiving hemodialysis during January 2010 (pre-PPS) and December 2011 (post-PPS). Standardized weekly mean epoetin dose administered pre- and post-PPS and adjustment in dose (titration) based on previous hematocrit level in each facility was disaggregated by profit status, chain membership and size.
Results
Major declines in epoetin use, dosing and achieved hematocrit levels were observed after PPS. Among the three largest dialysis chains, the decline in standardized epoetin dose was 29% at Fresenius, 47% at DaVita, and 52% at DCI. The standardized weekly epoetin dose among for profit and nonprofit facilities declined 38% and 42%, respectively. Changes in titration patterns suggest that a new hematocrit target of 30–33% was in place after PPS, replacing the erstwhile 33–36% hematocrit target used before PPS.
Conclusion
Historically, important differences in anemia management were evident by dialysis organizational status. However, the confluence of financial incentives bundling epoetin payments and mounting scientific evidence linking higher hematocrit targets and higher epoetin doses to adverse outcomes have culminated in lower access to epoetin and lower doses across all dialysis providers in the first year after PPS.
Keywords: erythropoietin, epoetin, end-stage renal disease, anemia, Prospective Payment System, bundling, financial incentives, dialysis organizational status, dialysis chains, profit status
INTRODUCTION
Epoetin or EPO (trade name EPOGEN®) cost Medicare more than $40 billion between 1989 and 2010. Seven years ago1 we examined the dosing of epoetin across U.S. hemodialysis facilities and found that large for-profit chains administered the highest doses of epoetin and targeted hematocrit levels beyond the FDA-recommended guidelines. In response to escalating epoetin doses, Congress mandated a new ESRD prospective payment system (PPS). This new system incorporates erstwhile separately billable services, including epoetin, into a single bundled payment. Following PPS there has been an overall decrease in epoetin use,2 but limited attention has been paid to changes in anemia management by organizational status. The purpose of this study is to examine the changes in epoetin use across the different types of dialysis providers in the first year after PPS implementation.
METHODS
The United States Renal Data System (USRDS) is a national system that includes demographic and clinical data on all ESRD patients and their institutional dialysis providers. We analyzed USRDS standard analytic files for December 2009 and January 2010 (one year before PPS), and November 2011 and December 2011 (one year after PPS). We selected January 2010 as our pre-PPS period because, in anticipation of PPS, providers appear to have already lowered epoetin doses in the months immediately preceding PPS.3
As in our previous work,1 adult Medicare-eligible ESRD patients receiving in-center hemodialysis and alive in December 2009 and January 2010, and November and December 2011 were eligible for inclusion in the study. The analyses excluded 0.3% of patient records with hematocrit values less than 15% or greater than 60%, epoetin dose outliers (above 99.9th percentile), and patients in 8% of facilities that did not originally ‘opt in’ to PPS (all facilities must fully comply with PPS provisions by 2014). Dialysis facilities were classified as freestanding or hospital-based; freestanding facilities were classified as either for-profit or not for-profit, and as either large chains (comprising two large for-profit chains, Fresenius and DaVita, and one nonprofit chain, Dialysis Clinic, Inc.) or smaller and/or non-chain affiliated facilities.4
The three measures of epoetin use that we compared pre- and post-PPS were: (1) the proportion of patients who received epoetin therapy; (2) the average weekly epoetin dose; and (3) the epoetin dose adjustments (titration) in the two-month period based on the hematocrit value at the start of the second month. Titration or dose adjustment is an indirect measure of the target hematocrit for each patient: a dose increase (positive change) suggests that the provider is aiming for a higher hematocrit than the level the patient currently has, a dose decrease (negative change) suggests a lower hematocrit target is desired, and no dose change (zero change) suggests the current hematocrit level is the desired goal. To adjust for differences in facility case mix, all three measures were standardized for the covariates listed in Table 1.5 95% confidence intervals for the standardized measures were obtained using a nonparametric bootstrap with 200 samples.
Table 1.
Anemia Management by Organizational Status Before and After ESRD Prospective Payment System (PPS)
| Pre-PPS, January 2010 (N=187,587)
|
Post-PPS, December 2011 (N=206,161)
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freestanding | HB | Freestanding | HB | |||||||||||||
|
| ||||||||||||||||
| Ownership | Chain Status | NP | Ownership | Chain Status | NP | |||||||||||
|
|
|
|||||||||||||||
| FP | NP | LDO
|
SDO/NC
|
FP | NP | LDO
|
SDO/NC
|
|||||||||
| FMA | DV | DCI | FP | NP | FMA | DV | DCI | FP | NP | |||||||
| Total patients1, n | 164,716 | 15,285 | 70,636 | 58,526 | 7,523 | 36,322 | 6,994 | 7,586 | 182,035 | 16,592 | 76,753 | 67,210 | 7,863 | 39,087 | 7,714 | 7,534 |
|
|
||||||||||||||||
| Total patients, % | 88 | 8 | 38 | 31 | 4 | 19 | 4 | 4 | 88 | 8 | 37 | 33 | 4 | 19 | 4 | 4 |
|
|
||||||||||||||||
| Hematocrit level, %2 | ||||||||||||||||
| <30% | 9 | 9 | 10 | 8 | 8 | 10 | 10 | 10 | 21 | 19 | 11 | 29 | 15 | 24 | 23 | 20 |
|
|
||||||||||||||||
| 30–33% | 19 | 15 | 19 | 18 | 13 | 21 | 17 | 20 | 38 | 37 | 26 | 47 | 36 | 38 | 37 | 34 |
|
|
||||||||||||||||
| 33–36% | 36 | 40 | 36 | 37 | 44 | 36 | 36 | 38 | 31 | 35 | 43 | 21 | 38 | 29 | 30 | 31 |
|
|
||||||||||||||||
| ≥36% | 35 | 36 | 35 | 37 | 35 | 33 | 37 | 32 | 10 | 10 | 20 | 3.3 | 11 | 8.7 | 9.2 | 14 |
|
|
||||||||||||||||
| Mean | 34.5 | 34.7 | 34.4 | 34.7 | 34.7 | 34.3 | 34.6 | 34.3 | 32.1 | 32.3 | 33.5 | 31.1 | 32.6 | 31.9 | 31.9 | 32.4 |
|
|
||||||||||||||||
| Epoetin therapy3 | ||||||||||||||||
| Percent receiving epoetin | 94 | 94 | 94 | 95 | 96 | 91 | 92 | 56 | 87 | 87 | 88 | 87 | 88 | 85 | 85 | 47 |
|
|
||||||||||||||||
| Mean dose (all patients) (U/wk) | 19,187 | 16,958 | 19,637 | 20,470 | 18,325 | 16,271 | 15,226 | 8,333 | 11,863 | 9,335 | 14,052 | 10,957 | 8,852 | 9,210 | 9,330 | 5,501 |
|
|
||||||||||||||||
| Mean dose (patients receiving epoetin) (U/wk) | 20,594 | 18,207 | 20,942 | 21,476 | 19,128 | 18,085 | 16,707 | 14,049 | 13,644 | 10,785 | 16,379 | 12,618 | 9,978 | 10,949 | 11,469 | 10,965 |
|
|
||||||||||||||||
| Darbepoetin therapy (% receipt) | 2 | 4 | 1 | 0 | 0 | 8 | 9 | 62 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 40 |
Abbreviation: ESRD, end-stage renal disease; FP, for-profit; NP, nonprofit; FMA, Fresenius; DV, DaVita; DCI, Dialysis Clinics, Inc.; HB, hospital-based LDO, large dialysis organizations; SDO/NC, small dialysis organizations/nonchains; U/wk, units/week.
Includes patients from dialysis facilites that opted into bundling.
Based on patients with a hematocrit value (patients receiving epoetin therapy are required to have hematocrit values reported in the claim).
Weighted average using prevalence of covariates including age, sex, race, region, cause of ESRD, cardivascular and noncardiovascular comorbidities, and duration of dialysis.
RESULTS
Our analysis includes 187,591 ESRD patients receiving hemodialysis services pre-PPS, and 206,163 post-PPS. The mean pre-PPS hematocrit was ~34.5% across all facility types (Table 1). The mean post-PPS hematocrit among large dialysis chains ranged from 31.1% at DaVita to 33.5% at Fresenius. Regardless of profit status, the mean post-PPS hematocrit for small dialysis organizations, nonchain facilities, and hospital-based facilities declined to ~32%. The proportion of patients receiving epoetin therapy decreased from 94–96% pre-PPS to 87–88% post-PPS among the three largest dialysis chains, and from 91% to 85% among the small chains (Table 1). The lower epoetin use in hospital-based facilities must be interpreted with caution because of their high use of darbepoetin, a derivative form of epoetin (Table 1).
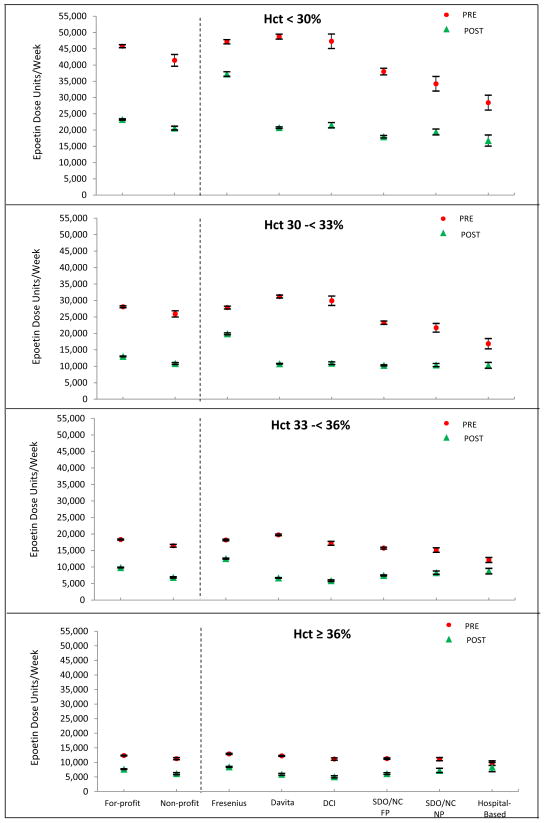
Compared with the pre-PPS dose, the average post-PPS epoetin dose declined 52% at DCI, 47% at DaVita, 29% at Fresenius, 43% at small for profit chains, and 39% at small nonprofit chains. Overall, the decline in epoetin dose was 38% among for profit facilities and 42% among nonprofit facilities (Table 1). Among patients receiving epoetin therapy, similar average epoetin dose declines can be seen for each type of organization, regardless of hematocrit level (Table 1 and Figure 1). For all facility types, the overall aggregate reduction in epoetin dose is inversely related to the hematocrit level (Figure 1). Unadjusted measures of epoetin use were found to be similar to standardized measures for all facility types because the patient case-mix was similar across all facility types (data not shown).
FIG 1.
Standardized mean epoetin dose and 95% confidence interval based on January 2010 (pre-PPS) and December 2011 (post-PPS) by hematocrit category.
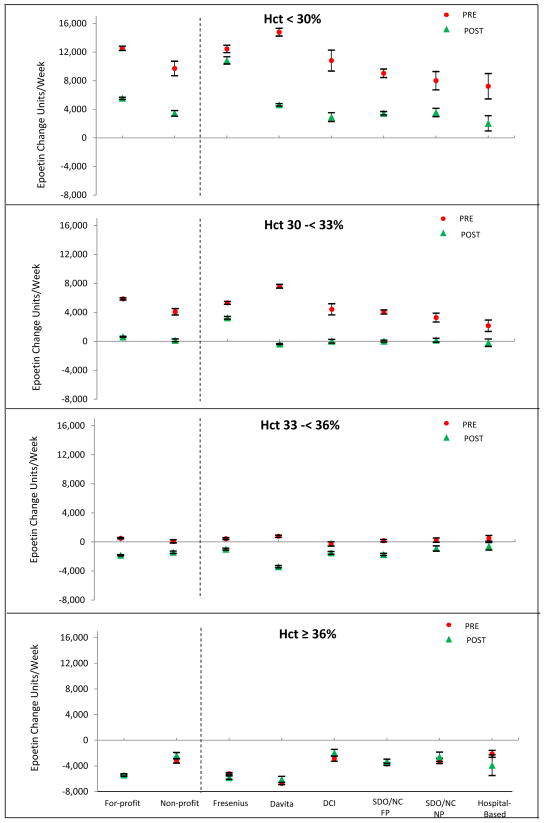
When assessing titration patterns in the pre-PPS period, facilities did not adjust epoetin dose among patients with hematocrit 33-<36% in all facility types (except Fresenius). However, in the post-PPS period, this maintenance of epoetin dose occurred in a lower hematocrit range, among patients with hematocrit 30-<33% (Figure 2). Patients with hematocrit <30% had dose increases both pre- and post-PPS. Patients with hematocrit 33-<36% had, on average, dose reductions in the post-PPS period and patients with hematocrit > 36% had dose reduction in both the pre-PPS and post-PPS periods.
FIG 2.
Standardized change in epoetin dose and 95% confidence interval between December 2009 and January 2010 (pre-PPS) and November 2011 and December 2011 (post-PPS) by hematocrit category.
CONCLUSIONS
Our study shows changes in anemia management across all dialysis facility types one year after implementation of PPS: 8% fewer patients received epoetin therapy and doses were reduced by one third to nearly one-half in the two chains that treat 70% of all dialysis patients. Titration patterns suggest that the hematocrit target changed from 33 – 36% before PPS to 30 – 33% after PPS. The concordance across facility types in response to PPS is notable. The exception is Fresenius, which had a smaller epoetin dose reduction compared to other facility types. However, an USRDS report6 (based on data not yet available to outside researchers) indicates that Fresenius abruptly decreased epoetin dose in 2012 to levels comparable with those of DaVita and DCI. The difference in timing of Fresenius’s response to PPS might be explained by a corporate decision for a smoother transition post-PPS7 or the expiration of vendor programs (e.g., epoetin volume discount) in place during 2011.8,9
Our results extend the findings of the other studies that have examined changes in anemia management by organizational status. Hirth et al. examined costs of all injectable drugs used during dialysis among five different types of facilities and found that use of expensive drugs such as epoetin declined substantially after PPS in comparison to lower cost iron substitutes.10 Similar to our findings, the authors also reported that Fresenius used higher epoetin doses compared with DaVita and DCI at the end of 2011. In another study, Brunelli et al. found that epoetin use and hemoglobin levels decreased after PPS in small dialysis organizations.11
Between 1991 and 2008, Medicare epoetin payments comprised the second largest source of facility income (~25%) among large for-profit chains before PPS.12,13 The mean epoetin dose increased four-fold despite increasing evidence that high hematocrit targets and high epoetin doses led to adverse outcomes,14, 15, 16 and the subsequent issuance of a FDA Black Box warning. During this pre-PPS period, providers were underpaid by Medicare for dialysis services17 and therefore injectable drugs like epoetin became profitable since they were reimbursed on a fee-for-service basis. Furthermore, dialysis providers belonging to large chains typically obtained volume discounts and rebates for epoetin linked to usage resulting in increased incentives to use higher doses.8 To rein in epoetin use, ESRD PPS was implemented in January 2011with two key components (a) bundling costs previously reimbursed separately on a fee-for-service basis, including epoetin therapy (providers now receive a fixed bundled payment per dialysis session, whether or not epoetin is administered); and (b) incorporating targeted incentives for quality care keyed to particular measured outcomes (e.g., in the first year of PPS, 2% of a facility’s 2012 Medicare payment could be withheld based on a high proportion of patients with a hematocrit < 30% or >36%; the former measure weighted more heavily). Notably, despite these potential financial penalties, 29% of all patients at DaVita had a hematocrit below 30% in December 2011, more than three times the proportion in January 2010. The lower hematocrit target penalty was subsequently rescinded in 2013 however to reflect the most recent FDA guidelines (issued in June 2011).18 GAO reports suggest that the June 2011 FDA guidelines resulted in further precipitous declines in epoetin utilization.19
Our study has limitations. First, we did not have information on the route of epoetin administration, although data suggest less than 3% subcutaneous administration (requiring less epoetin) in large and medium chains compared to 13% in small chains and independent in December 2011.20 Our results may overstate the relatively small differences in epoetin dosing found among small chains and independent facilities. Second, we have focused on the first year post-PPS. Future studies are warranted to examine if the trends we report continue or plateau.
In conclusion, the confluence of new financial incentives bundling epoetin payments and mounting scientific evidence linking higher epoetin doses to adverse outcomes culminated in fewer hemodialysis patients receiving epoetin therapy, lower epoetin doses administered and lower target hematocrit levels across nearly all dialysis providers in the first year after PPS. The effects of these changes in anemia management on mortality and quality of life among the dialysis population are uncertain and will require careful future assessment.
Acknowledgments
The data reported herein have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as the official policy or interpretation of the US government. Furthermore, there are no disclaimers for any of the authors to report. All authors participated in the design, analysis, interpretation, writing, and/or editing of this study and have seen and approved the final version. Dr. Mae Thamer, as first author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Financial Disclosure: None of the authors had any conflict of interest related to this paper.
There are no conflicts of interest to disclose for any of the authors.
Contributor Information
M Thamer, Email: mthamer@mtppi.org.
Y Zhang, Email: yz@mtppi.org.
J Kaufman, Email: James.Kaufman@va.gov.
O Kshirsagar, Email: onkar@mtppi.org.
D Cotter, Email: dcott@mtppi.org.
MA Hernán, Email: miguel_hernan@post.harvard.edu.
References
- 1.Thamer M, Zhang Y, Kaufman J, Cotter D, Hernan M. Dialysis facility ownership and epoetin dosing in patients receiving hemodialysis. Am J Kidney Dis. 2007;50(4):538–541. doi: 10.1053/j.ajkd.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Pisoni RL, Fuller DS, Bieber BA, Gillespie BW, Robinson BM. The DOPPS Practice Monitor for US dialysis care: trends through August 2011. Am J Kidney Dis. 2012;60(1):160–165. doi: 10.1053/j.ajkd.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Miskulin DC, Zhou J, Tangri N, et al. Trends in anemia management in US hemodialysis patients 2004–2010. BMC nephrology. 2013;14:264. doi: 10.1186/1471-2369-14-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duh MS, Mody SH, McKenzie RS, et al. Dosing patterns and treatment costs of erythropoietic agents in elderly patients with pre-dialysis chronic kidney disease in managed care organisations. Drugs & aging. 2006;23(12):969–976. doi: 10.2165/00002512-200623120-00004. [DOI] [PubMed] [Google Scholar]
- 5.Hernan MA, Robins JM. Causal Inference. Chapman & Hall/CRC; 2015. http://www.hsph.harvard.edu/miguel-hernan/causal-inference-book/ [Google Scholar]
- 6.U.S. Renal Data System. USRDS 2013 Annual Data Report. 2013 [Google Scholar]
- 7.Ravani P, Palmer SC, Oliver MJ, et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. Journal of the American Society of Nephrology : JASN. 2013 Feb;24(3):465–473. doi: 10.1681/ASN.2012070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goozner M. The making and selling of a star drug. [Accessed May 21, 2014];Chicago Tribune. 1999 http://articles.chicagotribune.com/1999-05-24/news/9905240171_1_epogen-amgen-dialysis-patients.
- 9.Beasley D. UPDATE 3-Amgen signs DaVita, Fresenius anemia drug deals. [Accessed May 21, 2014];Reuters. 2011 http://www.reuters.com/article/2011/11/18/amgen-davita-idUSN1E7AH0XA20111118.
- 10.Hirth RA, Turenne MN, Wheeler JR, et al. The initial impact of Medicare’s new prospective payment system for kidney dialysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013 Oct;62(4):662–669. doi: 10.1053/j.ajkd.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 11.Brunelli SM, Monda KL, Burkart JM, et al. Early trends from the Study to Evaluate the Prospective Payment System Impact on Small Dialysis Organizations (STEPPS) American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013 Jun;61(6):947–956. doi: 10.1053/j.ajkd.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 12.Nephrology ASo. Five Things Physicians and Patients Should Question. http://www.choosingwisely.org/doctor-patient-lists/american-society-of-nephrology/
- 13.Ludwig H, Fritz E. Anemia of cancer patients: patient selection and patient stratification for epoetin treatment. Seminars in oncology. 1998 Jun;25(3 Suppl 7):35–38. [PubMed] [Google Scholar]
- 14.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. The New England journal of medicine. 1998 Aug 27;339(9):584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 15.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. The New England journal of medicine. 2006 Nov 16;355(20):2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 16.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. The New England journal of medicine. 2006 Nov 16;355(20):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005 Jan 1–7;365(9453):82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 18.FDA. [Accessed May 20, 2014];FDA Drug Safety Communication: Modified dosing recommendations to improve the safe use of Erythropoiesis-Stimulating Agents (ESAs) in chronic kidney disease. 2011 http://www.fda.gov/drugs/drugsafety/ucm259639.htm.
- 19.GAO. Letter December 7, 2012 to Congressional Committees. End-Stage Renal Disease: Reduction in Drug Utilization Suggests BundledPayment Is Too High GAO-13-190R Trends in ESRD Drug Utilization.
- 20.Arbor Research Collaborative for Health. Dialysis Outcomes and Practice Pattern Study. [Accessed May 20, 2014];DOPPS Practice Monitor (DPM): Emerging Trends. 2014 May 8; http://www.dopps.org/DPM/DPM_webex.pdf.