Abstract
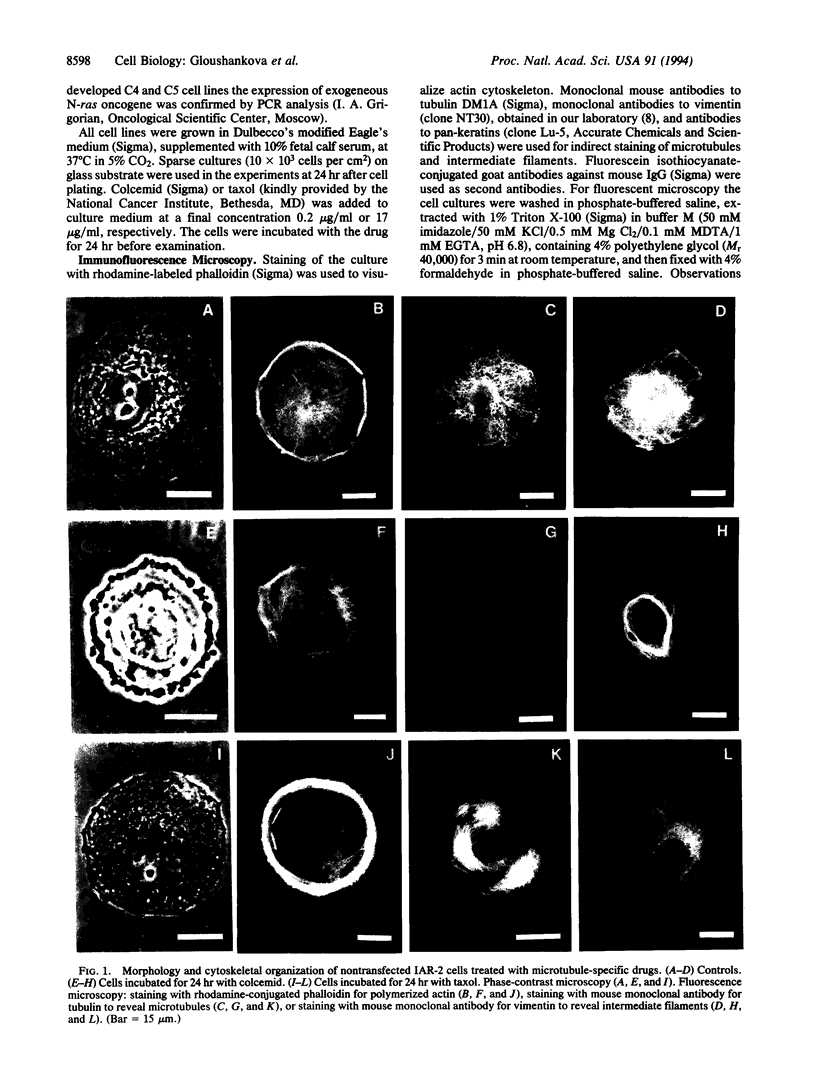
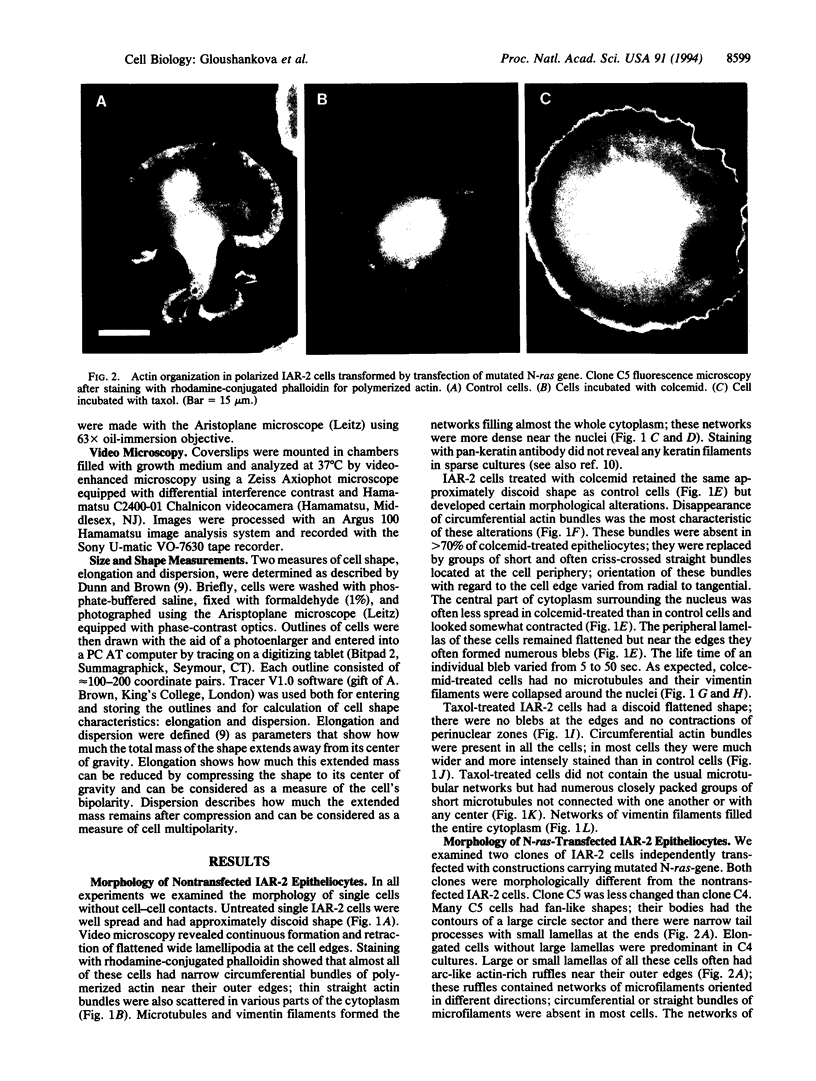
To understand better the role of the microtubular system in the development and maintenance of morphological organization of nonpolarized and polarized cells of the same origin we examined the effects of two microtubule-specific drugs, colcemid and taxol, on discoid cultured epithelial rat cells of the IAR-2 line and on polarized cells obtained from this line by transfection of mutated N-ras oncogene; morphometric, immunomorphologic, and videomicroscopic methods were used. Depolymerization of microtubules by colcemid did not cause major changes in the discoid shape of IAR cells but altered organization of actin cortex; in particular, it led to disappearance of circumferential bundle of actin microfilaments. Taxol reorganized the normal network of microtubules radiating from the perinuclear centers into numerous arrays of short microtubules not associated with any centers. Taxol-treated cells had wider circumferential bundles of microfilaments than control cells and morphometric analysis showed that their contours were closer to geometric circle than those of control or of colcemid-treated cells. These data show that function of the microtubular system is essential for maintenance of the characteristic morphological organization of discoid cells; we propose to name this function "contra-polarization." Contra-polarization is not prevented and is even promoted by taxol; this result suggests that a decentralized system of microtubules is sufficient for this function. In contrast, maintenance of polarized morphology of IAR-2 cells transfected by the N-ras oncogene is inhibited not only by colcemid but also by taxol and thus requires the presence of a normal centralized microtubular system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacallao R., Antony C., Dotti C., Karsenti E., Stelzer E. H., Simons K. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J Cell Biol. 1989 Dec;109(6 Pt 1):2817–2832. doi: 10.1083/jcb.109.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brabander M., Geuens G., Nuydens R., Willebrords R., De Mey J. Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of the centrosomes and kinetochores. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5608–5612. doi: 10.1073/pnas.78.9.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domnina L. V., Rovensky J. A., Vasiliev J. M., Gelfand I. M. Effect of microtubule-destroying drugs on the spreading and shape of cultured epithelial cells. J Cell Sci. 1985 Mar;74:267–282. doi: 10.1242/jcs.74.1.267. [DOI] [PubMed] [Google Scholar]
- Dunn G. A., Brown A. F. Alignment of fibroblasts on grooved surfaces described by a simple geometric transformation. J Cell Sci. 1986 Jul;83:313–340. doi: 10.1242/jcs.83.1.313. [DOI] [PubMed] [Google Scholar]
- Middleton C. A., Brown A. F., Brown R. M., Roberts D. J. The shape of cultured epithelial cells does not depend on the integrity of their microtubules. J Cell Sci. 1988 Nov;91(Pt 3):337–345. doi: 10.1242/jcs.91.3.337. [DOI] [PubMed] [Google Scholar]
- Montesano R., Saint Vincent L., Drevon C., Tomatis L. Production of epithelial and mesenchymal tumours with rat liver cells transformed in vitro. Int J Cancer. 1975 Oct 15;16(4):550–558. doi: 10.1002/ijc.2910160405. [DOI] [PubMed] [Google Scholar]
- Rodionov V. I., Gyoeva F. K., Tanaka E., Bershadsky A. D., Vasiliev J. M., Gelfand V. I. Microtubule-dependent control of cell shape and pseudopodial activity is inhibited by the antibody to kinesin motor domain. J Cell Biol. 1993 Dec;123(6 Pt 2):1811–1820. doi: 10.1083/jcb.123.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenenberger C. A., Zuk A., Kendall D., Matlin K. S. Multilayering and loss of apical polarity in MDCK cells transformed with viral K-ras. J Cell Biol. 1991 Mar;112(5):873–889. doi: 10.1083/jcb.112.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M., Gherardi E. Regulation of cell movement: the motogenic cytokines. Biochim Biophys Acta. 1991 Apr 16;1072(1):81–102. doi: 10.1016/0304-419x(91)90008-9. [DOI] [PubMed] [Google Scholar]
- Symons M. H., Mitchison T. J. Control of actin polymerization in live and permeabilized fibroblasts. J Cell Biol. 1991 Aug;114(3):503–513. doi: 10.1083/jcb.114.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanovsky S. M., Bannikov G. A., Montesano R., Vasiliev J. M. Density-dependent expression of keratins in transformed rat liver cell lines. Cell Biol Int Rep. 1986 Apr;10(4):263–270. doi: 10.1016/0309-1651(86)90073-1. [DOI] [PubMed] [Google Scholar]
- Vasiliev J. M. Polarization of pseudopodial activities: cytoskeletal mechanisms. J Cell Sci. 1991 Jan;98(Pt 1):1–4. doi: 10.1242/jcs.98.1.1. [DOI] [PubMed] [Google Scholar]