Abstract
BACKGROUND
Normal human prostate accumulates the highest levels of zinc of any soft tissue in the body. In contrast, the zinc level in prostate cancer is markedly decreased from the level detected in nonprostate tissues. Despite these relationships, the possible role of zinc in the growth of normal and malignant prostate has not been determined.
METHODS
Growth inhibition and various regulatory responses were investigated in two human prostate carcinoma cell lines (LNCaP and PC-3), treated with or without zinc.
RESULTS
Incubation of the prostate carcinoma cell lines with physiological levels of zinc resulted in the marked inhibition of cell growth. A lower 50% inhibition of cell growth (IC50) value for zinc (about 100 ng/ml) was detected in LNCaP cells, which are androgen-responsive, whereas androgen-independent PC-3 cells exhibited a higher IC50 for zinc (about 700 ng/ml). Incubation with 1 μg/ml zinc resulted in maximum inhibition of growth in both cell lines. These inhibitory effects of zinc correlated well with the accumulation of zinc in the cells. Simultaneously, cell flow cytometric analyses revealed a dramatic increase of the cell population in G2/M phase, in both LNCaP (2.3-fold vs. control) and PC-3 (1.9-fold vs. control), and a decreased proportion of cells in S phase (LNCaP, −51.4%; PC-3, −23%), indicating a G2/M phase arrest. The cell growth inhibition and G2/M arrest in these cells were accompanied by an increase in apoptosis, as demonstrated by the characteristic cell morphology and further confirmed by cellular DNA fragmentation. The specificity of zinc-induced apoptosis was identified by ethylenediamine-tetraacetic acid (EDTA)-chelation, which abolished the zinc effect on cellular DNA fragmentation. The zinc-induced G2/M phase arrest and apoptosis were accompanied by increased mRNA levels of p21Waf1/Cip1/Sdi1 in both LNCaP (p53+/+) and PC-3 (p53−/−) cells.
CONCLUSIONS
These results suggest that zinc inhibits human prostatic carcinoma cell growth, possibly due to induction of cell cycle arrest and apoptosis. There now exists strong evidence that the loss of a unique capability to retain high levels of zinc is an important factor in the development and progression of malignant prostate cells.
Keywords: zinc, prostate, cell cycle, apoptosis, p21Waf1/Cip1/Sdi1
INTRODUCTION
Normal human prostate accumulates the highest levels of zinc of any soft tissue in the body. This unique capability is retained in benign prostatic hyperplasia (BPH). In contrast, the zinc level in prostate adenocarcinoma (PCa) is markedly decreased from the level associated with typical nonprostate tissues. Such relationships indicate that, under natural in situ conditions, malignant prostate epithelial cells, in contrast to normal or BPH prostate epithelial cells, have lost the ability to accumulate high levels of zinc. Moreover, substantial information exists which implicates the changes in zinc accumulation in the development and progression of prostate malignancy. For a recent detailed review of these and other relationships of zinc in normal and malignant prostate, we refer the reader to Costello and Franklin [1].
Studies from different groups have provided some information regarding the effects of zinc on prostate cells, the mechanisms of zinc accumulation, and the regulation of zinc accumulation in normal or malignant human prostate cells [2–4]. Previous studies from this laboratory demonstrated that the accumulation of high zinc levels inhibits mitochondrial (m−) aconitase activity and citrate oxidation of prostate epithelial cells [5]. The accumulation of zinc in rat lateral prostate cells is upregulated by testosterone and by prolactin in vivo and in vitro [6]. Recent studies revealed that the malignant prostate cell lines LNCaP and PC-3 exhibit the capability to accumulate high zinc levels under in vitro culture conditions; and, like rat lateral prostate cells, prolactin and testosterone upregulate zinc accumulation in these cells (Costello et al., unpublished data).
In typical normal mammalian cell physiology, an optimal availability of zinc is essential for normal growth and proliferation of cells. However, accumulation of zinc in cells has varied effects from stimulation to inhibition of cell growth, depending upon the level of zinc and the cell type [7]. These relationships led us to investigate the possible effects of high zinc accumulation on the growth of malignant human prostate cells. For present purposes, we define “growth effects” as the ability of cells to increase in cell number in culture. Such an effect would be the sum of the rates of the proliferation and the death of cells. The present report reveals (for the first time, to our knowledge) that exposure of LNCaP and PC-3 cells to physiological levels of zinc results in inhibition of cell growth, and that this effect is due to the induction of cell cycle arrest and apoptosis.
MATERIALS AND METHODS
Cell Lines and Cultures
Human prostate cancer cell lines LNCaP and PC-3 (American Type Culture Collection, Rockville, MD) were maintained in RPMI-1640 medium with glutamine, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin, at 37°C in a humidified atmosphere with 5% CO2. The passages for both cell lines were within the range of 10–35.
Growth Experiments
Cells were subcultured in 100-mm dishes at a density of 5 × 105 cells in the same conditions as described above. Twenty-four hours before the zinc treatment, fresh serum-free medium was added to the cells, and followed by zinc treatment (ZnSO4) at 0, 100, 250, 500, 750, and 1,000 ng/ml, respectively. Fresh serum-free medium with zinc was replaced every 24 hr. Triplicate cultures were used for each dosage, and on day 3 of incubation with zinc, the cells that had attached to the culture dishes were harvested by trypsinization and counted through a blood cell-counter (Coulter-Z1, Coulter Electronics, Ltd., Luton, Beds., England). For each cell line, at least three individual experiments were performed.
Cellular Zinc Measurement
LNCaP and PC-3 cells harvested from each treatment were digested in trichloracetic acid/nitric acid (1:1) in a boiling water bath until the digestion mixture was clear (approximately 30 min). The digests were assayed for zinc by atomic absorption with a spectrophotometer 210 VGP (Buck Scientific, Inc., East Norwalk, CT). Aliquots of the cell preparations prior to digestion were assayed for protein by the method of Bradford [8]. Zinc values are reported as μg zinc/mg protein.
Flow Cytometric Analysis
For cell cycle analysis, cells were incubated in 100-mm plates and allowed to grow to subconfluence, and then treated with 1 μg/ml zinc. After 3, 6, 24, 48, and 72 hr of incubation with zinc, cells were harvested by trypsinization, washed with phosphate-buffered saline (PBS), and fixed with 70% ice-cold ethanol for 12 hr at 4°C. Fixed cells were then centrifuged and the cell pellets were resuspended and stained with 1 ml of a mixture of propidium iodide (PI, 50 μg/ml) and RNase A (1 mg/ml). The stained cells were subject to analysis by two-parameter flow cytometry [9].
Detection of DNA Fragmentation
DNA was extracted as described previously [10]. Briefly, cell pellets were lysed with 100 mM NaCl/10 mM Tris-HCL/25 mM EDTA, pH 8.0/0.5% sodium dodecyl sulfate/0.1 mg/ml proteinase K, followed by extraction with an equal volume of phenol/ chloroform/isoamyl alcohol (25:24:1) and by ethanol precipitation with 7.5 M ammonium acetate. Samples were then subjected to RNase A (40 μg/ml) digestion at 37°C for 1 hr, followed by phenol-extraction. Samples were then precipitated again as described above. The DNA (10 μg) was electrophoresed in a 1.5% agarose gel and stained with ethidium bromide, and the results were visualized under ultraviolet (UV) light and photographed.
Acridine Orange Staining for Detection of Cell Apoptosis
A cell suspension was adhered to a slide by cyto-spin and air-dried. The cells were then fixed with chilled acetone and stained with acridine orange solution (10 μg/ml in PBS) containing Dnase-free RNase A (1 mg/ml) for 15 min. The slides were rinsed with double distilled (dd) H2O, air-dried, and covered by coverslip with mounting fluid. The cells were examined under a microscope (Axiovert 10, Zeiss, Oberkochen, Germany) with a fluorescence attachment. Fluorescence was detected between 500–525 nm. Cells exhibiting brightly fluorescent condensed or fragmented nuclei were considered apoptotic.
Northern Blot Analysis
Cells were treated with 1 μg/ml zinc in 100-mm plates as described above, for 0, 12, and 24 hr. Total cellular RNA was prepared by acid guanidine thiocyanate-phenol-chloroform extraction, as previously described [11]. Northern blot analysis was carried out with 50 μg of total RNA for each sample, using di-goxigenin-labeled p21 cDNA probes (a gift of Dr. B. Volgelstein, Johns Hopkins University, Baltimore, MD), as described previously [12]. The intensity of corresponding 28S rRNA was used as an internal control to monitor the amount of loaded RNA. The intensities of specific hybridization bands were quantitated with an LKB Ultra Scan XL laser densitometer (Image Quant, Molecular Dynamics, Sunnyvale, CA). Relative mRNA levels were determined as the ratio of the integral intensity value of the target to the 28S rRNA bands.
RESULTS
Zinc Inhibits LNCaP and PC-3 Cell Growth in a Dose-Dependent Manner
The first studies were concerned with the effects of varying concentrations of zinc added to medium on the growth of LNCaP and PC-3 cells (Fig. 1). The growth of LNCaP cells was markedly inhibited (54% decrease) by medium containing 100 ng zinc/ml, and 86% with 1,000 ng zinc/ml (P < 0.01). The growth of PC-3 cells was significantly less sensitive to zinc than were LNCaP cells. At 100 ng zinc/ml medium, the growth of PC-3 cells was essentially unaffected (P > 0.05). For a 50% decrease in cell growth, LNCaP cells required about 100 ng zinc/ml, as opposed to 700 ng zinc/ml for PC-3 cells. The highest zinc concentration (1,000 ng/ml) utilized in this study induced a maximum growth inhibition observed in both cell lines.
Fig. 1.
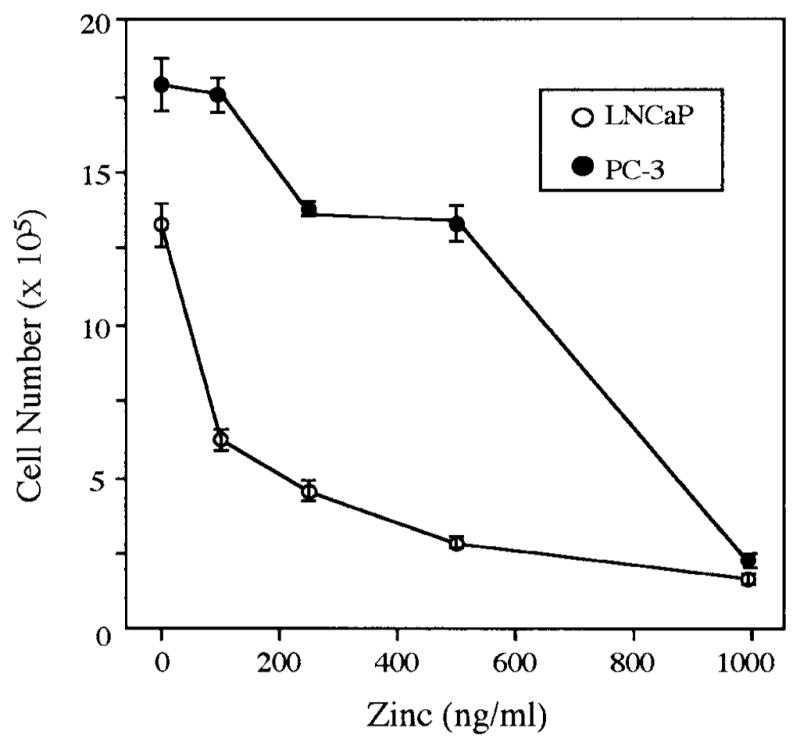
Dose-dependent inhibitory effects of Zinc on growth of LNCaP and PC-3 prostatic carcinoma cells treated with 100–1,000 ng/ml zinc for 72 hr. Data represent the mean of three experiments. Each individual experiment produced the same results, as represented here. Statistical analyses were conducted using StatView 4.5 program (Abacus, Berkeley, CA) by Student’s t-test (P < 0.01 in LNCaP cells; P < 0.05 in PC-3 cells, except for the group of PC-3 cells treated with 100 ng/ml zinc, P > 0.05).
Zinc Uptake Is Higher in LNCaP Than in PC-3 Cells
The greater sensitivity of growth inhibition of LNCaP to extracellular zinc correlates well with the level of intracellular zinc (Fig. 2). After 24-hr incubation with 100 ng/ml zinc, the accumulation of zinc was increased 52% in LNCaP cells but unchanged in PC-3 cells. Our data are in accordance with a previous zinc uptake study which showed that LNCaP cells have a higher basal endogenous zinc level than PC-3 cells, and consistently accumulate significant higher intracellular zinc levels than do PC-3 cells when both cell types are exposed to identical conditions (Costello et al., unpublished data). In contrast, zinc was found to have very little effect on the growth of squamous carcinoma cells, which do not accumulate high cellular levels of zinc (Costello et al., unpublished data). Consequently there exists a cell-specificity for the effect of zinc on malignant prostate cells, and the growth-inhibitory effect requires that the intracellular concentration of zinc is increased.
Fig. 2.
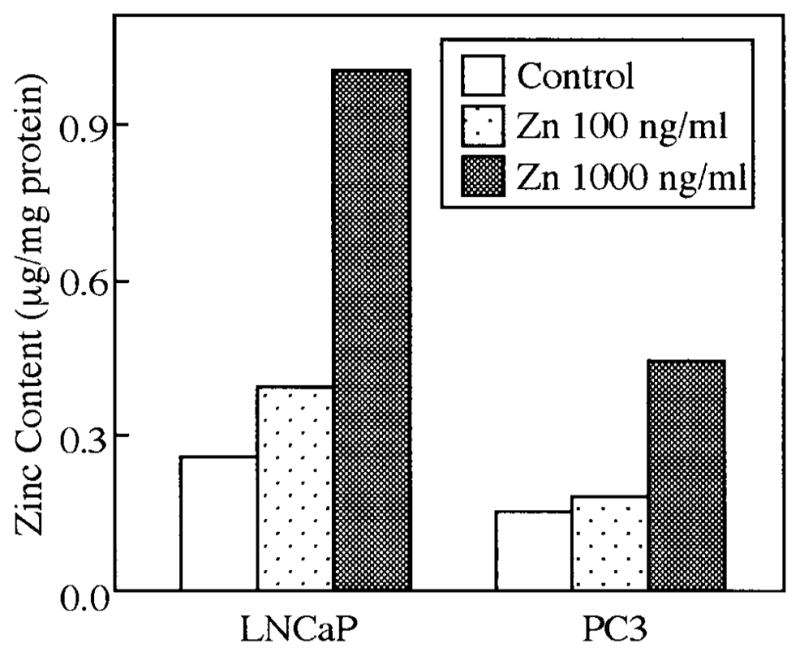
Zinc uptake in LNCaP and PC-3 cells treated without or with zinc at 100 or 1,000 ng/ml for 48 hr. Data represent the mean of three experiments. Each individual experiment produced the same results, as represented here.
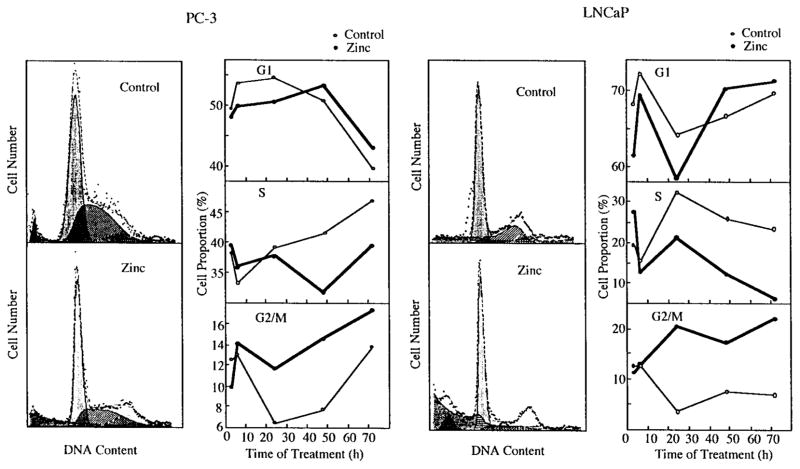
Zinc Induces Cell Cycle Arrest at G2/M Phase
In human prostatic carcinoma cells (Fig. 3), DNA content histograms from flow cytometry studies demonstrated that after 24-hr incubation with zinc (1,000 ng/ml), the number of cells containing G2/M phase DNA was dramatically increased in both LNCaP (4.9-fold) and PC-3 (1.8-fold) cells. This correlated well with the higher sensitivity of LNCaP cells to growth inhibition by zinc. Between 24–72 hr of zinc treatment, the significantly increased cell population in G2/M phase was associated with decreased cell numbers in S phase, as observed in both cell lines. The effects of zinc on G2/M phase were evident after 24 hr of zinc treatment and remained constant over the 72 hr of culture. However, total cell numbers were significantly reduced over the 72-hr incubation period due to the elimination of the apoptotic cells, which presumably resulted from those arrested in G2/M phase (Fig. 1). It was evident that zinc treatment increased the population of “floating dead cells” compared to cultures which had minimal cell death in the absence of zinc. These results suggest that zinc induces a G2/M phase arrest in human prostate cancer cells.
Fig. 3.
Induction of G2/M phase arrest by zinc in LNCaP and PC-3 cells. Logarithmically growing cells were treated with 1,000 ng/ml zinc or without zinc (controls). At different time points as indicated, cells were harvested and analyzed for DNA content by propidium iodine staining and flow cytometry. Left: Representative histographs show that zinc induces G2/M phase arrest appeared after 6-hr incubation compared with controls. Right: DNA distribution of the cell cycle was analyzed by the program mPLUS and mCYCLE from Phenix Flow Systems (San Diego, CA).
Zinc Induces Apoptosis in Human Prostate Carcinoma Cells
Cell apoptotic morphological features were observed in both LNCaP and PC-3 cells treated with zinc (1,000 ng/ml) for 24 hr, as shown in Figure 4. Apoptotic changes, including the appearance of cell membrane “blebbing,” nuclear chromosome condensation, and eventually the formation of apoptotic bodies, were significantly induced by zinc compared with controls. Apoptosis was further confirmed by genomic DNA fragmentation (Fig. 5). Multiple 180-bp DNA fragments, resulting from internucleosomal cleavage, were clearly visible in LNCaP and PC-3 cells treated with 500 and 1,000 ng/ml of zinc for 24 and 48 hr. The effect was evident in both cell lines after 24-hr treatment with 500 ng/ml of zinc, and was more pronounced with 1,000 ng/ml. Thus, the ability of zinc to inhibit the growth of these cells is due in part to zinc induction of apoptosis.
Fig. 4.
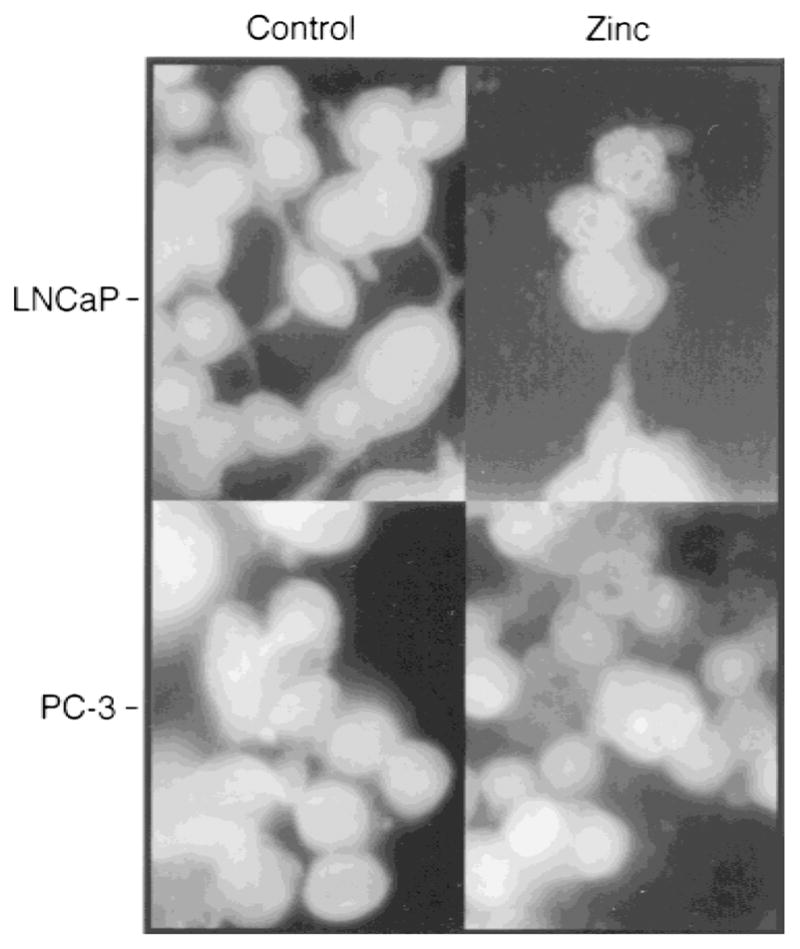
Zinc-induced apoptosis in LNCaP and PC-3 cells. Prostatic carcinoma cells were incubated in the presence or absence of zinc (1,000 ng/ml) for 24 hr. Cells were stained with acridine orange for detection of apoptosis and photographed under a fluorescence phase contrast microscope (Axiovert 10, Zeiss) with magnification ×100.
Fig. 5.
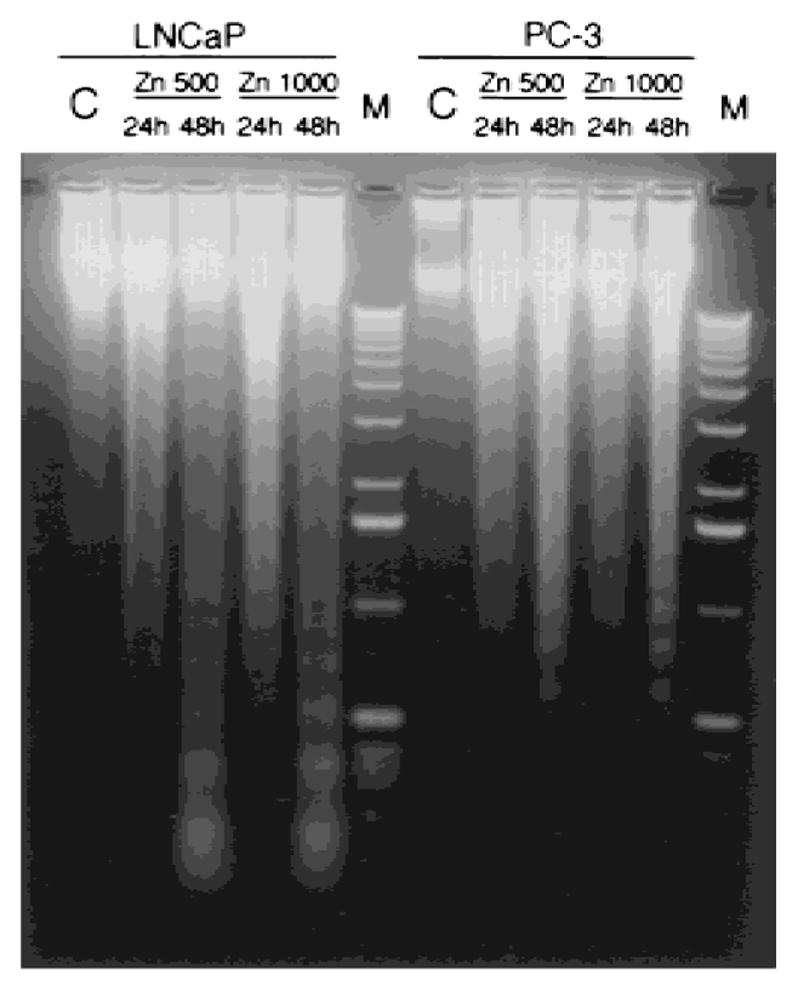
Effect of zinc on DNA fragmentation in LNCaP and PC-3 cells. Shown is representative electrophoretic fragmentation pattern of DNA isolated from LNCaP and PC-3 cells treated with or without zinc (500 and 1,000 ng/ml) for 24 and 48 hr, as indicated. Medium and the zinc treatment were replaced every 24 hr.
To further show that a mobile reactive form of zinc was specifically responsible for the inhibitory effects on prostate tumor cell growth, an experiment utilizing EDTA chelation of zinc was performed [13]. The results showed that chelation of zinc with Ca-EDTA (1 mM) abolished the apoptotic effect of zinc on LNCaP cells, and that EDTA itself has no effect on the cell apoptosis (Fig. 6).
Fig. 6.
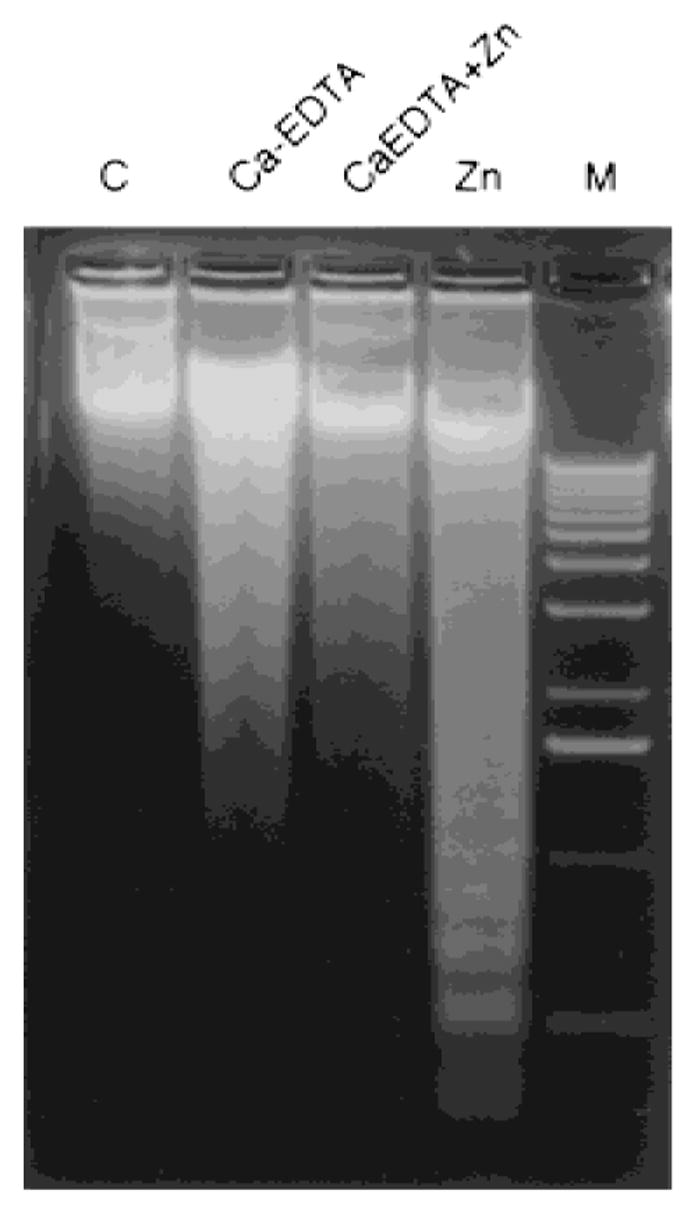
Induction of DNA fragmentation by zinc in LNCaP cells was abolished by chelation of zinc with Ca-EDTA. LNCaP cells were incubated without (control) or with zinc (1,000 ng/ml) in the presence and/or absence of Ca-EDTA (1 mM) for 24 hr. Cells were also treated with Ca-EDTA (1 mM) alone, as indicated. Cells were harvested and DNA was extracted as described in Materials and Methods.
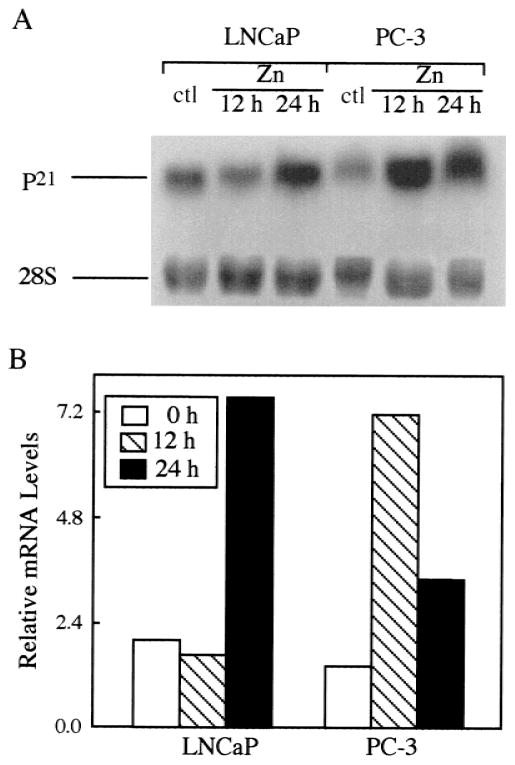
Alteration of p21Waf1/Cip1/Sdi1 Gene Expression During Zinc-Induced Apoptosis in Human Prostate Cancer Cells
It is believed that the initiation of apoptosis can take place at any phase in the cell cycle. The flow cytometric data (Fig. 3) demonstrated that zinc arrested both cell lines in G2/M phase, followed by apoptotic cell death. p21Waf1/Cip1/Sdi1, a cyclin-dependent kinase inhibitor, is known to be involved in G2/M phase arrest [14]. Thus, to further investigate whether p21Waf1/Cip1/Sdi1 is also implicated in zinc-induced inhibition of prostate cancer cell growth, p21Waf1/Cip1/Sdi1 gene expression was examined in both LNCaP and PC-3 cells following exposure to zinc. Northern blot analyses showed that p21Waf1/Cip1/Sdi1 mRNA levels were significantly up-regulated in both cell lines treated with zinc (Fig. 7). In LNCaP cells, an increase of p21Waf1/Cip1/Sdi1 mRNA levels was observed at 24-hr incubation with zinc (1,000 ng/ml); in contrast, in PC-3 cells the maximum elevation of p21Waf1/Cip1/Sdi1 mRNA was detected by 12 hr of incubation and had declined by 50% at 24 hr. The extent of p21Waf1/Cip1/Sdi1 mRNA induction was similar in LNCaP and PC-3 cells.
Fig. 7.
A: Northern blot analysis of p 21Waf11/Cip1/Sdi1 gene expression induced by zinc in LNCaP and PC-3 cells. Prostatic carcinoma cells were incubated with zinc (1,000 ng/ml) for different times, as indicated. Total RNA was extracted, and 50 μg of total RNA were loaded in each lane; the loading variation was adjusted by 28S rRNA signals. B: Relative p21Waf1/Cip1/Sdi1 mRNA levels were determined by the densitometry scanning.
DISCUSSION
Zinc is an essential trace element and plays important roles as a component of many cofactors and enzymes involved in gene regulation and/or protein synthesis [15–17]. Clearly, there exists in all cells an optimal zinc requirement for growth and function. There have been numerous and inconsistent reports concerning the involvement of zinc in the growth of cells [7]. Beyond this fundamental relationship, the effect of zinc on cell growth has been a controversial issue. The present study revealed that the growth of LNCaP and PC-3 cells was inhibited by exposure to physiological levels of extracelluar zinc. The plasma level is about 1 μg/ml, of which about 67% is mobile, transportable zinc available for uptake by cells [1]. This effect of zinc was associated with and dependent upon the unique ability of prostate cells to accumulate high intracellular zinc levels. Squamous carcinoma cells, which do not accumulate high intracellular zinc levels, exhibited no inhibited growth when exposed to physiological levels of extracellular zinc (data not shown).
Interpretations of the zinc effect on apoptosis have varied. Several studies reported that zinc inhibits apoptosis in various cell types [18–22]. This is in contradiction to other studies which showed that zinc increased apoptosis [23–25]. However, it is important to note that in most of these studies, unphysiological levels of zinc (mM range) were employed. Zinc concentrations in this range are much higher than circulating levels of zinc, and represent zinc concentrations that cells would never be subjected to in situ. In contrast, our data clearly showed that zinc treatment at lower, physiological concentrations induced internucleosomal cleavage and DNA fragmentation. In addition, the zinc effect on induction of apoptosis was abolished by treatment with Ca-EDTA. These results indicate that specific chelation of zinc was responsible for eliminating zinc-induced apoptosis.
The mechanism of the zinc inhibition of prostatic cell growth involved the effect of zinc on cell cycle progression (cells arrested in G2/M) and apoptosis. It is known that p21, a cyclin-dependent kinase inhibitor, can induce cell cycle arrest in G1 and/or G2 by inhibiting kinase activity [14]. Interestingly, we have now found that the zinc-induced elevation of p21 mRNA levels is associated with a G2/M cell cycle arrest in both LNCaP and PC-3 cells, with a time-course correlation.
As described previously, p21 is a nuclear factor which not only acts on cell cycle progression, but is also associated with apoptosis [26]. However, at present, the possible role of p21 in the regulation of apoptosis is still not clear. It has been shown that prolonged expression of p21 could induce apoptosis in breast carcinoma cells [27]. On the other hand, p21 has also been reported to decrease apoptosis [28,29]. Our study showed that in prostatic carcinoma cells, the elevation of zinc-induced p21 mRNA levels was detected prior to the onset of apoptosis. Thus, zinc-induced p21 gene expression is significantly consistent with the effect of zinc on the inhibition of prostate cancer cell growth.
While p21 has most often been implicated as a mediator of p53-induced growth inhibition, p21 gene expression is also regulated by p53-independent pathways [30]. That zinc-induced p21gene expression and growth arrest were observed in p53-negative PC-3 cells suggests that the inhibitory effect of zinc on prostatic carcinoma cell growth occurs through a p53-independent pathway.
Several reports have shown that G2/M arrest is associated with decreased activity of the Cdc2/cyclin complex [31–33]. Moreover, zinc acts as a potent inhibitor of Cdc2 kinase activity in Schizosaccharomyces pombe [34]. Thus, Cdc2 kinase is a possible target site of action for zinc in these studies. The inhibitory effect of zinc may be mediated by the interaction of zinc with the p13suc1 subunit of Cdc2. Zinc promotes inter-conversion among three forms of p13suc1 [35]. This interconversion of p13suc1 forms likely generates different binding sites for Cdc2 and may modulate the availability of functional Cdc2 kinase subunits at different stages of the cell cycle [35]. Therefore, these studies set the stage for more detailed studies of the mechanism of the zinc effect on growth at the molecular level in mammalian cells.
This report opens the door to investigate the mechanism of zinc inhibition of growth in prostate cancer cells. Zinc induction of cell cycle arrest in G2/M suggests that the transition from G2 to M is inhibited and/or that the mitotic process is inhibited. An important issue to resolve is the possibility that the increased apoptosis results from enhanced susceptibility of arrested cells to apoptosis. Alternatively, zinc may have distinct and separate effects on cell cycle progression and on apoptosis.
We have now described two significant effects of zinc on prostate cells. In addition to inhibitory effects on growth, zinc also inhibits citrate oxidation by virtue of its specific inhibitory effect on mitochondrial aconitase [5]. This metabolic effect has obvious implications in altering the energy metabolism and adenosine triphosphate (ATP) production of prostate cells. Cell cycle events and apoptosis involve energy-dependent reactions. In recent studies (unpublished findings), we observed that zinc treatment induces the mitochondrial release of cytochrome C and activation of caspase 3. Thus, zinc induces the proapoptotic actions of mitochondria. This suggests that the effect of zinc on growth may be linked to the metabolic-energy effect as well as the direct nuclear effect of zinc. We previously described the role of zinc in preventing the progression of prostate malignancy based on metabolic effects [1]. The present study further demonstrates the growth effect of zinc in two different types of prostatic carcinoma cells.
Acknowledgments
Grant sponsor: National Institute of Child Health and Human Development; Grant number: HD30818; Grant sponsor: National Institute of Diabetes and Kidney Disease; Grant numbers: DK28015, DK42839; Grant sponsor: National Cancer Institute; Grant number: CA71207.
References
- 1.Costello LC, Franklin RB. Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate. 1998;35:285–296. doi: 10.1002/(sici)1097-0045(19980601)35:4<285::aid-pros8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Habib FK. Zinc and the steroid endocrinology of the human prostate. J Steroid Biochem. 1978;9:403–407. doi: 10.1016/0022-4731(78)90608-8. [DOI] [PubMed] [Google Scholar]
- 3.Zaichick VY, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol. 1997;29:565–574. doi: 10.1007/BF02552202. [DOI] [PubMed] [Google Scholar]
- 4.Iguchi K, Hamatake M, Ishida R, Usami Y, Adachi T, Yamamoto H, Koshida K, Uchibayashi T, Hirano K. Induction of necrosis by zinc in prostate carcinoma cells and identification of proteins increased in association with this induction. Eur J Biochem. 1998;253:766–770. doi: 10.1046/j.1432-1327.1998.2530766.x. [DOI] [PubMed] [Google Scholar]
- 5.Costello LC, Liu Y, Franklin RB, Kennedy MC. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelia cells. J Biol Chem. 1997;272:28875–28881. doi: 10.1074/jbc.272.46.28875. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Franklin RB, Costello LC. Prolactin and testosterone regulation of mitochondrial zinc in prostate epithelial cells. Prostate. 1997;30:26–32. doi: 10.1002/(sici)1097-0045(19970101)30:1<26::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Fraker PJ, Telford WG. A reappraisal of the role of zinc in life and death decisions of cells. Proc Soc Exp Biol Med. 1997;215:229–236. doi: 10.3181/00379727-215-44132. [DOI] [PubMed] [Google Scholar]
- 8.Bradford M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Tonkinson JL, Marder P, Andis SL, Schultz RM, Gossett LS, Shin C, Mendelsohn LG. Cell cycle effects of antifolate antimetabolites: implications for cytotoxicity and cytostasis. Cancer Chemother Pharmacol. 1997;39:521–531. doi: 10.1007/s002800050608. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong DK, Kaufmann SH, Ottaviano YL, Furuya Y, Buckley JA, Isaacs JT, Davidson NE. Epidermal growth factor-mediated apoptosis of MDA-MB-468 human breast cancer cells. Cancer Res. 1994;54:5280–5283. [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Rao JN, Debeljuk L, Bartke A, Gao YP, Wilber JF, Feng P. The detection of thyrotropin-releasing hormone (TRH) and TRH receptor gene expression in Siberian hamster testes. Peptides. 1997;18:1217–1222. doi: 10.1016/s0196-9781(97)00183-6. [DOI] [PubMed] [Google Scholar]
- 13.Koh J-Y, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 14.Medema RH, Klompmaker R, Smits VAJ, Rijksen G. p21waf1 can block cells at two points in the cell cycle, but does not interfere with processive DNA-replication or stress-activated kinases. Oncogene. 1998;16:431–441. doi: 10.1038/sj.onc.1201558. [DOI] [PubMed] [Google Scholar]
- 15.Vallee BL, Galdes A. The metallobiochemistry of zinc enzymes. Adv Enzymol. 1984;56:283–430. doi: 10.1002/9780470123027.ch5. [DOI] [PubMed] [Google Scholar]
- 16.Epner DE, Herschman HR. Heavy metals induce expression of the TPA-inducible sequence (TIS) genes. J Cell Physiol. 1991;148:68–74. doi: 10.1002/jcp.1041480109. [DOI] [PubMed] [Google Scholar]
- 17.Richards RI, Heguy A, Karin M. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell. 1984;37:263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- 18.Sunderman FW., Jr The influence of zinc on apoptosis. Ann Clin Lab Sci. 1995;25:134–142. [PubMed] [Google Scholar]
- 19.Fernandes G, Nair M, Onoe K, Tanaka T, Floyd R, Good RA. Impairment of cell-mediated immunity functions by dietary zinc deficiency in mice. Proc Natl Acad Sci USA. 1979;76:457–461. doi: 10.1073/pnas.76.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin SJ, Mazdai G, Strain JJ, Cotter TG, Hannigan BM. Programmed cell death (apoptosis) in lymphoid and myeloid cell lines during zinc deficiency. Clin Exp Immunol. 1991;83:338–348. doi: 10.1111/j.1365-2249.1991.tb05639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flieger D, Reithmuller G, Ziegler-Heitbrock HWL. Zn++ inhibits both tumor necrosis factor-mediated DNA fragmentation and cytolysis. Int J Cancer. 1989;44:315–319. doi: 10.1002/ijc.2910440221. [DOI] [PubMed] [Google Scholar]
- 22.Iguchi K, Hamatake M, Ishida R, Usami Y, Adachi T, Yamamoto H, Koshida K, Uchibayashi T, Hirano K. Induction of necrosis by zinc in prostate carcinoma cells and identification of proteins increased in association with this induction. Eur J Biochem. 1998;253:766–770. doi: 10.1046/j.1432-1327.1998.2530766.x. [DOI] [PubMed] [Google Scholar]
- 23.Cohen JJ, Duke RC. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984;132:38–42. [PubMed] [Google Scholar]
- 24.Sellins KS, Cohen JJ. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987;139:3199–3206. [PubMed] [Google Scholar]
- 25.Shimizu T, Kubota M, Tanizawa A, Sano H, Kasai Y, Hashimoto H, Akiyama Y, Mikawa H. Inhibition of both etoposide-induced DNA fragmentation and activation of poly (ADP-ribose) synthesis by zinc ion. Biochem Biophys Res Commun. 1990;169:1172–1177. doi: 10.1016/0006-291x(90)92019-v. [DOI] [PubMed] [Google Scholar]
- 26.El-Deiry WS, Harper JW, O’Connor PM, Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill DE, Wang Y. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 27.Sheikh MS, Rochefort H, Garcia M. Overexpression of p21 waf1/cip1 induces growth arrest, giant cell formation and apoptosis in human breast carcinoma cell lines. Oncogene. 1995;11:1899–1905. [PubMed] [Google Scholar]
- 28.Wang J, Walsh K. Resistance to apoptosis conferred by Cdk inhibitors during myocyte differentiation. Science. 1996;273:359–361. doi: 10.1126/science.273.5273.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donato NJ, Perez M. Tumor necrosis factor-induced apoptosis stimulates p53 accumulation and p21WAF1 proteolysis in ME-180 cells. J Biol Chem. 1998;273:5067–5072. doi: 10.1074/jbc.273.9.5067. [DOI] [PubMed] [Google Scholar]
- 30.Zeng YX, El-Deiry WS. Regulation of p21waf1/cip1 expression by p53-independent pathways. Oncogene. 1996;12:1557–1564. [PubMed] [Google Scholar]
- 31.Lock RB, Ross WE. Possible role of p34cdc2 kinase in etoposide-induced cell death of Chinese hamster ovary cells. Cancer Res. 1990;50:3767–3771. [PubMed] [Google Scholar]
- 32.Tsao YP, D’Arpa P, Liu LF. The involvement of active DNA synthesis in camptothecin-induced G2 arrest: altered regulation of p34cdc2/cyclin B. Cancer Res. 1992;52:1823–1829. [PubMed] [Google Scholar]
- 33.O’Connor PM, Ferris DK, White GA, Pines J, Hunter T, Longo DL, Kohn KW. Relationships between cdc2 kinase, DNA cross-linking, and cell cycle perturbations induced by nitrogen mustard. Cell Growth Differ. 1992;3:43–52. [PubMed] [Google Scholar]
- 34.Endicott JA, Noble ME, Garman EF, Brown N, Rasmussen B, Nurse P, Johnson LN. The crystal structure of p13suc1, a p34cdc2-interacting cell cycle control protein. EMBO J. 1995;14:1004–1014. doi: 10.1002/j.1460-2075.1995.tb07081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birck C, Vachette P, Welch M, Swaren P, Samama JP. Is the function of the cdc2 kinase subunit proteins tuned by their propensities to oligomerize? Conformational states in solution of the cdc2 kinase partners p13suc1 and p9cksphy. Biochemistry. 1996;35:5577–5585. doi: 10.1021/bi952199b. [DOI] [PubMed] [Google Scholar]