Abstract
Hendra virus and Nipah virus are recently discovered and closely related emerging viruses that now comprise the genus henipavirus within the subfamily Paramyxoviridae and are distinguished by their broad species tropism and ability to cause fatal disease in a wide variety of mammalian hosts including humans. The high mortality associated with human and animal henipavirus infections has highlighted the importance and necessity of developing effective immunization strategies. The development of suitable animal models of henipavirus infection and pathogenesis has been critical for testing the efficacy of potential therapeutic approaches. Several henipavirus challenge models have been used and recent successes in both active and passive immunization strategies against henipaviruses have been reported which have all targeted the viral envelope glycoproteins.
1 Introduction
Hendra virus (HeV) and Nipah virus (NiV) are recently identified members of the family Paramyxoviridae (Eaton et al. 2007). The henipaviruses are distinguished from all other paramyxoviruses particularly by their broad species tropism and in addition to bats can infect and cause fatal disease in multiple vertebrate hosts including humans, monkeys, pigs, horses, cats, dogs, ferrets, hamsters, and guinea pigs, spanning six mammalian Orders (Bossart et al. 2009; Geisbert et al. 2010; Guillaume et al. 2009; Hooper et al. 1997b, 2001; Li et al. 2010; Marianneau et al. 2010; Middleton et al. 2007; Mungall et al. 2006; Rockx et al. 2010, 2011; Weingartl et al. 2005; Westbury et al. 1995, 1996; Wong et al. 2003). HeV appeared first in eastern Australia in 1994 and was transmitted to humans from infected horses (reviewed in Murray et al. 1998); NiV later emerged in 1998–1999 in peninsular Malaysia and was primarily transmitted to humans from infected pigs, but several other animal species also became infected (reviewed in Bishop and Broder 2008; Eaton et al. 2006). Thus, both viruses may be amplified and cause disease in animals and may in turn be transmitted to humans, where infection is manifested as a severe respiratory illness and/or febrile encephalitis with associated high case fatality rates (Selvey et al. 1995; Tan and Wong 2003; Wong et al. 2002).
Since their recognition in the mid to late 1990s, both HeV and NiV have continued to re-emerge. Occasional outbreaks of HeV occurred in the years immediately following its appearance in 1994, but in 2006 HeV began to cause spillover events on an annual basis with all occurring in horses in Australia and a total of seven human cases of which four have been fatal (Anonymous 2009; Playford et al. 2010). In 2011, however, (June to October) the dynamics of HeV spillover events changed considerably, and an unprecedented 18 independent outbreaks of HeV among horses in Australia were recorded, leading to the death or euthanasia of 23 horses, one dog and the monitoring of more than 60 people for possible HeV infection (Anonymous 2011; Smith et al. 2011). There has also been a somewhat surprising early appearance of HeV infection in a horse reported in the first week of January, 2012 (Anonymous 2012a). There have now been a total of 33 separate occurrences of HeV spillover and infection of horses since 1994 in Queensland and New South Wales. Similarly, nearly annual outbreaks of NiV infection, primarily in Bangladesh but also including India, have occurred since 2001 (13 total) since NiV was first recognized from the Malaysian outbreak in 1998. These events have been associated with significantly higher case fatality rates (ranging from 10 to 100%) among the people that have been infected since 2001 following the first outbreak in 1998. To date, there have been a total of 570 reported cases of NiV infection in people of which 305 have been fatal (reviewed in Luby et al. 2009; Pallister et al. 2011a; Anonymous 2012b).
The natural hosts of HeV and NiV have been identified as several species of fruit bats (flying foxes) in the genus Pteropus (Chua et al. 2002; Field et al. 2007; Halpin et al. 2000). Although the spillovers and outbreaks of HeV and NiV have all been limited to Australia and Malaysia, Bangladesh, and India; respectively, accumulating serological and limited nucleic acid evidence among a variety of different species of bats suggests that at least antigenically related henipaviruses are circulating in other regions including Thailand, Indonesia, China, Madagascar, and West Africa (Drexler et al. 2009; Hayman et al. 2008; Iehle et al. 2007; Li et al. 2008; Sendow et al. 2006, 2010; Wacharapluesadee et al. 2005).
In addition, serological evidence (cross-reactive antibodies to NiV glycoproteins) has also suggested the apparent transmission of some antigenically related henipaviruses to domestic pigs in West Africa is possible (Hayman et al. 2011). The routes of transmission to humans are also notably different for the henipaviruses, with HeV being transmitted from bats to horses and then to humans, whereas NiV transmission has included transmissions from bats to pigs and then to humans, from bats to humans and from humans to human (Bishop and Broder 2008; Field et al. 2010; Gurley et al. 2007; Homaira et al. 2010; Luby et al. 2009).
The unusually broad species tropism and highly pathogenic capacity of HeV and NiV, together with their uniquely large genome size led to their classification into the new genus henipavirus in the family Paramyxoviridae (Lamb et al. 2005). Given the high morbidity and mortality rates associated with henipavirus infection in both humans and livestock, their recognized natural reservoirs in nature and ease of propagation, and a lack of any licensed vaccines or therapeutics, HeV and NiV pose significant biosecurity threats and are classified as biosafety level 4 (BSL-4) pathogens. There are presently no approved or commercially available active or passive therapeutic measures available for preventing or treating henipavirus infection as a result of a natural outbreak, laboratory accident, or deliberate misuse. However, significant developments in effective active and passive immunization strategies against HeV and NiV infection applicable for both human and livestock protection have been reported in recent years. Here, we will summarize these countermeasure developments and the immunization and virus challenge models that have been used to test their efficacy.
2 Henipavirus Tropism and Pathogenesis
As with most paramyxoviruses, henipavirus infection of a susceptible host cell is mediated by an attachment glycoprotein and a fusion (F) glycoprotein, and HeV and NiV possess an attachment glycoprotein (G) (Eaton et al. 2006; Lamb and Parks 2007). The F glycoprotein is a type I transmembrane glycoprotein with an extracellular NH2-terminus and biologically active F consists of two disulfide-linked subunits, F1 and F2, that are generated by the proteolytic cleavage of an F0 precursor. Biologically active F mediates fusion between the viral and host cell membranes via a class I fusion mechanism involving two α-helical domains known as heptad repeats that mediate the formation of a six-helix bundle during or concomitant with membrane merger (reviewed in Lee and Ataman 2011). The attachment G glycoprotein is a type II transmembrane glycoprotein with its NH2-terminus oriented towards the cytoplasm and an extracellular COOH-terminus consisting of a stalk and globular head, and both the NiV and HeV head domain structures alone and in complex with ephrin receptors have recently been determined (Bowden et al. 2008, 2010; Colgrave et al. 2012; Xu et al. 2008, 2012). Henipaviruses require both G and F to mediate membrane fusion in a cooperative manner (reviewed in Dutch 2010).
Both HeV and NiV utilize the host cellular membrane proteins ephrin-B2 and ephrin-B3 as entry receptors via their attachment G glycoproteins (Bishop et al. 2007; Bonaparte et al. 2005; Negrete et al. 2005, 2006). Ephrin-B2 and ephrin-B3 are members of a large family of cell surface expressed ligands that bind to Eph receptor tyrosine kinases mediating important bidirectional cell–cell signaling events within the vascular, nervous, and skeletal systems playing critical roles in governing cell migration, attachment and repulsion (Lackmann and Boyd 2008; Pasquale 2010). Ephrin-B2 and ephrin-B3 are highly conserved proteins across vertebrate species (95–96% and 95–98% amino acid identity, respectively), including hosts known to be susceptible to henipavirus infection such as human, horse, pig, cat, dog, and flying foxes (Bossart et al. 2008). Ephrin-B2 is found in arteries, arterioles, capillaries in multiple organs, and tissues but appears absent from the venous components of the vasculature, whereas ephrin-B3 is more prominently found in the nervous system as well as the vasculature (Gale et al. 2001; Pasquale 2008; Su et al. 2004). The recognition of ephrin-B2 and ephrin-B3 as the virus entry receptors for HeV and NiV provided important insight into both the broad species tropisms of the viruses, because of the high sequence conservation of the molecules, as well as the distribution of viral antigen in arterial endothelial cells, smooth muscle, neurons, and some epithelial cells from infected hosts (reviewed in Hooper et al. 2001; Wong 2010).
2.1 Human Pathology
In people, acute henipavirus infection and pathogenesis results from a systemic infection that likely occurs via hematogenous spread of the virus from an undefined primary site of replication (Wong et al. 2002). The key findings of infection are a wide-spread vasculitis and endothelial cell tropism resulting in multinucleated syncytial cells which is considered to be diagnostic of henipavirus infection. There is also prominent parenchymal cell infection and pathogenesis of many, if not most, major organs with the brain, lung, heart, kidney, and spleen severely involved (Chua et al. 1999; Wong et al. 2002, 2009).
Clinically, severe henipavirus infection in humans will present as a severe respiratory disease, encephalitis or a combination of both. In humans, henipavirus infections can also result in a clinically quiescent period following an apparent recovery from an acute infection, which can later recrudesce as encephalitis. This was first observed in the second fatal human HeV case which occurred in an individual who experienced relapsed encephalitis 13 months after infection (O’Sullivan et al. 1997). Among the many more cases of human NiV infection during the initial Malaysian outbreak, it was noted that neurological disease could frequently present later (more than 10 weeks) after a recovery from an acute encephalitic or even an asymptomatic infection (Tan and Wong 2003). Relapsed encephalitis presented from several months to as late as 4 years after infection (Chong and Tan 2003; Tan et al. 2002). An analysis of the first two fatal human cases of HeV infection; one presenting as an acute respiratory disease and the other as relapsed encephalitis, revealed that HeV was neurotropic in each situation causing either acute encephalitis without apparent clinical symptoms or a relapsed encephalitis that resembled those cases of relapsed NiV encephalitis (Wong et al. 2009). These episodes of elapsed encephalitis are believed to be caused by the recrudescence of virus replication that is apparently restricted to central nervous system (CNS) (Tan et al. 2002; Wong et al. 2009). A disseminated endothelial cell infection, vasculitis, thrombosis, and CNS parenchymal cell infection all appear to play essential roles in the fatal outcome of human henipavirus infection (Eaton et al. 2007; Hooper et al. 2001; Wong et al. 2002).
2.2 Animal Pathology and Virus Challenge Models
The development of animal modeling systems of henipavirus infection has been essential for understanding their pathogenic processes and for the evaluation of potential antiviral approaches. A detailed review of all natural and experimental infections of various mammalian species, including their bat reservoir hosts, with either NiV or HeV or both is reviewed in a separate chapter within this series (Geisbert et al.). Here, we will only briefly summarize the salient features of henipavirus in vivo pathogenesis in those well established animal models that have been used in the evaluation of promising antiviral therapeutic strategies.
A major challenge faced by researchers in animal experimentation with the henipaviruses has been the restriction of live virus use to BSL-4 containment; nevertheless, remarkable progress has been made by a number of research teams over the past decade in evaluating the outcomes of experimentally infecting animals with the henipaviruses. It has also been essential that consistent outcomes from experimental animal infection experiments be possible to establish a successful modeling approach. For the henipaviruses, these challenges have been exacerbated by the early observations that neither HeV nor NiV could cause a productive infection with disease in several typical small animal model systems; including mice, rats, and rabbits (Westbury et al. 1995; Wong et al. 2003). Further, for agents such as HeV and NiV more than one accepted animal modeling platform would be required to move forward a potential vaccine or antiviral pharmaceutical for possible human use, falling under the “animal rule” requirement set forth by US food and drug administration (FDA) in 2002 as an alternative licensing pathway for therapeutics against highly pathogenic agents when human efficacy studies are not feasible or ethical (Crawford 2002) (recently reviewed in Aebersold 2012). Several animal model platforms of henipavirus infection have now emerged that essentially mirror the pathogenesis seen in either naturally infected humans or in economically important livestock (horses and pigs).
2.2.1 Pathogenic Natural and Experimental Henipavirus Infections
All occurrences of natural HeV infection in Australia have been in horses, whereas NiV was associated with pigs in Malaysia, although dogs, cats, and horses were also involved (reviewed in Eaton et al. 2006). The pathology caused by either virus in horses (natural or experimental infection with HeV or natural infection with NiV) is more severe than that caused by either virus in pigs (reviewed in Weingartl et al. 2009). The exact mode of transmission from bats to animal hosts is not known, but the most likely scenarios are that virus-contaminated residual fruit pulp spat out by flying foxes are ingested by horses or pigs (Yob et al. 2001) or that urine and or fetal tissues and fluids from infected and/or virus shedding bats contaminates pastures or other food sources for livestock (Halpin et al. 2000).
Naturally acquired HeV infection in horses is often associated with severe disease, and experimental infections are essentially uniformly fatal. The incubation period in naturally infected horses is between 8 and 11 days, and the animals initially become anorexic and depressed with general uneasiness and ataxia, with a developing fever with sweating. Respiration becomes rapid, shallow and labored with pulmonary edema and congestion with nasal discharge being a common terminal feature 1–3 days following the onset of clinical signs. Neurologic disease is also present but less frequent and noted in both terminally ill horses and in those that recovered from respiratory infection (Rogers et al. 1996; Williamson et al. 1998). Infection is wide spread with an endothelial cell tropism with syncytia (Hooper et al. 2001) and virus can be readily recovered from a number of internal organs (Hooper et al. 1997a; Marsh et al. 2011; Murray et al. 1995; Williamson et al. 1998). Experimental infection of horses with NiV has not been carried out, but the brain and spinal cord of one naturally infected horse was confirmed and revealed nonsuppurative meningitis (Hooper et al. 2001).
Experimental NiV infection of pigs has revealed the respiratory system as a primary target organ of virus replication and pathology (Hooper et al. 2001; Middleton et al. 2002), but virus was also present in the kidneys (Middleton et al. 2002) which was less common in naturally infected pigs (Hooper et al. 2001). The involvement of the CNS in pigs was less prevalent and meningitis or meningoencephalitis more common than encephalitis (Middleton et al. 2002). Other experimental NiV infections (landrace piglets) resulted in a mild clinical disease with fever and respiratory signs, but could cause neurological disease depending on the challenge route of infection (Weingartl et al. 2005). Recoverable virus was obtained from the respiratory, lymphatic and nervous systems, with virus shedding in nasal, pharyngeal and ocular fluids. Experimental HeV infection of pigs has also confirmed their susceptibility to disease, and again infection manifested primarily as a respiratory disease in both Landrace piglets and older Gottingen minipigs, with possible CNS involvement observed in minipigs, and virus shedding was noted in nasal, oral, rectal, and ocular fluids (Li et al. 2010). Both horses and pigs now serve as models for the testing of livestock vaccines against henipaviruses, discussed below.
2.2.2 Pathogenic Experimental Henipavirus Infections
Cats were recognized as a naturally susceptible host for NiV during the 1998–1999 Malaysian outbreaks (Hooper et al. 2001). Experimental infections of cats revealed they are highly susceptible to productive infection by both HeV and NiV and disease is severe. HeV infected cats develop fever and elevated respiratory rates, and there is rapid progression to severe illness and death within 24 h of the onset of clinical signs (Westbury et al. 1995, 1996). HeV disease in cats is quite similar to that seen in horses, with widespread vasculitis and parenchymal lesions in a wide range of organ systems particularly the lungs (Hooper et al. 1997b, 2001). Experimental NiV infection in the cat is essentially identical in outcome as compared to HeV infection and closely resembles most of the pathogenic processes seen in cases of henipavirus infection of people (Hooper et al. 2001; Middleton et al. 2002; Mungall et al. 2006, 2007). The cat model has been used in the testing of the first henipavirus subunit vaccine, discussed below.
The only successful small animal model for henipavirus infection and pathogenesis studies described is the golden hamster, carried out first with NiV challenge experiments (Wong et al. 2003) and more recently with HeV (Guillaume et al. 2009). Hamsters infected by an intranasal or intraperitoneal route with NiV revealed neurological disease signs that progressed rapidly followed by death in 5–8 days. Intranasally challenged animals succumbed to infection 9–15 days later. NiV infection is systemic and endothelial tropic and viral pathology is particularly prevalent in CNS and somewhat to a lesser extent in the lung (Wong et al. 2003). HeV infection of golden hamsters also resembled the pathology seen in acute human NiV cases, including both respiratory and brain pathology with widespread endothelial infection and vasculitis especially in the CNS (Guillaume et al. 2009; Rockx et al. 2011). The golden hamster model has been extensively used to evaluate vaccines and antivirals against henipaviruses, discussed below.
Ferrets have more recently emerged as a highly suitable model for both NiV and HeV infection and pathogenesis (Bossart et al. 2009; Pallister et al. 2009, 2011b). Ferrets challenged with NiV develop severe respiratory and neurological disease within 6–10 days, with generalized and widespread vasculitis and parenchymal lesions following oral nasal challenge with low doses of virus (Bossart et al. 2009; Pallister et al. 2009). Parenchymal lesions and virus antigen were found in the CNS including neurons and virus could be isolated from brain and a wide variety of organs. HeV challenged ferrets, by the oral nasal route results in essentially identical outcomes as reported for NiV challenge (Pallister et al. 2011b), and both models reproduce all the hallmarks seen in henipavirus-infected people. The ferret model has been used in the first human monoclonal antibody passive immunization strategy and also the subunit vaccination approach against henipaviruses, discussed below.
The first nonhuman primate models of NiV and HeV infection have also recently been reported in challenge studies using the African green monkey (AGM) (Geisbert et al. 2010; Rockx et al. 2010). Both NiV and HeV infection of AGMs result in a uniformly lethal disease with low dose challenge by intratracheal virus inoculation within 7–10 days postinfection. Subjects develop severe respiratory disease with congestion and hemorrhage along with fibrosis (Geisbert et al. 2010). There is widespread vasculitis and endothelial and arterial smooth muscle cell virus infection in most organs and tissues. The development of respiratory disease was seen within 7 days postinfection and the progression of lung pathology was observed by radiological examination following an intratracheal inoculation (Geisbert et al. 2010; Rockx et al. 2010). Neurological disease, along with vascular and parenchymal lesions in the brain including infection of neurons and the brainstem is significant in both NiV and HeV infected animals (Geisbert et al. 2010; Rockx et al. 2010). The severe respiratory and neurological disease along with the generalized vasculitis observed in henipavirus-infected AGMs provides the most accurate reflection of what has been reported in henipavirus-infected people. Indeed, for the purposes of moving potential therapeutics forward to human application, the development of the AGM model has been a significant milestone.
3 Active Immunization
The use of safe and efficacious vaccines for several important viral pathogens has been the mainstay of prevention strategies in humans. As with all paramyxoviruses the attachment and fusion glycoproteins are the principal antigens to which virtually all neutralizing antibodies are directed (Lamb and Parks 2007), and neutralizing antibodies are the key vaccine-induced protective mechanisms in the case of some well known paramyxovirus diseases of humans (mumps and measles viruses) (Griffin 1995; Pantaleo and Koup 2004). It is therefore likely that a successful henipavirus vaccine will be one that can elicit neutralizing antibodies.
Paramyxovirus F glycoproteins are homotrimers and the attachment glycoproteins including the henipavirus G glycoproteins are tetramers composed of disulfide-linked dimers (Lee and Ataman 2011), and the native oligomeric structures of viral glycoproteins can influence their antigenicity and immunogenicity (Broder et al. 1994; Wiley and Skehel 1987). For the henipaviruses, the development of vaccines and therapeutics has focused on targeting virus attachment and entry, processes facilitated by their oligomeric viral envelope glycoprotein spikes. Here, we will summarize the successful active and passive immunization and henipavirus challenge studies that have been reported to date (Table 1).
Table 1.
Immunization strategies against Nipah virus and Hendra virus trialed in one or more animal models
| Immunization strategy | Viral antigen used | Animal challenge model |
|---|---|---|
| Active immunization | ||
| Recombinant vaccinia virusa | Nipah F and/or G glycoprotein | Golden Hamster |
| Recombinant canarypox virusb | Nipah F and/or G glycoprotein | Pig |
| Glycoprotein subunit | Hendra or Nipah soluble G glycoprotein | Catc |
| Hendra soluble G glycoprotein | Ferretd African Green Monkeye Horsef |
|
| Passive immunization | ||
| Polyclonal antibodyg | Nipah F and/or G glycoproteins | Golden Hamster |
| Monoclonal antibodyh | Nipah F and/or G glycoproteins | Golden Hamster |
| Nipah/Hendra G glycoprotein | Ferreti African green monkeyj |
|
Nipah F and/or G glycoprotein encoding recombinant vaccinia viruses used to immunize hamsters protects against intraperitoneal NiV challenge (Guillaume et al. 2004)
Nipah F and/or G encoding recombinant canarypox viruses used to immunize pigs protects against intranasal NiV challenge (Weingartl et al. 2006)
Recombinant HeV-sG subunit, in CSIRO triple adjuvant (Montanide, Quil A, and DEAE-dextran) used to immunize cats (three doses of 100 μg, at three week intervals) protects against subcutaneous NiV challenge (Mungall et al. 2006); HeV-sG in CpG (ODN 2007) and Allhydrogel™ used to immunize cats (two doses of 50, 25 μg or 5 μg, day 0 and 21) protects against oronasal NiV challenge (McEachern et al. 2008)
Recombinant HeV-sG in CpG (ODN 2007) and Allhydrogel™ used to immunize ferrets (two doses of 100, 20 μg or 4 μg; day 0 and 20) protects against oronasal HeV challenge (Pallister et al. 2011b); or NiV challenge (Pallister, Middleton and Broder, unpublished)
Recombinant HeV-sG in CpG (ODN 2006) and Allhydrogel™ used to immunize African green monkeys can protect against intratracheal NiV challenge (Hickey et al. 2011); or HeV challenge (Geisbert and Broder, unpublished)
Recombinant HeV-sG used to immunize horses (two dose regime) protects against high dose oronasal HeV challenge and prevents virus replication and shedding (Middleton and Broder, unpublished) (Balzer 2011)
Polyclonal hamster serum against NiV F or G glycoprotein administered by intraperitoneal injection can protect against intraperitoneal NiV challenge (Guillaume et al. 2004)
Mouse mAbs against NiV F or G glycoprotein administered by intraperitoneal injection can protect against intraperitoneal NiV challenge (Guillaume et al. 2006), and mouse mAbs to NiV F administered by intraperitoneal injection pre and postexposure can protect against intraperitoneal HeV challenge (Guillaume et al. 2009)
A cross-reactive neutralizing human mAb against henipavirus G glycoprotein (m102.4) provides postexposure protection in ferrets by intravenous infusion following high dose oronasal NiV challenge (Bossart et al. 2009)
African green monkeys are protected against lethal intratracheal HeV challenge by postexposure intravenous infusion of human mAb m102.4 at 10, 24 or 72 h (Bossart et al. 2011); similar results were obtained against NiV challenge (Geisbert and Broder, unpublished)
3.1 Live Recombinant Vaccines
The first vaccination and challenge experiments were carried out with NiV in the hamster model using recombinant vaccinia viruses (Guillaume et al. 2004; Table 1). Here, NiV F and G encoding recombinant vaccinia viruses were examined individually and in combination by immunizing hamsters twice with a 1 month interval, using 107 PFU of either the F or G encoding recombinants or 5 × 106 of each recombinant together. Antibody titers measured by ELISA and virus neutralization following the second immunization were modest with the sera raised against the NiV G recombinant eliciting the strongest response (~1:4,000 and <1:50, respectively). All animals were completely protected following an intraperitoneal challenge of 1,000 PFU of NiV, regardless of whether they were immunized with the G or F or both vaccinia virus recombinants (Guillaume et al. 2004). However, both ELISA and neutralizing antibody titers against NiV were considerably elevated following virus challenge indicating virus replication and a humoral anamnestic response. Although the contribution of cell-mediated immunity to protection in this experiment cannot be excluded, passive transfer experiments with antisera, prepared using these recombinant vaccinia viruses, demonstrated protection (discussed below), and together these studies suggested a major role of specific neutralizing antibody in protection. However, even though the highly attenuated vaccinia virus strain, NYVAC, was employed, such a vaccine platform for use in humans is unlikely although there potential for use in livestock is possible although protection against HeV challenge in hamsters has not been reported.
The development of livestock vaccines for HeV and NiV has been a desirable goal because of the association of pigs as an amplifying host for NiV and the fact that all HeV outbreaks in Australia have occurred in horses. The first vaccine that was explored and tested was a recombinant canarypox-based vaccine candidate for swine (Weingartl et al. 2006; Table 1). Similar to the vaccinia virus constructs described above, the NiV F and G glycoprotein genes were used to produce recombinant canarypox virus (ALVAC) vaccine vectors. These ALVAC NiV F and G recombinants were used to immunize 4-week-old pigs twice with a 2 week interval. Similar to the study carried out in hamsters, each ALVAC recombinant was tested alone and in combination, and 108 PFU of either the F or G recombinant were employed or a 108 PFU dose of each. Piglets were challenged intranasally with a 2.5 × 105 PFU dose of NiV, which was divided between each nostril at 28 days post vaccination. Even though NiV disease in pigs is often less severe than in other susceptible mammalian hosts, the virus does replicate and disseminate to a variety of organ systems with significant levels of recoverable virus present in the respiratory system associated with virus shedding. This vaccination study aimed to prevent disease and also to impede or prevent virus shedding. The study demonstrated protection from NiV-mediated disease in all vaccinated animals by the G, F or combination of ALVAC recombinant vectors. Further, only low levels of viral RNA were detectable in only a few tissue samples and importantly no isolatable virus was observed in the vaccine recipients. In contrast, both high levels of viral RNA as well as isolatable virus were consistently obtained in the control challenged pigs, notably in the throat and nose. A more detailed description of the clinical and pathological findings of NiV infection in the pig model can be found in a separate review in this series (Geisbert, Feldmann and Broder). Neutralizing antibody was elicited by both vectors, with ALVAC-G yielding the strongest response approximately five-fold higher (~1:1,280). The combined ALVAC-F/G vaccination appeared to be only marginally better than that of G alone, all together the data indicated that either formulation could serve as a protective vaccine against NiV for swine (Weingartl et al. 2006). The ALVAC henipavirus vaccine use in protection against HeV challenge in pigs has not been reported.
3.2 Subunit Vaccines
In contrast to live or replication competent recombinant viral vaccines, subunit immunogen based vaccines represent a viable option for vaccine development, especially in the case for henipaviruses. These types of vaccines can be relatively quickly implemented and tested, are simple, and can be administered with no risk of infection. Soluble and secreted, oligomeric versions of the G glycoprotein from both HeV and NiV (sG) were generated in the course of analyzing henipavirus host cell interaction and virus entry mechanisms, and these reagents represented potential subunit vaccines that could be tested in vivo (Bossart et al. 2005). The Hendra virus sG glycoprotein subunit vaccine (Fig. 1) is an engineered secreted version of the molecule in which the transmembrane and cytoplasmic tail domains have been deleted from the N-terminal coding sequence. On expression, the molecule is produced and released from cells because it lacks its transmembrane anchor sequence, and sG has been shown to retain many native characteristics, such as its oligomerization into dimers and tetramers (Bossart et al. 2005), and ability to bind ephrin receptor (Bonaparte et al. 2005). HeV sG has typically been produced in mammalian cell culture expression systems, and is N-linked glycosylated at its predicted sites; recently shown to be variably occupied (between four and seven) depending on the recombinant expression platform utilized (Colgrave et al. 2012; Xu et al. 2012).
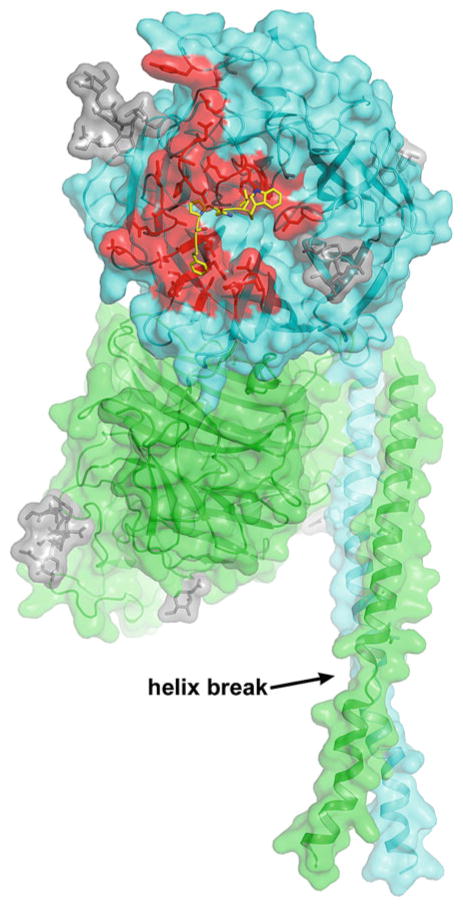
Fig. 1.
Hendra virus soluble G glycoprotein subunit vaccine. The recombinant HeV-sG glycoprotein subunit vaccine candidate is the entire predicted ectodomain (residues 76–604). Here, HeV-sG is shown as the dimer, with secondary structure and surface exposed elements modeled. HeV-sG dimer has been the purified form of HeV-sG used in all vaccine studies to date. One monomer in the dimer is colored cyan and the other is green. The secondary structure elements of the two globular head domains (cyan and green) are derived from the crystal structure of the HeV G head domain, which also revealed that all five predicted N-linked glycosylation sites (N306, N378, N417, N481 and N529) were occupied by carbohydrate moieties (Xu et al. 2012). The N-linked carbohydrate modifications are illustrated as gray sticks, but N378 was not modeled in the figure due to weak electron density. The G glycoprotein head domain folds as a six-bladed β-propeller. The structure of the entire HeV G stalk domain (residues 71–173) has not been determined, but here the stalk regions (residues 77–136) of each monomer are modeled (Kelley and Sternberg 2009) and are not a continuous helix (labeled helix break). There are two helical ranges, Thr-77 to Lys-95 and Thr-98 to Ser-135. The hydrophobic residues distribution (most hydrophobic side chains point to the same direction) suggests a bundling tendency. The HeV-sG stalk residues 98–135 appear equivalent to the HN glycoprotein stalk helix domain of the recently reported NDV structure (Yuan et al. 2011). Here, the position of HeV sG head dimer and stalks are oriented based on the alignment to the NDV structure. The ephrin receptor binding face of the cyan monomer is facing out and that of the green monomer is facing left. The ephrin receptor binding region is colored red in the cyan globular head, and an overlay of the ephrin-B2 G-H loop is shown in yellow
HeV sG was first shown to elicit a potent cross-reactive neutralizing (HeV and NiV) antibody response in rabbits (Bossart et al. 2005), suggesting that its application as a subunit vaccine would be promising. Rabbit anti-HeV G antibodies could neutralize both HeV and NiV in cell culture, displaying a slightly higher titer against the homologous virus; however, follow-up studies have indicated that Hendra virus sG appears to present more cross-reactive epitopes (anti-NiV G antibodies) as compared to sG from NiV (Bossart et al. 2007). Four neutralizing epitopes have been mapped on the globular head of the Hendra virus G protein. Two are located on the base of the head and two on the top, in locations resembling those identified as neutralizing sites in other paramyxoviruses (White et al. 2005). Further studies have indicated that there are at least three more independent neutralizing epitopes on HeV G (seven in total) and only one of the seven maps to the ephrin-B2/B3 receptor binding site (Hickey and Broder, unpublished).
Immunization and challenge studies using the HeV-sG subunit immunogen (produced by recombinant vaccinia virus in human cell culture) were first carried out in the cat model and a side-by-side comparison was made using a similarly designed and expressed NiV-sG. Both HeV-sG and NiV-sG could elicit a completely protective immune response against a lethal subcutaneous NiV challenge (NiV-Malaysia isolate) (Mungall et al. 2006; Table 1). Both HeV-sG and NiV-sG were formulated in CSIRO triple adjuvant (Montanide, Quil A, and DEAE-dextran) and three doses of 100 μg each were given at 3 week intervals. This protocol elicited a very strong neutralizing antibody response, and homologous serum neutralizing titers were greater than 1:20,000 and heterologous titers were greater than 1:20,000 to 16-fold lower. Immunized animals and two additional Naïve controls were challenged subcutaneously with 500 TCID50 of NiV. Naïve animals developed clinical disease (fever and respiratory distress) and succumbed to infection 6–13 days postnfection, while none of the immunized animals showed any sign of fever or disease. Taqman PCR analysis of samples from Naïve animals revealed high levels of NiV genome in a wide range of tissues, while genome was evident in only two animals and in only four samples with signals below the limit of accurate detection (Mungall et al. 2006). These data suggested that a single vaccine (HeV-sG) could be effective against both HeV and NiV. Further, an analysis of the antibody responses in sera from naturally infected or other immunized sources has also shown that HeV elicited high levels of NiV G cross-reactive antibodies; whereas NiV gave rise to limited cross-reactive antibodies to HeV G. Together, these data suggested that the HeV G stimulates a more crossreactive immune response (Bossart et al. 2007).
A follow-up study in the cat model explored the protection levels of prechallenge virus neutralizing titers in combination with a high oronasal challenge, the likely natural route of zoonotic exposure (McEachern et al. 2008). Here, two doses of 50, 25 μg or 5 μg of HeV-sG formulated with CpG and Alhydrogel™ were administered at day 0 and 21, and neutralizing antibody titers present at day 35 ranged from 1:32 (low vaccine dose) to 1:512 (high vaccine dose). Animals were challenged on day 42, and the study revealed that even low vaccine dose and just a two-dose immunization protocol could fully protect animals from a high dose oronasal challenge (50,000 TCID50 of NiV-Malaysia) with prechallenge neutralizing titers as low as 1:32 offering full protection.
During the vaccination and challenge studies carried out in the cat model, the ferret was explored as an alternative and more suitable henipavirus animal model and recently, a HeV-sG immunization study was carried out in the ferret with a HeV challenge (Pallister et al. 2011b). Here, ferrets (two in each group) were vaccinated twice (day 0 and 20) with either a 100, 20 μg or 4 μg dose of HeV-sG formulated in CpG and Alhydrogel™ using similar parameters as the second cat vaccination and challenge study. In this case, recombinant HeV-sG was produced by stable human 293-F cell culture (and all subsequent vaccination studies have since used human cell line derived HeV-sG). Here again, the strong immunogenic characteristics of HeV-sG were noted and at the time of the booster vaccination at day 20, significant neutralizing titers in sera were seen which correlated to the dose of HeV-sG used. Specifically, titers ranged from 1:8,192 in the 100 μg vaccination groups, to 1:1,024 and 1:2,048 in the 20 μg vaccination group, and 1:64 and 1:128 for the 4 μg vaccination group. Prior to challenge, neutralizing titers in all six vaccinated animals were 1:8,192 or greater. All vaccinated ferrets remained completely free of any signs of fever or clinical disease from HeV infection following an oronasal challenge with 5,000 TCID50 of a low passage isolate of HeV (Redlands 2008 isolate), whereas the control ferrets developed fever, depression, lack of grooming and tremors, and necropsy revealed HeV induced gross pathological lesions. Histological and immunohistological analysis of control animals revealed widespread systemic vasculitis and parenchymal lesions in many organs and tissues (Fig. 2). In HeV-sG vaccinated ferrets at postmortem, all were found to be gross pathologically and histopathologically normal (except one of four animals in the lose dose (4 μg) group. Further, there was no evidence of virus or viral genome in any tissues or body fluids in any animals in the 100 μg and 20 μg vaccination groups; whereas a low level of genome was detected in the nasal washes from one animal (of four) in the 4 μg vaccinated group; and no isolatable virus was recovered from any vaccinated ferrets. Together, these findings indicate that 100 or 20 μg doses of HeV-sG vaccine can completely prevent a productive HeV infection in the ferret, confirming in a second animal model of henipavirus challenge, that subunit protein vaccination strategy to prevent the infection and shedding of HeV is possible.
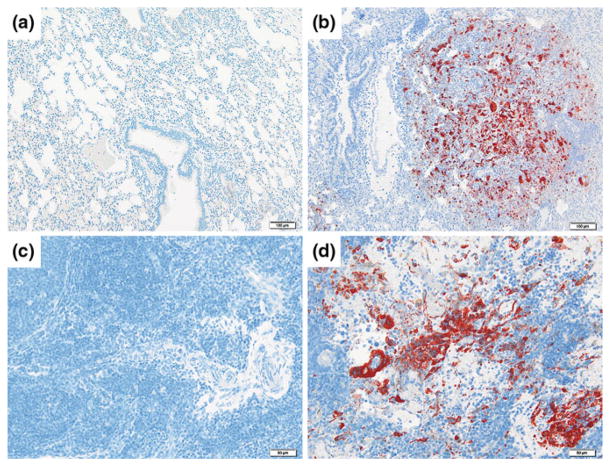
Fig. 2.
HeV sG subunit vaccine protection against HeV challenge in the ferret. Immunohistochemical analysis using a NiV nucleoprotein (N) specific rabbit polyclonal antibody following lethal HeV challenge: lung tissue (a, b) and lymph node tissue (c, d) in a ferret immunized with recombinant HeV-sG glycoprotein prior to challenge (left panel) and a control ferret (right panel) (Pallister et al. 2011b). Scale bar (a, b) = 100 μm, scale bar (c, d) = 50 μm
In addition to the cat and ferret HeV-sG vaccination studies, HeV-sG vaccination of nonhuman primates (AGMs) followed by intratrachealNiV challenge (Hickey et al. 2011) or intratracheal HeV challenge (Geisbert and Broder, unpublished) has been piloted and here, complete protection from henipavirus-induced disease was achievable. Follow-up experiments and further preclinical development of HeV-sG in vaccine formulations that could potentially be suitable for use in humans are in progress. Finally, because of these successes in HeV-sG vaccine-mediated protection in multiple animal challenge models, and the recent escalation and geographic spread of recurrent HeV outbreaks in Australia, the HeV-sG subunit was recently licensed by a multinational animal health company and is in commercial development as an equine vaccine for use in Australia. Preliminary horse HeV-sG vaccination and HeV challenge studies have recently been conducted in Australia at the high containment BSL-4 facilities of the Australian Animal health laboratories (AAHL) in Geelong. This research programhas been a collaborative effort between the Uniformed Services University and Henry M. Jackson foundation, AAHL and a corporate partner. Findings of these preliminary studies were reported at Australian veterinary association, Annual conference in Adelaide, in May 2011. The HeV-sG was used to immunize horses (two dose regime, 3 week interval) and a high dose and low dose antigen formulation was used, and prechallenge HeV neutralizing titers ranged from 1:128 to 1:2,048. Following a high dose oronasal challenge with 2 × 106 TCID50 of HeV, all vaccinated horses remained clinically disease-free, and there was no evidence of virus replication or virus shedding in any of the immunized horses (Balzer 2011). A target date for full registration of the equine HeV-sG subunit vaccine is mid 2013.
4 Passive Immunization
Traditionally, the antibody response has been the immunologic measure of vaccine efficacy. While the neutralizing antibodies elicited by a vaccine can be highly effective, purified neutralizing antibodies used to passively immunize infected individuals can be equally efficacious. Passive antibody therapy is routinely used as an effective antiviral therapy or as a prophylactic measure for hepatitis B, CMV, varicella, Respiratory syncytial virus (RSV) and rabies virus (Casadevall et al. 2004). The development of mouse-human chimeric mAbs, the ability to “humanize” murine mAbs (Kang et al. 1991; Wright et al. 1992) and the advancement of phage-displayed human antibody libraries has made passive antibody therapy more feasible. Here we will summarize the passive immunization studies that have been carried out as antiviral strategies against the henipaviruses.
4.1 Polyclonal Antibody
The first evidence of passive protection against a NiV challenge was shown using the hamster model discussed above (Guillaume et al. 2004; Table 1). Here, monospecific polyclonal antiserums against NiV F and G were prepared in hamsters by using recombinant vaccinia viruses and immunization with cell lysates prepared from BHK21 hamster cells expressing NiV F or G in complete Freund’s adjuvant. The protective efficacy of the various antisera to either NiV F or G or both mixed together, were tested in hamsters. Animals were given 200 μl of antisera followed 1 h later by challenge virus, and another 200 μl of antisera was administered at 24 h, all by intraperitoneal injection. Based on prior studies, the 1,000 PFU challenge of NiV was used and here all the antisera could provide protection (Guillaume et al. 2004) demonstrating the importance of the humoral response to the NiV envelope glycoproteins as a mechanism of protection.
4.2 Monoclonal Antibody
The polyclonal antisera protection studies in hamsters were followed-up using two murine mAbs against NiV F and NiV G as passive immunotherapies (Guillaume et al. 2006; Table 1). Here, the mAbs were examined as ascitic fluid preparations and delivered to hamsters by intraperitoneal administration. A series of experiments using various amounts of mAbs administered 24 h before NiV virus challenge and again at 1 h following challenge were conducted and hamsters were inoculated with 100 LD50 of NiV. Also, the efficacy of the anti-F or anti-G mAbs was examined as a postexposure therapy from 1 to 96 h after virus challenge. Hamsters that received mAb before and immediately following the intraperitoneal challenge of NiV were completely protected (Guillaume et al. 2006). However, only partial protection (50%) was observed with the anti-G mAbs when the animals were inoculated up to 24 h after challenge. Although F-specific mAb could protect, higher amounts were required in comparison to anti-G mAb. High levels of either anti-G or anti-F mAbs appeared to yield sterilizing immunity, when administered intraperitoneally with virus, while lower amounts of antibody could still protect against fatal infection but did result in measurable increases in anti-NiV antibodies following virus challenge. Similar studies using the hamster with a HeV challenge have also been conducted, and cross-reactive mouse mAbs specific for the NiV F glycoprotein could protect hamsters from HeV disease if given before virus challenge (Guillaume et al. 2009; Table 1). Together, these studies provided the proof-of-concept that passively immunotherapy against NiV infection by targeting the viral envelope glycoproteins is possible.
However, passive immunization using antibody administered systemically following virus infection would be the more likely scenario a person would encounter and this would require a more potent mAb therapeutic. Further the humanization of mouse mAbs is a considerable undertaking in order to formulate an acceptable therapeutic product suitable for human use, and also one without guaranteed success.
The development of molecular-based platforms for the identification and isolation of human-derived recombinant mAbs has accelerated the development of antibodies with the potential for human use. A major advance has been in the development of the phage display platform of combinatorial antibody libraries (Rader and Barbas 1997) that can encode human antibodies in the form of Fab fragments or single-chain variable region fragments (scFvs). Using recombinant antibody techniques, neutralizing human mAbs specific for the henipavirus G glycoprotein have been isolated and characterized (Zhu et al. 2006). These mAbs were generated by panning a large na human phage-display antibody library containing about 1010 different phage-displayed Fabs using recombinant HeV-sG (the subunit vaccine immunogen described above). In particular, two Fabs, m101 and m102, had significant neutralizing activities against live virus and m101 was converted to a full length human IgG1 antibody. IgG1 m101 was exceptionally potent in neutralizing infectious HeV; complete (100%) neutralization was achieved with 12.5 μg/ml and 98% neutralization with 1.6 μg/ml using a 96-well plate-based assay using 200 TCID50 of virus with Vero cells. The other mAb, m102, exhibited the highest level of cross-reactive neutralization of both NiV and HeV, and m102 was affinity maturated by light-chain shuffling combined with random mutagenesis of its heavy-chain variable domain and clones were reisolated using HeV-sG. One of the selected antibody Fab clones, m102.4, had improved affinity of binding to HeV-sG and it was converted to IgG1 format and tested against infectious NiV and HeV. The mAb m102.4 exhibited exceptionally potent and crossreactive inhibitory activity against NiV and HeV with 50% inhibitory concentrations below 0.04 and 0.6 μg/ml, respectively (Zhu et al. 2008). These were the first human mAbs identified against the henipaviruses and because of their potency in vitro, they have been piloted in vivo in NiV and HeV animal challenge studies.
The first in vivo efficacy study of human mAb m102.4 was carried out in the ferret model (Bossart et al. 2009; Table 1), and groups of three animals each were given a single 50 mg dose (~25 mg/kg) either 24 h before (pre-) or 10 h after (post-) NiV challenge. The mAb m102.4 was administered via intravenous catheter, but one animal was given m102.4 intraperitoneally because of a catheter block and control ferrets were given PBS. Ferrets were challenged by oronasal inoculation with a 5,000 TCID50 dose of NiV (ten-fold the minimal infectious dose, 50%). Control ferrets became febrile with signs of clinical illness by day 6 following challenge, and by day 8 were severely depressed with subcutaneous edema of the head and cutaneous hemorrhages and were euthanized. By day 8 one animal in the pregroup and all ferrets in the postgroup were febrile with variable levels of depression and suppression of play activity, but by day 10, fever in some animals started to fall, and these animals had moderate (pregroup) or mild (postgroup) edema of the throat; while one animal in each group remained febrile with no other clinical signs. By day 13, however, two of three ferrets in the pregroup were depressed and inappetant with cutaneous ecchymoses, and one animal had marked hind limb paresis and generalized tremor and both animals were euthanized. However, at day 13 all other ferrets (3/3 in the postgroup and 1/3 pregroup) were well and free of any disease signs and remained so until the end of the study at day 20 postchallenge. Gross and microscopic pathology in control animals revealed severe systemic pathology; however, in the two pregroup animals that experienced a delayed disease course, the frequency of pinpoint hemorrhagic lesions observed in the pulmonary parenchyma was reduced and lesions were much smaller suggesting the disease progression in the respiratory tract had been dampened, consistent with their survival to 13 dpi. There were no significant pathological abnormalities found in any of the surviving ferrets which included 3/3 animals that received a signal dose of m102.4 10 h following NiV challenge (Bossart et al. 2009). Additional testing of m102.4 also confirmed its potent cross-reactive neutralization activity against the NiV-Malaysia (Chua et al. 1999), the original HeV-1994 (Murray et al. 1995), the recent HeV-Redlands (Anonymous 2008), and NiV-Bangladesh isolates (Harcourt et al. 2005). Although this 10 h postexposure treatment scenario is relevant to a known exposure, such as a laboratory accident, the therapeutic window of m102.4 needed further characterization. In addition, the average distribution and elimination halftimes of m102.4 in ferrets were calculated to be 1.48 and 3.53 days; respectively, and although distribution was good, clearance of the human mAb in ferrets was rapid suggesting that more dosing could have had a beneficial effect especially in the pretreated animals. Nevertheless, this study represented the first human mAb therapy successfully evaluated in vivo for prevention of lethal henipavirus infection.
To meet the requirements for potential development and approved use of m102.4 in people, additional in vivo studies are required as well as a second animal model showing efficacy. To accomplish these goals, m102.4 was examined in the recently developed, lethal henipavirus infection model in a nonhuman primate (the AGM) (Geisbert et al. 2010; Rockx et al. 2010; Table 1). This first study focused on the potential real life scenario of requiring mAb therapy as a post-exposure treatment against virus infection and HeV was examined first, building on the prior results obtained with NiV challenge and m102.4 postexposure treatment. Fourteen AGMs were challenged intratracheally with a lethal dose of HeV and 12 animals were infused twice with a 100 mg dose (~20 mg/kg) of m102.4 beginning at either 10 and 24 h or 72 h p.i. and again approximately 48 h later. The presence of viral RNA, infectious virus and HeV-specific immune responses demonstrated that all subjects became infected following challenge; however, all 12 AGMs that received m102.4 survived infection; whereas the untreated control subjects succumbed to severe systemic disease on day 8 following virus challenge. It was noted that animals in the 72 h treatment group exhibited neurological signs of disease, but all these animals also recovered by day 16. In AGMs a distribution halftime of ~1 day and the elimination halftime of ~11 days of mAb m102.4 were calculated.
HeV-specific pathological changes were not observed in any of the m102.4-treated animals, whereas immunohistochemistry analysis revealed high levels of HeV antigen in the lung and brain of the control subjects along with high levels of HeV RNA in the lung, spleen, lymph nodes, and brain. HeV was recovered from numerous tissues, highlighting the extensive dissemination of HeV within the body of control subjects. Blood samples collected over the course of infection were assayed for infectious HeV and HeV RNA, and consistent with previous findings, a gradual rise in viral RNA over time was evident in the control subjects and HeV was isolated from blood of both control subjects. Only very low levels of viral RNA could be detected on days 6 and 10 in some m102.4-treated subjects and all blood samples from all m102.4-treated subjects were negative for HeV isolation. Tissue samples collected from all m102.4- treated subjects on necropsy were assayed for the presence of HeV RNA and infectious virus, and only occasionally, were very low levels of viral RNA detected (spleen, lung, and brain) and predominantly only in the late treatment cohort (72 h/d5). Importantly, no infectious HeV could be recovered from any of the tissues from m102.4-treated subjects. Together, these data demonstrated that mAb m102.4 could prevent widespread HeV dissemination in the challenged subjects. Lung, brain, and spleen tissues from surviving subjects were also assayed for the presence of HeV antigen. All tissue architecture appeared normal and all survivor tissues examined were negative for HeV antigen (Fig. 3), whereas control subject tissues showed significant HeV antigen staining. These results were the first successful postexposure in vivo therapy against HeV and highlight the importance of further developing human mAbs, such as m102.4, to combat emerging viral pathogens like the henipaviruses.
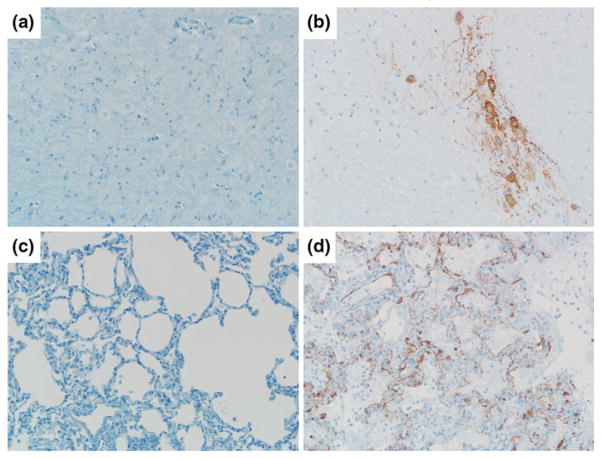
Fig. 3.
Passive Immunotherapy against HeV challenge in the African green monkey. Localization of HeV antigen by immunohistochemical stain in the brain stem (a, b) and lower lung (c, d) of m102.4 treated subject (72 h/d5; left panels) or control subject (right panels). Sections were stained with a NiV nucleoprotein (N) specific rabbit polyclonal antibody and images were obtained at an original magnification of at 20X. Figure is modified and reproduced from original data previously published, with permission (Bossart et al. 2011)
5 Concluding Remarks
NiV and HeV are the only examples of zoonotic paramyxoviruses that are capable of infecting and causing disease with a remarkably broad host range. At present, there are no approved commercially available vaccines or passive immunization therapeutics for preventing or treating henipavirus infection in humans or animals. Because of the risk from natural infection, laboratory accident or the potential of deliberate misuse of HeV and NiV, and the high morbidity and mortality associated with infection, development of effective countermeasures has been a priority. Over the past several years, a focus on developing viable henipavirus animal challenge models has often gone hand-in-hand with targeted research strategies on preventing henipavirus entry by neutralizing antibodies; and together these efforts have led to the development of highly effective active and passive immunization strategies.
At present, there is a cross-reactive human mAb, m102.4, which is capable of neutralizing all known isolates of HeV and NiV. The m102.4 mAb has been demonstrated to be exceptionally efficacious as a postexposure therapy by protecting both ferrets and nonhuman primates from lethal henipavirus disease, even under conditions of high dose virus challenge by oronasal or intratracheal routes; and in monkeys it can afford protection as late as 3 days following infection (Bossart et al. 2009, 2011). This human mAb has already been administered to healthy individuals on a compassionate use basis and no adverse reactions were seen. The m102.4 mAb is presently in preclinical development in both the United States and in Australia. As an active vaccination strategy, a recombinant, subunit vaccine that consists of entire ectodomain of the G glycoprotein of HeV has shown remarkable efficacy and is cross protective against HeV and NiV. HeV-sG vaccination can protect against henipavirus challenge in cats, ferrets, monkeys, and horses. Immunization protocols of only two doses with several adjuvant systems, elicits a cross-reactive neutralizing antibody response that can completely protect from high dose virus challenge, with no evidence of virus replication, or anamnestic humoral response. Further, there is no evidence of virus shedding in vaccinated animals. The HeV-sG subunit vaccine immunogen has been licensed for commercial development as a livestock vaccine and currently being developed in Australia for use in horses. Finally, the HeV-sG subunit vaccine could be further developed for potential use in humans, and preliminary data in nonhuman primates using sG formulations in Alhydrogel™ appear promising.
Acknowledgments
The views expressed in the manuscript are solely those of the authors, and they do not represent official views or opinions of the Department of Defense or The Uniformed Services University of the Health Science. Supported in part by the Department of Health and Human Services, National Institutes of Health, grants AI054715, AI077995, AI082121.
Footnotes
Conflict of Interest C.C.B is a United States federal employee; C.C.B is coinventor on patents relating to human monoclonal antibodies against Hendra and Nipah viruses and C.C.B and K.N.B are coinventors on patents relating to soluble forms of Hendra and Nipah envelope glycoproteins and vaccines; assignees are The United States of America as represented by the Department of Health and Human Services (Washington, DC), and the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (Bethesda, MD). All other authors declare no competing interests.
Contributor Information
Christopher C. Broder, Email: cbroder@usuhs.mil, Department of Microbiology and Immunology, Uniformed Services University, Bethesda, MD 20814, USA
Thomas W. Geisbert, Email: tom.geisbert@utmb.edu, Galveston National Laboratory and Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX 77550, USA
Kai Xu, Email: xuk@mskcc.org, Structural Biology Program, Memorial Sloan-Kettering Cancer Center, New York, NY 10065, USA.
Dimitar B. Nikolov, Email: nikolovd@mskcc.org, Structural Biology Program, Memorial Sloan-Kettering Cancer Center, New York, NY 10065, USA
Lin-Fa Wang, Email: Linfa.Wang@csiro.au, CSIRO Livestock Industries, Australian Animal Health Laboratory, 5 Portarlington Road, Geelong, VIC 3220, Australia.
Deborah Middleton, Email: Deborah.Middleton@csiro.au, CSIRO Livestock Industries, Australian Animal Health Laboratory, 5 Portarlington Road, Geelong, VIC 3220, Australia.
Jackie Pallister, Email: Jackie.Pallister@csiro.au, CSIRO Livestock Industries, Australian Animal Health Laboratory, 5 Portarlington Road, Geelong, VIC 3220, Australia.
Katharine N. Bossart, Email: kbossart@bu.edu, Department of Microbiology, Boston University School of Medicine, Boston, MA 02118, USA. National Emerging Infectious Diseases Laboratories Institute, Boston University School of Medicine, Boston, MA 02118, USA
References
- Aebersold P. FDA experience with medical countermeasures under the animal rule. Adv Prev Med. 2012;2012:507–571. doi: 10.1155/2012/507571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Hendra virus, human, equine. Australia (04), Queensland: Pro-MED-mail, International Society for Infectious Diseases; 2008. Jul 25, archive no. 20080725.2260. Available at www.promedmail.org. [Google Scholar]
- Anonymous. Human, Equine. Australia (04), Queensland Fatal: Pro-MED-mail, International Society for Infectious Diseases; 2009. Sep 3, archive no. 20090903.3098. Available at www.promedmail.org. [Google Scholar]
- Anonymous. Hendra Virus, Equine. Australia (28), Queensland, New South Wales: Pro-MED-mail International Society for Infectious Diseases; 2011. Oct 12, archive no. 20111013.3061. Available at www.promedmail.org. [Google Scholar]
- Anonymous. Hendra virus, equine. Australia, Queensland: Pro-MED-mail International Society for Infectious Diseases; 2012a. Jan 6, archive no. 20120106.1001359. Available at www.promedmail.org. [Google Scholar]
- Anonymous. Nipah encephalitis, human. Bangladesh, Jipurhat: Pro-MED-mail, International Society for Infectious Diseases; 2012b. Feb 7, archive no. 20120212.1040138. Available at www.promedmail.org. [Google Scholar]
- Balzer M. Hendra vaccine success announced. Aust Vet J. 2011;89(7):N2–N3. doi: 10.1111/j.1751-0813.2011.news_v89_i7.x. [DOI] [PubMed] [Google Scholar]
- Bishop KA, Broder CC. Hendra and Nipah: lethal zoonotic paramyxoviruses. In: Scheld WM, Hammer SM, Hughes JM, editors. Emerging infections. American Society for Microbiology; Washington: 2008. pp. 155–187. [Google Scholar]
- Bishop KA, Stantchev TS, Hickey AC, Khetawat D, Bossart KN, Krasnoperov V, Gill P, Feng YR, Wang L, Eaton BT, Wang LF, Broder CC. Identification of hendra virus G glycoprotein residues that are critical for receptor binding. J Virol. 2007;81(11):5893–5901. doi: 10.1128/JVI.02022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaparte MI, Dimitrov AS, Bossart KN, Crameri G, Mungall BA, Bishop KA, Choudhry V, Dimitrov DS, Wang LF, Eaton BT, Broder CC. Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc Natl Acad Sci U S A. 2005;102(30):10652–10657. doi: 10.1073/pnas.0504887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart KN, Crameri G, Dimitrov AS, Mungall BA, Feng YR, Patch JR, Choudhary A, Wang LF, Eaton BT, Broder CC. Receptor binding, fusion inhibition and induction of crossreactive neutralizing antibodies by a soluble G glycoprotein of hendra virus. J Virol. 2005;79(11):6690–6702. doi: 10.1128/JVI.79.11.6690-6702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart KN, McEachern JA, Hickey AC, Choudhry V, Dimitrov DS, Eaton BT, Wang LF. Neutralization assays for differential henipavirus serology using bio-plex protein array systems. J Virol Methods. 2007;142(1–2):29–40. doi: 10.1016/j.jviromet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Bossart KN, Tachedjian M, McEachern JA, Crameri G, Zhu Z, Dimitrov DS, Broder CC, Wang LF. Functional studies of host-specific ephrin-B ligands as henipavirus receptors. Virology. 2008;372(2):357–371. doi: 10.1016/j.virol.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Bossart KN, Zhu Z, Middleton D, Klippel J, Crameri G, Bingham J, McEachern JA, Green D, Hancock TJ, Chan YP, Hickey AC, Dimitrov DS, Wang LF, Broder CC. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute Nipah virus infection. PLoS Pathog. 2009;5(10):e1000642. doi: 10.1371/journal.ppat.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart KN, Geisbert TW, Feldmann H, Zhu Z, Feldmann F, Geisbert JB, Yan L, Feng YR, Brining D, Scott D, Wang Y, Dimitrov AS, Callison J, Chan YP, Hickey AC, Dimitrov DS, Broder CC, Rockx B. A neutralizing human monoclonal antibody protects african green monkeys from hendra virus challenge. Sci Transl Med. 2011;3(105):103–105. doi: 10.1126/scitranslmed.3002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden TA, Aricescu AR, Gilbert RJ, Grimes JM, Jones EY, Stuart DI. Structural basis of Nipah and Hendra virus attachment to their cell-surface receptor ephrin-B2. Nat Struct Mol Biol. 2008;15(6):567–572. doi: 10.1038/nsmb.1435. [DOI] [PubMed] [Google Scholar]
- Bowden TA, Crispin M, Harvey DJ, Jones EY, Stuart DI. Dimeric architecture of the hendra virus attachment glycoprotein: evidence for a conserved mode of assembly. J Virol. 2010;84(12):6208–6217. doi: 10.1128/JVI.00317-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder CC, Earl PL, Long D, Abedon ST, Moss B, Doms RW. Antigenic implications of human immunodeficiency virus type 1 envelope quaternary structure: oligomer-specific and sensitive monoclonal antibodies. Proc Natl Acad Sci U S A. 1994;91(24):11699–11703. doi: 10.1073/pnas.91.24.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol. 2004;2(9):695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- Chong HT, Tan CT. Relapsed and late-onset Nipah encephalitis, a report of three cases. Neurol J Southeast Asia. 2003;8:109–112. [Google Scholar]
- Chua KB, Goh KJ, Wong KT, Kamarulzaman A, Tan PS, Ksiazek TG, Zaki SR, Paul G, Lam SK, Tan CT. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354(9186):1257–1259. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- Chua KB, Lek Koh C, Hooi PS, Wee KF, Khong JH, Chua BH, Chan YP, Lim ME, Lam SK. Isolation of Nipah virus from Malaysian island flying-foxes. Microbes Infect. 2002;4(2):145–151. doi: 10.1016/s1286-4579(01)01522-2. [DOI] [PubMed] [Google Scholar]
- Colgrave ML, Snelling HJ, Shiell BR, Feng YR, Chan YP, Bossart KN, Xu K, Nikolov DB, Broder CC, Michalski WP. Site occupancy and glycan compositional analysis of two soluble recombinant forms of the attachment glycoprotein of hendra virus. Glycobiology. 2012;22(4):572–584. doi: 10.1093/glycob/cwr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford LM. New drug and biological drug products; evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. Office of the Federal Register, National Archives and Records Administration (NARA); Washington: 2002. pp. 37988–37998. [PubMed] [Google Scholar]
- Drexler JF, Corman VM, Gloza-Rausch F, Seebens A, Annan A, Ipsen A, Kruppa T, Muller MA, Kalko EK, Adu-Sarkodie Y, Oppong S, Drosten C. Henipavirus RNA in African bats. PLoS One. 2009;4(7):e6367. doi: 10.1371/journal.pone.0006367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutch RE. Entry and fusion of emerging paramyxoviruses. PLoS Pathog. 2010;6(6):e1000881. doi: 10.1371/journal.ppat.1000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton BT, Broder CC, Middleton D, Wang LF. Hendra and Nipah viruses: different and dangerous. Nat Rev Microbiol. 2006;4(1):23–35. doi: 10.1038/nrmicro1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton BT, Mackenzie JS, Wang L-F. Henipaviruses. In: Knipe DM, Howley PM, editors. Fields virology. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1587–1600. [Google Scholar]
- Field HE, Mackenzie JS, Daszak P. Henipaviruses: emerging paramyxoviruses associated with fruit bats. Curr Top Microbiol Immunol. 2007;315:133–159. doi: 10.1007/978-3-540-70962-6_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H, Schaaf K, Kung N, Simon C, Waltisbuhl D, Hobert H, Moore F, Middleton D, Crook A, Smith G, Daniels P, Glanville R, Lovell D. Hendra virus outbreak with novel clinical features Australia. Emerg Infect Dis. 2010;16(2):338–340. doi: 10.3201/eid1602.090780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM, Yancopoulos GD. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230(2):151–160. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- Geisbert TW, Daddario-DiCaprio KM, Hickey AC, Smith MA, Chan YP, Wang LF, Mattapallil JJ, Geisbert JB, Bossart KN, Broder CC. Development of an acute and highly pathogenic nonhuman primate model of Nipah virus infection. PLoS One. 2010;5(5):e10690. doi: 10.1371/journal.pone.0010690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE. Immune responses during measles virus infection. Curr Top Microbiol Immunol. 1995;191:117–134. doi: 10.1007/978-3-642-78621-1_8. [DOI] [PubMed] [Google Scholar]
- Guillaume V, Contamin H, Loth P, Georges-Courbot MC, Lefeuvre A, Marianneau P, Chua KB, Lam SK, Buckland R, Deubel V, Wild TF. Nipah virus: vaccination and passive protection studies in a hamster model. J Virol. 2004;78(2):834–840. doi: 10.1128/JVI.78.2.834-840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume V, Contamin H, Loth P, Grosjean I, Courbot MC, Deubel V, Buckland R, Wild TF. Antibody prophylaxis and therapy against Nipah virus infection in hamsters. J Virol. 2006;80(4):1972–1978. doi: 10.1128/JVI.80.4.1972-1978.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume V, Wong KT, Looi RY, Georges-Courbot MC, Barrot L, Buckland R, Wild TF, Horvat B. Acute hendra virus infection: analysis of the pathogenesis and passive antibody protection in the hamster model. Virology. 2009;387(2):459–465. doi: 10.1016/j.virol.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Gurley ES, Montgomery JM, Hossain MJ, Bell M, Azad AK, Islam MR, Molla MA, Carroll DS, Ksiazek TG, Rota PA, Lowe L, Comer JA, Rollin P, Czub M, Grolla A, Feldmann H, Luby SP, Woodward JL, Breiman RF. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg Infect Dis. 2007;13(7):1031–1037. doi: 10.3201/eid1307.061128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin K, Young PL, Field HE, Mackenzie JS. Isolation of hendra virus from pteropid bats: a natural reservoir of hendra virus. J Gen Virol. 2000;81(Pt 8):1927–1932. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- Harcourt BH, Lowe L, Tamin A, Liu X, Bankamp B, Bowden N, Rollin PE, Comer JA, Ksiazek TG, Hossain MJ, Gurley ES, Breiman RF, Bellini WJ, Rota PA. Genetic characterization of Nipah virus, Bangladesh 2004. Emerg Infect Dis. 2005;11(10):1594–1597. doi: 10.3201/eid1110.050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman DT, Suu-Ire R, Breed AC, McEachern JA, Wang L, Wood JL, Cunningham AA. Evidence of henipavirus infection in west african fruit bats. PLoS ONE. 2008;3(7):e2739. doi: 10.1371/journal.pone.0002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman DT, Wang LF, Barr J, Baker KS, Suu-Ire R, Broder CC, Cunningham AA, Wood JL. Antibodies to henipavirus or henipa-like viruses in domestic pigs in Ghana, West Africa. PLoS One. 2011;6(9):e25256. doi: 10.1371/journal.pone.0025256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey AC, Bossart KN, Rockx B, Feldmann F, Geisbert JB, Yan L, Feng YR, Feldmann H, Geisbert TW, Broder CC. Vaccination of nonhuman primates with a recombinant soluble henipavirus attachment G glycoprotein protects against lethal Nipah virus challenge. Am Soc Virol. 2011;40–2:195. [Google Scholar]
- Homaira N, Rahman M, Hossain MJ, Epstein JH, Sultana R, Khan MS, Podder G, Nahar K, Ahmed B, Gurley ES, Daszak P, Lipkin WI, Rollin PE, Comer JA, Ksiazek TG, Luby SP. Nipah virus outbreak with person-to-person transmission in a district of Bangladesh 2007. Epidemiol Infect. 2010;138(11):1630–1636. doi: 10.1017/S0950268810000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper PT, Ketterer PJ, Hyatt AD, Russell GM. Lesions of experimental equine morbillivirus pneumonia in horses. Vet Pathol. 1997a;34(4):312–322. doi: 10.1177/030098589703400407. [DOI] [PubMed] [Google Scholar]
- Hooper PT, Westbury HA, Russell GM. The lesions of experimental equine morbillivirus disease in cats and guinea pigs. Vet Pathol. 1997b;34(4):323–329. doi: 10.1177/030098589703400408. [DOI] [PubMed] [Google Scholar]
- Hooper P, Zaki S, Daniels P, Middleton D. Comparative pathology of the diseases caused by hendra and Nipah viruses. Microbes Infect. 2001;3(4):315–322. doi: 10.1016/s1286-4579(01)01385-5. [DOI] [PubMed] [Google Scholar]
- Iehle C, Razafitrimo G, Razainirina J, Andriaholinirina N, Goodman SM, Faure C, Georges-Courbot MC, Rousset D, Reynes JM. Henipavirus and tioman virus antibodies in pteropodid bats. Madagascar. Emerg Infect Dis. 2007;13(1):159–161. doi: 10.3201/eid1301.060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang AS, Jones TM, Burton DR. Antibody redesign by chain shuffling from random combinatorial immunoglobulin libraries. Proc Natl Acad Sci U S A. 1991;88(24):11120–11123. doi: 10.1073/pnas.88.24.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ. Protein structure prediction on the web: a case study using the phyre server. Nat Protoc. 2009;4(3):363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Lackmann M, Boyd AW. Eph, a protein family coming of age: more confusion, insight, or complexity? Sci Signal. 2008;1(15):re2. doi: 10.1126/stke.115re2. [DOI] [PubMed] [Google Scholar]
- Lamb RA, Parks GD. Paramyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields virology. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1449–1496. [Google Scholar]
- Lamb RA, Collins PL, Kolakofsky D, Melero JA, Nagai Y, Oldstone MBA, Pringle CR, Rima BK. Family Paramyxoviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus taxonomy: the classification and nomenclature of viruses. The eighth report of the international committee in taxonomy of viruses. Elsevier Academic Press; San Diego: 2005. pp. 655–668. [Google Scholar]
- Lee B, Ataman ZA. Modes of paramyxovirus fusion: a henipavirus perspective. Trends Microbiol. 2011;19(8):389–399. doi: 10.1016/j.tim.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang J, Hickey AC, Zhang Y, Wu Y, Zhang H, Yuan J, Han Z, McEachern J, Broder CC, Wang LF, Shi Z. Antibodies to Nipah or Nipah-like viruses in bats. China. Emerg Infect Dis. 2008;14(12):1974–1976. doi: 10.3201/eid1412.080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Embury-Hyatt C, Weingartl HM. Experimental inoculation study indicates swine as a potential host for hendra virus. Vet Res. 2010;41(3):33. doi: 10.1051/vetres/2010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby SP, Hossain MJ, Gurley ES, Ahmed BN, Banu S, Khan SU, Homaira N, Rota PA, Rollin PE, Comer JA, Kenah E, Ksiazek TG, Rahman M. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001–2007. Emerg Infect Dis. 2009;15(8):1229–1235. doi: 10.3201/eid1508.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marianneau P, Guillaume V, Wong T, Badmanathan M, Looi RY, Murri S, Loth P, Tordo N, Wild F, Horvat B, Contamin H. Experimental infection of squirrel monkeys with Nipah virus. Emerg Infect Dis. 2010;16(3):507–510. doi: 10.3201/eid1603.091346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh GA, Haining J, Hancock TJ, Robinson R, Foord A, Barr JA, Riddell S, Heine H, White JR, Crameri G, Field HE, Middleton D, Wang LF. Experimental infection of horses with hendra virus/Australia/Horse/2008/Redlands; implications for control of transmission to people and horses. Emerg Infect Dis. 2011;17(12):2232–2238. doi: 10.3201/eid1712.111162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachern JA, Bingham J, Crameri G, Green DJ, Hancock TJ, Middleton D, Feng YR, Broder CC, Wang LF, Bossart KN. Arecombinant subunit vaccine formulation protects against lethal Nipah virus challenge in cats. Vaccine. 2008;26(31):3842–3852. doi: 10.1016/j.vaccine.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton DJ, Westbury HA, Morrissy CJ, van der Heide BM, Russell GM, Braun MA, Hyatt AD. Experimental Nipah virus infection in pigs and cats. J Comp Pathol. 2002;126(2–3):124–136. doi: 10.1053/jcpa.2001.0532. [DOI] [PubMed] [Google Scholar]
- Middleton DJ, Morrissy CJ, van der Heide BM, Russell GM, Braun MA, Westbury HA, Halpin K, Daniels PW. Experimental Nipah Virus Infection in Pteropid Bats (Pteropus poliocephalus) J Comp Pathol. 2007;136(4):266–272. doi: 10.1016/j.jcpa.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Mungall BA, Middleton D, Crameri G, Bingham J, Halpin K, Russell G, Green D, McEachern J, Pritchard LI, Eaton BT, Wang LF, Bossart KN, Broder CC. Feline model of acute Nipah virus infection and protection with a soluble glycoprotein-based subunit vaccine. J Virol. 2006;80(24):12293–12302. doi: 10.1128/JVI.01619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungall BA, Middleton D, Crameri G, Halpin K, Bingham J, Eaton BT, Broder CC. Vertical transmission and fetal replication of Nipah virus in an experimentally infected cat. J Infect Dis. 2007;196(6):812–816. doi: 10.1086/520818. [DOI] [PubMed] [Google Scholar]
- Murray K, Selleck P, Hooper P, Hyatt A, Gould A, Gleeson L, Westbury H, Hiley L, Selvey L, Rodwell B, et al. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268(5207):94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- Murray K, Eaton B, Hooper P, Wang L, Williamson M, Young P. Flying foxes, horses, and humans: a zoonosis caused be a new member of the Paramyxoviridae. In: Scheld WM, Armstrong D, Hughes JM, editors. Emerging infections. ASM Press; Washington: 1998. pp. 43–58. [Google Scholar]
- Negrete OA, Levroney EL, Aguilar HC, Bertolotti-Ciarlet A, Nazarian R, Tajyar S, Lee B. EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature. 2005;436(7049):401–405. doi: 10.1038/nature03838. [DOI] [PubMed] [Google Scholar]
- Negrete OA, Wolf MC, Aguilar HC, Enterlein S, Wang W, Muhlberger E, Su SV, Bertolotti-Ciarlet A, Flick R, Lee B. Two key residues in Ephrinb3 are critical for its use as an alternative receptor for Nipah virus. PLoS Pathog. 2006;2(2):e7. doi: 10.1371/journal.ppat.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan JD, Allworth AM, Paterson DL, Snow TM, Boots R, Gleeson LJ, Gould AR, Hyatt AD, Bradfield J. Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet. 1997;349(9045):93–95. doi: 10.1016/s0140-6736(96)06162-4. [DOI] [PubMed] [Google Scholar]
- Pallister J, Middleton D, Crameri G, Yamada M, Klein R, Hancock TJ, Foord A, Shiell B, Michalski W, Broder CC, Wang LF. Chloroquine administration does not prevent Nipah virus infection and disease in ferrets. J Virol. 2009;83(22):11979–11982. doi: 10.1128/JVI.01847-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallister J, Middleton D, Broder CC, Wang LF. Henipavirus vaccine development. J Bioterr Biodef. 2011a:S1:005. doi: 10.4172/2157-2526.S1-005. [DOI] [Google Scholar]
- Pallister J, Middleton D, Wang LF, Klein R, Haining J, Robinson R, Yamada M, White J, Payne J, Feng YR, Chan YP, Broder CC. A recombinant hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal hendra virus challenge. Vaccine. 2011b;29(34):5623–5630. doi: 10.1016/j.vaccine.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don’t know, what we should know. Nat Med. 2004;10(8):806–810. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133(1):38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10(3):165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford EG, McCall B, Smith G, Slinko V, Allen G, Smith I, Moore F, Taylor C, Kung YH, Field H. Human hendra virus encephalitis associated with equine outbreak, Australia 2008. Emerg Infect Dis. 2010;16(2):219–223. doi: 10.3201/eid1602.090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader C, Barbas CF., 3rd Phage display of combinatorial antibody libraries. Curr Opin Biotechnol. 1997;8(4):503–508. doi: 10.1016/s0958-1669(97)80075-4. [DOI] [PubMed] [Google Scholar]
- Rockx B, Bossart KN, Feldmann F, Geisbert JB, Hickey AC, Brining D, Callison J, Safronetz D, Marzi A, Kercher L, Long D, Broder CC, Feldmann H, Geisbert TW. A novel model of lethal hendra virus infection in African green monkeys and the effectiveness of ribavirin treatment. J Virol. 2010;84(19):9831–9839. doi: 10.1128/JVI.01163-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B, Brining D, Kramer J, Callison J, Ebihara H, Mansfield K, Feldmann H. Clinical outcome of henipavirus infection in hamsters is determined by the route and dose of infection. J Virol. 2011;85(15):7658–7671. doi: 10.1128/JVI.00473-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RJ, Douglas IC, Baldock FC, Glanville RJ, Seppanen KT, Gleeson LJ, Selleck PN, Dunn KJ. Investigation of a second focus of equine morbillivirus infection in coastal Queensland. Aust Vet J. 1996;74(3):243–244. doi: 10.1111/j.1751-0813.1996.tb15413.x. [DOI] [PubMed] [Google Scholar]
- Selvey LA, Wells RM, McCormack JG, Ansford AJ, Murray K, Rogers RJ, Lavercombe PS, Selleck P, Sheridan JW. Infection of humans and horses by a newly described morbillivirus. Med J Aust. 1995;162(12):642–645. doi: 10.5694/j.1326-5377.1995.tb126050.x. [DOI] [PubMed] [Google Scholar]
- Sendow I, Field HE, Curran J, Darminto, Morrissy C, Meehan G, Buick T, Daniels P. Henipavirus in Pteropus vampyrus bats Indonesia. Emerg Infect Dis. 2006;12(4):711–712. doi: 10.3201/eid1204.051181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendow I, Field HE, Curran J, Darminto, Morrissy C, Daniels P. Screening for Nipah virus infection in west kalimantan province Indonesia. Zoonoses Public Health. 2010;57(7–8):499–503. doi: 10.1111/j.1863-2378.2009.01252.x. [DOI] [PubMed] [Google Scholar]
- Smith I, Broos A, de Jong C, Zeddeman A, Smith C, Smith G, Moore F, Barr J, Crameri G, Marsh G, Tachedjian M, Yu M, Kung YH, Wang LF, Field H. Identifying hendra virus diversity in pteropid bats. PLoS One. 2011;6(9):e25275. doi: 10.1371/journal.pone.0025275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101(16):6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CT, Wong KT. Nipah encephalitis outbreak in Malaysia. Ann Acad Med Singapore. 2003;32(1):112–117. [PubMed] [Google Scholar]
- Tan CT, Goh KJ, Wong KT, Sarji SA, Chua KB, Chew NK, Murugasu P, Loh YL, Chong HT, Tan KS, Thayaparan T, Kumar S, Jusoh MR. Relapsed and late-onset Nipah encephalitis. Ann Neurol. 2002;51(6):703–708. doi: 10.1002/ana.10212. [DOI] [PubMed] [Google Scholar]
- Wacharapluesadee S, Lumlertdacha B, Boongird K, Wanghongsa S, Chanhome L, Rollin P, Stockton P, Rupprecht CE, Ksiazek TG, Hemachudha T. Bat Nipah virus Thailand. Emerg Infect Dis. 2005;11(12):1949–1951. doi: 10.3201/eid1112.050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl H, Czub S, Copps J, Berhane Y, Middleton D, Marszal P, Gren J, Smith G, Ganske S, Manning L, Czub M. Invasion of the central nervous system in a porcine host by Nipah virus. J Virol. 2005;79(12):7528–7534. doi: 10.1128/JVI.79.12.7528-7534.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl HM, Berhane Y, Caswell JL, Loosmore S, Audonnet JC, Roth JA, Czub M. Recombinant Nipah virus vaccines protect pigs against challenge. J Virol. 2006;80(16):7929–7938. doi: 10.1128/JVI.00263-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl HM, Berhane Y, Czub M. Animal models of henipavirus infection: a review. Vet J. 2009;181(3):211–220. doi: 10.1016/j.tvjl.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Westbury HA, Hooper PT, Selleck PW, Murray PK. Equine morbillivirus pneumonia: susceptibility of laboratory animals to the virus. Aust Vet J. 1995;72(7):278–279. doi: 10.1111/j.1751-0813.1995.tb03549.x. [DOI] [PubMed] [Google Scholar]
- Westbury HA, Hooper PT, Brouwer SL, Selleck PW. Susceptibility of cats to equine morbillivirus. Aust Vet J. 1996;74(2):132–134. doi: 10.1111/j.1751-0813.1996.tb14813.x. [DOI] [PubMed] [Google Scholar]
- White JR, Boyd V, Crameri GS, Duch CJ, van Laar RK, Wang LF, Eaton BT. Location of, immunogenicity of and relationships between neutralization epitopes on the attachment protein (G) of hendra virus. J Gen Virol. 2005;86(Pt 10):2839–2848. doi: 10.1099/vir.0.81218-0. [DOI] [PubMed] [Google Scholar]
- Wiley DC, Skehel JJ. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Ann Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Williamson MM, Hooper PT, Selleck PW, Gleeson LJ, Daniels PW, Westbury HA, Murray PK. Transmission studies of hendra virus (equine morbillivirus) in fruit bats, horses and cats. Aust Vet J. 1998;76(12):813–818. doi: 10.1111/j.1751-0813.1998.tb12335.x. [DOI] [PubMed] [Google Scholar]
- Wong KT. Emerging epidemic viral encephalitides with a special focus on henipaviruses. Acta Neuropathol. 2010;120(3):317–325. doi: 10.1007/s00401-010-0720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KT, Shieh WJ, Kumar S, Norain K, Abdullah W, Guarner J, Goldsmith CS, Chua KB, Lam SK, Tan CT, Goh KJ, Chong HT, Jusoh R, Rollin PE, Ksiazek TG, Zaki SR. Nipah virus infection: pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am J Pathol. 2002;161(6):2153–2167. doi: 10.1016/S0002-9440(10)64493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KT, Grosjean I, Brisson C, Blanquier B, Fevre-Montange M, Bernard A, Loth P, Georges-Courbot MC, Chevallier M, Akaoka H, Marianneau P, Lam SK, Wild TF, Deubel V. A golden hamster model for human acute Nipah virus infection. Am J Pathol. 2003;163(5):2127–2137. doi: 10.1016/S0002-9440(10)63569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KT, Robertson T, Ong BB, Chong JW, Yaiw KC, Wang LF, Ansford AJ, Tannenberg A. Human hendra virus infection causes acute and relapsing encephalitis. Neuropathol Appl Neurobiol. 2009;35(3):296–305. doi: 10.1111/j.1365-2990.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- Wright A, Shin SU, Morrison SL. Genetically engineered antibodies: progress and prospects. Crit Rev Immunol. 1992;12(3–4):125–168. [PubMed] [Google Scholar]
- Xu K, Rajashankar KR, Chan YP, Himanen JP, Broder CC, Nikolov DB. Host cell recognition by the henipaviruses: crystal structures of the Nipah G attachment glycoprotein and its complex with ephrin-B3. Proc Natl Acad Sci U S A. 2008;105(29):9953–9958. doi: 10.1073/pnas.0804797105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Khetawat D, Rajashankar KR, Chan YP, Kolev MV, Broder CC, Nikolov DB. Crystal structures of the hendra virus G glycoprotein and its complex with ephrin-B2 reveal new insights into the virus attachment and entry process submitted. 2012 doi: 10.1371/journal.pone.0048742. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yob JM, Field H, Rashdi AM, Morrissy C, van der Heide B, Rota P, bin Adzhar A, White J, Daniels P, Jamaluddin A, Ksiazek T. Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emerg Infect Dis. 2001;7(3):439–441. doi: 10.3201/eid0703.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Swanson KA, Leser GP, Paterson RG, Lamb RA, Jardetzky TS. Structure of the newcastle disease virus hemagglutinin-neuraminidase (HN) ectodomain reveals a four-helix bundle stalk. Proc Natl Acad Sci U S A. 2011;108(36):14920–14925. doi: 10.1073/pnas.1111691108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Dimitrov AS, Bossart KN, Crameri G, Bishop KA, Choudhry V, Mungall BA, Feng YR, Choudhary A, Zhang MY, Feng Y, Wang LF, Xiao X, Eaton BT, Broder CC, Dimitrov DS. Potent neutralization of Hendra and Nipah viruses by human monoclonal antibodies. J Virol. 2006;80(2):891–899. doi: 10.1128/JVI.80.2.891-899.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Bossart KN, Bishop KA, Crameri G, Dimitrov AS, McEachern JA, Feng Y, Middleton D, Wang LF, Broder CC, Dimitrov DS. Exceptionally potent cross-reactive neutralization of Nipah and Hendra viruses by a human monoclonal antibody. J Infect Dis. 2008;197(6):846–853. doi: 10.1086/528801. [DOI] [PMC free article] [PubMed] [Google Scholar]