Abstract
Cbl-b is a member of the Cbl family of RING finger E3 ubiquitin ligases and polymorphisms and mutations in Cbl-b are associated with several autoimmune/inflammatory diseases in humans. Furthermore, gene targeting experiments in mice have provided proof of the in vivo effects of Cbl-b on T cell function and its involvement with these diseases. This brief review updates our understanding of Cbl-b in T cell tolerance, proallergic T cell development, and cancer immunity in light of the most recent advances, and their impact on autoimmune-/inflammatory diseases and cancer immunotherapy.
Keywords: Cbl-b, T cell tolerance, autoimmunity, proallergic T cells, asthma, NK cells, cancer
Introduction
Protein ubiquitination is one of fundamental regulatory post-translational modifications regulating intracellular signaling and plays potent roles in regulating a variety of signals in both innate and adaptive immune cells. The selectivity of the ubiquitin-26 S proteasome system for a particular substrate protein relies on the interaction between an E2 ubiquitin-conjugating enzyme and an E3 ubiquitin ligase. The unique feature of E3 ubiquitin ligases is to recognize substrate proteins and target them for ubiquitination [1]. The E3 ubiquitin ligase Cbl-b is a member of the highly conserved family of the Cbl (Casitas B lineage Lymphoma) proteins. They function as E3 ubiquitin ligases via their RING finger domain and as adaptor proteins via various protein-protein interaction domains [2]. Importantly Cbl-b is the only member of the Cbl family which has been documented to be crucial for T cell tolerance. In this brief review, we summarize the major work that we and others have carried out investigating Cbl-b in T cell tolerance and autoimmunity, proallergic T cell development, and cancer immunity.
Cbl-b in T cell tolerance and Autoimmunity
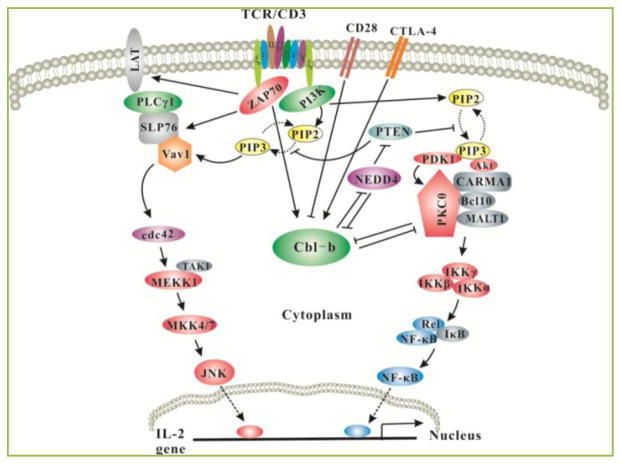
The genetic proof of the in vivo biological function of Cbl-b comes from gene targeting experiments in mice. Drs. Hua Gu and Penninger groups generated Cblb−/− mice independently in 2000. Both groups found that Cblb−/− mice are highly susceptible to autoimmunity [3, 4], suggests that Cbl-b plays a crucial in T cell tolerance induction. Consistent with this notion, we demonstrated that CD28 costimulation potentiates Cbl-b self-ubiquitination and degradation, whereas CTLA-4-B7 interaction induces Cbl-b expression [5, 6]. As both CD28 and CTLA-4 are critical for peripheral T cell tolerance, our findings strongly support that Cbl-b is one of the key molecules involved in T cell tolerance induction. In support of this, Cbl-b has been shown to be required for T cell anergy induction in vitro and in vivo [7, 8]. Cbl-b was suggested to target PLC-γ1 and PKC-θ for ubiquitination in anergic T cells [7, 8], although it is unclear how Cbl-b specifically induces PLC-γ1 and PKC-θ ubiquitination in anergic T cells since Cbl-b associates with PKC-θ and possibly PLC-γ1 in naïve T cells upon TCR/CD28 stimulation [9, 10]. Interestingly, Cbl-b appears to be important for the conversion of naïve CD4+CD25− T cells into CD4+Foxp3+ regulatory T cells induced by TGF-β in vitro, and for the peripheral generation of CD4+Foxp3+ regulatory T cells [11–13], a process that is regulated by the T cell activation threshold via an Akt-2-dependent mechanism [13]. In keeping with this observation, we found that Cblb−/− T cells display heightened Pten inactivation, and hyper-activation of Akt [14]. Therefore, Cbl-b regulates T cell tolerance via multiple mechanisms (Fig. 1). In support of the importance of Cbl-b in maintaining T cell tolerance, Cblb−/− mice are highly susceptible to experimental autoimmune encephalomyelitis (EAE), collagen-induced arthritis (CIA), and autoimmune diabetes [4, 8, 15]. These data are further supported by the evidence that Cbl-b polymorphisms or mutations have been identified in patients with multiple sclerosis and lupus [16, 17], although the cellular and molecular mechanisms for Cbl-b in the development of these diseases remain to be determined.
Figure 1. Model of Cbl-b in T-cell activation and tolerance.
Upon TCR stimulation, Pten is inactivated via Nedd4 which targets Pten for K63-linked polyubiquitination, and this process is inhibited by Cbl-b. Inactivation of Pten leads to the accumulation of PIP3 which recruits PDK-1, Vav-1 and Akt to the plasma membrane via its interaction with the PH domains within these molecules. Therefore, Vav1 links PKC-θ to PDK-1, the former coupling IKKs to the CBM complex. Activated Akt also facilitates the formation of the CBM complex possibly by phosphorylating CARMA1. Thus, Cbl-b inhibits NF-κB activation via PKC-and Akt. One of the important outcomes for Akt activation is that Akt-2 can phosphorylate Foxo1/3a which excludes them from the nucleus, thus inhibiting Foxp3 expression (not shown). In anergic T cells, Cbl-b targets PLC-γ1 and PKC-θ for ubiquitination, thus inhibiting T cell anergy induction. The expression of Cbl-b in T cells is controlled by CD28 and CTLA-4. CD28 costimulation induces Cbl-b ubiquitination and proteasomal degradation, while CTLA-4-B7 interaction induces Cbl-b expression.
As mice lacking Cbl-b are highly susceptible to Th17-mediated autoimmune diseases such as EAE and CIA, it was thought that Cbl-b might directly regulate Th17 cell differentiation. However, our recent study suggests that Cbl-b deficiency does not lead to a biased Th17 cell differentiation program [18]. Therefore, the increased Th17 responses observed during EAE developpment may not result from T cell-intrinsic loss of Cbl-b, but rather from the loss of Cbl-b in innate immune cells. It is envisioned that Cbl-b deficiency in innate immune cells, such as dendritic cells and macrophages, leads to heightened production of pro-inflammatory cytokines which promote Th17 cell development. This notion is supported by evidence that Cbl-b deficiency results in increased pro-inflammatory cytokine production by splenocytes upon LPS stimulation [19]. Therefore, it is important to use cell-type-specific Cblb knockout strain to dissect the role of Cbl-b in T cells vs. non-T cells.
Cbl-b in proallergic T cell development and allergic airway inflammation
The finding that Cbl-b was not required for Th17 cell differentiation was surprising. Using adoptive transfer of naive CD4+ T cells from wild type (WT) and Cblb−/− mice into Rag1−/− recipients, followed by immunization with cardiac myosin peptide in Complete Freund’s adjuvant (CFA), we found that T cell-intrinsic loss of Cbl-b may not account for heightened myocarditis (our unpublished data) in a mouse model which has been shown to be mediated by Th17 [20, 21].
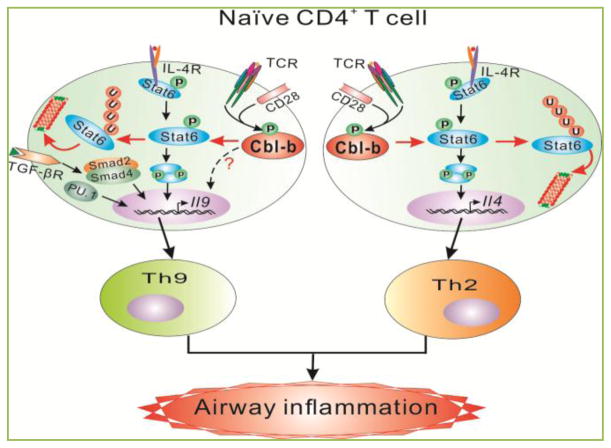
To further understanding the role of Cbl-b in Th cell differentiation, we performed extensive in vitro Th cell differentiation assays. Interestingly, although the in vitro generation of Th1 and Th17 cells are comparable between WT and Cblb−/−T cells, Th2 and Th9 cell differentiation is significantly higher in Cblb−/− mice compared to WT controls [18], suggesting that Cbl-b inhibits Th2 and Th9 cell differentiation. To demonstrate the in vivo biological relevance of this observation we utilized a well-established mouse model of allergic asthma which has been shown to be mediated by both Th2 and Th9 [22]. As expected, mice deficient for Cbl-b display severe airway inflammation accompanied with heightened Th2 and Th9 cytokines in branchoalveolar lavage fluid (BAL), and increased IgE in the serum [18]. These data strongly support that Cbl-b suppresses proallergic Th2 and Th9 development and allergic airway inflammation. At the molecular levels Cbl-b selectively binds to Stat6, a transcription factor which is critical for the development of both Th2 and Th9, and targets it for polyubiquitination (at K108 and K398) and subsequent degradation in the proteasome. Introducing Stat6 deficiency into Cblb−/− background completely abrogates Th2 responses, and greatly attenuates Th9 responses [18]. These data suggest that Cbl-b inhibits Th2 responses in a Stat6-dependent manner, while suppresses Th9 via both Stat-6-dependent and –independent mechanisms (Fig. 2). In addition, impaired inducible regulatory T cell development in the absence of Cbl-b may also account for this aberrant Th2 response in a mouse model of asthma [13]. In support of a crucial role of Cbl-b in allergic asthma, a Cblb D454A mutation has been identified as an asthma susceptibility gene variant in children [23].
Figure 2. Model of Cbl-b in proallergic T cell development and allergic asthma.
Upon stimulation with TCR/CD28 and IL-4, Cbl-b binds to Stat6 via its tyrosine residues and TKB domain with SH2 domain and phospho-tyrosine of Stat6, respectively. These interactions allow Cbl-b to induce Stat6 polyubiquitination at K108 and K398, which leads to the proteasome-mediated degradation. In the absence of Cbl-b, Stat6 ubuiquitination and degradation is impaired, which results in heightened Th2 and Th9 responses and allergic airway inflammation. Introducing Stat6 deficiency into Cblb−/− mice abrogates hyper-Th2 responses but only partially attenuates Th9 responses, suggesting that Cbl-b regulates Th2 cell differentiation via a Stat6-dependent mechanism but regulates Th9 cell differentiation via both Stat6-dependent and -independent mechanisms.
Cbl-b in anti-tumor immunotherapy
The enhanced T cell responses in the absence of Cbl-b prompted scientists to determine whether Cbl-b regulates anti-tumor immune responses. Deletion of Cbl-b confers spontaneous in vivo rejection of tumor cells that express human papilloma virus antigens [24]. Introduction of the Cbl-b deficiency into tumor-prone ataxia telangiectasia mutated–deficient mice markedly reduces the incidence of spontaneous thymic lymphomas [25]. Moreover, Cblb−/− mice develop significantly fewer ultraviolet B (UVB)- induced skin malignancies and reject UVB-induced skin tumors [24]. Cblb−/− mice also reject transplanted E.G7 and EL4 lymphomas [25]. The enhanced anti-tumor activity in the absence of Cbl-b is likely mediated by hyper- activation of CD8+ T cells which are resistant to the regulation by regulatory T cells or TGF-β [24, 25]. In support of these findings, abrogating Cbl-b in effector CD8+ T cells improves the efficacy of adoptive therapy of leukemia in mice [26].
Subcutaneous implantation of TC1 tumor cells, as well as induction of spontaneous tumors by UV irradiation, leads to a significantly delayed outgrowth of tumors in Cblb−/− mice when compared to WT mice [24]. Interestingly, subcutaneous injection of TC-1 tumor cells into Cblb−/−Rag2−/− mice, which lack T and B cells, causes a significant delay in tumor growth compared to Cblb+/+Rag2−/− littermates [27], suggesting that innate cells are involved in this process. Further analysis revealed that inactivation or deletion of Cbl-b licenses the natural killer (NK) NK cells to spontaneously reject metastatic tumors. The heightened anti-tumor activity by NK cells lacking Cbl-b is mediated by the TAM tyrosine kinase receptors Tyro3, Axl, and Mer. Treatment of WT NK cells with a newly-developed small molecule TAM kinase inhibitor confers therapeutic potential, efficiently enhancing anti-metastatic NK cell activity in vivo [27]. Therefore, Cbl-b appears to be a therapeutic target for cancer immunotherapy.
Conclusions
During the last 14 years, significant progress has been made towards our understanding of E3 ubiquitin ligase Cbl-b in T cell tolerance, autoimmunity, proallergic T cell development and allergic asthma, as well as cancer immunotherapy. These studies collectively indicate that Cbl-b is an excellent drug target for autoimmune disease, allergic asthma, and cancers. Future studies will be required to identify small molecules that could modify the enzymatic function of Cbl-b.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) R01 AI090901 to J.Z.
Footnotes
Conflict of Interests
The authors declare that there is no conflict of interests.
References
- 1.Malynn BA, Ma A. Ubiquitin makes its mark on immune regulation. Immunity. 2010;33:843–852. doi: 10.1016/j.immuni.2010.12.007. http://dx.doi.org/10.1016/j.immuni.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thien CB, Langdon WY. c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem J. 2005;391:153–166. doi: 10.1042/BJ20050892. http://dx.doi.org/10.1042/BJ20050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. http://dx.doi.org/10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 4.Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, et al. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. http://dx.doi.org/10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Bardos T, Li D, Gal I, Vermes C, Xu J, et al. Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J Immunol. 2002;169:2236–2240. doi: 10.4049/jimmunol.169.5.2236. http://dx.doi.org/10.4049/jimmunol.169.5.2236. [DOI] [PubMed] [Google Scholar]
- 6.Li D, Gal I, Vermes C, Alegre ML, Chong AS, Chen L, et al. Cutting edge: Cbl-b: one of the key molecules tuning CD28- and CTLA-4-mediated T cell costimulation. J Immunol. 2004;173:7135–7139. doi: 10.4049/jimmunol.173.12.7135. http://dx.doi.org/10.4049/jimmunol.173.12.7135. [DOI] [PubMed] [Google Scholar]
- 7.Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. http://dx.doi.org/10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 8.Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, et al. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. http://dx.doi.org/10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Qiao G, Li Z, Molinero L, Alegre ML, Ying H, Sun Z, et al. T-cell receptor-induced NF-kappaB activation is negatively regulated by E3 ubiquitin ligase Cbl-b. Mol Cell Biol. 2008;28:2470–2480. doi: 10.1128/MCB.01505-07. http://dx.doi.org/10.1128/MCB.01505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruber T, Hermann-Kleiter N, Hinterleitner R, Fresser F, Schneider R, Gastl G, et al. PKC-theta modulates the strength of T cell responses by targeting Cbl-b for ubiquitination and degradation. Sci Signal. 2009;2:ra30. doi: 10.1126/scisignal.2000046. http://dx.doi.org/10.1126/scisignal.2000046. [DOI] [PubMed] [Google Scholar]
- 11.Wohlfert EA, Gorelik L, Mittler R, Flavell RA, Clark RB. Cutting edge: deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-beta sensitivity in vitro and in vivo. J Immunol. 2006;176:1316–1320. doi: 10.4049/jimmunol.176.3.1316. http://dx.doi.org/10.4049/jimmunol.176.3.1316. [DOI] [PubMed] [Google Scholar]
- 12.Harada Y, Harada Y, Elly C, Ying G, Paik JH, DePinho RA, et al. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med. 2010;207:1381–1391. doi: 10.1084/jem.20100004. http://dx.doi.org/10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiao G, Zhao Y, Li Z, Tang PQ, Langdon WY, Yang T, et al. T cell activation threshold regulated by E3 ubiquitin ligase Cbl-b determines fate of inducible regulatory T cells. J Immunol. 2013;191:632–639. doi: 10.4049/jimmunol.1202068. http://dx.doi.org/10.4049/jimmunol.1202068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo H, Qiao G, Ying H, Li Z, Zhao Y, Liang Y, et al. E3 ubiquitin ligase Cbl-b regulates Pten via Nedd4 in T cells independently of its ubiquitin ligase activity. Cell Rep. 2012;1:472–482. doi: 10.1016/j.celrep.2012.04.008. http://dx.doi.org/10.1016/j.celrep.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gronski MA, Boulter JM, Moskophidis D, Nguyen LT, Holmberg K, Elford AR, et al. TCR affinity and negative regulation limit autoimmunity. Nat Med. 2004;10:1234–1239. doi: 10.1038/nm1114. http://dx.doi.org/10.1038/nm1114. [DOI] [PubMed] [Google Scholar]
- 16.Sanna S, Pitzalis M, Zoledziewska M, Zara I, Sidore C, Murru R, et al. Variants within the immunoregulatory CBLB gene are associated with multiple sclerosis. Nat Genet. 2010;42:495–497. doi: 10.1038/ng.584. http://dx.doi.org/10.1038/ng.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doniz-Padilla L, Martinez-Jimenez V, Nino-Moreno P, Abud-Mendoza C, Hernandez-Castro B, Gonzalez-Amaro R, et al. Expression and function of Cbl-b in T cells from patients with systemic lupus erythematosus, and detection of the 2126 A/G Cblb gene polymorphism in the Mexican mestizo population. Lupus. 2011;20:628–635. doi: 10.1177/0961203310394896. http://dx.doi.org/10.1177/0961203310394896. [DOI] [PubMed] [Google Scholar]
- 18.Qiao G, Ying H, Zhao Y, Liang Y, Guo H, Shen H, et al. E3 Ubiquitin Ligase Cbl-b Suppresses Proallergic T Cell Development and Allergic Airway Inflammation. Cell Rep. 2014;6:709–723. doi: 10.1016/j.celrep.2014.01.012. http://dx.doi.org/10.1016/j.celrep.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachmaier K, Toya S, Gao X, Triantafillou T, Garrean S, Park GY, et al. E3 ubiquitin ligase Cblb regulates the acute inflammatory response underlying lung injury. Nat Med. 2007;13:920–926. doi: 10.1038/nm1607. http://dx.doi.org/10.1038/nm1607. [DOI] [PubMed] [Google Scholar]
- 20.Ying H, Yang L, Qiao G, Li Z, Zhang L, Yin F, et al. Cutting Edge: CTLA-4-B7 Interaction Suppresses Th17 Cell Differentiation. J Immunol. 2010;185:1375–1378. doi: 10.4049/jimmunol.0903369. http://dx.doi.org/10.4049/jimmunol.0903369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. http://dx.doi.org/10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan MH. Th9 cells: differentiation and disease. Immunol Rev. 2013;252:104–115. doi: 10.1111/imr.12028. http://dx.doi.org/10.1111/imr.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeWan AT, Egan KB, Hellenbrand K, Sorrentino K, Pizzoferrato N, Walsh KM, et al. Whole-exome sequencing of a pedigree segregating asthma. BMC Med Genet. 2012;13:95. doi: 10.1186/1471-2350-13-95. http://dx.doi.org/10.1186/1471-2350-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeser S, Loser K, Bijker MS, Rangachari M, van der Burg SH, Wada T, et al. Spontaneous tumor rejection by cbl-b-deficient CD8+ T cells. J Exp Med. 2007;204:879–891. doi: 10.1084/jem.20061699. http://dx.doi.org/10.1084/jem.20061699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang JY, Jang IK, Hodes R, Gu H. Ablation of Cbl-b provides protection against transplanted and spontaneous tumors. J Clin Invest. 2007;117:1029–1036. doi: 10.1172/JCI29472. http://dx.doi.org/10.1172/JCI29472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stromnes IM, Blattman JN, Tan X, Jeevanjee S, Gu H, Greenberg PD. Abrogating Cbl-b in effector CD8+ T cells improves the efficacy of adoptive therapy of leukemia in mice. J Clin Invest. 2010;120:3722–3734. doi: 10.1172/JCI41991. http://dx.doi.org/10.1172/JCI41991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paolino M, Choidas A, Wallner S, Pranjic B, Uribesalgo I, Loeser S, et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014;507:508–512. doi: 10.1038/nature12998. http://dx.doi.org/10.1038/nature12998. [DOI] [PMC free article] [PubMed] [Google Scholar]