Abstract
A high-fat (HF) diet inducing hyperlipidemia has been associated with the pathophysiology of major diseases, such as atherosclerosis and osteoporosis. A HF diet has significant adverse effects on bone, including lower bone density, volume and strength. Statins, drugs that lower serum cholesterol levels have beneficial effects on bone metabolism. Since host’s bone quantity, quality and healing potential play a crucial role in osseointegration of dental implants, we hypothesized that hyperlipidemia may negatively affect implant osseointegration. In the present study, we evaluated the effects of hyperlipidemia on implant osseointegration in mice. Atherosclerosis susceptible C57BL/6J male mice were randomly placed on a control chow or an HF diet. After 12 weeks on the diet, each mouse received a titanium implant in the proximal metaphysis of the femur. The animals were sacrificed at 4 or 8 weeks after the implant surgery. Results showed that the HF diet fed mice had significantly increased implant loss as well as decreased formation and strength of bone-to-implant interface. These results support the hypothesis that an HF diet can significantly compromise osseointegration, causing poor outcome in dental implant therapy.
Keywords: high-fat diet, hyperlipidemia, dental implant, osseointegration
INTRODUCTION
The atherogenic or high-fat (HF) diet induces hyperlipidemia, characterized by an elevation of lipids in the bloodstream. Hyperlipidemia is widespread in our society, with total cholesterol levels above 200 mg/ml for over 45.0% of people 20 years or older(Kuklina, Yoon et al. 2009). HF diet is associated with the pathophysiology of major diseases, including atherosclerosis and osteoporosis(Tintut, Morony et al. 2004; Corwin, Hartman et al. 2006; Huang, Morony et al. 2007). Interestingly, both hyperlipidemia and atherosclerosis have been linked to periodontal disease(Lowe 2001; Solomon, Avorn et al. 2005; Fentoglu and Bozkurt 2008).
A HF diet has significant adverse effects on bone health, leading to lower bone mineral density and to higher risk of osteoporosis and bone fracture(Corwin 2003; Pirih, Lu et al. 2012). Statins, HMG-CoA reductase inhibitors that lower cholesterol levels, have beneficial effects on bone metabolism by inducing bone formation and mineral density as well as decreasing the risk of hip fractures(Mundy, Garrett et al. 1999; Edwards, Hart et al. 2000; Meier, Schlienger et al. 2000; Montagnani, Gonnelli et al. 2003). At the cellular level, osteoblasts are capable of oxidizing low-density lipoproteins, possibly increasing the local concentration of oxidized reactive products in bone milieu(Brodeur, Brissette et al. 2008). Oxidative stress generated in hyperlipidemic conditions inhibits the differentiation of bone cells(Mody, Parhami et al. 2001; Almeida, Ambrogini et al. 2009). Statins enhance osteoblastic differentiation and mineralization(Maeda, Matsunuma et al. 2001) and suppress osteoclastogenesis(Ayukawa, Yasukawa et al. 2009). Furthermore, around implants, statins increase osteogenesis, suppress osteoclast formation and increase bone volume(Moriyama, Ayukawa et al. 2010).
The effects of hyperlipidemia on bone health may also interfere with dental implant therapy since the host’s bone quantity, quality and healing potential play an important role in osseointegration(Fedele, Sabbah et al. 2011; Gaetti-Jardim, Santiago-Junior et al. 2011; Olivares-Navarrete, Raines et al. 2012). Currently, the role of hyperlipidemia in implant osseointegration is unknown. Because of the deleterious effects of hyperlipidemia in bone, we hypothesized that hyperlipidemia negatively affects implant osseointegration. The present study evaluates implant osseointegration in hyperlipidemic mice at four and eight weeks after implant placement.
MATERIALS & METHODS
Mice and diets
Four-week-old C57BL/6J male mice (atherosclerosis susceptible strain, The Jackson Laboratories, Bar Harbor ME) were randomly divided into two groups: control chow diet and an atherogenic HF diet (TD 90221, Harlan Teklad, Madion, WI; including 1.25% cholesterol, 15.8% fat and 0.5% cholate)(Parhami, Tintut et al. 2001; Sage, Lu et al. 2011; Pirih, Lu et al. 2012). Animals were started on the diet 12 weeks prior to the implant placement (figure 1A). The experimental protocols were reviewed and approved by the Chancellor’s Animal Research Committee of UCLA.
Figure 1.
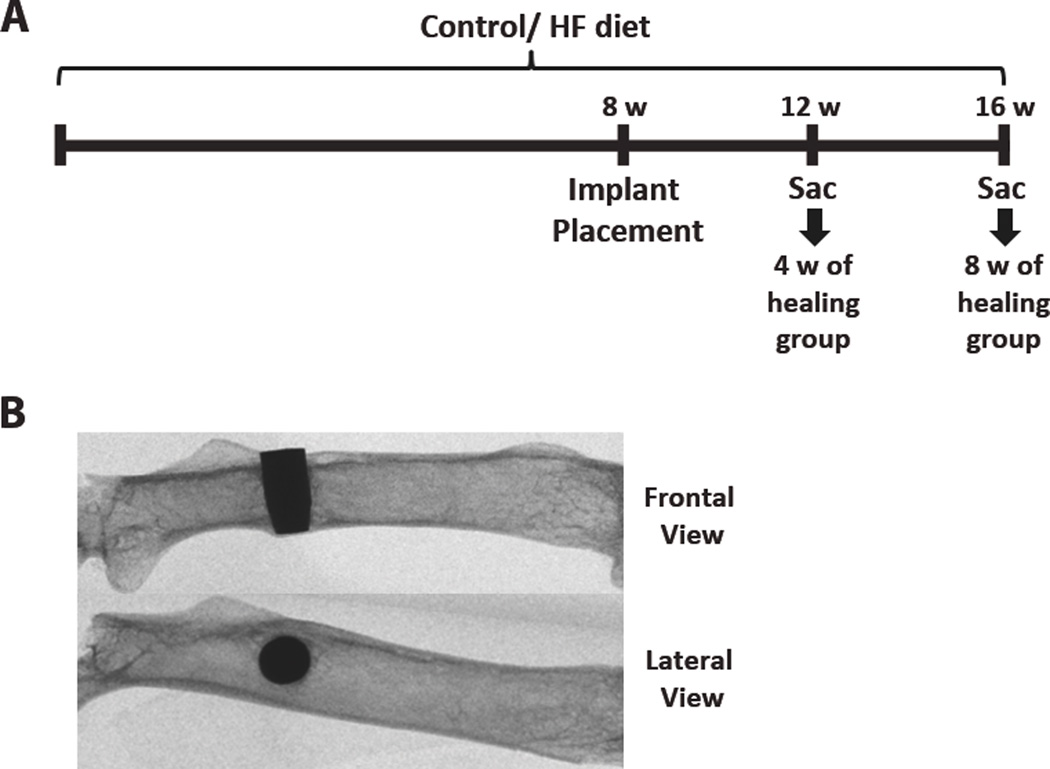
(A) Schematic diagram of the timeline for the diet and implant placement. HF = high-fat; W = weeks; Sac = sacrifice. (B) X-ray images showing the implant placement location (distal to the 3rd trochanter) on a frontal and lateral view.
Implant placement
Under general gas anesthesia, an incision in the lateral aspect of the femur was created, and a pilot hole was made using a 1/2 mm round carbide burr with high speed. The sterile custom-made cylindrical smooth-type tapered titanium implants (1 × 2 mm) were placed by push-fit in the diaphysis of the left femur, perpendicular to its long axis (bicortically) (figure 1B), and the wound was sutured. Animals were given antibiotics (sulfamethoxazole and trimethoprim oral suspension, USP; 850 μg/170 μg per mL) for 7 days. The animals were then sacrificed at 4 or 8 weeks after implant placement (Figure 1A).
Serum analysis
Blood was collected by heart puncture, and serum was sent to the UCLA Division of Laboratory Animal Medicine to analyze levels of cholesterol, triglyceride and glucose.
Evaluation of implant osseointegration
At necropsy, femurs were procured and fixed for 48 hours in 10% formalin. The femurs were scanned by micro-computed tomography (μCT; SkyScan 1172; Kontich, Belgium)(Aghaloo, Kang et al. 2011) .The entire implant site was selected as the region-of-interest (ROI).
All quantitative measurements were performed on the midsection of the implant, and analysis was conducted on its proximal, distal, lateral, and medial sides. The bone-to-implant contact (BIC) was considered the sum of all regions of contact between bone (two coronal and two sagittal) and implant (μm)/whole implant length from the first to the last BIC (μm)×100, and measured using the Dolphin 11.0 Imaging software (Chatsworth, CA, USA).
Implant push-in test
The implant push-in test was performed as described by Ogawa et al (Ogawa, Ozawa et al. 2000). In brief, femurs were embedded in autopolymerizing resin in a custom made mold, in which the bottom flat surface was parallel to the top surface of the implant. The Instron machine (Instron 5544 Electro-mechanical Testing System, Instron, Canton, MA, USA) contained a steel-pushing rod (0.8mm diameter) that applied a load on the implant at a speed of 1 mm/min while simultaneously recording values. The push-in value was determined as the breakpoint load, which is the maximum load prior to a drop in the load-displacement curve (Ogawa, Ozawa et al. 2000).
Statistical analysis
Two-tailed Student’s t-test was performed to compare the effects of the chow diet and the HF diet groups. Results are presented as the mean ± SEM of measurements, considering statistically significant values of p < 0.05.
RESULTS
High fat diet
Serum levels of total cholesterol, triglycerides and glucose were measured. The HF diet significantly increased the total cholesterol levels 2-fold at both time points (p<0.0001) (Table 1). The HF diet had an opposite effect in the triglycerides levels, which was significantly higher in the chow diet (p<0.001) (Table 1). Glucose was also higher in the chow diet, but only in the 4-week group (p<0.05) (Table 1).
Table 1.
Effects of the HF diet on serum cholesterol (n ≥ 9/group), triglycerides (n ≥ 8/group) and glucose (n ≥ 8/group).
| 4 weeks | 8 weks | |||
|---|---|---|---|---|
| (mg/dl) | Control Diet | HF Diet | Control Diet | HF Diet |
| Cholesterol | 102.67±3.97 | 206.30±14.00**** | 113.33±3.27 | 223.70±10.71**** |
| Triglycerides | 150.25±13.15 | 64.00±6.17**** | 142.38±15.23 | 71.40±9.89*** |
| Glucose | 299.20±30.79 | 210.00±14.02* | 230.78±8.05 | 244.20±22.43 |
Values are mean ± SEM. Significant difference when compared to respective control:
p<0.05,
p<0.01,
p<0.001,
p<0.0001.
Hyperlipidemia and Osseointegration
Timeline of the diet and implant placement as well as location of the implant are shown in Figure 1. After 4 or 8 weeks of healing, mice were euthanized and scanned using micro-CT to determine the remaining percentage of osseointegrated implants. The percentage of implant survival was The percentage of implants lost was greater in the HF groups compared to their respective control groups (chow diet) at both time points (Figure 2A).
Figure 2.
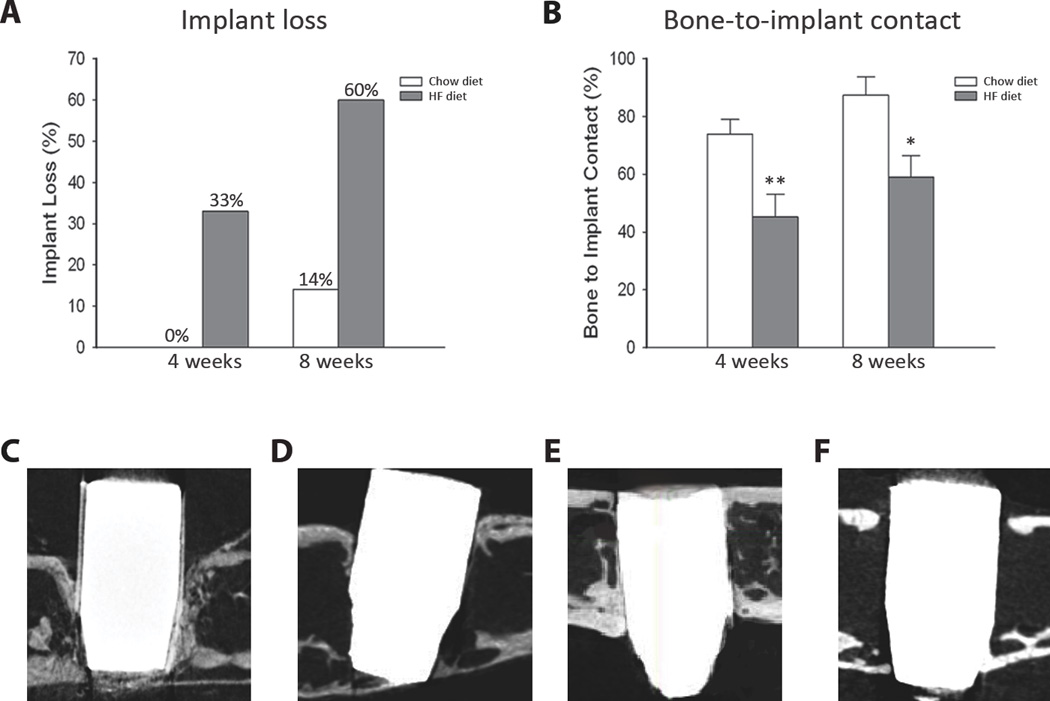
Effects of the HF diet on implant osseointegration at 4 and 8 weeks after implant placement. (A) Percent of implant loss at 4 weeks and 8 weeks after implant placement (n ≥ 6/group). (B) Percent of bone to implant contact throughout in the entire implant length (n ≥ 6/group). Significant difference when compared to respective control: *p<0.05, **p<0.01. (C–F) Representative µCT images of the implant in each group: (C) 4 week chow diet, (D) 4 week HF diet, (E) 8 week chow diet, (F) 8 week HF diet. No statistically significant differences were found when comparing chow diet and HF diet groups within the respective time points.
To determine whether the there were differences in bone-to-implant contact (BIC) in the control versus the HFD, micro-CT analysis was performed. BIC was significantly higher in the chow diet mice as compared to the HFD at the respective time point (p<0.01 at 4 weeks and p<0.05 at 8 weeks) (Figure 2B). However, no statistically significant difference was found when comparing the BIC from 4 and 8 week time points within the respective diet groups (chow diet at 4 weeks compared to chow diet at 8 weeks and HFD at 4 weeks compared to HFD at 8 weeks (Figure 2C–F).
Biomechanical evaluation of osseointegration, with the push-in test, revealed that the HF diet group required a lower load to break the bone-to-implant interface compared to the chow diet, in both time points (p<0.01 at 4 weeks and p<0.05 at 8 weeks) (Figure 3). Statistical significant difference was also found when comparing the average load between high fat mice at 4 and 8 weeks (p<0.05) However, no statistical difference was observed between the chow diet mice at 4 and 8 weeks.
Figure 3.
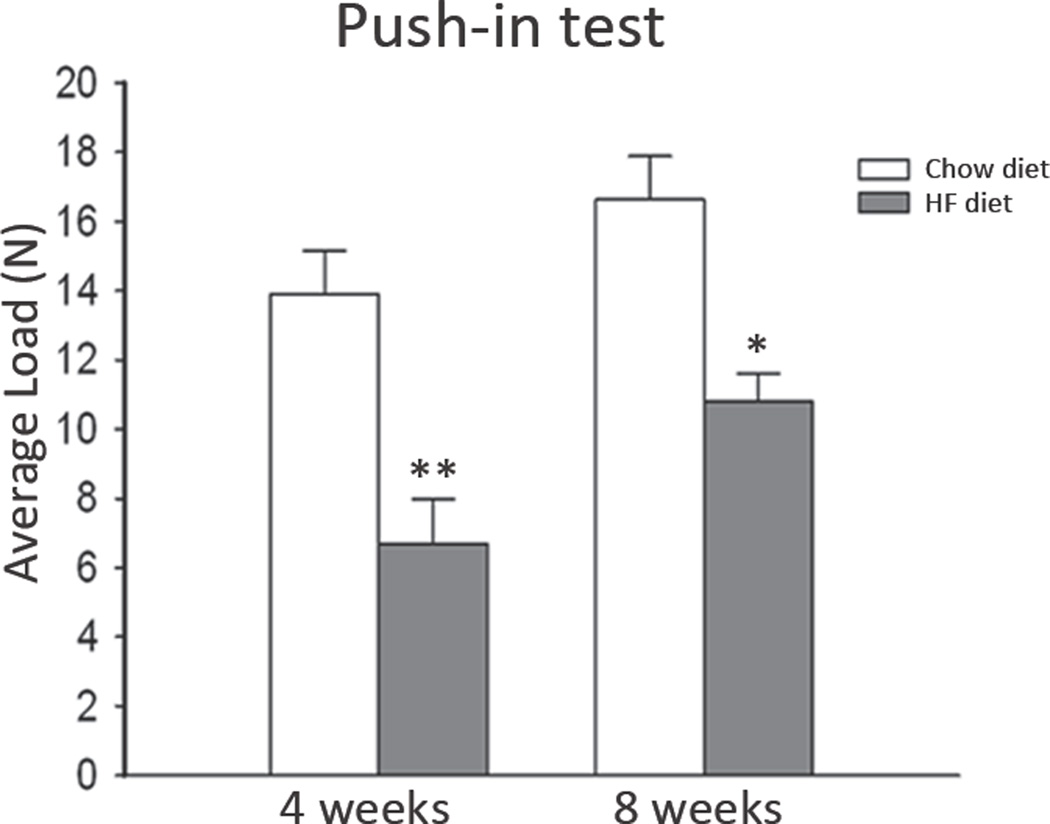
Load (force) necessary to break the bone to implant interface (n ≥ 3/group). Significant difference when compared to respective control: *p<0.05. No statistically significant differences were found when comparing chow diet and HF diet groups within the respective time points.
DISCUSSION
Successful osseointegration is a major determinant of implant success. The lack of osseointegration is a major concern for patients, especially when another implant placement cannot be easily performed. The ultimate consequence of the failure to obtain osseointegration is the loss of the implant. It was demonstrated that the degree of bone loss before implant placement is a significant factor that causes implant loss(Berglundh, Persson et al. 2002). Osseointegration is also critical since 36% of the patients who had lost a prior implant lost a second one in another occasion(Roos-Jansaker, Lindahl et al. 2006).
The results of the present study show that a high-fat diet significantly reduced implant osseointegration and bone volume in the femurs of mice. Implant loss increased and load necessary to break the bone-to-implant interface decreased in mice on an HF diet, which has been shown to significantly reduce bone density and mineral content, thus altering morphological and mechanical properties (Parhami, Tintut et al. 2001; Ionova-Martin, Do et al. 2010; Sage, Lu et al. 2011). More than one mechanism can account for the deleterious effects of an HF diet in the osseointegration shown in the present study. At a cellular level, hyperlipidemic conditions lead to inhibition of osteogenic signaling(Huang, Morony et al. 2007), decrease of the formation of mature osteoblasts, a higher expression of molecular markers of bone remodeling(Sage, Lu et al. 2011), enhance of osteoclast differentiation and activity(Tintut, Morony et al. 2004), and the increase of bone resorption(Sage, Lu et al. 2011; Lange, Barz et al. 2013). Moreover, hyperlipidemia significantly impairs bone healing, thus decreasing bone surface and volume(Pirih, Lu et al. 2012).
The present study also demonstrated that a HF diet decreases the bone-to-implant interface. The peri-implant bone tissue formation and mineralization by osteoblasts is similar to the process of bone fracture repair. During fracture repair, osteoblasts deposit a collagenous matrix(Marco, Milena et al. 2005). It has been demonstrated that a HF diet disrupts collagen processing (Parhami, Tintut et al. 2001) as well as orientation, causing loss of local alignment (Pirih, Lu et al. 2012), which may reduce mechanical integrity and quality of bone. The HF diet-induced loss of bone strength, bending stiffness, and fracture toughness(Ionova-Martin, Do et al. 2010; Pirih, Lu et al. 2012) may also be attributable to inhibitory effects on differentiation(Mody, Parhami et al. 2001) and mineralization of osteoblasts and stimulatory effects on differentiation and resorption of osteoclasts(Tintut, Morony et al. 2004).
Testing orthopedic or dental implants in mouse femoral bones to study bone-implant interaction, microstructure, composition and bone remodeling accompanies some limitations. Although bone remodeling in resorption cavities in mice has been shown to be similar to the Haversian remodeling in larger animals, mouse cortical bones do not undergo Haversian remodeling as humans do(Nunamaker 1998). Femurs also have a larger marrow space in comparison to jaws, causing less remodeling to take place in femurs compared with jaws. Lack of load on the implant should be also considered a limitation because dental implants in general are osseointegrated to withstand loading pressure (Hoshaw, Brunski et al. 1997). However, there are also advantages of using mice as a model to study bone regeneration and development of drug therapies. These include availability of a wide variety of mouse models with genetic mutations and cost effectiveness in handling and drug consumption(Histing, Garcia et al. 2011).
In conclusion, the present in vivo study demonstrates that a hyperlipidemia significantly increases implant loss and decreases the formation and strength of the bone-to-implant interface in the mouse femur. Human clinical correlation is required to determine the effects of hyperlipidemia on dental implant survival and success. No large clinical studies exist to evaluate hyperlipidemia in dental implant failure. Studies that evaluate patients with coronary artery disease and implant failure, include mostly patients with treated hyperlipidemic conditions by cholesterol lowering medications (Moy, Medina et al. 2005). However, this study is important as we continuously attempt to identify medical risk factors associated with implant success, including bone strength and bone to implant contact. However, increased implant failure, decreased osseointegration, and poor mechanical strength suggest that untreated hyperlipidemia may be a risk factor in this implant model system.
ACKNOWLEDGEMENTS
We thank Elisa Atti for the assistance with the micro-CT scanning and analysis. This work was supported in part by an AAID RF Student research grant (A.K.), AAID RF research grant (F.P. T.A), a scholarship from CNPq – Brazil (A.B.), NIH grants R21-DE023901 (FP), T90-DE022734 (SH) and NIDDK R01-DK081346 (YT).
Footnotes
We declare no conflicts of interest with respect to the authorship and/or publication of this article.
REFERENCES
- Aghaloo TL, Kang B, et al. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res. 2011;26(8):1871–1882. doi: 10.1002/jbmr.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Ambrogini E, et al. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem. 2009;284(40):27438–27448. doi: 10.1074/jbc.M109.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayukawa Y, Yasukawa E, et al. Local application of statin promotes bone repair through the suppression of osteoclasts and the enhancement of osteoblasts at bone-healing sites in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(3):336–342. doi: 10.1016/j.tripleo.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Berglundh T, Persson L, et al. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J Clin Periodontol. 2002;29(Suppl 3):197–212. doi: 10.1034/j.1600-051x.29.s3.12.x. discussion 232-193. [DOI] [PubMed] [Google Scholar]
- Brodeur MR, Brissette L, et al. Influence of oxidized low-density lipoproteins (LDL) on the viability of osteoblastic cells. Free Radic Biol Med. 2008;44(4):506–517. doi: 10.1016/j.freeradbiomed.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Corwin RL. Effects of dietary fats on bone health in advanced age. Prostaglandins Leukot Essent Fatty Acids. 2003;68(6):379–386. doi: 10.1016/s0952-3278(03)00062-0. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Hartman TJ, et al. Dietary saturated fat intake is inversely associated with bone density in humans: analysis of NHANES III. J Nutr. 2006;136(1):159–165. doi: 10.1093/jn/136.1.159. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Hart DJ, et al. Oral statins and increased bone-mineral density in postmenopausal women. Lancet. 2000;355(9222):2218–2219. doi: 10.1016/s0140-6736(00)02408-9. [DOI] [PubMed] [Google Scholar]
- Fedele S, Sabbah W, et al. Common oral mucosal diseases, systemic inflammation, and cardiovascular diseases in a large cross-sectional US survey. Am Heart J. 2011;161(2):344–350. doi: 10.1016/j.ahj.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Fentoglu O, Bozkurt FY. The Bi-Directional Relationship between Periodontal Disease and Hyperlipidemia. Eur J Dent. 2008;2(2):142–146. [PMC free article] [PubMed] [Google Scholar]
- Gaetti-Jardim EC, Santiago-Junior JF, et al. Dental implants in patients with osteoporosis: a clinical reality? J Craniofac Surg. 2011;22(3):1111–1113. doi: 10.1097/SCS.0b013e3182108ec9. [DOI] [PubMed] [Google Scholar]
- Histing T, Garcia P, et al. Small animal bone healing models: standards, tips, and pitfalls results of a consensus meeting. Bone. 2011;49(4):591–599. doi: 10.1016/j.bone.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Hoshaw SJ, Brunski JB, et al. Mechanical Loading of Branemark Implants Affects Interfacial Bone Modeling and Remodeling. Int Oral Maxillofac Implants. 1997;9:345–360. [Google Scholar]
- Huang MS, Morony S, et al. Atherogenic phospholipids attenuate osteogenic signaling by BMP-2 and parathyroid hormone in osteoblasts. J Biol Chem. 2007;282(29):21237–21243. doi: 10.1074/jbc.M701341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionova-Martin SS, Do SH, et al. Reduced size-independent mechanical properties of cortical bone in high-fat diet-induced obesity. Bone. 2010;46(1):217–225. doi: 10.1016/j.bone.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklina EV, Yoon PW, et al. Trends in high levels of low-density lipoprotein cholesterol in the United States, 1999–2006. JAMA. 2009;302(19):2104–2110. doi: 10.1001/jama.2009.1672. [DOI] [PubMed] [Google Scholar]
- Lange J, Barz T, et al. Gene expression profile in bone of diabetes-prone BB/OK rats fed a high-fat diet. Genes Nutr. 2013;8(1):99–104. doi: 10.1007/s12263-012-0299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe GD. The relationship between infection, inflammation, and cardiovascular disease: an overview. Ann Periodontol. 2001;6(1):1–8. doi: 10.1902/annals.2001.6.1.1. [DOI] [PubMed] [Google Scholar]
- Maeda T, Matsunuma A, et al. Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun. 2001;280(3):874–877. doi: 10.1006/bbrc.2000.4232. [DOI] [PubMed] [Google Scholar]
- Marco F, Milena F, et al. Peri-implant osteogenesis in health and osteoporosis. Micron. 2005;36(7–8):630–644. doi: 10.1016/j.micron.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Meier CR, Schlienger RG, et al. Statin drugs and the risk of fracture. JAMA. 2000;284(15):1921–1922. [PubMed] [Google Scholar]
- Mody N, Parhami F, et al. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31(4):509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- Montagnani A, Gonnelli S, et al. Effect of simvastatin treatment on bone mineral density and bone turnover in hypercholesterolemic postmenopausal women: a 1-year longitudinal study. Bone. 2003;32(4):427–433. doi: 10.1016/s8756-3282(03)00034-6. [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Ayukawa Y, et al. Local application of fluvastatin improves peri-implant bone quantity and mechanical properties: a rodent study. Acta Biomater. 2010;6(4):1610–1618. doi: 10.1016/j.actbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- Moy PK, Medina D, et al. Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants. 2005;20(4):569–577. [PubMed] [Google Scholar]
- Mundy G, Garrett R, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286(5446):1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- Nunamaker DM. Experimental models of fracture repair. Clin Orthop Relat Res. 1998;(355 Suppl):S56–S65. doi: 10.1097/00003086-199810001-00007. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Ozawa S, et al. Biomechanical evaluation of osseous implants having different surface topographies in rats. J Dent Res. 2000;79(11):1857–1863. doi: 10.1177/00220345000790110701. [DOI] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Raines AL, et al. Osteoblast maturation and new bone formation in response to titanium implant surface features are reduced with age. J Bone Miner Res. 2012;27(8):1773–1783. doi: 10.1002/jbmr.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhami F, Tintut Y, et al. Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner Res. 2001;16(1):182–188. doi: 10.1359/jbmr.2001.16.1.182. [DOI] [PubMed] [Google Scholar]
- Pirih F, Lu J, et al. Adverse effects of hyperlipidemia on bone regeneration and strength. J Bone Miner Res. 2012;27(2):309–318. doi: 10.1002/jbmr.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos-Jansaker AM, Lindahl C, et al. Nine- to fourteen-year follow-up of implant treatment. Part I: implant loss and associations to various factors. J Clin Periodontol. 2006;33(4):283–289. doi: 10.1111/j.1600-051X.2006.00907.x. [DOI] [PubMed] [Google Scholar]
- Sage AP, Lu J, et al. Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J Bone Miner Res. 2011;26(6):1197–1206. doi: 10.1002/jbmr.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon DH, Avorn J, et al. Lipid levels and bone mineral density. Am J Med. 2005;118(12):1414. doi: 10.1016/j.amjmed.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Tintut Y, Morony S, et al. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24(2):e6–e10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]


