Abstract
Purpose
Persistent uncertainty over the clinical significance of various pathological continuous electroencephalography (cEEG) findings in the intensive care unit (ICU) has prompted efforts to standardize ICU cEEG terminology and an ensuing debate. We set out to understand the reasons for, and a satisfactory resolution to, this debate.
Method
We review the positions for and against standardization, and examine their deeper philosophical basis.
Results
We find that the positions for and against standardization are not fundamentally irreconcilable. Rather, both positions stem from conflating the three cardinal steps in the classic approach to EEG, which we term “description”, “interpretation”, and “prescription”. Using real-world examples we show how this conflation yields muddled clinical reasoning and unproductive debate among electroencephalographers that is translated into confusion among treating clinicians. We propose a middle way that judiciously uses both standardized terminology and clinical reasoning to disentangle these critical steps and apply them in proper sequence.
Conclusion
The systematic approach to ICU cEEG findings presented herein not only resolves the standardization debate but also clarifies clinical reasoning by helping electroencephalographers assign appropriate weights to cEEG findings in the face of uncertainty.
Keywords: Electroencephalography, Critical care, Intensive care, Long-term monitoring, Standardization, Nomenclature
1. Introduction
There is ongoing debate within the electroencephalographic community over the place of standardized terminology in intensive care unit continuous electroencephalogram (ICU cEEG) recordings.1–4 We first review the main arguments and then through case studies show that disagreement arises from uncertainty over the significance of cEEG findings coupled with conflation of basic steps in the clinical approach to EEG. We then revisit our cases to propose a structured approach that disentangles these steps and strategically incorporates standardization to deal with uncertainty over ICU cEEG monitoring and management.
2. The standardization debate
Terminology standardization proponents advocate uniform nomenclature to facilitate communication among electroencephalographers and end-user physicians for clinical and research purposes. Clinically, cEEG volume is growing dramatically, updates are demanded rapidly and frequently, and findings need to be clearly and consistently communicated to clinicians from a variety of medical backgrounds. Standardization may reduce uncertainty by reducing inter-reader variability and providing an unbiased description. For research, standardization is seen as a necessary foundation for investigating the significance of ICU cEEG findings, with the ultimate goal of reducing uncertainty in the field. Standardization efforts have led to the American Clinical Neurophysiology Society (ACNS) ICU cEEG terminology framework, and various proposed criteria for electrographic status epilepticus.5–7
Standardization opponents resist uniform nomenclature based on some variation of the objection that ‘one size does not fit all’: the schema may not be comprehensive enough, too comprehensive, or contain elements unsatisfactory in both regards. More restrictive schemas assume it is acceptable to disregard certain differences between cEEG patterns because there are only a limited number of possible distinct clinical responses to ICU cEEG findings, and choosing among them usually does not depend on distinctions beyond those provided by standardized terminology. But standardization opponents argue that subtle differences may still matter, and “lumping” subtly different cEEG patterns under a single standardized term risks encouraging over-treatment of benign patterns and under-treatment of malignant patterns. On the other hand, more granular schemas may include descriptive features that seem arbitrary or lacking proven clinical relevance. Such criticisms have already been raised against the ACNS ICU cEEG terminology.4
Even if one could strike a perfectly balanced schema, a more fundamental philosophical objection remains: a one-size-fits-all system, no matter how well-refined, risks downplaying the role of clinical judgment. Standardization opponents may argue that instead of being free to craft a report customizable to any clinical presentation, the electroencephalographer would be unduly constrained by a pre-determined set of terms.
But standardization proponents may have their own philosophical objection: unconstrained inter-physician (and possibly intra-physician) variation in cEEG reporting amidst significant uncertainty leaves clinical reasoning vulnerable to subconscious cognitive biases. Cognitive biases are increasingly recognized as important and avoidable sources of medical error.8,9 For instance, probability adjustment is the act of changing one’s assessment of data in order to make it fit better with other data. The narrative fallacy is believing something to be true simply because it makes for a coherent story.10 The substitution heuristic replaces a difficult or unanswerable question with an easier question. Standardization proponents maintain that uniform nomenclature is necessary for minimizing the influence of heuristics and biases by forcing electroencephalographers to use available neutral terminology to form an unbiased description of the recording.
3. The conflation trap
At face value, these philosophical objections appear irreconcilable. Resolution lies in acknowledging the larger issue that both sides are liable to conflating the basic steps in the classic approach to EEG: “description” of the record, subsequent “interpretation”, and finally electroclinical correlation.11–14 But in the ICU, furnishing a traditional laundry list of “compatible” differential diagnoses does not suffice because ICU patients require prompt translation of the cEEG into a concrete actionable “prescription”. Thus, the basic steps in approaching ICU cEEG are “description”, “interpretation”, and “prescription”.
At one extreme, overzealous standardization proponents may conflate description with interpretation and prescription if they use only uniform nomenclature because standardized terminology can only render a description. A report with neither interpretation nor prescription represents an abdication of responsibility on the part of the electroencephalographer. At the other extreme, conflation also occurs when overzealous standardization opponents use only free text. In the face of uncertainty, free text risks allowing other information (e.g. clinical history) to bias description when this information is only relevant in formulating an interpretation. Free text also allows ad hoc inferences formed while reviewing the recording to bias the description. If the cEEG description is bent to fit interpretative biases, then this is an instance of conflating description with interpretation. Similarly, if the biases are prescriptive in nature, then this represents conflation of description with prescription.
Strictly adhering to either a standardized or unconstrained free text approach results in conflation and confusion in the ICU. Instead of standing alone, we believe that both standardized description and the clinical reasoning behind free text need to be incorporated into a “middle way” that rediscovers EEG first principles by disentangling the oft-conflated cardinal steps of description, interpretation, and prescription into distinct obligations shared between the electroencephalographer and treating clinicians (Fig. 1). More explicitly, we propose an approach that uses standardized terminology to first describe the ICU cEEG without knowledge of (bias from) the history. This then sets the stage for clinical reasoning to generate a cEEG interpretation that finally yields a cEEG prescription. We believe this “three-fold separation” approach not only resolves the standardization debate, but is also clinically useful by allowing patient-specific assignment of proper weight to cEEG findings among the total aggregate of data available to clinicians caring for critically ill patients.
Fig. 1.
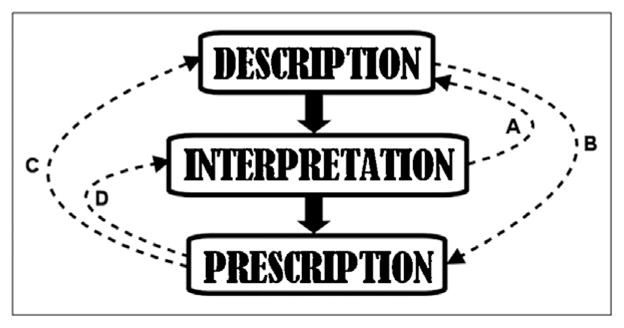
Proposed three-fold separation approach to critical care cEEG as denoted by solid vertical arrows. Dotted arrows denote potential routes of three-way conflation between the distinct steps of description, interpretation, and prescription. (A) Conflation of interpretation with description: e.g. letting blood-work bias one’s interpretation of a triphasic wave as being indicative of metabolic encephalopathy. (B) Conflation of description with prescription: e.g. because EEG patterns are stimulus-induced, AEDs are automatically out of the question no matter the situation. (C) Conflation of prescription with description: e.g. because one does not want to prescribe AEDs in CJD, one alters one’s initial impression of nonconvulsive seizures on the EEG. (D) Conflation of prescription with interpretation: e.g. because one does not want to prescribe AEDs in a case of devastating postanoxic brain injury, one dismisses the significance of any EEG findings no matter how alarming.
4. Case studies
In this section, we illustrate the standardization debate as it unfolds in common ICU clinical scenarios with actual specialist discussions about reporting and clinical management. In each case, we demonstrate that disagreement is rooted in implicit conflation of the three cardinal steps in approaching ICU cEEG. In a later section, we revisit each case to show that reconciliation lies in firmly disentangling these steps and executing them in proper sequence using our proposed three-fold separation approach.
For concreteness, we will use ACNS ICU cEEG standardized terminology and the Young electrographic seizure diagnostic criteria as they are among the best known attempts at ICU cEEG terminology standardization to date (Table 1).4,7 Nevertheless we emphasize from the outset that we are not here defending any specific standardization schema per se. Rather, we focus on the principles motivating terminology standardization in general, and clarify their proper place in the overall approach to ICU cEEG.
Table 1.
Diagnostic criteria for nonconvulsive seizure.a
Any EEG pattern which satisfies both of the following:
|
Primary criteria
|
Source: adopted from Ref. 7.
If criteria are not fulfilled, nonconvulsive seizure is not ruled out.
Evolution in amplitude alone is not sufficient.
4.1. Triphasic waves – conflating description and interpretation
A 40-year-old dialysis-dependent female with uremia developed acute obtundation. She remained awake but mute. cEEG demonstrated prominent discharges (Fig. 2a) designated by some electroencephalographers as “triphasic waves (TPWs)”, and by others as “generalized periodic epileptiform discharges (GPEDs)”. Proponents of the TPW designation argued she likely had metabolic encephalopathy from known uremia and the discharges were representative of this condition: triphasic morphology, symmetric bifrontally predominant distribution, anterior-to-posterior phase lag, and intermittent character.15–17 In contrast, GPED proponents argued that the discharges appeared too sharp and too periodic for metabolic encephalopathy, and were more consistent with nonconvulsive status epilepticus (NCSE). In response, TPW proponents argued that the term GPED would lead to treatment with antiepileptic drugs (AEDs) that could worsen metabolic encephalopathy by way of polypharmacy. Citing a suggestion in the literature6, GPED proponents countered that an AED trial was exactly what the patient needed.
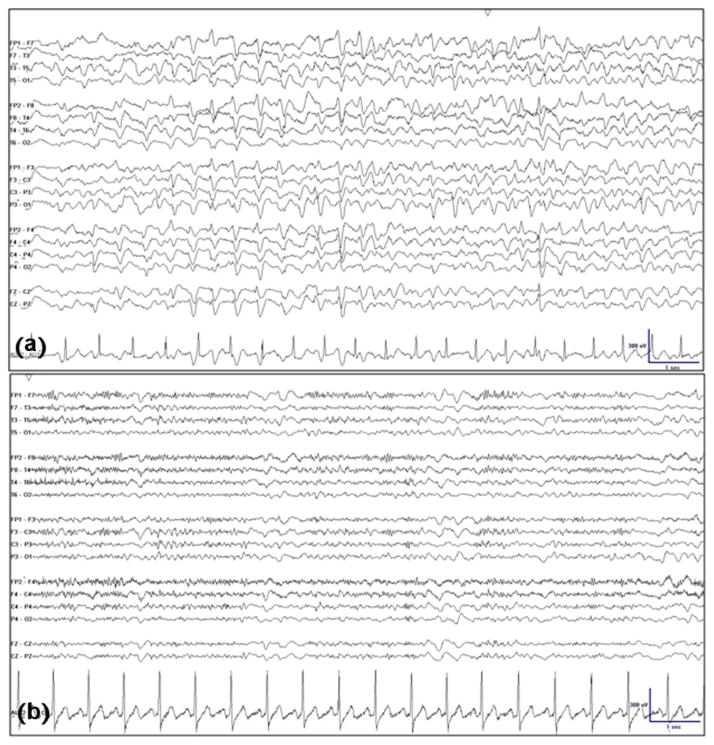
Fig. 2.
(a) Generalized periodic discharges with characteristic triphasic (three-lobed) morphology, symmetric bifrontally predominant distribution, typical anterior-to-posterior phase lag, and intermittent nature. (b) Resolution of generalized periodic discharges (“triphasic waves”) concomitant with dramatic clinical improvement minutes after administration of intravenous Lorazepam (4 mg).
At first glance, conflict was seemingly over terminology (TPW versus GPED). But on closer examination, the disagreement actually arose from deeper argument over cEEG interpretation that represents conflation of interpretation with description. The history of uremia and dialysis-dependence led TPW proponents to diagnose metabolic encephalopathy (interpretation) leading to the decision that cEEG discharges must be TPWs (description) because of their classical association with metabolic encephalopathy. For GPED proponents, sharpness and periodicity of the discharges appeared incompatible with metabolic encephalopathy (interpretation) and they were designated with a term implying higher epileptiform valence (description). Distinct from either “TPW” or “GPED”, the neutral standardized ACNS descriptive term is simply GPD (“generalized periodic discharge”) – a term devoid of association with an epileptic state or indication for AEDs that the extra E in GPED (“epileptiform”) may imply. As we shall see, using a neutral term prior to interpretation and prescription smoothed the way forward.
4.2. Electrographic seizures – conflating description and prescription
A healthy 40-year-old male developed rapidly progressive dementia over 3 months. As he deteriorated, cEEG demonstrated 1.5 Hz periodic sharp waves (Fig. 3a) and neuroimaging demonstrated prominent cortical ribboning with hyperintense basal ganglia (Fig. 3b). Toward end of hospital stay, there was a confident diagnosis of Creutzfeldt–Jakob disease (CJD), later confirmed on autopsy, and blunted periodic discharges evolved into continuous slow spike-and-wave complexes. On many occasions, these discharges displayed discrete evolution and devolution without any apparent change in clinical state (Fig. 3c). Using standardized diagnostic criteria (Table 1), some electroencephalographers designated the episodes as electrographic seizures. Others avoided the term ‘seizure’ for fear of prompting futile aggressive AED therapy. Seizure proponents suggested that an empiric AED trial would reasonable to test whether the electrographic events were truly asymptomatic. Seizure opponents objected to using AEDs in this clinical context, and the “blind mechanical” application of standardized criteria that designated the electrographic patterns as seizures. They believed that this exposed not only a critical error in the standardization schema per se but the very notion of standardizing ICU cEEGs.
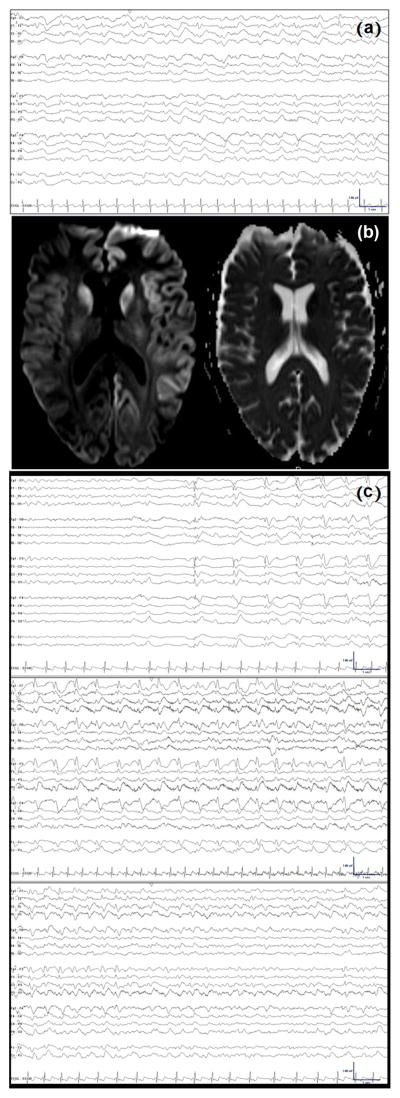
Fig. 3.
(a) Interictal generalized periodic 1 Hz triphasic sharp waves. (b) Axial DWI (left) and ADC (right) MRI slices demonstrating diffusion restriction within the caudate nuclei bilaterally and also diffusely within the left hemispheric cortical ribbon. (c) Electrographic seizure with generalized spike-wave complexes evolving out of quiescent background and increasing in frequency from 0.5 Hz to 2 Hz prior to abrupt termination and resumption of background activity.
In this case, conflict appeared to revolve around terminology: whether to report a paroxysmal pattern as electrographic seizure. But this disagreement arose over conflation of cEEG prescription with description, as seizure proponents cited a possible empiric AED trial (prescription) to defend the term electrographic seizure (description) while seizure opponents avoided the term electrographic seizure (description) due to the belief that aggressive AED therapy had no place in a terminally ill patient (prescription). Without acknowledging this web of conflations, the discussion stalled at an impasse. But as we shall see, standardized terminology provided the starting point for moving forward.
4.3. Postanoxic patterns – conflating interpretation and prescription
A 40-year-old male suddenly collapsed in front of witnesses. Paramedics found him in pulseless electrical activity and restored spontaneous circulation in 10 min. Therapeutic hypothermia began 90 min post-arrest. ICU cEEG monitoring began 16 h post-arrest during cooling and initially demonstrated diffuse low amplitude delta background slowing. During re-warming, GPDs emerged without motor manifestations while he remained comatose (Fig. 4a). After re-warming was complete, GPDs became higher, spikier, and more persistent (Fig. 4b), which led some electroencephalographers to argue they were consistent with NCSE. Others objected that GPDs after anoxic brain injury may represent epiphenomena instead of NCSE and they favored a watch-and-wait approach for clinical correlate in order to “prove” clinical significance. GPD proponents countered that as it is unknown whether postanoxic GPDs are epiphenomena, one should err on the side of caution and use the NCSE designation in order to prompt aggressive therapy targeted to electrographic burst suppression.
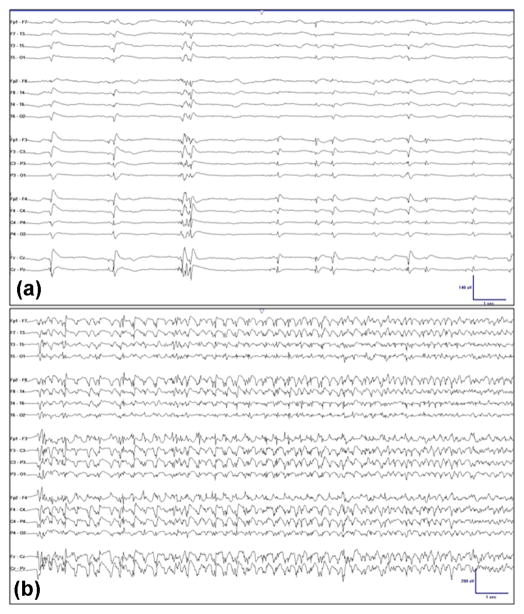
Fig. 4.
(a) Generalized periodic discharges following anoxic brain injury. (b) Evolution of generalized periodic discharges into spike-wave discharges consistent with nonconvulsive seizure following anoxic brain injury.
Here, conflict was again superficially over how to interpret GPDs, that is, whether they represent NCSE with the main desiderata being treatment response desired from responding clinicians. But the impasse actually arose from conflation of cEEG prescription with interpretation, as AED opponents believed postanoxic epileptiform discharges do not require treatment (prescription) because they are epiphenomena (interpretation), while AED proponents believed they merit aggressive intervention (prescription). Also implicit in both positions is conflation between description and prescription, implicit in the apparent believe that designating a pattern as NCSE necessarily demands aggressively treating with AEDs. Without addressing these conflations, there was a large chance of fruitless debate and little chance of reaching consensus. Again as we shall see, starting with standardization provided the way forward.
5. A middle way
The first step in disentangling the cEEG conflations described above is to acknowledge the uncertain significance of most cEEG patterns by describing them in neutral standardized terminology devoid of any implied clinical significance or management ramifications. There is no a priori way of knowing from cEEG alone whether GPDs actually represent metabolic encephalopathy versus an AED-responsive (epileptic) state, whether electrographic seizures actually demanded treatment, or whether postanoxic GPDs are epiphenomena or causative of secondary neural injury. Attempting to extract a definitive answer in the absence of clinical correlation or guidance from clinical trials is an exercise in futility that leads to confusion and relapse into an unproductive standardization debate. Using standardized terminology focuses the arguments by first requiring the electroencephalographer to agnostically describe what is seen. Description should be performed without knowledge (bias from) the history. Only then is the stage set for clinical reasoning to interpret the cEEG description in clinical context in order to rationally generate recommendations (prescription). With these points in mind, we now revisit each case using this “middle way” three-fold separation approach.
5.1. Triphasic waves – disentangling description and interpretation
The first step is to acknowledge that there are no failsafe morphological criteria at present to distinguish metabolic TPWs from similar-appearing cEEG patterns considered to be epileptic.16,18–21 As a result, one resorts to standardized terminology to agnostically describe “sharp GPDs with triphasic morphology”. Subsequent cEEG interpretation required coordinating an AED trial with treating clinicians. Our patient received 4 mg of intravenous lorazepam and abruptly began conversing coherently, coincident with disappearance of the cEEG pattern and substantial normalization of the background (Fig. 2b). This unequivocal electroclinical response yielded a cEEG interpretation (and diagnosis) of NCSE with the resultant prescription of AED treatment. Had the AED trial been equivocal, additional clinical factors would have required consideration. For instance, if there were no evidence of metabolic encephalopathy and the patient could tolerate AEDs, then a period of empiric treatment would be reasonable. But if the patient had overwhelming metabolic encephalopathy or could not tolerate AEDs, then AEDs (including an empiric trial) may be deferred to concentrate on resolving metabolic derangements instead. None of these scenarios require the objectionable practice of tailoring cEEG description to the electroencephalographer’s personal management style and biases, perceived clinical significance, or desire to guide the responding clinician’s ensuing management. Instead, the three-fold separation approach insists only upon an initial standardized description, leaving ample room for subsequent clinical correlation and advisement.
5.2. Electrographic seizures – disentangling description and prescription
Similarly, in this case the first step forward is acknowledging significant uncertainty and active research surrounding concepts of electrographic seizures that vary from pathophysiology to AED clinical response.6,22–24 While the epilepsy community has worked hard to vest the word “seizure” with particular salience vis-à-vis need for treatment, at present it remains unclear exactly whether, which, and to what extent ICU cEEG patterns termed electrographic seizures cause brain damage and neuronal injury.25,26 Thus, while uncertainty remains, standardized criteria for designating cEEG patterns as ‘electrographic seizures’ (as distinct from electroclinical seizures) without regard to clinical correlation, clinical significance, or required treatment at least afford consistent classification of certain cEEG patterns as electrographic seizures. A standardized definition provides an operational starting point for the electroencephalographer to formulate the significance (interpretation) of these patterns by discussing the clinical scenario with treating clinicians.
In our case, the diagnosis of CJD was well-founded, there was rapid clinical decline, and there was no indication that the cEEG patterns were symptomatic. In this context, recurrent electrographic seizures were felt to be of minimal clinical significance because the patient would be unlikely to benefit from treatment and aggressive measures were felt more likely to do harm than good. This risk–benefit consideration led to a prescription of foregoing AEDs. Decoupling the cEEG description of electrographic seizures from subsequent interpretation and prescription removes the temptation to “soften” the report based on subconscious conflations. Within the three-fold separation approach, there is nothing incoherent about the idea that cEEG findings described as electrographic seizures in the setting of CJD may be clinically insignificant and may not warrant treatment – much like how a p-value result can be statistically significant but, at the same time, clinically irrelevant.
5.3. Postanoxic patterns – disentangling interpretation and prescription
Moving beyond terminological debate in this case begins by acknowledging that there is no way at present of knowing from inspection of the cEEG pattern alone whether postanoxic GPED-appearing cEEG patterns represent epiphenomena. As a result, one agnostically describes them as GPDs using standardized terminology to set the stage for interpretation vis-à-vis each patient’s clinical circumstances. For example, if the patient is judged to have a meaningful chance of recovery and can tolerate AEDs, a reasonable prescription may be to attempt aggressive AED treatment for some time because the cEEG pattern could still be potentially damaging and worsen the probability of neurological recovery.27–29 But if the patient is judged to have an unequivocally poor prognosis, it may be reasonable to conclude (without altering the cEEG description) that treatment is indeed futile and then prescribe no intervention.30,31 Similarly, if the patient cannot tolerate AEDs, then it may also be reasonable to forego empiric treatment.
5.4. New onset refractory status epilepticus – the middle way in action
As a final example illustrating the benefits of the three-fold-separation approach, we discuss a new case of a 20-year-old male with cryptogenic new onset refractory status epilepticus (NORSE). He initially required barbiturate-induced burst suppression to subdue relentless unequivocal clinical seizures. Whenever pharmacological sedation was lifted, the cEEG was described as showing recurrent GPDs with polyspike morphology, and it was noted they were consistently time-locked with myoclonic jerks (Fig. 5a). To decide whether myoclonic jerks were caused by cortical spikes or represented simply muscle artifact, neuromuscular blockade was carried out to help with interpretation (an alternative method being jerk-locked back-averaging). Although the neuromuscular blocker stopped the myoclonus, it did not stop the polyspikes (Fig. 5b), indicating a cerebral origin, leading to a diagnosis (interpretation) of convulsive SE (CSE). The ensuing prescription was to maintain burst suppression until maintenance AEDs alone could prevent resumption of myoclonic CSE upon lightening of pharmacological burst suppression.
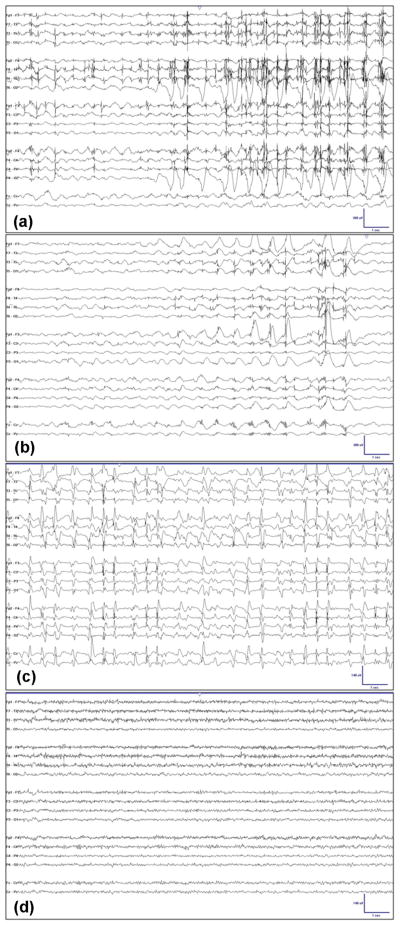
Fig. 5.
(a) Time-locked myoclonus to generalized periodic polyspikes. (b) Persistence of generalized periodic polyspikes despite treatment with Cisatracurium to abolish myogenic artifact. (c) Upon lightening of burst suppression, generalized periodic sharp waves without myoclonus. This EEG pattern is consistent with nonconvulsive status epilepticus. (d) Resolution of epileptiform activity.
After months of empiric AED trials, burst suppression was lifted without resumption of myoclonus, though GPDs persisted unabated (Fig. 5c). While the cEEG description remained unchanged (as GPDs), the clinical interpretation did not. Having previously been shown to firmly correlate with CSE, GPDs now became consistent with a clinical diagnosis (interpretation) of NCSE because they no longer possessed clinical (i.e. myoclonic) correlate. The freedom to change clinical interpretation of cEEG findings without altering their description, afforded by the uncoupling of cEEG description from its interpretation, opened the way for discussions about the clinical risk–benefit ratio of continued aggressive treatment to suppress NCSE. As the potential for neuronal injury and overall morbidity may be relatively less for NCSE than for CSE, this led some to consider tolerating the remaining GPDs in order to reduce the potential for iatrogenic complications.23,32,33 While there is no unequivocal right answer to this debate at present, it was nonetheless important that individual clinicians’ interpretation and prescription on this topic were not complicated by also arguing over what the cEEG record showed (description). In this case, the group prescription was to continue lightening pharmacological burst suppression despite ongoing GPDs. After a few days, the GPDs resolved (Fig. 5d) and the patient eventually regained a clinically significant degree of independence.
Had there not been a three-fold separation approach, the initial cEEG finding of GPDs may have been conflated with the clinical diagnosis of CSE into a single composite diagnosis of simply “status epilepticus” – convulsive or not. This conflation might have compelled treating physicians to continue aggressive treatment as long as GPDs remained. As an aside, conflation may also lead to under-aggressive treatment, such as in cases of late CSE with electroclinical dissociation. Returning to this case, given its long duration, there is a further risk that different electroencephalographers rotating through cEEG reporting duty may knowingly or unwittingly engage in “terminology wars” against one another. For instance, some may describe “simply” GPDs to “permit” the treating physicians to reduce aggressiveness of treatment. Others may favor NCSE to “encourage” more aggressive treatment. This to and fro often leads to substantial confusion among treating clinicians and patient families. Such ad hoc gross descriptive manipulations to influence interpretation and management represent a conflation of the descriptive, interpretative, and prescriptive elements of the ICU cEEG. Conflation leads to muddled thinking, cognitive dissonance, unwarranted confidence in clinical narratives, and irrational management decisions. In contrast, a clear, rigorous, and structured three-fold separation approach, which strategically incorporates standardized terminology as a common starting point prior to the judicious use of thoughtful clinical reasoning, in our case led to successful diagnosis and management of initial unrelenting myoclonic CSE followed by worrisome NCSE. This middle way ultimately facilitated successful emergence out of prolonged pharmacological burst suppression in an extremely challenging case of NORSE.
6. Conclusion
A three-fold separation approach that disentangles the oft-conflated steps of cEEG description, interpretation, and prescription is a middle way in the standardization debate that strategically uses standardized terminology as a descriptive starting point for a process that continues with clinical reasoning to interpret cEEG findings in their clinical context and formulating clinical management strategies based on risk–benefit considerations. This approach combats the natural human tendency to fall prey to cognitive biases in the face of prevailing uncertainty over the clinical significance of ICU cEEG findings. It also satisfies the need for objectivity and inter-rater agreement in communicating between clinicians from a wide variety of backgrounds in the ICU. Rather than substituting for clinical judgment, standardized terminology when used as part of a systematic three-fold separation approach ultimately empowers it. This middle way also refocuses unproductive debate into a rational discourse on the real issues: the clinical significance of cEEG findings for an individual patient, and the need to refine our scientific understanding of cEEG findings in order to begin chipping away at the uncertainty in the field.
Footnotes
Author contributions
Westover and Ng contributed in study concept and design. Ng, O’Rourke, and Westover contributed in acquisition of data. Ng, Westover, and O’Rourke contributed in analysis and interpretation of data. Ng and Westover contributed in drafting of the manuscript. Ng, Westover, Gaspard, Bianchi, Cole, Hoch, Rosenthal, Cash, Chu, and O’Rourke helped in critical revision of the manuscript for important intellectual content.
Conflict of interest statement
None of the authors have any relevant disclosures to report.
Contributor Information
Marcus C. Ng, Email: mng2@hsc.mb.ca.
Nicolas Gaspard, Email: nicolas.gaspard@yale.edu.
Andrew J. Cole, Email: ajcole1@mgh.harvard.edu.
Daniel B. Hoch, Email: dhoch@mgh.harvard.edu.
Sydney S. Cash, Email: scash@mgh.harvard.edu.
Matt Bianchi, Email: mtbianchi@mgh.harvard.edu.
Deirdre A. O’Rourke, Email: daorourke@mgh.harvard.edu.
Eric S. Rosenthal, Email: erosenthal@mgh.harvard.edu.
Catherine J. Chu, Email: cjchu@mgh.harvard.edu.
M. Brandon Westover, Email: mwestover@mgh.harvard.edu.
References
- 1.Abend NS, Gutierrez-Colina A, Zhao H, Guo R, Marsh E, Clancy RR, et al. Interobserver reproducibility of electroencephalogram interpretation in critically ill children. J Clin Neurophys. 2011;28(1):15–9. doi: 10.1097/WNP.0b013e3182051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerber PA, Chapman KE, Chung SS, Drees C, Maganti RK, Ng YT, et al. Interobserver agreement in the interpretation of EEG patterns in critically ill adults. J Clin Neurophys. 2008;25(5):241–9. doi: 10.1097/WNP.0b013e318182ed67. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch LJ, Brenner RP, Drislane FW, So E, Kaplan PW, Jordan KG, et al. The ACNS subcommittee on research terminology for continuous EEG monitoring: proposed standardized terminology for rhythmic and periodic EEG patterns encountered in critically ill patients. J Clin Neurophys. 2005;22(2):128–35. doi: 10.1097/01.wnp.0000158701.89576.4c. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophys. 2013;30(1):1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 5.Beniczky S, Hirsch LJ, Kaplan PW, Pressler R, Bauer G, Aurlien H, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013;54(S6):28–9. doi: 10.1111/epi.12270. [DOI] [PubMed] [Google Scholar]
- 6.Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophys. 2005;22(2):79–91. doi: 10.1097/01.wnp.0000158699.78529.af. [DOI] [PubMed] [Google Scholar]
- 7.Young BG, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47(1):83–9. doi: 10.1212/wnl.47.1.83. [DOI] [PubMed] [Google Scholar]
- 8.Kahneman D. Thinking, fast and slow. 1. New York: Farrar, Straus and Giroux; 2011. [Google Scholar]
- 9.Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124–31. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- 10.Taleb NN. The Black Swan: the impact of the highly improbable. 2. New York: Random House Inc; 2010. [Google Scholar]
- 11.American Clinical Neurophysiology Society. Guideline 7: guidelines for writing EEG reports. Am J Electroneurodiagn Technol. 2006;46(3):231–5. [PubMed] [Google Scholar]
- 12.Kaplan PW, Benbadis SR. How to write an EEG report: dos and don’ts. Neurology. 2013;80(1 Suppl 1):S43–6. doi: 10.1212/WNL.0b013e3182797528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noachtar S, Binnie C, Ebersole J, Mauguière F, Sakamoto A, Westmoreland B. A glossary of terms most commonly used by clinical electroencephalographers and proposal for the report form for the EEG findings. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:21–41. [PubMed] [Google Scholar]
- 14.Benbadis SR. Just like EKGs! Should EEGs undergo a confirmatory interpretation by a clinical neurophysiologist? Neurology. 2013;80(S1):47–51. doi: 10.1212/WNL.0b013e3182797539. [DOI] [PubMed] [Google Scholar]
- 15.Faigle R, Sutter R, Kaplan PW. Electroencephalography of encephalopathy in patien c disorders. J Clin Neurophys. 2013;30(5):505–16. doi: 10.1097/WNP.0b013e3182a73db9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulanger JM, Deacon C, Lécuyer D, Gosselin S, Reiher J. Triphasic waves versus nonconvulsive status epilepticus: EEG distinction. Can J Neurol Sci. 2006;33(2):175–80. doi: 10.1017/s0317167100004935. [DOI] [PubMed] [Google Scholar]
- 17.Schomer DL, Lopes da Silva FH. Niedermeyer’s electroencephalography: basic principles, clinical applications, and related fields. 6. Philadelphia: Lippincott Williams & Wilkins, a Wolters Kluwer business; 2011. [Google Scholar]
- 18.Brigo F, Storti M. Triphasic waves. Am J Electroneurodiagn Technol. 2011;51(1):16–25. [PubMed] [Google Scholar]
- 19.Kaya D, Bingol CA. Significance of atypical triphasic waves for diagnosing nonconvulsive status epilepticus. Epilepsy Behav. 2007;11(4):567–77. doi: 10.1016/j.yebeh.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Sheridan PH, Sato S. Triphasic waves of metabolic encephalopathy versus spike-wave stupor. J Neurol Neurosurg Psychiatry. 1986;49(1):108–9. doi: 10.1136/jnnp.49.1.108-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Xanthopoulos P, Liu CC, Bearden S, Uthman BM, Pardalos PM. Real-time differentiation of nonconvulsive status epilepticus from other encephalopathies using quantitative EEG analysis: a pilot study. Epilepsia. 2010;51(2):243–50. doi: 10.1111/j.1528-1167.2009.02286.x. [DOI] [PubMed] [Google Scholar]
- 22.Cash SS. Status epilepticus as a system disturbance: is status epilepticus due to synchronization or desynchronization? Epilepsia. 2013;54(S6):37–9. doi: 10.1111/epi.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson M, Bianchi MT, Sutter R, Rosenthal ES, Cash SS, Kaplan PW, et al. Calculating the risk–benefit equation for aggressive treatment of non-convulsive status epilepticus. Neurocrit Care. 2013;18(2):216–27. doi: 10.1007/s12028-012-9785-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopp JL, Sanchez A, Krumholz A, Hart G, Barry E. Nonconvulsive status epilepticus: value of a benzodiazepine trial for predicting outcomes. Neurologist. 2011;17(6):325–9. doi: 10.1097/NRL.0b013e31822f688c. [DOI] [PubMed] [Google Scholar]
- 25.Aminoff MJ. Do nonconvulsive seizures damage the brain?—no. Arch Neurol. 1998;55(1):119–20. doi: 10.1001/archneur.55.1.119. [DOI] [PubMed] [Google Scholar]
- 26.Young GB, Jordan KG. Do nonconvulsive seizures damage the brain?—yes. Arch Neurol. 1998;55(1):117–9. doi: 10.1001/archneur.55.1.117. [DOI] [PubMed] [Google Scholar]
- 27.Blondin NA, Greer DM. Neurologic prognosis in cardiac arrest patients treated with therapeutic hypothermia. Neurologist. 2011;17(5):241–8. doi: 10.1097/NRL.0b013e318224ee0e. [DOI] [PubMed] [Google Scholar]
- 28.Busl KM, Greer DM. Pitfalls in the diagnosis of brain death. Neurocrit Care. 2009;11(2):276–87. doi: 10.1007/s12028-009-9231-y. [DOI] [PubMed] [Google Scholar]
- 29.Rossetti AO, Oddo M, Liaudet L, Kaplan PW. Predictors of awakening from postanoxic status epilepticus after therapeutic hypothermia. Neurology. 2009;72 (8):744–9. doi: 10.1212/01.wnl.0000343006.60851.62. [DOI] [PubMed] [Google Scholar]
- 30.Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010;67(3):301–7. doi: 10.1002/ana.21984. [DOI] [PubMed] [Google Scholar]
- 31.Wijdicks EFM, Hijdra A, Young GB, Bassetti CL, Wiebe S. Quality Standards Committee of the American Academy of Neurology, Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203–10. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 32.Arif H, Hirsch LJ. Treatment of status epilepticus. Semin Neurol. 2008;28(3):342–54. doi: 10.1055/s-2008-1079339. [DOI] [PubMed] [Google Scholar]
- 33.Jirsch J, Hirsch LJ. Nonconvulsive seizures: developing a rational approach to the diagnosis and management in the critically ill population. Clin Neurophysiol. 2007;118(8):1660–70. doi: 10.1016/j.clinph.2006.11.312. [DOI] [PubMed] [Google Scholar]