Abstract
Aims
In young people with Type 1 diabetes, depressive symptoms and shared responsibility for management of diabetes impact upon diabetes management and control. However, the simultaneous effects of both depressive symptoms and parental involvement on diabetes self-care and glycaemic control have not been examined. Thus, the aim of the current study was to examine the relationships between parental involvement and adolescent depressive symptoms in predicting blood glucose monitoring and glycaemic control.
Methods
One hundred and fifty young people with Type 1 diabetes (mean age 15.3 years) and their parents completed responsibility sharing and depressive symptom assessments, meter assessment of blood glucose monitoring and HbA1c at baseline and then 6, 12 and 18 months.
Results
Parental involvement affected HbA1c through blood glucose monitoring only at low levels of adolescent depressive symptoms (score ≤ 6), which made up only 20% of the sample. In the presence of more depressive symptoms, parental involvement no longer was related to HbA1c through blood glucose monitoring. This was the relationship in the majority of the sample (80%).
Conclusions
While most young people in this sample are not showing evidence of high levels of depressive symptoms, even modest levels of distress interfere with parental involvement in diabetes management. By addressing adolescent depressive symptoms, interventions promoting parental involvement in these families may be more effective.
Introduction
Young people with Type 1 diabetes and their families engage in a demanding treatment regimen designed to maximize glycaemic control and prevent adverse diabetes-related outcomes [1]. The daily management regimen includes multiple blood glucose checks, insulin administration, and coordination with dietary intake and physical activity. Caregivers have critical roles in daily diabetes management and either perform or supervise multiple tasks [1-3]. Although Type 1 diabetes management is demanding across the age spectrum, the adolescent years produce a number of significant and unique challenges.
During adolescence, young people frequently take on more independence with diabetes management and families must find new ways to share the responsibility for management. For example, young people may independently manage their diabetes regimen when they are at social events, but still receive guidance and support about their implementation at these events when they return home [4-6]. As a result of the challenges associated with managing diabetes during adolescence, and the common deterioration observed in glycaemic control during this developmental period [7,8], studies have examined a number of factors, both family and individual, which affect diabetes-related outcomes (i.e. glycaemic control) through the mediator of diabetes management. One collection of studies on family factors indicates that, during adolescence, more parental involvement (e.g. direct monitoring or sharing of diabetes management) is associated with optimal diabetes control through the mediator of adequate diabetes management [9]. Adolescents whose parents stay involved and find new ways to supervise and support diabetes management tend to experience improved glycaemic control [10,11].
A second group of studies focuses on individual factors and one individual factor that has garnered much attention is the extent to which young people experience depressive symptomatology. Most studies indicate that depressive symptoms are elevated and more likely to co-occur in young people with Type 1 diabetes than in young people without diabetes [12,13]. Further, higher levels of depressive symptoms have been linked with poorer diabetes management, such as less frequent blood glucose monitoring and worse glycaemic control [14,15]. Depressive symptoms could affect one’s ability to adhere to the diabetes regimen by negatively impacting energy, motivation, concentration and problem-solving abilities, all which are essential for effective diabetes management [16]. Given that depressive symptoms have been linked with poor family functioning among young people without chronic health problems [17,18], it may be important to examine how these two factors (i.e. parental involvement, depressive symptoms) relate when young people and their families are faced with the added challenge of managing a chronic illness such as Type 1 diabetes.
Currently, there are two separate literatures documenting the relationships between parental involvement and diabetes management/glycaemic control and between depressive symptoms and diabetes management/glycaemic control. However, the interacting influences of family factors (e.g. parental involvement) with individual adolescent psychological factors (e.g. depressive symptoms) on diabetes management and glycaemic control have seldom been examined [19]. This gap limits our understanding of the potential impact of mood symptoms on families’ experiences with everyday diabetes management. For example, we would expect that the presence of depressive symptoms in an adolescent would disrupt the parent’s ability to stay fully involved in diabetes management. This could be attributable to fewer diabetes management tasks actually being carried out because depressive symptoms are causing decreased motivation and engagement in diabetes management; or parents having to focus more on the young person’s mood and motivation related to depressive symptoms and thus diabetes becomes a lower priority. Understanding the mechanisms involving psychological factors, diabetes management and glycaemic control will have implications for targets of intervention.
In summary, for young people with Type 1 diabetes, it is unknown whether and to what extent depressive symptoms moderate the relationship between parental involvement and glycaemic control through the mediator of diabetes management. Thus, the aims of the current study were to use a moderated mediation analytic technique to examine the effects of depressive symptoms on parental involvement in predicting glycaemic control through the mediator of a critical diabetes management task, blood glucose monitoring.
Patients and methods
Young people with Type 1 diabetes (n = 150) and their caregivers were recruited for the current study from a tertiary care diabetes centre at a children’s hospital in the Midwestern region of the USA. Young people were eligible if they had Type 1 diabetes according to the American Diabetes Association standards [1], had no psychiatric or neurocognitive disorder hindering study participation, or no medical disease other than Type 1 diabetes. Controlled thyroid disorders and caeliac disease were not exclusionary criteria given their common co-occurrence with Type 1 diabetes. Participants completed the study procedures and surveys in English.
Out of 166 young people approached about the study. 150 agreed to participate (participation rate 90%). After obtaining written informed consent/assent, participants were asked to complete study questionnaires at frou timepoints over the course of 18 months (baseline and 6, 12 and 18 months). A trained research assistant administered study questionnaires in the diabetes clinic before or after the medical visit. All study procedures were approved by the hospital’s institutional review board. Of the 150 young people, 133 completed the measures used in the current study at every time point (attrition rate 11%). Young people who dropped out prior to completing all four time points were significantly older (i.e. mean age 16.3 years, sd 1.0). There were no other significant differences between those included in the current analyses and those who dropped out on demographic characteristics or the variables of interest in the current study (i.e. parental involvement, adolescent depressive symptoms).
Measures
Family sharing of responsibility for diabetes-related tasks was assessed using the Diabetes Family Responsibility Questionnaire (DFRQ) [5], a 17-item measure with separate adolescent- and parent-report forms. The DFRQ has established validity and reliability (α =0.69–0.85) [5]. Scores range from 17 (adolescent has primary responsibility) to 54 (caregiver has primary responsibility), with a score of 34 indicating equal sharing of responsibility between adolescent and caregiver. The current analysis used the DFRQ completed by young people and their caregivers at the baseline study visit. Correlational analyses indicated that scores on this measure were highly correlated over time (r = 0.49-0.70, P < 0.001 adolescent report; r = 0.59–0.68, P < 0.001 parent report).
To assess for adolescent depressive symptoms, the Children’s Depression Inventory self-report (CDI) and parent-report (CDI:P) forms [20] were administered. The 27-item self-report form and the 17-item parent-report form have established validity and good reliability (CDI α = 0.84, CDI:P α = 0.86). Scores on the self-report form range from 0 to 54 (clinical cut-off = 13) and on the parent-report form range from 0 to 51 (clinical cut-off = 17), with higher scores indicating higher levels of depressive symptoms [13,20,21]. The current analysis used CDI forms completed by young people and their caregivers at the baseline study visit. The CDI is reported to have high test-retest reliability (e.g. r = 0.54-0.77 for test intervals of at least 1 month) [19]. Correlational analyses indicated that CDI and CDI:P scores were highly correlated across time points in this sample (r = 0.50-0.69, P < 0.001 CDI; r = 0.75-0.78, P < 0.001 CDI:P).
Diabetes management was assessed by calculating frequency of daily blood glucose monitoring. At the clinic visit, meters were downloaded and used to calculate the mean daily frequency using the past 14 days of data. In the absence of meter downloads, chart reviews or self report were used. Both methods show high correspondence with meter downloads in this patient population [22]. For this analysis, blood glucose monitoring data at 12 months were used. At that time point, 58% (n = 77) were from meter downloads, 33% (n = 44) were from chart review and 9% (n = 12) were based on self report. Frequency of blood glucose monitoring was also highly correlated across time points (r = 0.51-0.66, P < 0.001).
HbA1c was measured with the DCA+ 2000 (reference range 23-39 mmol/mol (4.3-5.7%), Bayer Inc., Tarrytown, NY, USA) and used as the indicator of glycaemic control. The 18-month study visit value was used as the outcome in this analysis.
Data analysis
Descriptive statistics (means, standard deviations, frequencies) and bivariate correlations between parental involvement, child depressive symptoms, blood glucose monitoring frequency, and HbA1c were calculated. The influence of child depressive symptoms (child- and parent-reported) on parental involvement’s prediction of HbA1c through blood glucose monitoring frequency was assessed using a SPSS macro that tests moderated mediation [23]. Moderated mediation was used so that the specific influence of depressive symptoms on the relationships between parental involvement, blood glucose monitoring frequency and glycaemic control could be examined within a single analytic procedure. This analytic procedure was selected because of the limitations in statistical power and potential for a type II error when using traditional methods of establishing mediation [24]. For instance, there are cases where moderated mediation models, such as the one that was tested, may be statistically significant when the traditional mediation steps would not detect this.
This model was examined using child- and parent-reported parental involvement and child depressive symptoms and, thus, four separate analytic models were tested (one for child report, one for parent report and two with child and parent reports). Baseline HbA1c was controlled for in all models. To control for a type I error (i.e. rejecting the null hypothesis when it should not be rejected), a Bonferroni correction was applied to the model results (P = 0.05/4 = 0.0125) for determining statistical significance. Significant interactions were plotted so that the nature of the moderation was displayed pictorially. Follow-up analyses to determine the levels of CDI symptoms that significantly affect the relationship between parental involvement on HbA1c through blood glucose monitoring were conducted using bootstrapping [23]. All analyses were run using SPSS 17.0 [25]. Only those models which were statistically significant will be presented in the Results.
Results
Participant demographic characteristics and descriptive statistics for the 133 participants providing data at all time points are displayed in Table 1. As noted earlier, the only significant difference between study completers and those who did not complete all assessments (n = 17) was participant age. Of the 133 participants, 51% were female, mean age was 15.3 years (range 13.1-18.5 years) and mean duration of Type 1 diabetes was 5.9 years. Approximately 64% of the participants were on insulin pumps. At baseline, 31 participants were at or above the CDI cut-off of 13 (i.e. self report) and 37 participants were at or above the CDI:P cut-off of 17 (i.e. parent report). Bivariate correlations between study variables are presented in Table 2.
Table 1.
Demographic characteristics and descriptives for study variables (mean ± SD, unless otherwise noted; n = 133)
| Demographic characteristics | |
|---|---|
| Age | 15.3 ± 1.4 years |
| % male | 49% |
| Insulin (% pump) | 64% |
| Diabetes duration | 5.9 ± 3.8 years (median 5.5 years) |
| % minority | 12% |
| % public insurance | 14% |
| Caregiver, % married | 77% |
| Caregiver, % ≥ high-school diploma |
45% |
| Descriptives for study variables | |
| Baseline | |
| CDI—child | 7.9 ± 7.1 (median 6.0) |
| CDI—parent | 12.9 ± 7.1 (median 13.0) |
| Parental involvement—child | 29.6 ± 3.8 |
| Parental involvement—parent | 33.3 ± 4.1 |
| HbA1c | 72 ± 20 mmol/mol (8.7 ± 1.8%) Median 68 mmol/mol (8.4%) |
| 12 months | |
| Blood glucose monitoring frequency |
3.3 ± 1.8 |
| 18 months | |
| HbA1c | 76 ± 21 mmol/mol (9.1 ± 1.9%) Median 69 mmol/mol (8.5%) |
Table 2.
Bivariate correlations
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| 1. CDI—child | — | 0.524 (P < 0.001) |
−0.189 (P = 0.029) |
−0.003 (P = 0.974) |
−0.251 (P = 0.004) |
0.124 (P = 0.156) |
| 2. CDI—parent | — | −0.157 (P = 0.071) |
0.118 (P = 0.174) |
−0.242 (P = 0.005) |
0.229 (P = 0.008) |
|
| 3. Parent involvement—child report | — | 0.453 (P < 0.001) |
0.169 (P = 0.052) |
−0.035 (P = 0.693) |
||
| 4. Parent involvement—parent report | — | 0.210 (P = 0.015) |
0.037 (P = 0.676) |
|||
| 5. Blood glucose monitoring frequency (12 months) | — | −0.343 (P < 0.001) |
||||
| 6. HbA1c (18 months) | — |
Models
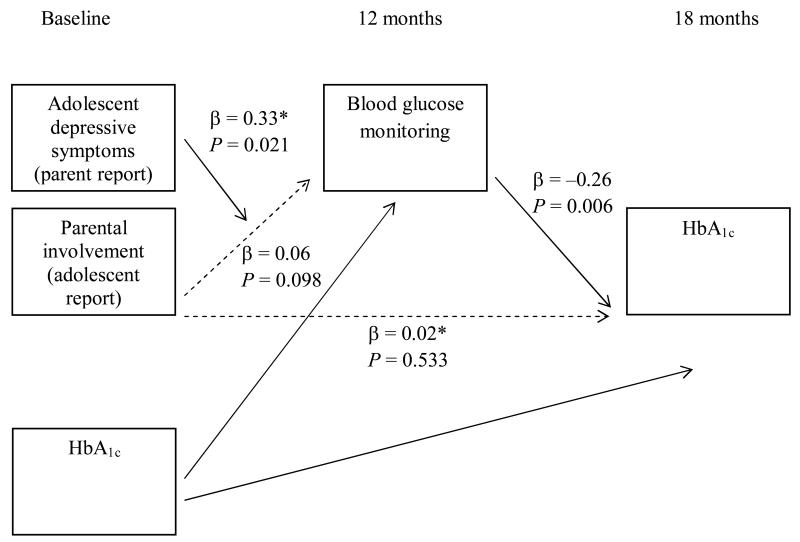
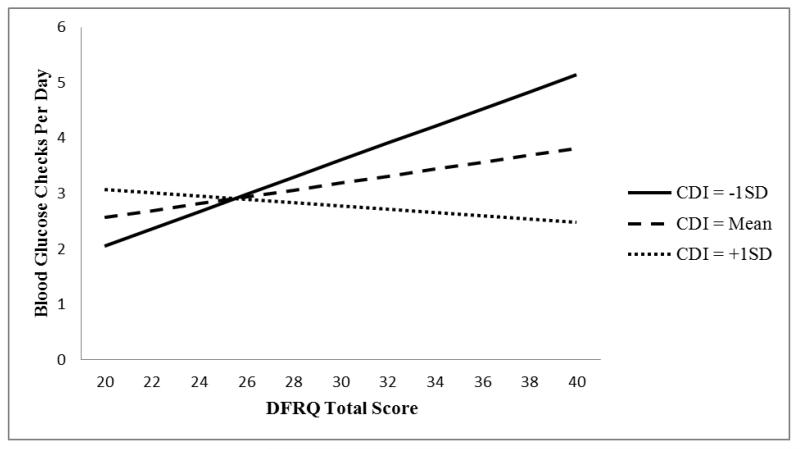
Of the four models examining the effect of child depressive symptoms on the relationship between parental involvement and HbA1c through blood glucose monitoring frequency, one model was statistically significant (Fig. 1). Specifically, adolescent depressive symptoms (reported by the parent; CDI:P) affected the relationship between parental involvement, blood glucose monitoring frequency and HbA1c (i.e. the influence of depressive symptoms on the relationship between parental involvement and HbA1c through blood glucose monitoring; β = −0.013, se = 0.005, t = −2.62, P = 0.010). At low levels of adolescent depressive symptoms (score ≤ 6; n = 26, 19.5%), parental involvement was related to HbA1c through blood glucose monitoring. However, when CDI:P scores were 7 or higher (n = 107, 80.5%), indicating more depressive symptomatology, parental involvement was no longer related to HbA1c through blood glucose monitoring. In order to provide a pictorial representation of these results, the prediction of blood glucose monitoring from parental involvement at three different levels of depressive symptoms [−1 sd (CDI:P = 5.8), mean (CDI:P = 12.9) and +1 sd (CDI:P = 20.0)] is depicted in Fig. 2. Figure 2 illustrates that the significant relationship between parental involvement and blood glucose monitoring is present only at lower levels of depressive symptoms. Specifically, the prediction of blood glucose monitoring from parental involvement is not present at the mean (CDI:P = 12.9) and +1 sd (CDI:P = 20.0) levels of depressive symptoms. However, at the −1 sd (CDI:P = 5.8) depressive symptom level, there is a significant relationship between parental monitoring and blood glucose monitoring.
Figure 1.
Model of the interacting influences between parental involvement and depressive symptoms. *Values when depressive symptoms were mean centred. Relationships that are not statistically significant are denoted using dashed lines.
Figure 2.
Blood glucose monitoring predicted from parental involvement by level of depressive symptoms. CDI, Children’s Depression Inventory; DFRQ, Diabetes Family Responsibility Questionnaire.
Discussion
The current results indicate that relationships between parental involvement, blood glucose monitoring frequency and glycaemic control are present only when young people have few depressive symptoms. The presence of higher levels of depressive symptoms appears to interrupt this family-mediated process around management for a large proportion of young people (i.e. 80% in the current sample). These results represent the first study to examine the impact of depressive symptoms on these relationships [10,19]. These data extend the prior literature on the link between adolescent depressive symptoms or parental involvement and diabetes management and control [9,14,15] by demonstrating how these individual and family factors interact longitudinally. The current results also have implications for clinical interventions and research [14,15].
The interaction of depressive symptoms and parental involvement may be a function of several scenarios. First, depressive symptoms leading to low motivation, concentration difficulties and problem-solving deficiencies could prevent young people and families from making effective decisions about parental involvement with the diabetes regimen. For example, irritability, a hallmark depressive symptom in adolescence, could serve as a barrier to family members agreeing on each person’s responsibility for certain management tasks. Second, depressive symptoms can fluctuate [26], causing frequent changes to family behaviours, including to parental involvement. Consequently, parental involvement could also change frequently and not have detectable, enduring influences on blood glucose monitoring and glycaemic control. Third, because depressive symptoms are known to be more common among parents of depressed young people [27], parents with depressive symptoms may be more likely to place a lower priority on or may be less able to maintain involvement in their adolescents’ daily diabetes management.
Fourth, problems and stress associated with depressive symptoms (e.g. negative mood causing frequent conflicts) and caregiving for an adolescent with depressive symptoms could be more pressing for families than those associated with parental involvement. Further, when parents perceive their adolescent to have depressive symptoms, they may view the adolescent as less capable of managing their diabetes regimen and, thus, parents may be less willing to share diabetes management responsibilities.
With these potential mechanisms and the broader results in mind, there are several implications for the clinical care of young people with Type 1 diabetes. As noted, the complex and demanding nature of Type 1 diabetes management is more effectively navigated when there is collaboration within the family about management tasks. Typically, there are efforts made by the diabetes care team to gradually increase a young person’s responsibility for self-management, considering developmental level, maturity, interest and parental involvement. The current study’s findings are consistent with existing recommendations to monitor and consider emotional health as another critical variable when promoting healthy parental involvement and the adolescent’s ability to ‘self-manage’ diabetes [1]. It is possible that, by targeting these aspects of emotional functioning (i.e. depressive symptoms), or by fostering more supportive and collaborative family environments, adherence to blood glucose monitoring guidelines and glycaemic control may improve. As a result, both efficient screening practices for depressive symptoms and effective and accessible interventions are needed for this population.
In order to assess adolescent depressive symptoms and prioritize treatment goals, routine clinic screening followed by triaging of patients to appropriate treatment may be useful [28]. As highlighted by the present results, obtaining parent and adolescent report of depressive symptoms is important. Currently well-established and efficacious treatments for depression in young people (e.g. cognitive behavioural therapy) [29] could be tailored for young people with Type 1 diabetes and for young people with subclinical levels of depressive symptoms [2,30].
If depressive symptoms are a focus of treatment, it may be important to also include negotiating level of parental involvement for the diabetes regimen as a treatment goal, given the links among parental involvement, diabetes management and glycaemic control [10,31]. To this end, interventions targeting family collaboration on diabetes regimen tasks have demonstrated feasibility and effectiveness in clinic settings [32,33]. For example, family interventions teach problem-solving techniques to address barriers to effective diabetes regimen management. Also, interventions promote family use of effective communication strategies which, combined with problem solving, can facilitate discussions about each family member’s responsibilities for management of the diabetes regimen [32,33].
The current study has several strengths, including the longitudinal design and use of statistical techniques allowing tests of how depressive symptoms influence the relationship between a common target of treatment for young people with diabetes (i.e. parental involvement), diabetes management and glycaemic control. In addition, the current study used multiple reporters (i.e. youth and parent report) of parental involvement and depressive symptoms. The results indicated that it is parent perceptions of young people’s depressive symptoms and young people’s perceptions of parental involvement that predict diabetes management and glycaemic control outcomes. When examining these results in the context of the other models tested, the findings highlight the importance of assessing perceptions of mood symptoms and parental involvement from multiple members of the family. Specifically, when examining the relationships between both individual factors (e.g. depressive symptomatology) and family factors (e.g. parental involvement), it may be particularly important to incorporate perceptions of these constructs from the multiple individuals involved (i.e. both parent and child). This may be why the analyses using only one report, the young person’s or the parent’s report, were not statistically significant. Also, diabetes management and control are most likely to be impacted when parents perceive higher adolescent mood symptoms, perhaps because young people are less likely to endorse low levels of mood symptoms, and young people’s perceptions of parental involvement are taken into account.
Study limitations include a relatively homogenous sample in terms of racial and socioeconomic diversity and thus the current results need to be replicated in more diverse patient populations. In addition, the current study took place within a specific (i.e. American) diabetes care delivery system. The results should therefore be replicated by future studies that include patients from healthcare systems outside of the USA, where care practices may differ (e.g. age at which young people do not have to involve parents in their care). Although the participant retention rate was reasonable, those who did not complete all assessments were significantly older. Also, the current study examined blood glucose monitoring as an indicator of diabetes management. While blood glucose monitoring is one of the most robust predictors of glycaemic control amongst the host of management behaviours [34,35], diabetes management is a multidimensional construct.
Future studies need to investigate other aspects of the regimen for a more complete assessment of diabetes management. For example, insulin administration and dietary adjustments may be similarly impacted by perceptions of parental involvement and young people’s depressive symptoms, but this remains to be tested. Future research should also include assessment of the qualitative components to parent-adolescent relationships around diabetes management, such as perceived support and display of positive (e.g. warmth) and negative (e.g. highly critical) communication. The current study utilized a measure of parental involvement in diabetes management that focused on quantity of parent involvement. Related to clinical management, future work is needed to adapt and test interventions targeting depressive symptoms and parental involvement for young people with Type 1 diabetes, and to create guidelines for selecting the most appropriate interventions for young people with varying levels of depressive symptoms.
In summary, the current results indicate that assessing for the presence of depressive symptoms is an important first step when working with young people with Type 1 diabetes and their parents to organize and structure level of parental involvement around diabetes management. If present, depressive symptoms may need to be addressed and there are a host of cognitive behavioural treatments that could be used. After evaluating and treating adolescent depressive symptoms, other interventions for negotiating the delicate balance of level of parental involvement may have a more robust impact on diabetes management and control. Implementation of interventions that are well timed and most relevant to families will ultimately lead to improved health outcomes.
Acknowledgments
Funding sources
This research was supported by a career development award from the National Institutes of Health to KKH (K23, DK077340) and a training grant from the National Institutes of Health supporting YPW (T32HD068223).
Abbreviations
- CDI
Children’s Depression Inventory self report
- CDI:P
Children’s Depression Inventory parent report
- DFRQ
Diabetes Family Responsibility Questionnaire
Footnotes
Competing interests
None declared.
References
- 1.Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, et al. Care of children and adolescents with type 1 diabetes. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 2.Wysocki T, Buckloh LM, Lochrie AS, Antal H. The psychologic context of pediatric diabetes. Pediatr Clin North Am. 2005;52:1755–1778. doi: 10.1016/j.pcl.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 3.La Greca AM, Auslander WF, Greco P, Spetter D, Fisher EB, Santiago JV. I get by with a little help from my family and friends: adolescents’ support for diabetes care. J Pediatr Psychol. 1995;20:449–476. doi: 10.1093/jpepsy/20.4.449. [DOI] [PubMed] [Google Scholar]
- 4.Anderson B, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. J Pediatr. 1997;130:257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- 5.Anderson BJ, Auslander WF, Jung KC, Miller JP, Santiago JV. Assessing family sharing of diabetes responsibilities. J Pediatr Psychol. 1990;15:477–492. doi: 10.1093/jpepsy/15.4.477. [DOI] [PubMed] [Google Scholar]
- 6.Palmer DL, Berg CA, Wiebe DJ, Beveridge RM, Korbel CD, Upchurch R, et al. The role of autonomy and pubertal status in understanding age differences in maternal involvement in diabetes responsibility across adolescence. J Pediatr Psychol. 2004;29:35–46. doi: 10.1093/jpepsy/jsh005. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson A, Hauser S, Lavori P, Willett J, Cole C, Wolfsdorf J, et al. Family environment and glycemic control: a four-year prospective study of children and adolescents with insulin-dependent diabetes mellitus. Psychosom Med. 1994;56:401–409. doi: 10.1097/00006842-199409000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Morris AD, Boyle DIR, McMahon AD, Greene SA, MacDonald TM, Newton RW. Adherence to insulin treatment, glycaemic control, and ketoacidosis in insulin-dependent diabetes mellitus. Lancet. 1997;350:1505–1510. doi: 10.1016/s0140-6736(97)06234-x. [DOI] [PubMed] [Google Scholar]
- 9.Ellis DA, Podolski C-L, Frey M, Naar-King S, Wang B, Moltz K. The role of parental monitoring in adolescent health outcomes: impact on regimen adherence in youth with type 1 diabetes. J Pediatr Psychol. 2007;32:907–917. doi: 10.1093/jpepsy/jsm009. [DOI] [PubMed] [Google Scholar]
- 10.Helgeson VS, Reynolds KA, Siminerio L, Escobar O, Becker D. Parent and adolescent distribution of responsibility for diabetes self-care: links to health outcomes. J Pediatr Psychol. 2008;33:497–508. doi: 10.1093/jpepsy/jsm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vesco AT, Anderson BJ, Laffel LMB, Dolan LM, Ingerski LM, Hood KK. Responsibility sharing between adolescents with type 1 diabetes and their caregivers: importance of adolescent perceptions on diabetes management and control. J Pediatr Psychol. 2010;35:1168–1177. doi: 10.1093/jpepsy/jsq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantzer C, Swendsen J, Maurice-Tison S, Salamon R. Anxiety and depression in juvenile diabetes: a critical review. Clin Psychol Rev. 2003;23:787–800. doi: 10.1016/s0272-7358(03)00069-2. [DOI] [PubMed] [Google Scholar]
- 13.Grey M, Whittemore R, Tamborlane W. Depression in type 1 diabetes in children: natural history and correlates. J Psychosom Res. 2002;53:907–911. doi: 10.1016/s0022-3999(02)00312-4. [DOI] [PubMed] [Google Scholar]
- 14.McGrady ME, Laffel L, Drotar D, Repaske D, Hood KK. Depressive symptoms and glycemic control in adolescents with type 1 diabetes. Diabetes Care. 2009;32:804–806. doi: 10.2337/dc08-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whittemore R, Kanner S, Singleton S, Hamrin V, Chiu J, Grey M. Correlates of depressive symptoms in adolescents with type 1 diabetes. Pediatr Diabetes. 2002;3:135–143. doi: 10.1034/j.1399-5448.2002.30303.x. [DOI] [PubMed] [Google Scholar]
- 16.McGrady ME, Hood KK. Depressive symptoms in adolescents with type 1 diabetes: associations with longitudinal outcomes. Diabetes Res Clin Pract. 2010;88:e35–e37. doi: 10.1016/j.diabres.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole DA, McPherson AE. Relation of family subsystems to adolescent depression: implementing a new family assessment strategy. J Fam Psychol. 1993;7:119–133. [Google Scholar]
- 18.Sheeber L, Hops H, Alpert A, Davis B, Andrews J. Family support and conflict: prospective relations to adolescent depression. J Abnorm Child Psychol. 1997;25:333–344. doi: 10.1023/a:1025768504415. [DOI] [PubMed] [Google Scholar]
- 19.Berg CA, Wiebe DJ, Beveridge RM, Palmer DL, Korbel CD, Upchurch R, et al. Mother-child appraised involvement in coping with diabetes stressors and emotional adjustment. J Pediatr Psychol. 2007;32:995–1005. doi: 10.1093/jpepsy/jsm043. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs M. Children’s Depression Inventory Manual. Multi-Health Systems; North Tonawanda, NY: 1992. [Google Scholar]
- 21.Hood KK, Huestis S, Maher A, Butler D, Volkening L, Laffel LMB. Depressive symptoms in children and adolescents with type 1 diabetes. Diabetes Care. 2006;29:1389–1391. doi: 10.2337/dc06-0087. [DOI] [PubMed] [Google Scholar]
- 22.Guilfoyle SM, Crimmins NA, Hood KK. Blood glucose monitoring and glycemic control in adolescents with type 1 diabetes: meter downloads versus self-report. Pediatr Diabetes. 2011;12:560–566. doi: 10.1111/j.1399-5448.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- 23.Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivariate Behav Res. 2007;42:185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- 24.Preacher K, Hayes A. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 25.SPSS I. SPSS 17. Statistical Package for the Social Sciences; Chicago, IL: 2009. [Google Scholar]
- 26.Dumenci L, Windle M. A latent trait-state model of adolescent depression using the Center for Epidemiologic Studies-Depression scale. Multivariate Behav Res. 1996;31:313–330. doi: 10.1207/s15327906mbr3103_3. [DOI] [PubMed] [Google Scholar]
- 27.Rice F, Harold G, Thapar A. The genetic aetiology of childhood depression: a review. J Child Psychol Psychiatry. 2002;43:65–79. doi: 10.1111/1469-7610.00004. [DOI] [PubMed] [Google Scholar]
- 28.Hilliard ME, Herzer M, Dolan LM, Hood KK. Psychological screening in adolescents with type 1 diabetes predicts outcomes one year later. Diabetes Res Clin Pract. 2011;94:39–44. doi: 10.1016/j.diabres.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurt MD, Crowley SL. How effective are treatments for child and adolescent depression?: a meta-analytic review. Clin Psychol Rev. 2002;22:247–269. doi: 10.1016/s0272-7358(01)00089-7. [DOI] [PubMed] [Google Scholar]
- 30.Hood KK, Nansel TR. Commonalities in effective behavioral interventions for children and adolescents with type 1 diabetes: a review of reviews. Diabetes Spectr. 2007;20:251–254. [Google Scholar]
- 31.Anderson BJ, Holmbeck G, Iannotti RJ, McKay SV, Lochrie A, Volkening LK, et al. Dyadic measures of the parent-child relationship during the transition to adolescence and glycemic control in children with type 1 diabetes. Fam Syst Health. 2009;27:141–152. doi: 10.1037/a0015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laffel LM, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. J Pediatr. 2003;142:409–416. doi: 10.1067/mpd.2003.138. [DOI] [PubMed] [Google Scholar]
- 33.Nansel TR, Anderson BJ, Laffel LMB, Simons-Morton BG, Weissberg-Benchell J, Wysocki T, et al. A multisite trial of a clinic-integrated intervention for promoting family management of pediatric type 1 diabetes: feasibility and design. Pediatr Diabetes. 2009;10:105–115. doi: 10.1111/j.1399-5448.2008.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helgeson VS, Honcharuk E, Becker D, Escobar O, Siminerio L. A focus on blood glucose monitoring: relation to glycemic control and determinants of frequency. Pediatr Diabetes. 2011;12:25–30. doi: 10.1111/j.1399-5448.2010.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rausch JR, Hood KK, Delamater A, Shroff Pendley J, Rohan JM, Reeves G, et al. Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care. 2012;35:1219–1224. doi: 10.2337/dc11-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]