Abstract
A new design for glucose monitoring with “smart” materials based on self assembly, competitive binding, and resonance energy transfer (RET) is presented. The basic transduction principle is changing RET efficiency from fluorescein isothiocyanate (FITC) to tetramethylrhodamine isothiocyanate (TRITC), as FITC-dextran is displaced from TRITC-Concanavalin A (Con A) with the addition of glucose. Nanoscale fabrication by self-assembly of Con A/dextran into multilayer films, followed by polymer multilayers. The advantages of this approach include physical localization and separation of sensing molecules from the environment via entrapment of the biosensor elements in a semi-permeable polymeric shell, and only functional molecules are included in the sensors. To realize these nanostructures, dissolvable resin microparticles were coated with FITC-dextran+TRITC-Con A multilayers, followed by polyelectrolyte multilayers, and the core particles were then dissolved to yield hollow capsules. The nanoassembly process was studied using microbalance mass measurements, fluorescence spectroscopy, confocal fluorescence microscopy, and zeta-potential measurements. The key findings are that the specific binding between Con A and dextran can be used to deposit ultrathin multilayer films, and these exhibit changing RET in response to glucose. Fluorescence spectra of a microcapsules exhibited a linear, glucose-specific, 27% increase in the relative fluorescence of FITC over the 0–1800 mg/dL range. These findings demonstrate the feasibility of using self-assembled microcapsules as optical glucose sensors, and serve as a basis for work toward better understanding the properties of these novel materials.
Keywords: Self-assembly, microcapsules, resonance energy transfer, glucose sensing
INTRODUCTION
In recent years, interest towards developing biosensors for continuous monitoring with high specificity has increased. Biosensors for monitoring blood glucose are of special interest, because the incidence of diabetes mellitus is increasing uncontrollably worldwide [1,2]. To prevent the complications of this disease, frequent measurements, followed by appropriate action to regulate blood glucose level, have been prescribed [2]. However, patients typically do not comply with these guidelines due to the pain and bother of current testing procedures. Therefore, there is an urgent need for a non-invasive glucose sensor to help improve the lives of many.
To date, some minimally-invasive methods have been developed for sensing blood glucose concentrations, but all of the accurate devices on the market still require extraction of blood samples. Fully-implantable systems with wireless communication are an ideal solution; however, while many such devices are under development, the stability of enzyme-electrochemical systems remains a difficult obstacle to overcome. Sensors based on optical measurements are an attractive alternative, as they may transduce chemical concentration information into an optical signal which may be analyzed spectroscopically [3–7]. Glucose sensors based on fluorescence spectroscopy, due to the high sensitivity and potentially superior specificity based on molecular recognition, are being investigated by many groups [3–8]. One of the more thoroughly investigated approaches for monitoring glucose with fluorescence is based on competitive binding and fluorescence resonance energy transfer (RET). Schultz has shown that the FITC-dextran and TRITC-Concanavalin A (Con A) association is sensitive to glucose concentration, and has reported several variations of sensors based on the same basic principle: with the addition of glucose, dextran is displaced from Con A, resulting in measurable changes in energy transfer [1,3,9–14]. Thus, glucose concentration can be estimated from RET efficiency, and the ratiometric nature of the RET analysis method allows variations in instrumental parameters, assay component concentrations, and measurement configuration to be internally compensated [15–17]. Similarly Lakowicz [4,18–20], and Pickup [7,21] investigated glucose assays based on affinity binding between Con A and dextran using fluorescence lifetime measurements. The best reported Con A/dextran systems for sensing glucose based on competitive binding and RET, had the sensitivity in the range of ~10−4 ratio units/(mg/dL)[12].
The above-described systems have been demonstrated in solution, and using fiber probes, wherein the assay chemistry was entrapped within dialysis tubing such that glucose may diffuse through membrane walls and displace dextran bound to Con A. A more direct approach, better suited to clinical application, is to immobilize the assay chemistry (TRITC-Con A and FITC-dextran) in a biocompatible polymeric carrier. Coté and Pishko proposed a solution based on entrapment of FITC-dextran in poly(ethylene glycol) (PEG) hydrogels with TRITC-Con A covalently bound to the crosslinked gel. The glucose response in this system was demonstrated, and the sensitivity was controlled by varying the Con A:dextran ratio inside the hydrogel [6,22]. While several problems with the glucose sensor materials have not been overcome, additional work on modeling skin optics and instrumentation has shown the promise of this concept for transdermal monitoring of implanted fluorescent microspheres, even for the short-wavelength FITC-TRITC RET pair [23,24].
In this paper, we discuss a novel approach for construction of “smart” glucose sensitive nanoassemblies using layer-by-layer (LbL) self-assembly, which allows for precise construction of composite materials using principles of molecular recognition and electrostatic attraction. The LbL fabrication technique, often resulting in nanocomposite materials with a “fuzzy” structure due to interpenetration of adjacent layers [25], has recently been elaborated to produce microcapsules by depositing the multilayer films on spherical microtemplates. This paper employs a novel combination of materials and assembly conditions to create multilayer films of the glucose-binding protein (Con A) and glucose analog (dextran), each labeled with one fluorophore in a RET pair. The resulting nanostructured microdevices comprise an optical glucose sensor allowing measurements based on fluorescence spectroscopy, utilizing the same basic concept of competitive binding introduced by Schultz in 1979 [9]. It is believed that this is the first demonstration of such a system, wherein a fluorescent assay for glucose is produced by encapsulating Con A and dextran molecules within a nanoengineered polymeric microcapsule. The system retains the advantages of the competitive binding approach: selectivity to the analyte of interest can be ensured, reaction byproducts are eliminated, and there is no consumption of the analyte during the sensing process.
It has been shown that the sensitivity and detection range of the sensor are dependent on the donor-acceptor pairs, and their starting concentrations [7,26]; these factors can be precisely controlled using the recognition properties of Con A for the glucose residues of dextran. Thus, we aim to build precise nanoassemblies of protein/polysaccharide multilayers to control the total sample per unit area, by exploiting the multiple binding sites of Con A and the multiple glucose groups of dextran [27,28]. It was hypothesized that this assembly approach would be successful based on the previous work done on multilayer films of Con A/glycogen [29] and Con A/glycolipids [30] built due to affinity between the molecules and not due to the electrostatic attractions.
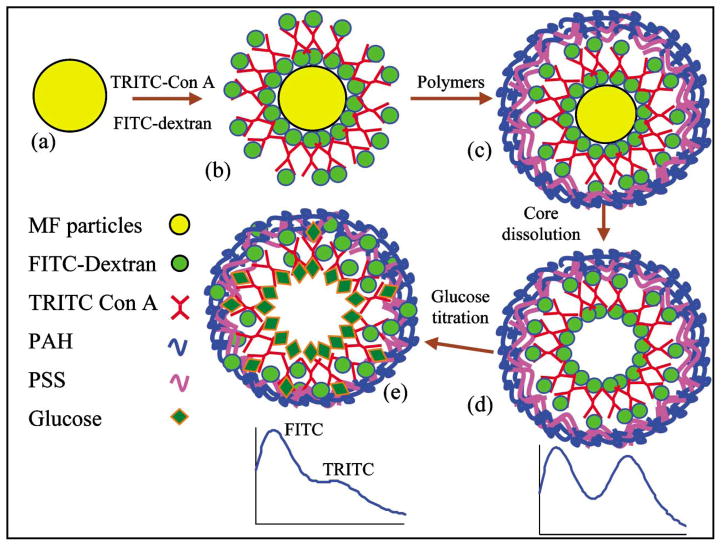
This report expands on previous work by elaborating the precise assembly of Con A/dextran multilayers on planar substrates to microcapsules, and packaging of these dynamic nanoassemblies within permeable polymeric capsules, as shown in Fig. 1. This illustration depicts the encapsulated Con A/dextran thin films inside a membrane of self-assembled polymers. In this arrangement, glucose may diffuse into the capsule interior (under equilibrium conditions) and displace dextran from Con A. By careful selection of polymers, physical entrapment of the large dextran and Con A molecules along with rapid glucose transport is possible.
Fig. 1.
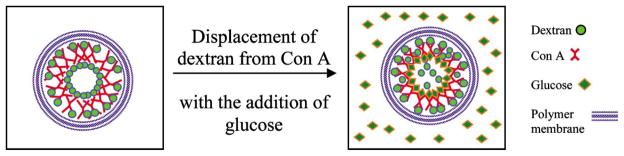
Encapsulation of Con A/dextran assemblies in a capsule with polymeric membrane, and dissociation of nanoassemblies with the addition of glucose. The shape and size of the components are not intended to describe the true properties.
This study was completed to characterize the self assembly properties and, in particular, the glucose sensitivity of self-assembled Con A/dextran multilayer films. To assess the association and dissociation of the ultrathin films, mass deposition, molecular association, surface charge, and spectral distribution of the Con A/dextran films were quantified using quartz crystal microbalance, confocal microscopy, zeta-potential analysis, and fluorescence spectroscopy, respectively. Furthermore, the glucose sensitivity of the TRITC-Con A/FITC-dextran-coated particles and capsules was demonstrated by measuring changes in RET between FITC and TRITC resulting from titration of glucose. Such micro/nano scale systems containing Con A/dextran multilayers may potentially be used for in-vivo sensing glucose, if appropriately designed to eliminate concerns over Con A toxicity [23]. In addition, in the future, such a system may be used in other applications, such as drug delivery, wherein the Con A-dextran dissociation determines release in response to glucose [31]. More importantly, however, this demonstration suggests that the approach of self-assembling sensing elements based on molecular recognition may be more generally useful—similar methods could be used in the construction in a broad array of sensing systems just by selecting the appropriate set of associating molecules and fluorophore RET pairs.
Sensor Design
A schematic of the glucose microsensor fabrication process is shown in Fig. 2. To produce hollow capsules containing the assay components, soluble microparticles are coated with multilayers of FITC-dextran and TRITC-Con A thin films, followed by sequential deposition of oppositely charged polyelectrolytes. In the final step of fabrication, the particle core is dissolved, resulting in hollow capsules with immobilized Con A and dextran as the interior wall. The advantage of hollow capsules is the decreased diffusion barrier provided by the interior, which should give rise to rapid equilibrium and more uniform distribution of glucose in the films. As shown in Fig. 2, strong fluorescence peaks due to both FITC and TRITC are present after assembly of the films, indicating there is considerable energy transfer. With the addition of glucose, dextran is displaced from Con A, resulting in a decrease in energy transfer efficiency as indicated (Fig. 2) by a stronger FITC peak relative to TRITC. The details of experiments investigating these properties are given in the following sections.
Fig. 2.
Fabrication process flow diagram (LbL assembly) of the biosensor (a) Microparticle as positively-charged substrate (b) Deposition of dextran (anionic) and Con A layers alternatively (c) Adsorption of the polymer multilayers (d) Dissolution of core to yield capsules (e) Titration of glucose to displace dextran from Con A. These illustrations depict the structure and dynamics of the system in a conceptual way; the exact physical structure of the films during interaction with glucose is not yet clear.
EXPERIMENTAL SECTION
Materials
Poly(allylamine hydrochloride) (PAH, 15 kDa), poly(dimethyldiallyl ammonium chloride) (PDDA, MW 200 kDa), and poly(styrene sulfonate) (PSS, MW 70 kDa) from Aldrich were used as polyelectrolytes. Solutions of 2 mg/mL in DI water were prepared for each polymer, and these were used at neutral pH. An aqueous dispersion 10 wt% of 5-μm diameter melamine formaldehyde (MF) dissolvable resin particles was obtained from Microparticles Gmbh (Germany). FITC Dextran (MW 9 kDa, 150 kDa and 2 MDa) and Succinyl-Con A (MW 54 kDa) were purchased from Aldrich and EY Labs, respectively. Aqueous stock solutions of 0.5 mg/mL and 1 mg/mL were prepared in DI water for dextran and Con A, respectively, and the pH was adjusted to 8.5 by titrating NaOH. Tetramethylrhodamine isothiocyanate (TRITC, Molecular Probes) was used to label succinyl-Con A using a standard amine-labeling protocol [32]. Glucose, from Sigma, was dissolved in DI water at neutral pH, to prepare a 100 mg/mL stock solution. All solutions used in the experiments involving Con A contained 1 mM concentrations of calcium chloride (CaCl2) and magnesium chloride (MgCl2) salts to preserve Con A and glucose binding [33].
Instrumentation
A quartz crystal microbalance (QCM, USI-System, Japan) was used to study the self-assembly of Con A/dextran thin films. Absorbance spectra were collected using a UV-Vis absorbance spectrometer (Perkin Elmer Lambda 45). Zeta-potential measurements were taken using a ZetaPlus photon correlation spectroscopy and microelectrophoresis instrument (Brookhaven Instruments). A scanning fluorescence spectrometer (QM1, Photon Technology International) was used to collect fluorescence emission spectra from the sample using excitation at 488 nm. The microparticles and capsules containing FITC-dextran/TRITC-Con A were imaged using a confocal microscope (Leica Microsystems).
Assembly on QCM Resonator
To confirm the ability to form multilayer films from dextran and Con A, the LbL assembly of Con A/dextran was first monitored with QCM by constructing multilayer films on a quartz resonator. When a mass is adsorbed on the resonator there is a shift in resonant frequency, which is related to the adsorbed mass by the Sauerbrey equation [30]. According to this relationship, the change in frequency (ΔF, Hz) is related to total mass of the adsorbed thin film layer (Δm, ng) as Δm = −0.87ΔF.
QCM resonators were used as substrates for alternate assembly of FITC-dextran /Succinyl-Con A thin films. The resonator was a silver electrode, with surface area of 0.16 cm2 and natural resonance frequency of 9 MHz. During the assembly procedure, each resonator was dipped in the solution of polymer to be deposited for 15 min, which was determined through previous tests to be sufficient for adsorption saturation on the resonator surface. The resonance frequency, related to the adsorbed mass, was measured after rinsing the crystal with water and drying with nitrogen. Precursor films of (PAH/PSS)2/PDDA were deposited to obtain a smooth and uniformly charged substrate prior to the addition of the polysaccharide/protein multilayers. For FITC-dextran (MW 2 MDa) and succinyl-Con A deposition, the resonator was immersed in bulk solution for 25 min to allow enough time for the adsorption to reach steady state. The change in resonance frequency of the QCM was monitored for each adsorption step.
Following the completion of the layering process, the resonator was used to test the sensitivity of the Con A/dextran thin films to glucose by immersing the resonator in 1.5 mL of 100 mg/mL glucose solution. The frequency change was monitored every hour after dipping the resonator in the glucose solution for a period of 7.5 hr. After completing the assembly process on the resonator, absorbance measurements were performed on all the solutions used for deposition, including glucose solution, to assess the desorption of the layered thin film components into the bulk solutions.
Assembly on Microspheres
The techniques used for successful assembly of Con A/dextran multilayers on the planar surfaces of QCM resonators were applied to form Con A/dextran multilayers on the surface of 5 μm MF particles. FITC-dextran (2 MDa, 0.5 mL) was added to 10 μL of the MF particle suspension. After allowing 20 min for complete adsorption saturation, the sample was rinsed using DI water and centrifuged at 4000 rpm for 8 min. This rinse process was repeated twice for each assembly step. Then, 0.5 mL of 0.5 mg/mL TRITC-Con A was added to the sample, and after 20 min exposure, the sample was again rinsed twice. This assembly process was repeated to deposit multiple bilayers of TRITC-Con A/FITC-dextran, such that the outer surface was FITC-dextran.
To assess the charge of the adsorbed material and, therefore, determine the relative contribution of electrostatic attraction to the assembly process, multiple surface-potential measurements were taken after depositing and rinsing each layer. Fluorescence emission spectra were also collected from the suspension after depositing each layer, allowing tracking of the layering process and observation of RET efficiency with each additional component of the nanofilms. Finally, the layered microparticles were imaged using a confocal microscope after each bilayer addition of FITC-Dextran/TRITC-Con A. A 5 μL drop of the suspension was pipetted onto a glass microscope slide, and sequential confocal images were taken with a 63× oil objective by exciting FITC at 488 nm and TRITC at 543 nm using Ar/ArKr and green HeNe lasers, respectively.
Glucose Effect on Con A/Dextran-Coated MF Particles
After completing the assembly of FITC-dextran and TRITC-Con A multilayers, fluorescence spectra of the particles and surrounding medium were collected. This was performed to determine the relative contribution of particles to fluorescence signals, and to observe whether significant amounts of fluorophore-tagged macromolecules were present in the solution in free form. To assess the glucose-binding properties of the assembly, glucose was added to the continuously-stirred particle suspension in an attempt to displace FITC-dextran from TRITC-Con A. After each addition, fluorescence emission measurements were performed using 488 nm excitation to observe the change in energy transfer. After the final step of adding glucose, the particles were centrifuged and the fluorescence was observed to assess the effect of the dissociation of FITC-dextran from TRITC-Con A on film stability. A comparison of the supernatant fluorescence spectra before and after addition of glucose was used to demonstrate the sensitivity of the Con A/dextran nanoassemblies to glucose. These experiments were completed in parallel to the studies with QCM measurements, such that the two sets of data could confirm the assembly and dissocation of dextran/Con A multilayers both on planar surfaces and spherical microtemplates.
Encapsulation and Hollow Capsule Formation
Following successful demonstration of dextran/Con A multilayer glucose sensitivity, and observation of the partial decomposition of these films, an ultrathin polymer shell was constructed around the Con A/dextran-coated particles to stabilize the localization of the polysaccharide and protein molecules. As the outer layer was FITC-dextran, and zeta-potential measurements confirmed that the surface charge was anionic, the coated microparticles were first suspended in a solution of the polycation PAH. After 15 min of exposure, the sample was rinsed twice, and surface potential was measured. This procedure was followed to deposit a total of four bilayers of PAH and PSS, plus one additional outer layer of PAH. Thus, the final film architecture on the surface of the particles at the conclusion of the assembly was {(FITC-Dextran/TRITC-Con A)3/FITC-dextran/(PAH/PSS)4/PAH}. Following the completion of polymer layer deposition, MF core particles were dissolved by adding 0.5 mL of HCl at pH 1.1 to the particle suspension [27,34]. Finally, the capsules were centrifuged and rinsed with DI water to remove residual MF monomer.
Glucose Effect on Capsules containing Con A/ Dextran Multilayers
After encapsulation of the dextran/Con A films within the polymeric shells and removal of the solid MF cores, the glucose response of the capsules was studied using the same procedures applied to the coated particles. The key comparisons between the two cases (dextran/Con A films on solid particles versus hollow capsules with dextran/Con A multilayers inside a shell of polyelectrolyte multilayers) were the change in RET following exposure to glucose, and the decomposition of the dextran/Con A layers, releasing molecules to the surrounding medium. To observe the glucose sensitivity of the microcapsules, 20 μL additions of 100 mg/mL glucose stock solution were given to 1 mL of a continuously-stirred capsule suspension (200 mg/dL per step). To observe the change in energy transfer, fluorescence spectra were collected after each addition of glucose by exciting the sample at 488 nm and collecting the emission spectra from 500 nm to 650 nm. After the final step of glucose addition (200 μL total added), the capsules were centrifuged and the supernatant fluorescence was measured to observe if displaced dextran was released from the capsules. Confocal microscopy was also used to observe the change in RET within the capsule due to addition of glucose, and to determine whether FITC-dextran was released into the interior of the capsule when displaced from Con A.
RESULTS AND DISCUSSION
QCM Results
Mass measurements performed after depositing each layer are shown in Fig. 3. This graph presents, in the first phase, the change in adsorbed mass due to deposition of the following multilayer architecture:{(PAH/PSS)2/PDDA/(TRITC-Con A/FITC-Dextran)3/Glucose}. The precursor polymer layers exhibited the expected linear stepwise growth. The average stepwise increase in mass for Con A and dextran addition, not quite linear, corresponds to an average of ~ 60 ng (~1011 molecules) and ~ 40 ng (~109 molecules), respectively. In spite of having a higher MW compared to that of Con A, the average mass of dextran deposited per step was found to be less. This was expected, because the multiple glucose residues of dextran allow association with glucose binding sites on multiple Con A molecules. Thus, the dextran may assemble into a configuration more parallel to the surface, effectively covering more surface area per molecule. While the behavior of the assemblies in this context is important, the structure of these films is not critical for this discussion, and will be the subject of other studies.
Fig. 3.
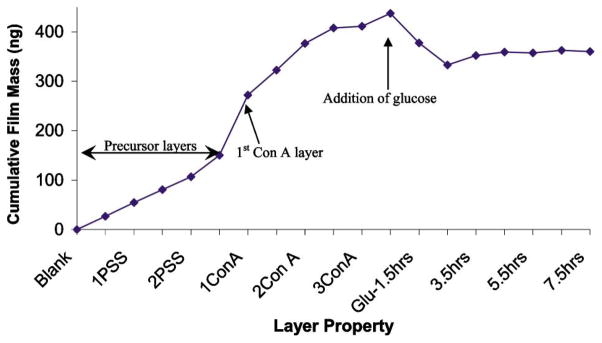
Mass deposited and released during adsorption of polyelectrolytes, FITC-dextran, and succinyl-Con A on the QCM resonator, and exposure of multilayer films to glucose. The increasing mass indicates film assembly, and decreasing mass indicates decomposition of surface-immobilized films due to glucose.
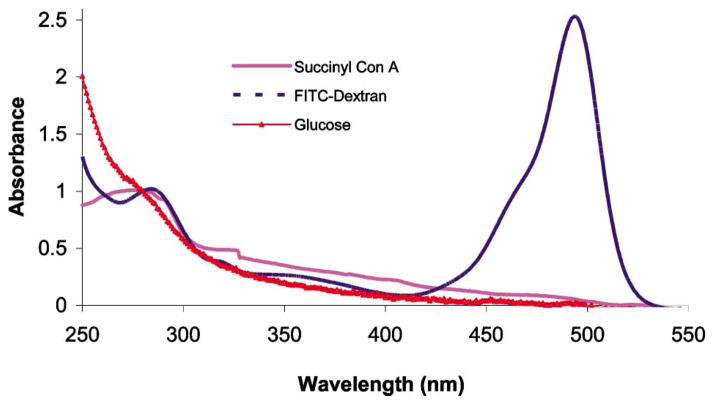
Due to the small number of glucose binding sites on Con A molecules, and the repulsive electrostatic force present between dextran and Con A, it is expected that the adsorption of Con A onto dextran films would be weakly enforced. Thus, it was also hypothesized that dextran could pull some Con A from the resonator surface when the films with Con A outer layers were exposed to dextran solution. To test this theory, absorbance measurements of the solutions used for layering QCM resonator were performed. These measurements, presented in Fig. 4, show that the absorbance spectrum of FITC-dextran assembly solution, after exposure to films with an outer layer of Con A, possessed an absorbance peak at 280 nm. This confirms that some protein was removed from the surface into the solution during this immersion period and, thus, it is likely that the dextran was causing some desorption of Con A. In contrast, the Con A solution, following exposure to films containing a FITC-dextran outer layer, did not contain an absorbance peak at 490 nm. Thus, the opposite effect (Con A removing dextran) was negligible; this is also logical, as the dextran molecules likely bind with multiple Con A molecules on the surface, and these multiple parallel associations per molecule make the adsorption much more stable.
Fig. 4.
Normalized absorbance spectra of stock FITC-dextran and succinyl-Con A solutions used for layering on QCM resonator. The absorbance spectrum of the glucose solution used to test the sensitivity of the Con A/dextran assemblies, after use, is also shown. The FITC-dextran, succinyl-Con A, and glucose solution supernatant spectra are scaled with factors of 12.5×, 25×, and 1000×, respectively.
In experiments to assess the glucose response of the films, the QCM resonators with dextran/Con A multilayers were exposed to 100 mg/mL glucose solution for 7.5 hr. Over this immersion period, the quartz resonator exhibited an increase in resonance frequency with time, corresponding to a decrease in adsorbed mass (Fig. 3). This was expected behavior, as glucose has been shown to displace dextran in competitive binding [10], and these experiments demonstrate saturation (plateau) of the mass change after approximately 2.5 hr, which corresponds to equilibration of the competition between glucose and dextran for available binding sites on Con A molecules. It is interesting that the response is so slow to equilibrate; this cannot currently be explained with confidence, but may be related to the diffusion barrier provided by the surface and the total mass of glucose present. However, the response was observed to be much more rapid for particle suspensions, which is the emphasis of this report.
Quantitatively, the total decrease in the film mass was 78.3 ng, which must correspond to the difference in glucose mass bound to the films and the dextran and/or Con A removed from the surface. In this case, glucose apparently displaced dextran from Con A, as absorbance measurements of the glucose solution after immersion of the QCM resonator (shown in Fig. 4) possessed weak absorbance at 490 nm. In addition, an absorbance peak at 280 nm suggests the presence of Con A in solution as well. This was also expected, as glucose displacement of dextran from all glucose binding sites of a Con A molecule could free it from the film if other attractive forces are negligible. Thus, the QCM studies demonstrated the affinity-binding nanoassemblies of dextran/Con A behaved as expected, and further investigation was warranted.
Microparticle Coating Results
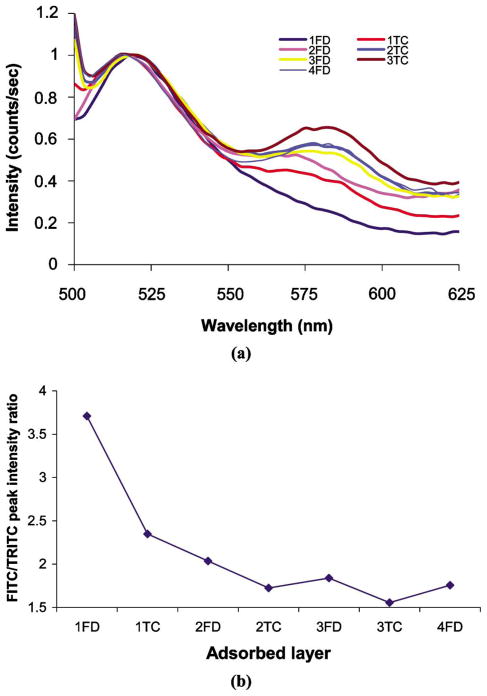
The surface potential of MF particles was measured to determine the contribution of electrostatic forces to dextran-Con A association for deposition of the multilayers with the architecture: {(FITC-Dextran/TRITC-ConA)3/FITC-Dextran/(PAH/PSS)4/PAH}. The average and standard deviation of the multiple zeta potential measurements for the particles after addition of each layer are given in Fig. 5. The first layer of FITC-dextran (anionic) was bound to the MF (cationic) particles’ surfaces, primarily due to electrostatic forces of attraction. However, the continuous negative potential values measured for Con A/dextran multilayers (1 FD–4 FD in Fig. 5) correspond to the adsorption of materials with negative charge. These values do not confirm successful deposition of the materials; however, the QCM data (Fig. 3) suggest film assembly, and the fluorescence spectra of particles (Figure 6a) directly confirm alternate assembly of Con A/dextran in multilayers. Thus, taken together, these data confirm that layer-by-layer assembly of FITC-Dextran and TRITC-Con A can be performed under neutral conditions, in spite of both molecules carrying net negative charge, and the association between Con A and dextran molecules is due to binding affinity. The attractive forces involved in this association are sufficient to overcome electrostatic repulsion. It can be observed from Fig. 6b that the FITC/TRITC peak intensity ratio decreases with the addition of each layer, up to two bilayers of FITC-dextran and TRITC-Con A, indicating that there is increase in energy transfer from FITC to TRITC. After two bilayers, the peak intensity ratio remained constant, suggesting that a constant level of energy transfer is achieved due to consistent association of dextran and Con A molecules with similar average distance between FITC and TRITC. This is an interesting finding, and will be studied in the future within the context of characterizing the structure and function of these novel ultrathin films.
Fig. 5.
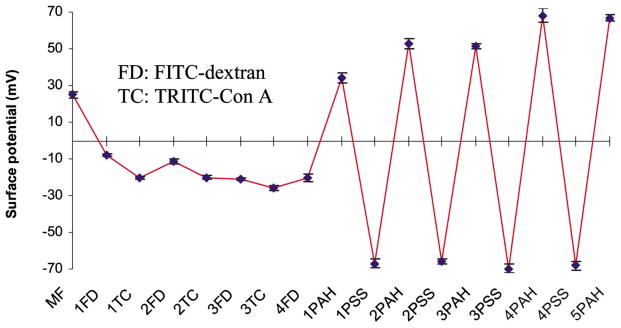
Mean and standard deviation values for zeta potential measurements of coated MF particles. Film architecture: {(FITC-Dextran/TRITC-Con A)3/FITC-Dextran (PAH/PSS)4 (PAH)}.
Fig. 6.
(a) Normalized fluorescence spectra of particle suspension after depositing fluorescent materials FITC-dextran (FD) and TRITC-Con A (TC); (b) FITC/TRITC peak intensity ratios after adsorbing each layer on MF particles.
To observe if free dextran and Con A molecules were present in the sample, the fluorescence of the coated particles was compared with the supernatant fluorescence following separation by centrifugation. It can be observed from Fig. 7a that the supernatant fluorescence had a prominent FITC peak compared to TRITC, and the total emission intensity was an order of magnitude less than the suspension fluorescence. In comparing the fluorescence of the particles and the supernatant after adding glucose (Fig. 7b), the relative FITC fluorescence of the supernatant increased significantly. Thus, it is apparent that when glucose was added to the sample, it was bound to Con A and displaced dextran into the bulk solution. By combining these observations with the QCM data presented earlier, the glucose interaction process may be described as follows: as dextran is released, the films partially decompose, and Con A-glucose complexes are also released. The absence of TRITC fluorescence in the supernatant after adding glucose indicates that the TRITC-Con A released into the supernatant are associated with glucose, not dextran, and therefore significant RET is not present.
Fig. 7.
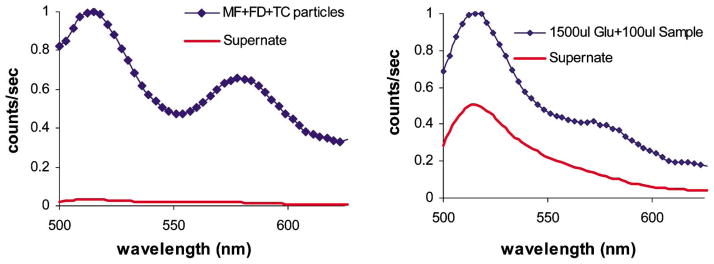
Fluorescence spectra of MF particle suspension coated with {(FITC-dextran/TRITC-Con A)3/FITC-dextran}, along with supernatant a) prior to addition of glucose, and b) after glucose titration.
Because ultrathin films based on electrostatic LbL self assembly are known to swell with the increase in salt concentration [35], additional experiments were performed to assess whether the observed changes in RET and release of FITC-dextran could have resulted from nonspecific effects of ionic strength or other properties of the environment. Fluorescence spectra of the particles (MF particles with FITC-dextran and TRITC-Con A multilayer films, without polymer coatings) and supernatant, following the addition of NaCl (up to 0.5 M) and dilution with water showed that there was no change in the FITC/TRITC peak intensity ratio. Therefore, the change in RET observed from the nanoassembled Con A/dextran multilayer films likely arises specifically due to glucose and, thus, the displacement of dextran from Con A is believed to be a result of competitive binding of glucose rather than a nonspecific change in the films. It is expected that these assemblies will have the same selectivity for glucose over other sugars as has been shown in other work [10], though this property is not investigated here.
Polymer-Coated Microcapsules
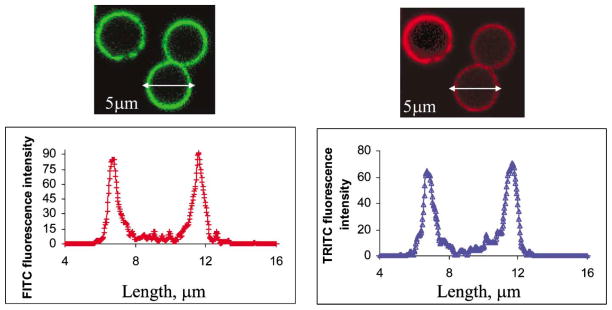
The charged polymers were deposited on top of the Con A-dextran multilayer films via electrostatic self assembly, and the charge was varied from positive to negative in alternate fashion. The alternation of surface potential values (Fig. 5, 1 PAH–5 PAH) corresponds to the adsorption of the positive (PAH) and negative (PSS) species, respectively. Confocal fluorescence images of the 5-μm capsules formed by MF dissolution are shown in Fig. 8, from which it can be observed that the capsule walls remain intact after core dissolution. In addition, there does not appear to be significant amounts of free fluorescent molecules inside or outside the capsules, indicating that the Con A/dextran assemblies remain mostly intact. The fluorescence intensity line scans of the capsules show the spatial distribution of fluorescence emitted from the image plane for both FITC and TRITC. These data clearly show the dimension of the capsules is approximately 5 μm, and the fluorescence arising from the walls is much stronger than the background from the capsule interior and exterior.
Fig. 8.
Confocal microscope images of capsules with {(FITC-dextran/TRITC-Con A)4/FITC-dextran/ (PAH/PSS)4/PAH} as shell structure, and corresponding intensity line scans of capsules, showing FITC (left) and TRITC (right) intensities.
It was hypothesized that the dextran in these capsules would be displaced from Con A by glucose. By this process, increasing glucose concentration in the suspension of hollow capsules would result in an increase of free FITC-dextran concentration and, hence, increasing FITC fluorescence was expected from the suspension. Correspondingly, the distance between dextran and Con A was expected to increase, resulting in a decrease in the energy transfer between FITC and TRITC, due to which the relative fluorescence of TRITC would decrease [11].
To test these expectations, fluorescence emission spectra of polymer-coated capsules were monitored with increasing glucose concentration. The FITC/TRITC peak intensity ratio plotted versus glucose concentration is shown in Fig. 9. It can be observed that the FITC fluorescence did, in fact, increase relative to the TRITC fluorescence, up to approximately 1200 mg/dL glucose. Over this range, the sensitivity curve shows approximately linear increase in FITC:TRITC fluorescence peak intensity ratio with glucose concentration. The slope of the sensitivity curve in the linear region was calculated to be 4 × 10−4 ratio units/(mg/dL), which corresponds to ~7–10% of the total ratio change for each step of 100 mg/dL of glucose. Above the linear range, the signal appears to plateau, indicating the saturation of Con A binding sites with glucose. This sensitivity is near to that of the best case reported in the literature using intensity measurements of RET, and was achieved without any attempt to optimize sensitivity or signal-to-noise ratio; therefore, these results are encouraging and suggest further work may lead to improvements in the system properties related to measurement performance.
Fig. 9.
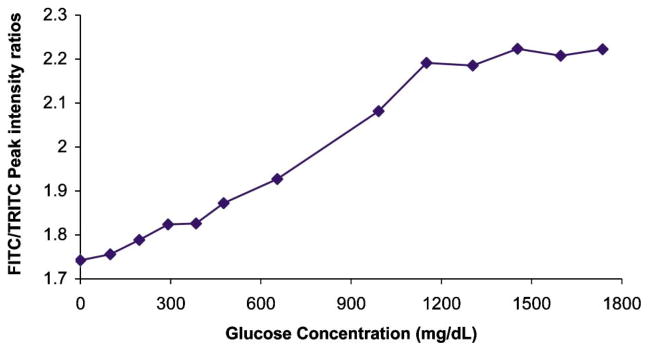
Effect of glucose titration into suspension of capsules comprising {(FITC-dextran/TRITC-Con A)4/FITC-dextran/(PAH/PSS)4/PAH} as shell layers.
In addition to testing response to glucose measured by changing RET, the stability of the structures was investigated. After completing the addition of glucose, the capsules were centrifuged and the supernatant was collected to determine loss of fluorescent molecules from the microcapsules. The emission intensity of the supernatant was two orders of magnitude lower than the emission from capsules and, because the peak ratio was identical to that of the capsules, even this small signal can be attributed to inefficient separation of capsules from supernatant. This is in contrast to the findings when assessing FITC-dextran/TRITC-Con A assemblies on MF particles without the polymer shell, as noted above, where significant loss of FITC-dextran was seen in the supernatant. These results highlight the improved stability of the polysaccharide/protein films encapsulated by polymer nanofilms, and supports further development of this technology. However, further investigation will focus on quantitatively determining whether FITC-dextran or TRITC-Con A is released from the microcapsules.
The results of the response of the microcapsule suspension to glucose, taken together, indicate that the presence of glucose did result in separation of Con A and dextran, such that the energy transfer between FITC and TRITC was decreased. Using the Förster distance for FITC and TRITC fluorophores as 55 Å [36], the estimated change in the average distance between FITC and TRITC molecules was found to be 3.5 Å with the addition of ~0.1 M glucose, which is a reasonable number given the size of a glucose molecule (~10 Å). These small displacements could easily be achieved without significant rearrangement of the ultrathin films, and could be a result of movement of glucose residues on dextran to accommodate for glucose molecules binding to the Con A sites.
Further confirmation of the stability of the microcapsules was provided by the confocal microscope images of the capsules after adding glucose. Still, these did not show any significant fluorescence inside or outside the capsules. There could be several reasons for not observing free molecules after glucose addition. The dextran and Con A molecules may be moving apart such that there is change in RET, but they remain immobilized within the films. Thus, the films are sufficiently deformable that they can accommodate this change. This is reasonable, given the calculation of 3.5Å for average displacement given above, which would not be resolved with microscopic measurements. Alternatively, the dextran and Con A molecules may be completely separated, but the free FITC-dextran molecules may be stuck in the polymer layers due to the large size. Regardless of the true physical arrangement of the molecules in the films, which will be the subject of further studies, the results here demonstrate the concept of RET-based glucose sensing using dextran-Con A multilayers, and further work to investigate these novel nanostructured materials is warranted.
CONCLUSION
In this paper, a novel glucose sensing system based on the LbL self assembly technique, competitive binding, and RET is demonstrated. Glucose-sensitive Con A/dextran multilayer films were assembled on planar substrates and microparticles due to affinity between molecules, and RET between the FITC and TRITC labels can be tailored by adjusting the number of Con A/dextran multilayers. Hollow capsules containing Con A/dextran multilayers showed a decrease in RET with the addition of glucose, with a detection range of this sensor system is from 0 to 1800 mg/dL and a sensitivity of 4 × 10−4 ratio units/(mg/dL). It was estimated from the data that the addition of ~0.1 M glucose solution to the capsule suspension, the Con A and dextran molecules moved apart by an average distance of approximately 3.5 Å, which corresponds to a 27% increase in the FITC:TRITC intensity ratio. Further experiments need be performed to understand how the number of Con A/dextran multilayers, labeling ratios, and other properties can be controlled to obtain maximum sensitivity, but these early results are encouraging. These findings show that the use of RET, competitive binding, and LbL principles together is a promising approach to build sensors, and this should be more generally useful in building many other biosensor elements beyond the model Con A-dextran system. Similar approaches to constructing microcapsules with species-specific response could potentially be used to realize drug carriers for controlled delivery, depending upon the structural dynamics of the ultrathin films in response to the analyte.
References
- 1.Mansouri S, Schultz JS. A miniature optical glucose sensor based on affinity binding. Biotechnology. 1984;2:885–890. [Google Scholar]
- 2.Griffiths DG, Hall G. Biosensors—what real progress is being made? Trends Biotechnol. 1993;111:122–130. doi: 10.1016/0167-7799(93)90086-O. [DOI] [PubMed] [Google Scholar]
- 3.Meadows DL, Schultz JS. Fiber-optic biosensors based on fluorescence energy transfer. Talanta. 1988;35(2):145–150. doi: 10.1016/0039-9140(88)80053-5. [DOI] [PubMed] [Google Scholar]
- 4.Cesare ND, Lakowicz JR. Wavelength ratiometric fluorescent probes for glucose. Proc SPIE. 2002;4625:152–159. [Google Scholar]
- 5.Pickup JC, McCartney L, Rolinski OJ, Birch DJS. In vivo glucose sensing for diabetes management: Progress towards non-invasive monitoring. BMJ. 1999;319:1–4. doi: 10.1136/bmj.319.7220.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell RJ, Pishko MV, Gefrides CC, McShane MJ, Cote GL. A fluorescence-based glucose biosensor using Concanavalin A and dextran encapsulated in a poly(ethylene glycol) hydrogel. Anal Chem. 1999;71(15):3126–3132. doi: 10.1021/ac990060r. [DOI] [PubMed] [Google Scholar]
- 7.Rolinski OJ, Birch DJS, McCartney L, Pickup JC. Molecular distribution sensing in a fluorescence resonance energy transfer based affinity assay for glucose. Spectrochim Acta, Part A: Mol Biomol Spectroscopy. 2001;57(11):2245–2254. doi: 10.1016/s1386-1425(01)00475-9. [DOI] [PubMed] [Google Scholar]
- 8.McShane MJ. Potential for glucose monitoring with nano-engineered fluorescent biosensors. Diabetes Technol Ther. 2002;4:533–538. doi: 10.1089/152091502760306625. [DOI] [PubMed] [Google Scholar]
- 9.Schultz JS, Sims G. Affinity sensors for individual metabolites. Biotechnol Bioeng Symp. 1979;9:65–71. [PubMed] [Google Scholar]
- 10.Schultz JS, Mansouri S, Goldstein IJ. Affinity sensor: A new technique for developing implantable sensors for glucose and other metabolites. Diabetes Care. 1982;5(3):245–253. doi: 10.2337/diacare.5.3.245. [DOI] [PubMed] [Google Scholar]
- 11.Meadows DL, Schultz JS. Miniature fiber optic sensor based on fluorescence energy transfer. SPIE. 1992;1648:202–211. [Google Scholar]
- 12.Meadows DL, Schultz JS. Design, manufacture and characterization of an optical fiber glucose affinity sensor based on an homogeneous fluorescence energy transfer assay system. Anal Chim Acta. 1993;280:21–30. [Google Scholar]
- 13.Ballerstadt R, Schultz JS. Competitive-binding assay method based on fluorescence quenching of ligands held in close proximity by a multivalent receptor. Anal Chim Acta. 1997;345:203–212. [Google Scholar]
- 14.Ballerstadt R, Schultz JS. Kinetics of dissolution of Concanavalin A/dextran sols in response to glucose measured by surface plasmon resonance. Sens Actuators, B. 1998;46:50–55. [Google Scholar]
- 15.Grant SA, Xu J, Bergeron E, Mroz J. Development of dual receptor biosensors: An analysis of FRET pairs. Biosens Bioelectron. 2001;16(4–5):231–237. doi: 10.1016/s0956-5663(00)00147-0. [DOI] [PubMed] [Google Scholar]
- 16.Chantal M, Lebrun C, Prats M. Fluorescence resonance energy transfer (FRET): Theory and experiments. Biochem Educ. 1998;26(4):320–323. [Google Scholar]
- 17.Wu PG, Brand L. Resonance energy transfer: Methods and applications. Anal Biochem. 1994;218:1–13. doi: 10.1006/abio.1994.1134. [DOI] [PubMed] [Google Scholar]
- 18.Tolosa L, Szmacinski H, Rao G, Lakowicz JR. Lifetime-based sensing of glucose using energy transfer with a long lifetime donor. Anal Biochem. 1997;250:102–108. doi: 10.1006/abio.1997.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolosa L, Malak H, Rao G, Lakowicz JR. Optical assay for glucose based on the luminescence decay time of the long wavelength dye Cy5™. Sen Actuators B. 1997;45:93–99. doi: 10.1016/S0925-4005(97)00275-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakowicz JR, Maliwal B. Optical sensing of glucose using phase-modulation fluorimetry. Anal Chim Acta. 1993;271(1):155–164. [Google Scholar]
- 21.Rolinski OJ, Birch DJS, McCartney LJ, Pickup JC. Fluorescence resonance energy transfer from allophycocyanin to malachite green. Chem Phys Lett. 1999;309:395–401. [Google Scholar]
- 22.Ibey BL, Meledeo MA, Gant VA, Yadavalli V, Pishko MV, Cote GL. In vivo monitoring of blood glucose using poly(ethylene glycol) microspheres. Proc SPIE. 2003;4965:1–6. [Google Scholar]
- 23.McShane MJ, Rastegar S, Pishko MV, Coté GL. Monte Carlo modeling for implantable fluorescent analyte sensors. IEEE Trans Biomed Eng. 2000;47:624–632. doi: 10.1109/10.841334. [DOI] [PubMed] [Google Scholar]
- 24.McShane MJ, Russell RJ, Pishko MV, Coté GL. Towards minimally-invasive glucose monitoring using implanted fluorescent microspheres. IEEE-EMBS Mag. 2000;19:36–45. doi: 10.1109/51.887244. [DOI] [PubMed] [Google Scholar]
- 25.Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 1997;277:1232–1237. [Google Scholar]
- 26.Birch DJS, Rolinski OJ. Fluorescence resonance energy transfer sensors. Resour Chemi Intermed. 2001;27(4–5):425–446. [Google Scholar]
- 27.McShane MJ, Brown JQ, Guice KB, Lvov YM. Polyelectrolyte microshells as carriers for fluorescent sensors: Loading and sensing properties of a ruthenium-based oxygen indicator. J Nanosci Nanotechnol. 2002;2:411–416. doi: 10.1166/jnn.2002.118. [DOI] [PubMed] [Google Scholar]
- 28.Lvov YM, Ariga JK, Ichinose I, Kunitake T. Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J Am Chem Soc. 1995;117(22):6117–6123. [Google Scholar]
- 29.Lvov YM, Ariga JK, Ichinose I, Kunitake T. Layer-by-layer architectures of Concanavalin A by means of electrostatic and biospecific interactions. J Chem Soc Chem Commun. 1995:2313–2314. [Google Scholar]
- 30.Ebara Y, Okahata Y. A kinetic study of Concanavalin A binding to glycolipid monolayers by using quartz crystal microbalance. J Am Chem Soc. 1994;116(25):11209–11212. [Google Scholar]
- 31.Tanna S, Taylor MJ, Adams G. Insulin delivery governed by covalently modified lectin-glycogen gels sensitive to glucose. J Pharm Pharmacol. 1999;51:1093–1098. doi: 10.1211/0022357991776778. [DOI] [PubMed] [Google Scholar]
- 32.Svensson HP, Kadow JF, Vrudhula VM, Wallace PM, Senter PD. Monoclonal antibody-b-lactamase conjugates for the activation of a cephalosporin mustard prodrug. Bioconjugate Chem. 1992;3(2):176–181. doi: 10.1021/bc00014a013. [DOI] [PubMed] [Google Scholar]
- 33.Bittiger H, Schnebli HP. Concanavalin A as a Tool. Wiley; London: 1976. [Google Scholar]
- 34.Sukhorukov GB, Dähne L, Hartmann J, Donath E, Möhwald H. Controlled precipitation of dyes into hollow polyelectrolyte capsules based on colloids and biocolloids. Adv Mater. 2000;12:112–115. [Google Scholar]
- 35.Ibarz G, Dahne L, Donath E, Möhwald H. Smart micro- and nanocontainers for storage, transport, and release. Langmuir. 2001;9(2):481–486. [Google Scholar]
- 36.Lakowicz JR. Principles of Fluorescence Spectroscopy. 2. Kluwer Academic/Plenum; New York: 1999. [Google Scholar]