Abstract
Most studies of psychosocial predictors of disease progression in HIV have not considered norepinephrine (NE), a neurohormone related to emotion and stress, even though NE has been related to accelerated viral replication in vitro and impaired response to ART. We therefore examine NE, cortisol, depression, hopelessness, coping, and life event stress as predictors of HIV progression in a diverse sample. Participants (n = 177) completed psychological assessment, blood draws (CD4, viral load (VL)), and a 15-hour urine sample (NE, cortisol) every 6 months over 4 years. HLM was used to model slope in CD4 and viral load controlling for ART at every time point, gender, age, race, SES, and initial disease status. NE (as well as depression, hopelessness, and avoidant coping) significantly predicted a greater rate of decrease in CD4 and increase in VL. Cortisol was not significantly related to CD4, but predicted VL increase. To our knowledge, this is the first study relating NE, in vivo, to accelerated disease progression over an extended time. It also extends our previous 2 year study by relating depressed mood and coping to accelerated disease progression over 4 years.
Keywords: HIV/AIDS, disease progression, norepinephrine, coping, stress
Introduction
There is considerable unexplained variation in the course of HIV (1, 2, 3, 4). It is plausible that psychological (5, 6, 7, 8, 9) and physiological (2, 10, 11, 12) correlates of stress, distress and coping may account for some of this unexplained variation, directly and/or via behavioral pathways (13). Individuals living with HIV, particularly women, have disproportionately high rates of trauma exposure and PTSD (14). Many studies have found associations between trauma exposure (15), PTSD (16), and immune and endocrine alterations, which may also account for variability in HIV progression (17). Stress has been shown to predict more rapid CD4 cell decline (18, 17) and viral load increase (19), as well as the presentation of clinical symptoms (20), and progression to AIDS (10). Stressful life events have been found to be inversely related to medication adherence (17) and greater levels of perceived stress predict reduced effectiveness of antiretrovirals (7). Similarly, depression has been related to poorer trajectory of CD4 cells and viral load (3, 19, 21, 22, 23, 24), AIDS onset, and mortality (1, 3, 17, 25). Prospective coping studies have related avoidant coping responses to stress to greater CD4 decline (19), progression to AIDS (20, 2), and mortality (20). The more recent prospective studies have established these relationships with HIV disease progression even in the era of widespread availability of highly active antiretroviral therapy (HAART), in diverse samples, and accounting for medication adherence (26, 19, 27), but have yet to be established over longer follow-up periods.
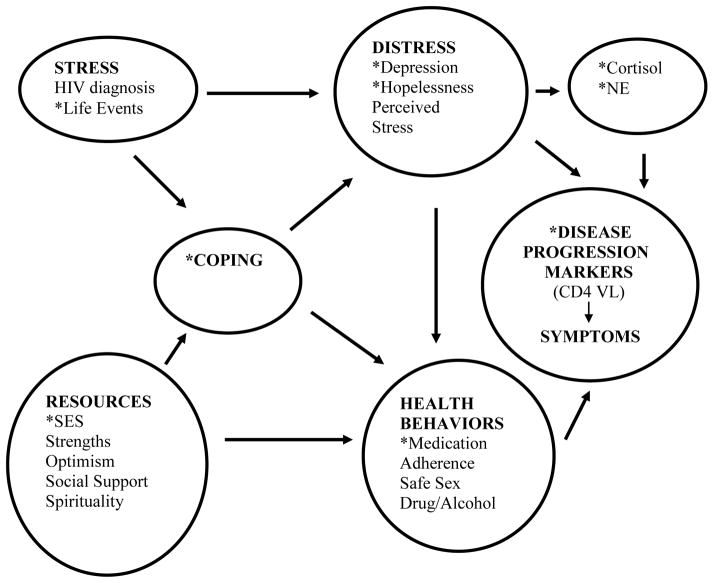
The physiological mechanisms linking psychological stress responses to disease outcomes in HIV remain largely unknown (4, 28). It has been suggested that the stress hormones cortisol and norepinephrine (NE) may represent potential pathways linking psychological stress response to health outcomes in HIV (29). This is illustrated in Figure 1. (Figure 1 also includes other pathways, not tested in this paper, such as health behaviors, which are part of our overarching model). Both cortisol and NE can directly reach HIV-infected cells via the plasma, and can interact with receptors on macrophages and lymphocytes, thereby influencing the immune response (30). Interestingly, in vitro studies have shown that high levels of NE also enhance viral entry into target lymphocytes and increase viral replication, via the mechanisms of upregulated cell surface expression of the viral coreceptors CXCR4 and CCR5, and enhanced transcription of HIV genes by cellular transcription factors (31, 32, 33). One such study reported a ten-fold HIV replication rate in NE-exposed cells (34). In animal models of HIV, social stress is associated with greater viral replication, via increased density of sympathetic innervation of lymph nodes (35). In addition, higher levels of NE have also been associated with impaired response to HAART (36, 7). However, no one has determined whether levels of NE predict faster disease progression in vivo. Cortisol has been shown to predict disease progression in HIV (2, 10) and has been associated with down regulation of the immune system (37) and increased HIV infection of lymphocytes (38). However, in vitro virology studies show minimal impact of glucocorticoids on viral replication rates (38, 39), and the 2008 review by Cole notes that there is mixed evidence of glucocorticoid regulation on HIV replication in vitro (30), thus further study is necessary for the predictive ability of cortisol.
Figure 1.
Hypothesized associations between psychosocial and neurohormonal predictors of HIV disease progression.
* indicates the variables included in this analysis
For simplicity, not all arrows are included. There would be arrows from stress and coping to neurohormones and disease progression markers.
Thus, the main purpose of this study was to examine the prospective relationships between the stress hormones cortisol and especially NE (since there is much less literature about it and good in vitro evidence of its importance in HIV) and HIV disease progression. In addition, the prospective relationships between psychological responses to stress (i.e., depression, hopelessness, avoidant coping, and life event stress) and change in the biological markers of HIV disease (CD4 cells and viral load) over 4 years of follow-up were also examined and were extended to 4 years of follow-up. As antiretroviral medication and adherence to these medications substantially influence the course of HIV a comprehensive examination of these variables is also presented.
Methods
Participants
Participants were a paid volunteer sample recruited through physician offices, specialty clinics, service organizations and hospitals. Subjects were included in this study if they were HIV positive and in the mid range of disease (CD4 cells 150 – 500). Subjects were excluded if they had ever experienced an AIDS defining (Category C) symptom, ever had CD4 cells below 75, were under age 18, had other life threatening illnesses (e.g. cancer), were actively psychotic or suicidal, had dementia or current alcohol or drug dependence.
Design
This study used a longitudinal prospective design where participants were assessed every six months for a period of four years. The accrual period lasted 2.5 years, and the study period was from 1997 to 2004.
Procedures
At baseline, participants completed written informed consent, psychosocial questionnaires, a clinical assessment interview and blood draw for CD4 and viral load assay, and provided a 15 hour urine sample for cortisol and NE assay. Blood draws were completed in the morning to control for diurnal variation. Follow-up visits, repeated every 6 months, included the questionnaire battery, brief interview and blood draw and urine collection. Study procedures, including informed consent were approved by the IRB. Subjects were paid $50 for each assessment.
Measures
Disease Progression Markers
CD4 lymphocyte count (CD3+CD4+) was determined by whole-blood 4-color direct immunofluorescence using a XL-MCL flow cytometer (Beckman/Coulter, Miami, FL). In order to convert the % of total lymphocyte values generated by the flow cytometer to absolute count for each subset, the total lymphocyte count was determined using a MaxM electronic hematology analyzer (Beckman/Coulter, Miami, FL). Serum viral load was measured by determining the number of HIV-1 virions per milliliter (ml) of peripheral blood plasma using the Cobas Amplicor HIV-1 Monitor RT/PCR assay (version 1.5, Roche Molecular Systems, Branchburg, NJ). The lower limit of sensitivity of this assay was 400 copies of HIV-1 RNA/ml of plasma. Flow cytometry for CD4 was completed the same day and an aliquot of plasma was frozen and stored at −70°C for viral load assay, which was batched and run weekly.
Collection of Urinary Stress Hormones
At study entry and each 6-month assessment, participants collected 15-hour urines (from 6 p.m. until the first void the following morning). The 15 hour overnight collection time was used to maximize compliance, avoid a confound with employment status, and to capture nighttime stress hormones which is the period most sensitive to chronic stress (40). Participants stored their urine in a container with 1 gram of the preservative sodium metabisulfite which they were asked to refrigerate. Samples with volumes of less than 250 ml were excluded from analysis. Two samples were aliquoted in tubes (10ml each for the cortisol and NE assays). Concentrated hydrochloric acid (100μl) was added to the tube for catecholamine assay. The vials are stored and frozen at −70°C.
Urinary cortisol was determined by radioimmunoassay (Diagnostic System Laboratories, Webster, Texas). This assay used a 50 μl of a 500 μl aliquot of urine which was extracted in 500 μl of dichloromethane and then evaporated to dryness under nitrogen. One ml of I125 labeled cortisol was added to tubes coated with antibodies, incubated for 45 minutes, decanted, and radioactivity quantified for 1 minute using a gamma counter calibrated for I125. Cortisol levels in the sample were estimated using a standard calibration curve. Cortisol levels were expressed as μg/100 ml of urine. The intraassay and interassay coefficient of variance were 8.2 and 9.3% respectively.
Urinary NE levels were determined through the following procedure. Urine was purified and extracted using disposable columns filled with Biorex-70 resin (a cationic-exchange column) and assayed using High Performance Liquid Chromatography (HPLC; Manufacturer ESA) (41). The catecholamines from the extract are separated on a HPLC-CoulArray system using reverse phase C18, 5ì column and determined by a coulometric system. NE levels are expressed as pg/ml of urine. The intrassay and interassay CV were 3.4 and 7.1 % respectively.
Psychosocial Measures
The Beck Depression Inventory (42), measured depressive symptoms and included two subscales of depression: cognitive/affective and somatic. Scores above 18 represent moderate depressive symptom levels (43). The Beck Hopelessness Scale (44), a 20-item scale was used to measure hopelessness. The COPE (45) measured coping with two subscales combined to produce an avoidant coping composite (denial and behavioral disengagement). Life event stress was assessed by the Life Events Scale (46) and the measure used was the sum of negative events weighted by their impact. Prescribed medications and adherence was assessed through interviewer administered AIDS Clinical Trials Group Adherence measure (47). The adherence measure used was the percentage of missed doses over the past three days dichotomized to identify those achieving an average of 95% adherence or greater. Demographics and background information were assessed by self-report. Cumulative psychosocial measures were averaged over the first four time points.
Statistical Analysis
The main analyses used Hierarchical Linear Modeling (HLM; 48, 49) to model CD4 and viral load change. HLM was used because it permits control for antiretroviral use at each time point, allows for prediction of slope of change rather than a single endpoint, and allows for the calculation of expected changes in CD4 and viral load for each predictor. In the HLM approach variance in disease progression markers is separated into two levels: Level-1 represents a growth model for each individual capturing within-person change in CD4 and (log) viral load over repeated measurements. Level-2 represents a model of inter-individual differences in parameters of individual change and uses between-person characteristics (e.g. depressive symptoms) to predict change. Thus, systematic variability of the slopes at level-1 is modeled by predictors at level-2. Each predictor (psychosocial or neurohormonal) was individually tested over and above the covariates described in the next paragraph, and not over and above all of the other predictors).
Covariate Selection: Level 1 covariates included prescribed antiretroviral medication (as a time dependent covariate), time since baseline, and the interactions of these terms (for the CD4 growth model only). Antiretroviral medications were dummy coded (as HAART1 and HAART2) at each time point reflecting three levels: no medication, combination therapy not including HAART, or HAART. The demographic covariates and initial disease status (baseline CD4 or viral load) were included, a priori, in the Level 2 model for testing the slope (the outcome of interest) and remained in both CD4 and viral load models for all subsequent analysis. The demographic variables of race (coded 1 = non-Hispanic Caucasian, 0 = other), gender (coded 1 = male, 0 = female), age, education level (coded 0 = less than high school, 1 = some high school, 2 = high school graduate, 3 = trade school or some college, 4 = college graduate, 5 = graduate degree) were included as a priori covariates relevant to HIV (48, 49). Education level was used as a relatively unbiased indicator of SES since income and employment may be affected by advancing HIV disease. All continuous variables in the model were centered and all categorical variables were coded with zero as the lowest level. As the cortisol and viral load distributions were skewed, their distributions were transformed using log10 transformations.
Results
This sample (n = 177) of people with HIV was diverse with respect to gender, age, race, and other sociodemographic variables. The sample was 70% male (n = 124) and the average age was 38 years (S.D. = 8.88). The racial/ethnic groups were well represented: African American 36% (n = 64), Non-Hispanic Caucasian 30% (n = 54), Hispanic 28% (n = 50), and Other 5%. Just over half of the sample (n = 97) identified themselves as gay or bisexual. This was a well-educated sample with 68% (n =121) of the sample reporting educational experiences beyond high school although 18% (n = 32) did not graduate high school. Approximately one third of the sample was employed at study entry (19% (n = 33) fulltime and 13% (n = 24) part-time), 42 % (n = 75) of the sample were on disability and 15% (n = 27) were unemployed. Sixty-two percent (n = 110) reported an annual income of $10,000 or less. At study entry the average CD4 count was 297 and mean HIV viral load was 44,861.
At study entry 23.2% (n = 41) of the sample were not taking any antiretroviral medication although during the follow-up period 10% (n =17) of patients had not taken antiretroviral medications during at least one assessment time point. The mean urinary cortisol and NE concentration levels (averaged over the first two assessments) were 43.52 ug/100 ml (S.D. = 40.47) and 46.31 ug/100 ml (S.D. = 27.64) respectively. The demographic and medical characteristics of this sample have been described in an earlier study examining the 2-year prospective relationships between psychosocial (but not neurohormonal) variables and HIV disease progression (19).
The mean BDI score for the sample at study entry was 11.13 (S.D. = 8.87) placing the sample in the mild range for depressed symptoms (43). The average score on the Beck Hopelessness Scale was 4.29 (S.D = 4.34), well below the clinical cutoff for suicide risk in psychiatric outpatients (50). The mean score for avoidant coping was 5.76 (S.D = 2.45) suggesting that on average participants endorsed avoidant coping strategies with respect to HIV related stressors “not at all” to “a little bit”. The mean score for life event stress was -5.05 (S.D = 5.18) indicating that on average our sample had multiple stressful life events in the past 6 months.
Prediction to CD4 Change over Time
Basic Model
Table Ia describes the growth curve models estimating the change in CD4 cells over 4 years. The Level 1 parameters provide the structure of the model identifying the Intercept, Slope and the impact of HAART across each of the 9 assessment time points (baseline and every 6 months thereafter). The model indicates that average CD4 level at study entry (γ00) is 277.54 controlling for other covariates in the model. There is a significant linear decrease in CD4 over time (γ10) of 3.00 CD4 cells/month (about 36 cells/year). There is also significant individual variation in CD4 change over time (χ2 (171) = 542.8, p < .001). Being on combination therapy (γ20) or HAART (γ30) was also significantly associated with higher CD4 cell count. The level 2 parameters provide the estimates of the significance of the covariates to the slope of CD4. At level-2, higher baseline CD4 and higher education level buffered CD4 decline. Age, gender, and ethnicity were not significantly related to change in CD4 cell number over time.
Table I.
Basic Model including coefficients and significance tests for Level 1 and Level 2 Covariates in Prediction of CD4 (a) and Viral Load (b) Slope over 4 Years
| (a) CD4 Model | (b) Viral LoadLOG10 Model | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Fixed Effects | Coefficient | df | t-ratio | Coefficient × 10−2 | df | t-ratio |
| CD4/VL Intercept, γ00 | 277.54 | 176 | 18.71** | 445.00 | 176 | 51.97** |
| CD4/VL Slope(per month),γ10 | −3.00 | 171 | −2.84** | 1.32 | 171 | 2.97** |
| Baseline CD4/VL, γ11 | 0.01 | 171 | 2.82** | 0.06 | 171 | 0.34 |
| Education, γ12 | 0.88 | 171 | 3.07** | −0.39 | 171 | −2.74** |
| Age, γ12 | 0.02 | 171 | 0.49 | −0.00 | 171 | −0.10 |
| Gender, γ13 | −0.36 | 171 | −0.41 | 0.07 | 171 | 0.17 |
| Ethnicity, γ14 | −1.03 | 171 | −1.24 | 0.34 | 171 | 0.78 |
| aHAART 1 Increment, γ20 | 67.90 | 1020 | 3.65** | −98.07 | 1007 | −8.84** |
| HAART 2 Increment, γ30 | 35.08 | 1020 | 2.35* | −114.28 | 1007 | −11.37** |
|
| ||||||
| Random Effects | Variance | df | χ2 | Variance | df | χ2 |
|
| ||||||
| Intercept, U0 | 9,655.93 | 176 | 662.9** | 80.02 | 176 | 860.00** |
| Slope, U1 | 13.90 | 171 | 542.8** | 0.03 | 171 | 298.50** |
| Error, R | 6,619.60 | 40.47 | ||||
The interaction terms for HAART1x Time and HAART2 x Time which were included in the Level 1 model for estimating the CD4 slope (19) were not significant in combination and have been omitted from the table.
Psychosocial Predictors
Faster CD4 decline was predicted from baseline depressive symptoms, hopelessness, and avoidant coping, but not from life event stress (See Table IIa). Recall that each predictor (psychosocial or neurohormonal) was individually tested over and above the covariates, and not over and above all of the other predictors). When the BDI was restricted to the cognitive/affective subscale only, the significance of the relationship maintained (γ16 = -.130, t [170] = −2.56, p = .012). Cumulative depressive symptoms, hopelessness, avoidant coping, and life event stress were all significantly related to CD4 change over time (see Table IIa). The cumulative cognitive/affective subscale of the BDI was also significantly related to CD4 decline ((γ16 = −.21, t [170] = −3.27, p = .05), as were both denial (γ16 = −0.88, t [170] = −3.12, p = .003) and behavioral disengagement (γ16 = −0.92, t = [170] = −2.38, p = .019) coping.
Table II.
Prediction from Baseline Psychosocial and Neurohormonal Variables and Cumulative Psychosocial Variables to CD4 Slope (a) with additional control for Antiretroviral Medication Adherence (b)
| Psychosocial Predictors | (a) Main Analyses (n = 177) | (b) Main Analyses with additional control for Medication Adherence (n = 160) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Baseline Measures | γ16 Gamma Coefficient | t-ratio | p-value | Decline Ratioa | γ17 Gamma Coefficient | t-ratio | p-value |
| Depression | −0.11 | −3.21 | .002 | 1.51 | −0.11 | −3.02 | .003 |
| Hopelessness | −0.20 | −2.41 | .017 | 1.99 | −0.19 | −2.06 | .041 |
| Avoidant Coping | −0.34 | −2.53 | .013 | 1.55 | −0.33 | −2.09 | .038 |
| Life Events Stress | 0.11 | 1.53 | .128 | N/A | 0.10 | 1.38 | .171 |
| Cumulative Measures | |||||||
| Depression | −0.19 | −4.38 | <.001 | 2.92 | −0.18 | −3.97 | <.001 |
| Hopelessness | −0.27 | −3.29 | .002 | 1.55 | −0.24 | −2.67 | .009 |
| Avoidant Coping | −0.57 | −2.89 | .005 | 1.89 | −0.54 | −2.58 | .011 |
| Life Events Stress | 0.25 | 2.04 | .043 | 1.33 | 0.24 | 1.84 | .067 |
|
| |||||||
| Neurohormonal Predictors | |||||||
|
| |||||||
| Cortisol log10 | −0.35 | −0.99 | .322 | N/A | −0.38 | −1.33 | .185 |
| Norepinephrine | −0.03 | −2.11 | .036 | 1.50 | −0.03 | −2.01 | .046 |
Decline Ratio (DR) = [γ10 + γ16(75th percentile score – mean)]/ [γ10 + γ15 (25th percentile score – mean)], where γ10 is the average rate of CD4 decline controlling for other covariates in the model and γ16 is the increment in CD4 decline for every point above or below the mean of the psychological variable.
Neurohormonal Predictors
Urinary cortisol log10 concentration was not significantly related to the rate of change in CD4 over time. However, urinary NE concentration predicted a significantly faster rate of decline in CD4 cells over 4 years.
Clinical Translation
Decline ratios were calculated to compare the impact of the high and low levels (75th and 25th percentile) of each predictor on CD4 change and these ratios are presented in Table II. Those scoring high in baseline hopelessness experienced almost twice the rate of decline in CD4 cells compared to those scoring low in hopelessness. Those scoring high in cumulative depressive symptoms lost CD4 cells at three times the rate of those scoring low in cumulative depressive symptoms. High initial levels of NE predicted a rate of decrease in CD4 cells that was 1.5 times fasters than those with low NE.
Prediction to Viral Load Change over Time
Basic Model
Viral load significantly increased over time (γ10) controlling for covariates (See Table Ib). Patients had an average of 4.58 viral load log units at study entry, and increased .013 units/month (0.156 log units/year). Individual variation around the slope of viral load (change) was also significant (χ2 (171) = 298.50, p < .001) Antiretroviral medications were significantly associated with lower levels of viral load. (γ20, γ30). Education was the only Level 2 covariate that was significantly related to viral load slope (t [171] = −2.74, p = .029). Viral load at baseline was not significantly related to change in viral load over time.
Psychosocial Predictors
Higher baseline depressive symptoms, hopelessness, and avoidant coping predicted greater viral load increase over 4 years (See Table IIIa), as did both the subscales of avoidant coping; denial (γ16 = .25 × 10−2, t [170] = 2.68, p = .008) and behavioral disengagement (γ16 = .26 × 10−2, t [170] = 2.07; p = .04). Baseline levels of life event stress were not significantly related to viral load change. Cumulative depressive symptoms, hopelessness, and avoidant coping maintained their significant association with viral load change. The cumulative cognitive/affective subscale of the BDI (γ16 = .077 × 10−2, t [170] = 2.01, p = .045) and the cumulative avoidant coping subscale of denial (γ16 = .31 × 10−2, t [170] = 2.01, p = .045) were significantly related to viral load change although behavioral disengagement (γ16 = .29 × 10−2, t [170] = 1.70, p = .09) showed only a trend. Cumulative measures of life events stress were not significantly related to viral load change.
Table III.
Prediction from Baseline Psychosocial and Neurohormonal Variables and with Cumulative Psychosocial Variables of Viral Load Slope (a) with additional control for Antiretroviral Medication Adherence (b)
| Psychosocial Predictors | (a) Main Analyses (n = 177) | (b) Main Analyses with additional control for Medication Adherence (n = 160) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Baseline Measures | γ16 Gamma Coefficient × 10−2 | t-ratio | p-value | Increase Ratio | γ17 Gamma Coefficient × 10−2 | t-ratio | p-value |
| Depression | 0.04 | 2.29 | .023 | 1.42 | 0.05 | 2.34 | .021 |
| Hopelessness | 0.11 | 2.82 | .006 | 1.61 | 0.10 | 2.50 | .014 |
| Avoidant Coping | 0.18 | 2.77 | <.007 | 1.74 | 0.18 | 2.49 | .014 |
| Life Events Stress | −0.05 | −1.42 | .158 | N/A | −0.04 | −1.16 | .249 |
| Cumulative Measures | |||||||
| Depression | 0.07 | 2.69 | .008 | 2.00 | 0.06 | 2.55 | .012 |
| Hopelessness | 0.14 | 2.94 | .004 | 1.63 | 0.11 | 2.41 | .017 |
| Avoidant Coping | 0.20 | 2.03 | .043 | 1.58 | 0.18 | 1.84 | .067 |
| Life Events Stress | −0.12 | −1.89 | .060 | N/A | −0.11 | −1.61 | .109 |
|
| |||||||
| Neurohormonal Predictors | |||||||
|
| |||||||
| Cortisol log10 | 0.0065 | 1.98 | .049 | 1.30 | .0072 | 2.06 | .041 |
| Norepinephrine | 0.02 | 3.63 | .001 | 2.50 | 0.02 | 3.45 | .001 |
Neurohormonal Predictors
Both urinary cortisol concentration and higher urinary NE concentration predicted a significantly faster increase in viral load over 4 years.
Investigation of Possible Mediation
Our first step in investigating whether the neurohormonal variables might mediate the relationship between the psychosocial variables and disease progression was to correlate cortisol and norepinephrine with the psychosocial variables. None of the correlations with life events stress, depression, hopelessness, or avoidant coping were significant (smallest p=.18). Therefore mediation was not possible.
Clinical Translation
Those with high cumulative depressive symptom scores experienced close to a two fold increase in viral load as compared to those with low scores. The largest increase ratio (2.50) was observed for NE indicating that those high in NE had two and a half times the rate of increase in viral load compared to those low in NE. The increase ratios for each predictor are presented in Table III.
Medication Adherence
Because of the central role of adherence in optimal management of HIV the main analyses were rerun to determine whether the significance of the results were independent of adherence. Cumulative self-reported medication adherence significantly buffered the decline in CD4 cells (γ16 = 1.89, t [153] = 2.50, p = .014) and the increase in viral load (γ16 × 10−2 = −1.14, t [153] = −2.89, p = .005). Controlling for medication adherence did not alter the significance of any of the relationships found in the main analyses (See Tables IIb and IIIb) with the exception that cumulative Life Events Stress which had significantly predicted CD4 change and cumulative avoidant coping which had significantly predicted viral load change now only showed trends toward significance (p’s < .1).
Discussion
This is the first study of which we are aware to relate baseline levels of the stress hormone, NE, to decline of CD4 cells and an increase in HIV viral load over 4 years of follow-up, suggesting that higher initial levels of NE predict accelerated disease progression in people living with HIV. These results maintained controlling for the effects of antiretroviral medications at each of 9 assessment time points and for the cumulative effects of medication adherence. In addition, these effects cannot be explained by age, gender, SES, ethnicity or initial disease status. Those with high initial levels of NE lost CD4 cells 1.5 times faster and increased viral load 2.5 times faster than those low in NE. These findings are consistent with the in vitro studies showing that high levels of NE enhance HIV viral replication, viral gene expression (31, 33) and endothelial adhesion by HIV-infected leukocytes (51), and are related to impaired response to protease inhibitors (7) and to HAART (36). Cortisol was not significantly related to CD4 but was related to viral load change over time. This contrasts with the findings of Leserman and colleagues who reported that serum cortisol levels significantly predicted the development of AIDS and mortality in a sample followed for 9 years (2; 10). The interpretation of these results is unclear although possible explanations for these discrepant findings may lie in the fact that the current study differed from the Leserman study in several important ways: the current study utilized 15 hour urinary cortisol, collected from a larger diverse sample, recruited at the mid-range of disease, and utilized disease progression markers. Whereas the Leserman study used serum cortisol, in a group of gay/bisexual men, many of whom were at an earlier stage of disease progression, and predicted the development of AIDS. As cortisol is dysregulated in HIV infection (52, 53, 54) its predictive power may be increased by recruiting participants at earlier stages of disease. Consistent with our findings, however, the literature reviewed in the introduction (especially Cole’s work and review) suggests that NE may have more of an effect in HIV than cortisol.
Our psychosocial findings indicate that baseline and cumulative measures of depressive symptoms, hopelessness, and avoidant coping predict accelerated HIV disease progression over 4 years. Cumulative measures of life event stress also significantly predicted greater decrease in CD4 cells over the same time period although this effect was no longer significant when medication adherence was controlled. As with the neurohormonal findings these results are strengthened by the careful control for the effects of antiretroviral medication at every time point and by the additional control for adherence and sociodemographic characteristics. These results extend our previous findings that established the psychosocial relationships over 2 years of follow-up (19) and extend the results of previous studies (2, 10) to a cohort recruited and followed entirely during the widespread availability of HAART. Our finding that high cumulative depressive symptoms predicted three times the rate of CD4 depletion and twice the rate of increase in viral load underscores the importance of effective treatments for depression in HIV not just to improve quality of life but also for its potential impact on disease progression. In addition, as NE pathways are involved in mood (and anxiety) disorders, their effective treatment may be particularly indicated in people with HIV especially as the incidence of depressive disorders in people living with HIV is very high (55, 56). An increasing number of psychopharmacological (57, 58), psychosocial (59, 60) treatments have been shown to be effective in treating depressive disorders in people with HIV. Although it is possible that the treatment of mood disorders in people living with HIV could be informed by these findings, in that antidepressants that impact the noradrenergic pathways may have unanticipated consequences for HIV disease progression. Medications that result in increased NE may hasten disease progression, whereas those that block adrenergic pathways (e.g. beta-blockers) may slow disease progression. However, more data is needed before any such suggestions could be made. Conversely, cognitive-behavioral stress management interventions in HIV have produced significant reduction in distress, and NE may be particularly indicated (61). A recent meta-analysis of 15 controlled trials indicates that cognitive behavioral interventions, particularly those which teach stress management and provide at least 10 sessions, are effective in reducing depression, anxiety, stress, and anger in people living with HIV, but had no effect on CD4 count (60). These findings parallel those of another meta-analysis of 35 randomized controlled trials of stress management interventions, which reduced depression, anxiety, stress, and fatigue, and improved quality of life in people with HIV, but had no influence on CD4, viral load, or cortisol (62). In contrast, a single study showed that in those beginning with detectable viral load, a cognitive behavioral stress management intervention was able to impact significantly on viral load (63). However, these null findings pertaining to immune and endocrine outcomes may be due to the relatively short study duration and follow-up period of studies examined, as well as relatively homogenous participants (largely White males) treated primarily in group format, thus future work should address these limitations, as well as examine ART adherence, which was an additional limitation of many of these studies.
Although our study found that both psychosocial and neurohormonal variables predicted disease progression, the neurohormones did not mediate the relationship between psychosocial variables and disease progression, as hypothesized in our starting model. Contrary to our expectations the psychosocial variables were not correlated significantly with the neurohormones. While this is difficult to interpret, several possibilities could account for these findings. They may not truly be related. However, our previous work has shown relationships between psychosocial variables and neurohormones, such as spirituality and low cortisol (66) and perceived stress and NE (7) and changes in symptoms of PTSD and both cortisol and NE (67). This suggests that perhaps we have not measured the correct variables in this paper, or our timing needs to be in closer proximity to the stressor.
The current study is limited in that although hormonal and psychosocial variables were related to important markers of disease progression (i.e., CD4, viral load) that are known to predict clinical outcomes (64, 65) future studies could usefully examine these relationships with morbidity and mortality in HIV. Further, several of the psychosocial measures used in this study were based upon self-report and are vulnerable to the biases of that methodology. However, the psychosocial measures have established reliability and validity and self-reported medication adherence was predictive of both viral load and CD4 change in this sample providing some measure of its validity.
In summary, these findings demonstrated that higher levels of stress hormones and higher levels of psychological stress each contribute to poorer disease course in HIV over 4 years, even in the present era of HAART medication. In particular, high NE, feelings of hopelessness/depressed mood, and avoidant coping predict an accelerated decline in CD4 cells and greater increase in HIV viral load.
Acknowledgments
This research was graciously supported by the National Institute of Mental Health Grant (grant numbers R01MH53791, R01MH066697), and the Action for AIDS Foundation (grant number T32MH18917). Principal Investigator: Gail Ironson. We wish to thank our participants with HIV for their time and effort in making this analysis possible.
References
- 1.Mayne TJ, Vittinghoff E, Chesney MA, Barrett DC, Coates TJ. Depressive affect and survival among gay and bisexual men infected with HIV. Arch Intern Medicine. 1996;156:2233–38. [PubMed] [Google Scholar]
- 2.Leserman J, Petitto JM, Golden RN, et al. Impact of stressful life events, depression, social support, coping and cortisol on progression to AIDS. Am J Psychiatr. 2000;157(8):1221–28. doi: 10.1176/appi.ajp.157.8.1221. [DOI] [PubMed] [Google Scholar]
- 3.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: Longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285:1460–65. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 4.Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biol Psychiatr. 2003;54(3):295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 5.Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, Coates TJ. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. JAMA. 1993;270(21):2568–73. [PubMed] [Google Scholar]
- 6.Blashill AJ, Perry N, Safren SA. Mental health: a focus on stress, coping, and mental illness as it relates to treatment retention, adherence, and other health outcomes. Curr HIV/AIDS Rep. 2011;8:215–22. doi: 10.1007/s11904-011-0089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ironson G, Balbin E, Stieren E, et al. Perceived stress and norepinephrine predict the effectiveness of response to protease inhibitors in HIV. Int J Behav Med. 2008;15:221–26. doi: 10.1080/10705500802219606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Temoshok LR, Waldstein SR, Wald RL, et al. Type C coping, alexithymia, and heart rate reactivity are associated independently and differentially with specific immune mechanisms linked to HIV progression. Brain Behav Immun. 2008;22(5):781–92. doi: 10.1016/j.bbi.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Pence BW, O’Donnell JK, Gaynes BN. Falling through the cracks: The gaps between depression prevalence, diagnosis, treatment, and response in HIV care. AIDS. 2012;26(5):656–8. doi: 10.1097/QAD.0b013e3283519aae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leserman J, Petitto JM, Gu H, et al. Progression to AIDS, a clinical AIDS condition and mortality: Psychosocial and physiological predictors. Psych Med. 2002;32(6):1059–73. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- 11.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta- analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fekete EM, Antoni MH, Lopez C, et al. Stress buffering effects of oxytocin on HIV status in low-income ethnic minority women. Psychoneuroendocrinology. 2011;36(6):881–90. doi: 10.1016/j.psyneuen.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain D. Stress, immunity, and health: Research findings and implications. IJPR. 2010:94–100. [Google Scholar]
- 14.Machtinger EL, Wilson TC, Haberer JE, Weiss DS. Psychological Trauma and PTSD in HIV-Positive Women: A Meta-Analysis. AIDS Behav. 2012;16:2091–2100. doi: 10.1007/s10461-011-0127-4. [DOI] [PubMed] [Google Scholar]
- 15.Rose RC, House AS, Stepleman LM. Intimate partner violence and its effects on the health of African American HIV-positive women. Psychol Trauma. 2010;2:311–17. [Google Scholar]
- 16.Ironson G, Cruess D, Kumar M. Immune and neuroendocrine alterations in post-traumatic stress disorder. Psychoneuroimmunology. (4) 2004;25(1):531–47. [Google Scholar]
- 17.Leserman J, Ironson G, O'Cleirigh C, Fordiani JM, Balbin E. Stressful life events and adherence in HIV. AIDS Patient Care STDS. 2008;22:403–11. doi: 10.1089/apc.2007.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson TL, Semple SJ, Temoshok LR, et al. Stress and depressive symptoms prospectively predict immune change among HIV-seropositive men. Psychiatry. 1995;58(4):299–312. doi: 10.1080/00332747.1995.11024735. [DOI] [PubMed] [Google Scholar]
- 19.Ironson G, O’Cleirigh C, Fletcher MA, et al. Psychosocial factors predict CD4 and viral load change in men and women in the era of HAART. Psych Med. 2005;67(6):1013–21. doi: 10.1097/01.psy.0000188569.58998.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ironson G, Friedman A, Klimas N, et al. Distress, denial, and low adherence to behavioral interventions predict faster disease progression in gay men infected with human immunodeficiency virus. Int J Behav Med. 1994;1:90–105. doi: 10.1207/s15327558ijbm0101_6. [DOI] [PubMed] [Google Scholar]
- 21.Remor E, Penedo FJ, Shen BJ, Schneiderman N. Perceived stress is associated with CD4+ cell decline in men and women living with HIV/AIDS in Spain. AIDS Care. 2007;19(2):215–219. doi: 10.1080/09540120600645570. [DOI] [PubMed] [Google Scholar]
- 22.Ghebremichael M, Paintsil E, Ickovics JR, et al. Longitudinal association of alcohol use with HIV disease progression and psychological health of women with HIV. AIDS Care. 2009;21:834–41. doi: 10.1080/09540120802537864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrico AW, Riley ED, Johnson MO, et al. Psychiatric risk factors for HIV disease progression: The role of inconsistent patterns of anti-retroviral therapy utilization. J AIDS. 2011;56(2):146–50. doi: 10.1097/QAI.0b013e318201df63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alemu H, Mariam DH, Tsui A, Ahmed S, Shewamare A. Effect of depressive symptoms and social support on weight and CD4 count increase at HIV clinic in Ethiopia. AIDS Care. 2012;24:866–76. doi: 10.1080/09540121.2011.648160. [DOI] [PubMed] [Google Scholar]
- 25.Antelman G, Kaaya S, Wei R, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr. 2007;44:470–77. doi: 10.1097/QAI.0b013e31802f1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouhnik AD, Preau M, Vincent E, et al. Depression and clinical progression in HIV-infected drug users treated with highly active antiretroviral therapy. Antivir Ther. 2005;10(1):53–61. [PubMed] [Google Scholar]
- 27.Chida Y, Vedhara K. Adverse psychosocial factors predict poorer prognosis in HIV disease: a meta-analytic review of prospective investigations. Brain Behav Immun. 2009;23:434–45. doi: 10.1016/j.bbi.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Kopnisky KL, Stoff DM, Rausch DM. Workshop report: The effects of psychological variables on the progression of HIV-1 disease. Brain Behav Immun. 2004;18:246–61. doi: 10.1016/j.bbi.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Schneiderman N, Ironson G, Siegel S. Stress and Health: Psychological, behavioral and biological determinants. Annu Rev Clin Psychol. 2005;1:607–28. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole SW. Psychosocial influences on HIV-1 disease progression: Neural, endocrine, and virologic mechanisms. Psychosom Med. 2008;70:562–68. doi: 10.1097/PSY.0b013e3181773bbd. [DOI] [PubMed] [Google Scholar]
- 31.Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. J Immunol. 1998;161:610–16. [PubMed] [Google Scholar]
- 32.Cole SW, Jamieson BD, Zack JA. cAMP externalizes lymphocyte CXCR4: Implications for chemotaxis and HIV infection. J Immunol. 1999;162:1392–1400. [PubMed] [Google Scholar]
- 33.Cole SW, Kemeny ME, Fahey JL, Zack JA, Naliboff BD. Psychological risk factors for HIV pathogenesis: Mediation by the autonomic nervous system. Biol Psychiatry. 2003;54:1444–56. doi: 10.1016/s0006-3223(02)01888-7. [DOI] [PubMed] [Google Scholar]
- 34.Collado-Hidalgo A, Sung C, Cole S. Adrenergic inhibition of innate anti-viral response: PKA blockade of Type I interferon gene transcription mediates catecholamine support for HIV-1 replication. Brain Behav Immun. 2006;20:552–63. doi: 10.1016/j.bbi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. J Neurosci. 2007;27:8857–65. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole SW, Naliboff BD, Kemeny ME, Griswold MP, Fahey JL, Zack JA. Impaired response to HAART in HIV-infected individuals with high autonomic nervous system activity. Proc Natl Acad Sci. 2001;98:12695–700. doi: 10.1073/pnas.221134198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munck A, Guyre PM. Glucocorticoids and immune function. In: Ader R, Felton DL, Cohen N, editors. Psychoneuroimmunology. 2. San Diego, CA: Academic Press; 1991. [Google Scholar]
- 38.Markham PD, Salahuddin SZ, Veren K, Orndorff S, Gallo R. Hydrocortisone and some other hormones enhance the expression of HTLV-III. Int J Cancer. 1986;37:67–72. doi: 10.1002/ijc.2910370112. [DOI] [PubMed] [Google Scholar]
- 39.Kino T, Kopp JB, Chrousos GP. Glucocorticoids suppress human immunodeficiency virus type-1 long terminal repeat activity in a cell type-specific, glucocorticoid receptor-mediated fashion: direct protective effects at variance with clinical phenomenology. J Steroid Biochem Mol Biol. 2000;75:283–90. doi: 10.1016/s0960-0760(00)00187-4. [DOI] [PubMed] [Google Scholar]
- 40.Mellman TA, Kumar AM, Kulick-Bell R, Kumar M, Nolan B. Nocturnal/daytime urine noradrenergic measures and sleep in combat related PTSD. Biol Psychiatr. 1995;38:174–9. doi: 10.1016/0006-3223(94)00238-X. [DOI] [PubMed] [Google Scholar]
- 41.Kumar AM, Kumar M, Fernandez JB, Mellman TA, Eisdorfer C. A simplified HPLC-ECD technique for measurement of urinary free catecholamines. J Liq Chromatogr. 1991;14:3547–57. [Google Scholar]
- 42.Beck AT. Depression: Causes and treatment. Philadelphia, PA: University of Pennsylvania Press; 1976. [Google Scholar]
- 43.Kendall PC, Hollon SD, Beck AT, Hammen CL, Ingram RE. Issues and recommendations regarding use of the Beck Depression Inventory. Cognitive Therapy and Research. 1987;11(3):289–99. [Google Scholar]
- 44.Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: The Hopelessness Scale. J Consult Clin Psychol. 1974;42:861–65. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- 45.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: A theoretically based approach. J Pers Soc Psychol. 1989;56:267–83. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- 46.Sarason IG, Johnson J, Siegel J. Assessing the impact of life changes: Development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46:932–46. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 47.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient Care Committee and Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 48.Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. 2. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 49.Raudenbush SW, Bryk AS, Cheong YF, Congdon RC. HLM5: Hierarchical linear and nonlinear modeling. Lincolnwood, IL: Scientific Software International; 2002. [Google Scholar]
- 50.Beck AT, Brown G, Berchick RJ, Stewart BL, Steer RA. Relationship between hopelessness and ultimate suicide: A replication with psychiatric outpatients. Am J Psychiatry. 1990;147:190–95. doi: 10.1176/ajp.147.2.190. [DOI] [PubMed] [Google Scholar]
- 51.Sundstrom JB, Martinson D, Mosunjac M, et al. Norepinephrine enhances adhesion of HIV-1-infected leukocytes to cardiac microvascular endothelial cells. Exp Biol Med. 2003;228:730–40. doi: 10.1177/153537020322800613. [DOI] [PubMed] [Google Scholar]
- 52.Christeff N, Gherbi N, Mammes O, et al. Serum cortisol and DHEA concentrations during HIV infection. Psychoneuroendocrinology. 1997;22(suppl 1):S11–S18. doi: 10.1016/s0306-4530(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 53.Mayo J, Collazos, Martinez E, Ibarra S. Adrenal function in the HIV infected patient. Arch Intern Med. 2002;162(10):1095–8. doi: 10.1001/archinte.162.10.1095. [DOI] [PubMed] [Google Scholar]
- 54.Chittiprol S, Kumar AM, Shetty KT, et al. HIV-1 clade C infection and progressive disruption in the relationship between cortisol, DHEAS and CD4 cell numbers: A two-year follow-up study. Clinica Chimica Acta. 2009;409:4–10. doi: 10.1016/j.cca.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asch SM, Kilbourne AM, Gifford AL, et al. Underdiagnosis of depression in HIV: Who are we missing? J Gen Intern Med. 2003;18:450–60. doi: 10.1046/j.1525-1497.2003.20938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5:163– 71. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- 57.Markowitz J, Kocsis J, Fishman B, et al. Treatment of depressive symptoms in HIV-positive patients. Arch Gen Psychiatry. 1998;55:452–57. doi: 10.1001/archpsyc.55.5.452. [DOI] [PubMed] [Google Scholar]
- 58.Rabkin J, Wagner G, Rabkin R. Fluoxetine treatment for depression in patients with HIV and AIDS: A randomized, placebo-controlled trial. Am J Psychiatry. 1999;156:101–7. doi: 10.1176/ajp.156.1.101. [DOI] [PubMed] [Google Scholar]
- 59.Safren S, Hendriksen E, Mayer K, Mimiaga M, Pickard R, Otto M. Cognitive behavioral therapy for HIV medication adherence and depression. Cog Behav Pract. 2004;11:415–23. [Google Scholar]
- 60.Crepaz N, Passin WF, Herbst JH, et al. Meta-analysis of cognitive-behavioral interventions on HIV-positive persons' mental health and immune functioning. Health Psychol. 2008;27:4–14. doi: 10.1037/0278-6133.27.1.4. [DOI] [PubMed] [Google Scholar]
- 61.Antoni MH, Cruess DG, Cruess S, et al. Cognitive-behavioral stress management intervention effects on anxiety, 24-hr urinary norepinephrine output, and T-cytotoxic/suppressor cells over time among symptomatic HIV-infected gay men. J Consult Clin Psychol. 2000;68:31–45. doi: 10.1037//0022-006x.68.1.31. [DOI] [PubMed] [Google Scholar]
- 62.Scott-Sheldon LA, Kalichman SC, Carey MP, Fielder RL. Stress management interventions for HIV+ adults: A meta-analysis of randomized controlled trials, 1989 to 2006. Health Psychol. 2008;27:129–39. doi: 10.1037/0278-6133.27.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antoni MH, Carrico AW, Durán RE, et al. Randomized clinical trial of cognitive behavioral stress management on human immunodeficiency virus viral load in gay men treated with highly active antiretroviral therapy. Psychosom Med. 2006;68:143–51. doi: 10.1097/01.psy.0000195749.60049.63. [DOI] [PubMed] [Google Scholar]
- 64.Saag MS, Holodnly M, Kuirzkes DR, et al. HIV viral load markers in clinical practice. Nat Med. 1996;2:625–29. doi: 10.1038/nm0696-625. [DOI] [PubMed] [Google Scholar]
- 65.Powderly WG, Landry A, Lederman M. Recovery of the immune system with antiretroviral therapy; the end of opportunism? JAMA. 1998;280:72–7. doi: 10.1001/jama.280.1.72. [DOI] [PubMed] [Google Scholar]
- 66.Ironson G, Solomon S, Balbin E, et al. The Ironson-Woods Spirituality/Religiousness Index is associated with long survival, health behaviors, less distress and low cortisol in people with HIV/AIDS. Annals of Behavioral Medicine. 2002;24:34–48. doi: 10.1207/S15324796ABM2401_05. [DOI] [PubMed] [Google Scholar]
- 67.Ironson G, Cruess D, Kelsch CB, et al. Posttraumatic stress symptoms, intrusive thoughts, and disruption are longitudinally related to elevated cortisol and catecholamines following a major hurricane. JABR. 2014;19:24–52. [Google Scholar]