Abstract
Heat and cold exposure can decrease and increase total peripheral resistance, respectively, in humans. With unique access to human skeletal muscle feed arteries, we sought to both characterize these vessels and determine the interaction between temperature and α1-adrenergic receptor responsiveness. We hypothesized that α1-mediated vasocontraction of human feed arteries would be attenuated in response to 39 or 35°C. Skeletal muscle feed arteries were harvested from thirty-two human volunteers and studied using isometric technique. Vessel function was assessed using potassium chloride (KCl), sodium nitroprusside (SNP), phenylephrine (PE), and acetylcholine (ACh) dose response curves (DRCs) to characterize non-receptor and receptor mediated vasocontraction and vasorelaxation. Single doses of PE (1mM) and KCl (100mM) were administered at 37°C and then, in a balanced design, repeated at both 35° and 39°C. The KCl and PE DRCs elicited significant vasocontraction (2009±407; 1974±508 developed tension (mg), respectively), whereas SNP and ACh induced the expected vasorelaxation (102±6; 73±10 %relaxation, respectively). Altering temperature had no effect on inherent smooth muscle function (KCl response), but both a reduction (35°C) and an increase (39°C) in temperature decreased the vasocontractile response to 1mM PE (37°C: 1478 ± 338, 35°C: 546 ± 104, and 39°C: 896 ± 202 mg; p<0.05), or across PE dose (p<0.05; 35 and 39°C vs. 37°C). Despite clear heterogeneity between both the human volunteers and the feed arteries themselves, this novel approach to the procurement of human vessels revealed a robust “inverted U” response to altered temperature, such that α1-mediated vasocontraction was attenuated with either warming or cooling.
Keywords: Temperature, α1-adrenergic receptors, skeletal muscle, feed arteries
INTRODUCTION
In rodent skeletal muscle it has been established that a primary control point for regulating total muscle blood flow during exercise is the feed artery (Williams & Segal, 1993; Segal, 2000). Human skeletal muscle feed arteries, while relatively large compared to the equivalent vessels in rodents are by human standards very small (1–2% of aortic diameter), have a similar anatomical location. Therefore human feed arteries also likely contribute significantly to blood flow regulation by varying vascular resistance prior to entry into the muscle bed. Although difficult to obtain, human skeletal muscle feed arteries can, in fact, be harvested during certain surgical procedures and studied in vitro. However, not only are the vasocontraction and vasorelaxation characteristics of human feed arteries not well characterized, the impact of temperature upon vasoconstrictor agents important in the regulation of muscle blood flow in response to stress, such as norepinephrine, is unknown.
The specific effect of temperature on vasoconstrictor responsiveness has been extensively studied in vitro in vessels from animals. However, potentially, at least in part, due to methodological differences the results have been equivocal, with alterations in temperature leading to an increase (Padilla et al., 1998; Massett et al., 1999), a decrease (Vanhoutte & Lorenz, 1970; Cooke et al., 1984; Kregel & Gisolfi, 1990; Ryan & Gisolfi, 1995; Massett et al., 1998b; Mustafa et al., 2004), or no change (Massett et al., 1998a; Kluess et al., 2005; Siddegowda et al., 2007) in α-adrenergic mediated vasoconstriction. While there are no human in vitro studies with which to compare, prior in vivo animal and human studies on this topic reveal a general reduction in α-adrenergic vascular reactivity during local heating (Wilson et al., 2002) or systemic increases in core temperature (Kregel et al., 1997; Massett et al., 2000; Wilson et al., 2002; Brothers et al., 2009). However, recent work performed by Keller et al. (Keller et al., 2010) suggested that adrenergic responsiveness is maintained during heat stress localized to the leg. Such in vivo measurements represent the summation of the net vascular response and thus with such an approach it is currently not possible to differentiate the contribution of various levels of the arterial tree. An in vitro assessment of temperature and adrenergic responsiveness in human vessels recognized to regulate blood flow could provide greater clarity to this issue.
Thus, with the novel approach of harvesting human skeletal muscle feed arteries and an interest in α1-receptor responsiveness in humans, utilizing an isolated in vitro model, we sought to a) assess the vasocontraction and vasorelaxation characteristics of these human feed arteries and b) determine if changes in temperature exert an effect on the α1-adrenergic receptor responsiveness of these human feed arteries. Specifically, with implications for blood flow regulation in the face of both heating and cooling, we hypothesized that both an increase or a decrease in temperature from 37°C would attenuate the vasocontractile response to the α1-agonist phenylephrine.
METHODS
Subjects and General Procedures
A heterogeneous group of 32 subjects (20 males and 12 females, 37–93 yrs old) agreed to have their vessels used in this study (Table 1). Although medical conditions and medications were noted, by means of medical records, (Table 1), there were no exclusions based on this information. All subjects included in this study had not received chemotherapy, as this was a contraindication for surgery. All protocols were approved by the Institutional Review Boards of the University of Utah and the Salt Lake City VA Medical Center, and written informed consent was obtained by all subjects prior to vessel harvesting.
Table 1.
Characteristics of the subject population (n = 32).
| Mean ± SE | Normal Range | |
|---|---|---|
| Age (yr) | 59.00 ± 4.2 | -- |
| Height (cm) | 174.00 ± 3.5 | -- |
| Weight (kg) | 89.50 ± 6.1 | -- |
| Systolic Blood Pressure | 135.60 ± 6.4 † | -- |
| Diastolic Blood Pressure | 75.10 ± 2.4 † | -- |
| Glucose (mg/dl) | 109.10 ± 9.3 | 65 – 110 |
| Blood Urea Nitrogen (mg/dl) | 16.39 ± 1.8 | 6 – 21 |
| Creatinine (mg/dl) | 1.03 ± 0.1 | 0.52 – 0.99* |
| Albumin (g/dl) | 4.17 ± 0.3 | 3.3 – 4.8 |
| Bilirubin (mg/dl) | 0.37 ± 0.2 | 0.2 – 1.3 |
| Lactate Dehydrogenase (U/L) | 494.45 ± 36.3 | 313 – 618 |
| Hemoglobin (g/dl) | 14.40 ± 0.7 | 12 – 16 |
| WBC (K/uL) | 8.12 ± 0.9 | 3.6 – 10.6 |
| RBC (M/uL) | 4.61 ± 0.2 | 4 – 5.2 |
| Platelets (K/uL) | 238.22 ± 20.1 | 150 – 400 |
| Hematocrit (%) | 41.90 ± 1.9 | 36 – 46 |
| Lymphocyte (%) | 30.30 ± 3.9 | 24 – 44 |
| Monocyte (%) | 7.97 ± 1.2 | 0 – 12 |
| Neutrophil (%) | 59.75 ± 3.0 | 36 – 66 |
| Eosinophil (%) | 2.07 ± 0.4 | 0 – 5 |
| Basophil (%) | 0.56 ± 0.1 | 0 – 5 |
| Medications (users/n) | ||
| Cardiovascular | ||
| Diuretic | 2/32 | |
| Ca++ Channel Blocker | 2/32 | |
| Statin | 1/32 | |
| ACE inhibitor | 2/32 | |
| Beta Blocker | 1/32 | |
| Thyroid | ||
| Levothyroxine | 1/32 | |
| Other | ||
| Insulin | 1/32 | |
| Antibiotic | 1/32 |
Average outside of normal range
Data obtained during pre-operative examination
Vessel Harvest
Human skeletal muscle feed arteries from the axillary and inguinal regions were obtained during elective surgeries for melanoma at the Huntsman Cancer Hospital, University of Utah. Patients were anaesthetized using a standard protocol including: propofol, fentanyl, benzodiazepines, and succinylcholine. After removal of sentinel lymph nodes, skeletal muscle feed arteries in the axillary (e.g. serratus anterior, or latissimus dorsi) or inguinal (e.g. hip adductors, or quadriceps femoris) regions were identified and classified as feed arteries based on entry into a muscle bed, structure, coloration, and pulsatile bleed pattern. The vessels were ligated, excised, and immediately placed in iced isotonic saline and brought to the laboratory within 15 minutes of harvesting. Vessels were dissected under a stereo microscope at room temperature in normal physiological saline solution (NPSS) (125 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2, 18 NaHCO3, 0.026 Na2EDTA, and 11.2 Glucose mM). All NPSS solutions and drugs were newly prepared on the morning of the experiment. Vessel internal diameter was measured using a calibrated micrometer eyepiece and reported in micrometers (µM). Perivascular adipose tissue was dissected from the feed arteries. NPSS was continuously aerated with carbogen gas (95% oxygen, 5% carbon dioxide), and pH was monitored at regular intervals and maintained at pH 7.35 – 7.45 by altering the amount of aeration (Orion 3 Star, Thermo Scientific, Waltham MA). Vessels were dissected into four rings measuring approximately 2 mm in length, and mounted in wire myography chambers (700 MO, DMT Systems, Aarhus, DK). Once mounted vessel chambers were also aerated with the same carbogen gas mixture, and chamber NPSS was exchanged at 10 minute intervals, except during cumulative drug dose responses. Vessel chambers were warmed to 37°C over a 30 minute equilibration period prior to the protocol.
Vessel Function Protocols
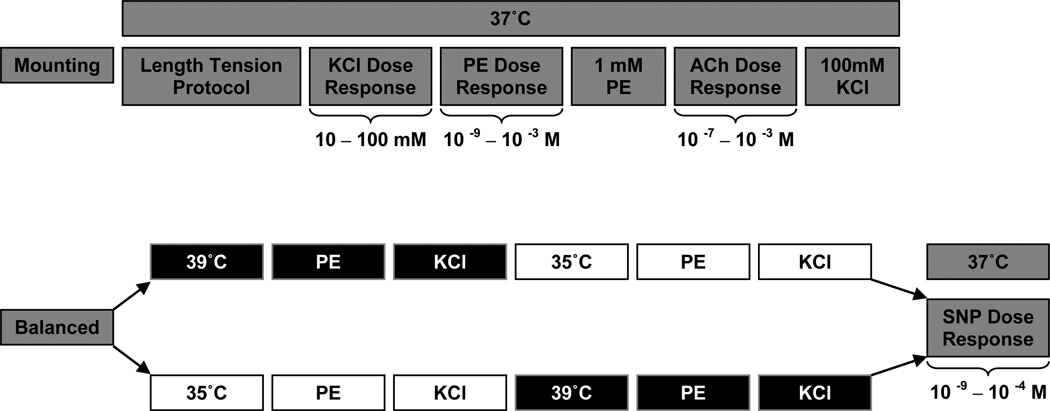
The experimental timeline is illustrated in Figure 1. All vessel segments underwent length tension procedures at 37°C to determine the length at which the vessels produced the greatest tension in response to a single dose of 100mM KCl (Lmax) (Symons et al., 2000). Lmax was operationally defined as less than a 10% improvement in developed tension in response to 100mM KCl. The viability of all vessels was characterized using KCl (10–100mM), phenylephrine (PE; 10−9 – 10−3 M), and acetylcholine (ACh; 10−7 – 10−3 M) dose response curves to determine non-receptor and receptor mediated vasocontraction and vasorelaxation at 37°C. All vasorelaxation responses are expressed as percent relaxation (%) from PE pre-contraction. Temperature changes were achieved by changing myograph chamber and solution temperature utilizing multiple water baths set to the corresponding temperatures. Temperatures of 35, 37, and 39°C were chosen as they can be achieved under physiologic conditions, either during cold exposure, normothermia, or during exercise (Saltin & Hermansen, 1966; Myrer et al., 1998). All feed arteries started experimentation at 37°C to confirm normal vessel function (via KCl, PE, and ACh dose response curves) prior to further experimental procedures (Figure 1). Single doses of PE (10−3 M) and KCl (100mM) were administered at 37°C, and were then, in a balanced design, randomized to either 35, or 39°C. All vessel segments were exposed to all temperatures, though to minimize the potential for an ordering effect, the temperature perturbations away from 37°C were balanced with an equal number of vessels starting with either 35 or 39°C, and were then crossed over to the remaining temperature. Each experimental protocol was separated by 30 minutes. To determine endothelium independent vasorelaxation, a sodium nitroprusside (SNP; 10−9 – 10−4 M) dose response curve was performed in all vessels at 37°C, and was conducted last in every case because of its known effect of abolishing vessel reactivity in vitro.
Figure 1. Experimental Timeline.
Potassium Chloride (KCl), Phenylephrine (PE), Acetylcholine (ACh) & Sodium Nitroprusside (SNP) concentrations in millimolar (mM) or log molar (10−x M).
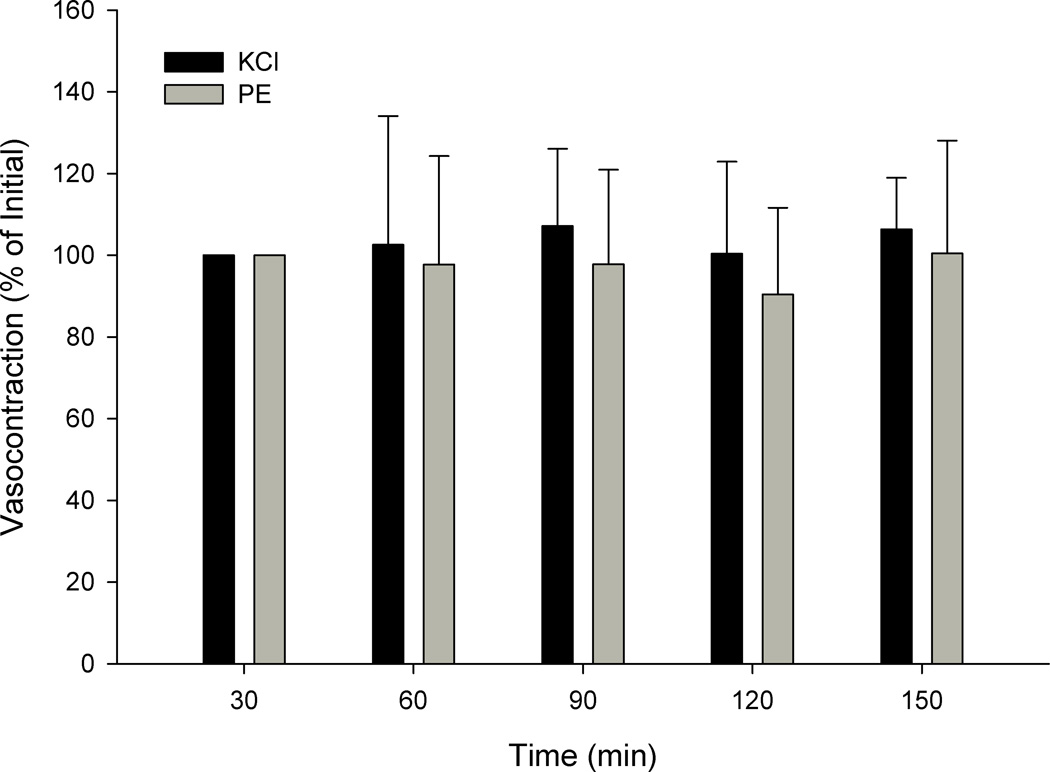
In addition to these main vessel function studies, three supplemental studies were performed. In the first, complete dose response curves for PE were performed across each temperature in a longitudinal design, as in the main vessel function studies (Figure 1). In the second, complete dose response curves for PE were again performed at 35°, 37°, and 39°C, but this time in a cross sectional design, where each vessel only experienced a single temperature. The developed tension (current tension – baseline tension) at each PE dose, for each condition, was expressed as a percent of the maximal tension developed at 37°C. Finally a third supplemental study examined stability of the preparation and the potential for tachyphylaxis with repeated contractions. Specifically, time control experiments were performed using repeated single doses of 1 mM PE and 100mM KCl, each separated by at least 30 minutes to replicate the timing employed in the main studies in which each experimental protocol was separated by 30 minutes. All the vasocontraction responses were expressed as percent of initial tension. For each of these supplemental studies subject and vessel characteristics were also assessed.
All data were acquired at 4Hz using an analog to digital data acquisition system (Biopac Systems, Goleta, CA) to monitor vessel tensions and allow later offline analyses. In the majority of cases (including supplemental studies) the data represents the average response of vessel rings in four chambers (average number of baths was 3.2 ±0.4 for each patient; CV across rings = 10–14%).
Statistical Analyses
Statistical analyses were performed using commercially available software (SPSS v. 17, Chicago, IL). A non-linear slope analysis and one-way repeated measures Analysis of Variance (ANOVA) were performed on the PE dose response data to determine the effect of temperature on PE-induced vasocontraction across doses. Two-way Repeated Measures ANOVA were utilized to determine if an interaction existed between order (2 levels; 35 or 39°C) and temperature (3 levels; 37, 39, 35°C) on baseline tension and in the vasocontraction response to phenylephrine and KCl. One-way repeated measures ANOVA were used to determine if time control data differed over time and if vasocontraction was different between 35 and 39°C when expressed as a percent change from 37°C. Significant differences were further analyzed using Tukeys’ Least Significant Difference post hoc test to make pair wise comparisons. Intraclass correlation coefficient analyses were performed on the time control experiments to determine the stability of the preparation for both PE and KCl mediated vasocontraction. The level of significance was set to p < 0.05. All data are reported as mean +/− standard error (SE).
RESULTS
Subject Characteristics
The subject characteristics, attained by medical records for all studies (main and supplemental), are listed in Table 1. The subjects were varied in terms of demographics (e.g. age), and tended to be overweight and with evidence of systolic hypertension, although it should be noted that these blood pressures were obtained during pre-operative examination. Given the blood chemistry and complete blood count (CBC) results (Table 1), which were on average within normal range except for creatinine, which was slightly elevated, the subjects appeared otherwise to be relatively healthy.
Vessel Characteristics
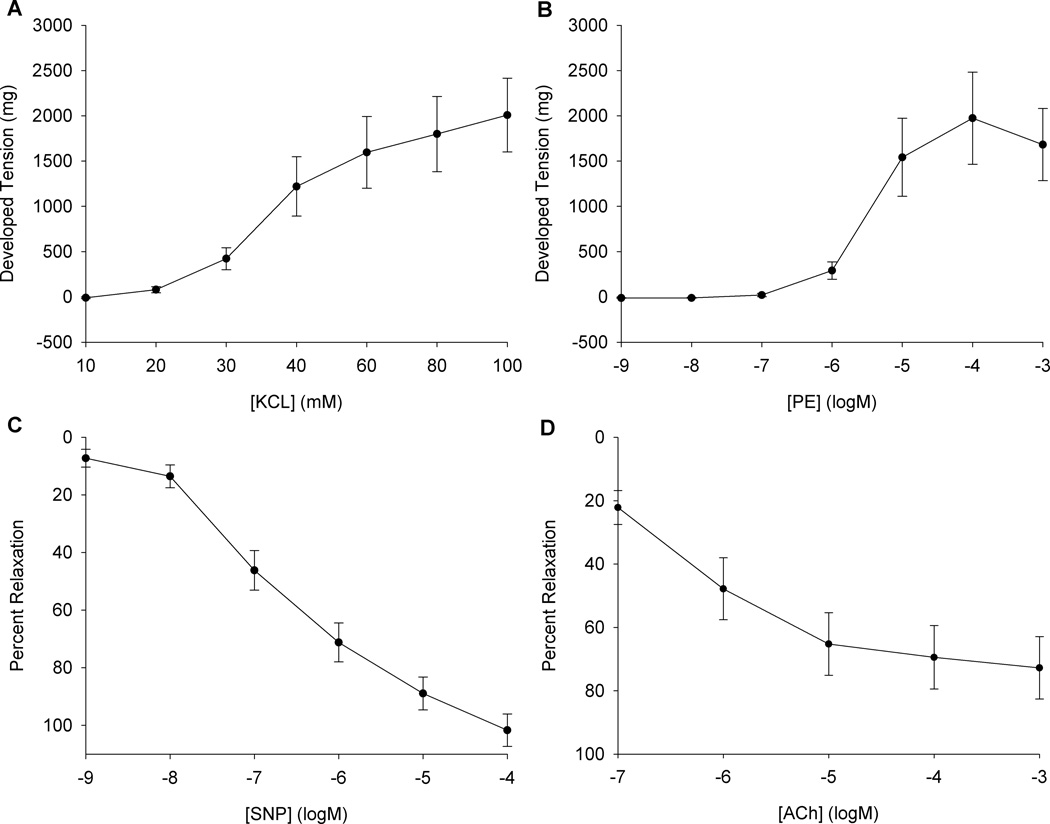
Thirty two human arteries were successfully harvested (19 inguinal, 13 axial). No statistical differences in Lmax, vasocontraction or vasorelaxation responses were observed in terms of anatomical location (axial vs. inguinal) or gender (male vs. female). Consequently, responses from all vessels were combined. The average unpressurized internal diameter for these feed arteries was 500 ± 200 µM. Baseline tensions prior to KCl and PE contractile function curves were 105 ± 29 mg and 106 ± 33 mg, respectively. Vessel function protocols revealed robust vasocontraction (peak KCl 2009 ± 407 mg and peak PE 1974 ± 508 mg) (Figure 2, panel A and B). Vessels were pre-contracted to 1460 ± 330 mg of tension prior to Ach and SNP dose response curves, corresponding to approximately 70% of the maximal PE response, and from this point feed artery segments achieved significant vasorelaxation (max SNP 102 ± 6 and max ACh 73 ± 10 % relaxation) (Figure 2, panel C and D). Taken together, these results indicate the feed arteries had functional smooth muscle, α1-adrenergic receptors, and an intact endothelium. Interestingly, there was no relationship between α1-adrenergic responsiveness, KCL-induced contraction, or the ratio of these two variables and age.
Figure 2. Human skeletal muscle feed artery functional characteristics assessed at normothermia (37°C).
A) Non-receptor mediated vasocontraction dose response for KCl. B) Phenylephrine (PE) dose response for α1-adrenergic mediated vasocontraction. C) Sodium Nitroprusside (SNP) dose response (% relaxation from PE pre-contraction). D) Acetylcholine (ACh) dose response (% relaxation from PE pre-contraction). Data are presented as mean +/−SE (n=32).
Temperature and α1-mediated vasocontraction
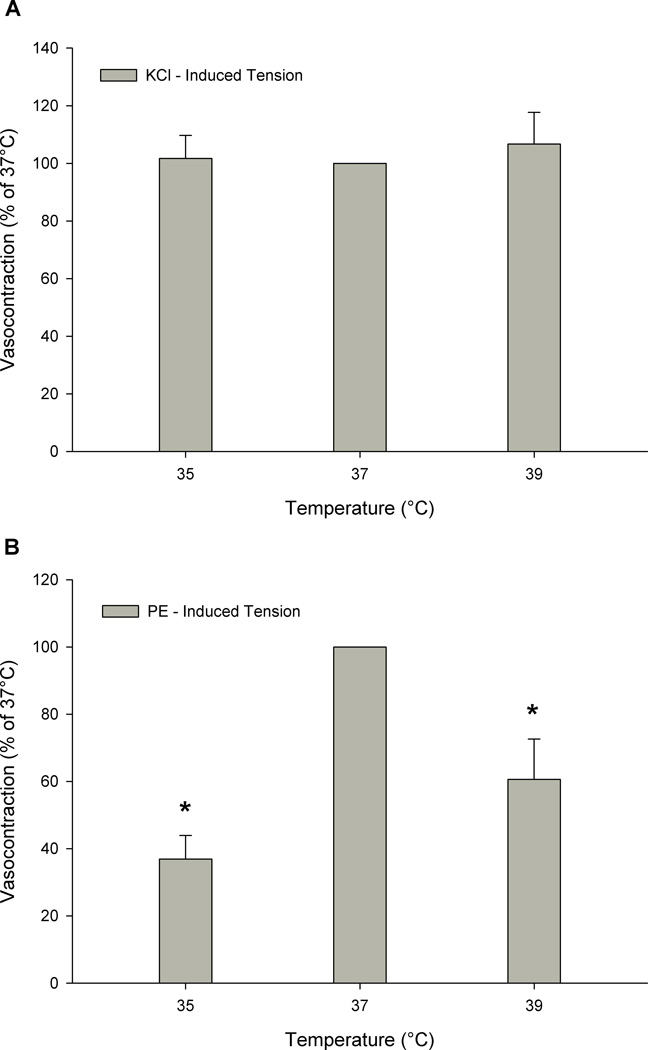
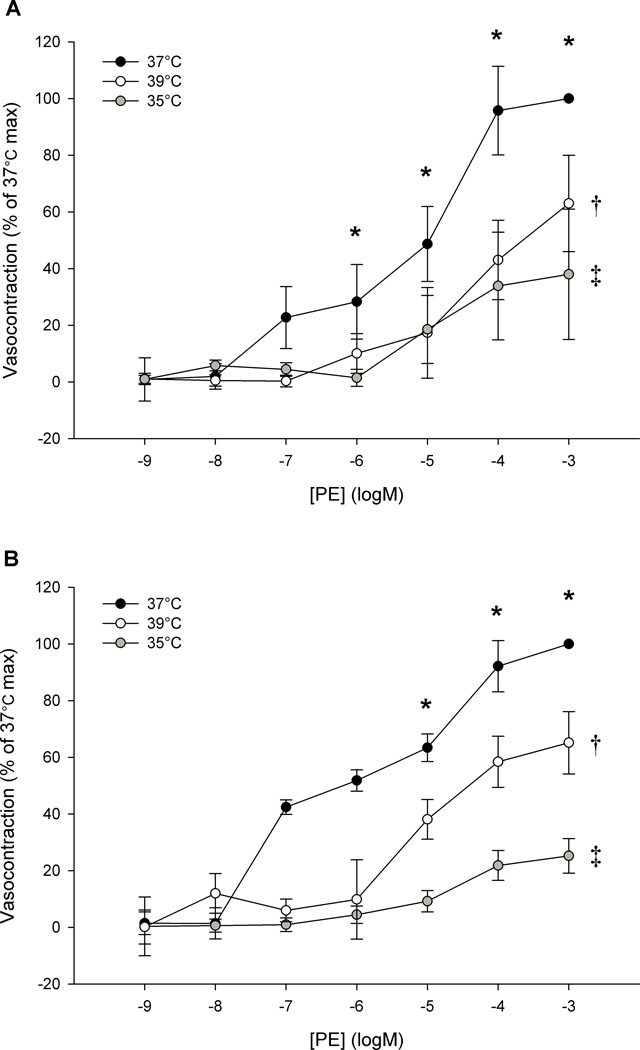
In the main protocol, baseline tensions were lower with a temperature of 35°C (111± 42 mg) or 39°C (85± 39 mg) as compared to 37°C (158 ± 42 mg) (p < 0.05). There was a decreased vasocontractile response to a single dose of PE as a consequence of altering temperature from 37°C (1478 ± 338 mg), to either 35°C (546 ± 104 mg) or 39°C (896 ± 202 mg) (p < 0.05 vs. 37°C) (Figure 3, panel B). Cooling tended to attenuate vasocontraction more so than heating, however this trend did not achieve statistical difference (PE-induced vasocontraction at 35 vs. 39°C; p = 0.07). However, when expressed as percent change from 37°C, the vasocontraction at 35°C was significantly more attenuated than that at 39°C (p < 0.05; 53.5 ± 7.1 vs. 20.6 ± 12.3 %, respectively). The supplemental studies also indicated that altering temperature prior to a phenylephrine dose response curve, significantly reduced α1-mediated vasocontraction at both 35 and 39°C (Figure 4; p < 0.05 35 and 39°C vs. 37°C) utilizing either a longitudinal (panel A) or cross sectional (panel B) study design. Additionally, standard curve analysis revealed a reduced Hillslope at 35 and 39°C, as compared to 37°C, indicating a reduced α1-receptor sensitivity with altered temperature.
Figure 3. The effect of temperature (35°, 37°, and 39° C) on the contractile response to (A) KCl and (B) a single dose of phenylephrine (PE) (1 mM) in human skeletal muscle feed arteries.
* p < .05 vs. 37°C. Data are presented as mean +/−SE (n = 14).
Figure 4. Phenylephrine Dose Response Curves at 35°, 37°, and 39°C in human skeletal muscle feed arteries assessed in both a longitudinal (A) and cross sectional design (B).
(Temperature effect: †p < 0.05, 39 vs. 37°C; ‡p < 0.05, 35 vs. 37°C; * dose effect: p < 0.05 vs. baseline). Note: These data represent an additional 6 feed arteries not represented in figures 3, or 5. Data are presented as mean +/−SE (n = 6 per panel).
Smooth Muscle Function
In the main protocol baseline tensions prior to KCl were lower with a temperature of 35°C (127 ± 46mg) or 39°C (107 ± 40mg), as compared to 37°C (207± 35mg) (p < 0.05). The effects of temperature were not due to altered inherent smooth muscle function, as measured by KCl mediated vasocontraction, evidenced by a lack of effect (p = 0.96) of changing temperature from 37°C (Figure 3, panel A). Additionally, the temperature effects did not appear to be the result of the passage of time, as the time control experiments revealed significant intraclass correlation coefficients for repeated single doses of KCl (0.964, p < 0.001) and PE (0.873, p < 0.01) over time (Figure 5), and responses were not different across time (p > 0.10). These time intervals were similar to those employed in the protocols that did reveal differences in PE-induced tension at different temperatures. Furthermore, all vessels achieved complete relaxation in response to SNP at the end of the experiment providing additional evidence of intact inherent smooth muscle function after exposure to different temperatures. Taken together these data provide evidence in support of the stability of the preparation, and indicate that the effect of temperature on α1-mediated vasoconstriction is not the result of altered inherent smooth muscle function.
Figure 5. Time control experiments for KCl and PE mediated vasocontraction expressed as developed tension (mg) at 37°C in human skeletal muscle feed arteries.
Note: These data represent an additional 6 feed arteries not represented in figures 3, or 4. Data are presented as mean +/−SE (n = 6).
DISCUSSION
The successful harvesting of human skeletal muscle feed arteries, their characterization, and subsequent use in an in vitro isolated vessel study documents the usefulness of this approach to better understand human vascular control. Beyond this novel approach, the main finding of this study was that a change in temperature from the homeostatic set point (37°C), reduced α1-mediated vasocontraction in human skeletal muscle feed arteries. Of importance, this observation was determined to not be a consequence of altered inherent smooth muscle function. Thus, in combination, these data reveal an “inverted U” response, such that α1-receptor responsiveness in human skeletal muscle feed arteries is temperature dependent. The potential implications of this finding are discussed.
Hyperthermia and inherent smooth muscle function
Elevated temperature may alter inherent smooth muscle function, as some in vitro findings have also revealed an altered depolarization-induced contraction (Padilla et al., 1998; Massett et al., 1999), although not all agree (Vanhoutte & Shepherd, 1970). This clearly has the potential to confound findings that suggest heating results in altered receptor-mediated vasoconstriction. Although, the current study did reveal an altered baseline tension, indicating an altered basal smooth muscle tone, this change was diminutive, particularly in light of such large reductions in α1-mediated vasocontraction, and stable KCl-mediated vasocontraction across temperature. In addition, the seminal works of Vanhoutte and Shepard (Vanhoutte & Shepherd, 1970) concluded that temperature does not exert its effect through reduced inherent smooth muscle contractility, which is in accordance with the results of the current study (Figure 3, panel A).
Effects of hyperthermia on vasoconstrictor agents
Hyperthermia has previously been demonstrated to attenuate adrenergic mediated vasoconstriction in vivo in humans (Wilson et al., 2002; Brothers et al., 2009) as well as in animal studies utilizing in vivo or in vitro approaches (Roberts et al., 1989; Kregel et al., 1997; Massett et al., 1998a; Massett et al., 1998b; Massett et al., 1999; Massett et al., 2000). However, not all agree, with recent evidence from Keller and colleagues (Keller et al., 2010) indicating that adrenergic responsiveness may be preserved during isolated heat stress in humans in vivo. Indeed, Keller et al. (2010) concluded that passive leg heating increases muscle blood flow and that, despite an apparent intact adrenergic sensitivity, whole limb vascular conductance remained significantly elevated with local hyperthermia in the face of clear sympathomimeticly-induced vasoconstriction in the femoral artery. In contrast, in vitro, utilizing the isolated rat femoral artery, Kluess et al. (2005) found that heating had no effect on PE-induced vasocontraction, but reduced purinergic-mediated vasocontraction (Kluess et al., 2005). As already indicated, Keller et al. (Keller et al., 2010) found no such effect, indicating, not surprisingly, that the femoral is not involved in the generation of resistance in vivo. In this context, the negative findings of Kluess and colleagues, again studying the femoral, but this time in rats, are also not surprising (Kluess et al., 2005). In fact, the present study appears to be the first to indicate that local heating can reduce α1-adrenergic responsiveness in human feed arteries, an important site of blood flow regulation (Williams & Segal, 1993; Segal, 2000). As the skin, although likely effected by differing mechanisms, has been extensively studied it is interesting to note that the current data from feed arteries respond in a similar fashion to cutaneous vessels with respect to heating (Wilson et al., 2002).
While not directly assessed in the current study, the effect of elevated temperature on agonist-receptor interaction may, in fact, be non-selective (Kregel & Gisolfi, 1990; Massett et al., 1998b), although not all agree (Kluess et al., 2005). Indeed, Massett and colleagues (Massett et al., 1998b) found, in vitro, in rat vessels that increasing temperature not only reduced norepinephrine mediated vasocontraction, but also the vasocontractile response to endothelin-1, and angiotensin II. Therefore, it is likely that heating results in an attenuation of receptor mediated vasoconstriction that may be indiscriminant of receptor type. It appears that attenuated temperature-dependent receptor-mediated vasoconstriction, as demonstrated here in human feed arteries with phenylephrine (Figures 3 and 4), may serve as an autoregulatory mechanism that increases perfusion to metabolically active skeletal muscle.
Temperature, α1-mediated responsiveness, and functional sympatholysis
The precise factor or combination of factors that contribute to functional sympatholysis has yet to be identified in humans. Indeed, during exercise many variables likely contribute to functional sympatholysis (e.g. K+, nitric oxide, ATP, H+, etc.), however a local elevation in temperature is also a potential candidate (Buckwalter & Clifford, 2001; Kluess et al., 2005) which has not been thoroughly examined, especially not in human feed arteries. The increase in skeletal muscle metabolism, associated with exercise, releases copious amounts of heat as a by-product. Indeed, as much as 65% of the energy conversion in the metabolic pathways may be lost as heat (Krustrup et al., 2003), elevating intramuscular temperature to ~39°C during moderate intensity exercise (Saltin & Hermansen, 1966; Magnusson et al., 2000). Mechanistically, increased temperature may contribute to functional sympatholysis through the direct or indirect attenuation of α1-adrenergic vasoconstriction, decreasing vascular resistance and enhancing blood flow to the active skeletal muscle (Buckwalter & Clifford, 2001; Kluess et al., 2005).
Effect of Hypothermia on Vascular Responsiveness
Previous in vitro animal studies have revealed that cooling alters receptor mediated vasocontraction in both cutaneous veins and arteries in skeletal muscle (Flavahan et al., 1985; Faber, 1988). Faber (1988), found that cooling appeared to exert a direct effect on basal vasomotor tone such that, upon cooling, vessels exhibited a transient vasorelaxation, revealing a suppressant effect of cooling on vascular tone. In terms of α-adrenergic stimulation, it appears that cooling selectively attenuates α1-mediated vasocontraction, whereas α2-induced vasocontraction remains intact or increased perhaps to compensate for reduced α1 responsiveness (Flavahan et al., 1985; Faber, 1988). More recently, another elegant series of experiments performed by Flavahan and colleagues (Chotani et al., 2000; Chotani et al., 2004; Bailey et al., 2005; Chotani et al., 2005; Eid et al., 2007; Eid et al., 2008) has provided convincing evidence, in cells and cutaneous arteries, that cold exposure induces translocation of previously quiescent α2c-adrenergic receptors, in part mediated by reactive oxygen species, towards the cell surface which increases vasoconstriction to selective α2c-adrenergic agonists. However, this effect was not apparent with α1-adrenergic agonists (Chotani et al., 2000), as the response to PE was unaffected by cold exposure which contrasts somewhat with the current finding of reduced α1-adrenergic responsiveness with cooling. Although, in vivo cold exposure is well known to stimulate a reduction in cutaneous blood flow to maintain core temperature, very little is known regarding the specific effect of a fall in temperature on skeletal muscle blood flow.
Teleologically, and in a not too dissimilar scenario to voluntary exercise and heating, it is possible that cold mediated sympatholysis plays a role in supporting the metabolic demand associated with shivering thermogenesis during cold stress (Colquhoun et al., 1990). Indeed, cold exposure in humans has been documented to increase cutaneous vascular resistance which resulted in a stroke volume-induced increase in cardiac output and prevented any decrement in MAP, despite a significantly reduced total peripheral resistance (Raven et al., 1970). While blood flow to the thermogenically active skeletal muscle was not measured in this study, the current data suggest that during cold exposure, blood flow to deep skeletal muscle may increase to support shivering thermogenesis, ultimately reducing resistance in the skeletal muscle vasculature. However, this observation may only be evident in arteries feeding deep skeletal muscle, such as those feed arteries used in the current study, is likely only to be of significance under prolonged and severe cooling conditions that cannot be overcome by shivering thermogenesis, but these hypotheses need to be confirmed in vivo. Potential explanations for the observed reduction in α1-adrenergic responsiveness in the current study (Figures 3 and 4) include reduced membrane permeability, decreased diffusional capacity, or a conformational change in the adrenergic receptor as a consequence of cooling (Fick, 1855; Krogh, 1919). Reduced adrenergic responsiveness could be a mechanism by which cold induced vasodilation occurs (Flouris & Cheung, 2009) and may augment pathological hypotension during hypothermia (Vanamoorthy et al., 2010).
Origin of the Vessel
The subjects who took part in this study were certainly heterogeneous in terms of age, gender, and health, but, although exhibiting a tendency to be overweight and some evidence of systolic hypertension (although obtained during pre-operative examination), they were taking minimal medications and had normal blood chemistry and CBC data (Table 1). However, it should also be recognized these subjects were undergoing prophylactic surgical treatment for melanoma and vessels were harvested during sentinel node dissection, although most lymph nodes were found to be negative for melanoma metastasis via Polymerase Chain Reaction analysis. In addition, it is of note the lactate dehydrogenase values (considered to be a crude indicator of metastasis), were within the normal range for all subjects and subjects were not undergoing any chemotherapy.
Despite rather varied origin, the novel approach of harvesting these human arteries during such surgeries yielded the expected receptor mediated and non-receptor mediated vasocontraction and vasorelaxation characteristics (Figure 2), suggestive of normal physiology. With our historical interest in limb specific vascular differences (Wray et al., 2010) and each harvest site (axial and inguinal) being associated with either the upper or lower extremities, it was anticipated that vessel characteristics may vary by limb proximity. However, although the magnitude of within site structural heterogeneity was of interest, this variation was too great to reveal any limb specific information. Therefore, despite a group of heterogeneous subjects, varied vessel harvest location (i.e. axial and inguinal) and potential pathology, the notion that temperature exhibited a common effect speaks to the robust nature of this response as it relates to the regulation of sympathetically mediated vasoconstriction. Specifically, independent of age, gender, or disease status, altering temperature from the homeostatic set-point significantly attenuates α1-mediated vasocontraction.
Limitations
While the novel approach of harvesting human skeletal muscle feed arteries affords a unique experimental paradigm, it was not achieved without limitation. The human feed arteries used in the current study were inarguably heterogeneous (i.e. harvest location, vessel size, age, gender, medications, health, etc.) and this could be considered a limitation of this study. However, a repeated measures design was employed specifically to reduce the impact of inter-individual variation. Additionally, although relatively small, some tachyphylaxis was observed in the time control studies (Figure 5), and thus may have contributed to the reduction in PE-induced tension development observed during measurements at subsequent temperatures. However, in supplemental studies, when PE dose responses at the different temperatures were performed in a cross sectional design, to avoid any time/tachyphylaxis effects, essentially the same temperature-dependent results were observed (Figure 4, panel B). Additionally, it is also acknowledged that in these supplemental and time control studies tension development was, on average, lower than the main protocol. This was attributed to differences in vessel caliber and arterial ring length and an unavoidable consequence of limited human feed artery availability. However, despite these differences, it is important to note that the functional characteristics of the vessels included in these supplemental studies were not different from those utilized in the main protocol. Finally, a reduction in baseline tension over time was also observed, which may have altered the contractile response to PE. However, it should be noted that KCl-induced tension remained stable across temperature, and the magnitude of the baseline shift (~70 mg tension) is highly unlikely to account for the dramatic reduction in tension (~500 mg tension) observed during the temperature perturbations.
Conclusion
Altering temperature, by either cooling or heating, reduces the contractile response of human skeletal muscle feed arteries to the α1-adrenergic receptor agonist phenylephrine in vitro. Importantly, these findings were independent of alterations in inherent smooth muscle function, as measured by KCl-mediated vasocontraction and have implications for human blood flow regulation in both hyperthermic and hypothermic conditions.
ACKNOWLEDGEMENTS
The authors would like to thank the gracious participation of the subjects and financial support by the National Institute of Health grant PO1 HL 091830-01.
Footnotes
DISCLOSURES: No conflicts of interest are reported by the author(s).
REFERENCES
- Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. American Journal of Physiology - Heart and Circulatory Physiology. 2005;289:H243–H250. doi: 10.1152/ajpheart.01305.2004. [DOI] [PubMed] [Google Scholar]
- Brothers RM, Wingo JE, Hubing KA, Del Coso J, Crandall CG. Effect of whole body heat stress on peripheral vasoconstriction during leg dependency. J Appl Physiol. 2009;107:1704–1709. doi: 10.1152/japplphysiol.00711.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter J, Clifford P. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exercise and Sport Sciences Reviews. 2001;29:159. doi: 10.1097/00003677-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent α2C-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. American Journal of Physiology - Heart and Circulatory Physiology. 2000;278:H1075–H1083. doi: 10.1152/ajpheart.2000.278.4.H1075. [DOI] [PubMed] [Google Scholar]
- Chotani MA, Mitra S, Eid AH, Han SA, Flavahan NA. Distinct cAMP signaling pathways differentially regulate α2C-adrenoceptor expression: role in serum induction in human arteriolar smooth muscle cells. American Journal of Physiology - Heart and Circulatory Physiology. 2005;288:H69–H76. doi: 10.1152/ajpheart.01223.2003. [DOI] [PubMed] [Google Scholar]
- Chotani MA, Mitra S, Su BY, Flavahan S, Eid AH, Clark KR, Montague CR, Paris H, Handy DE, Flavahan NA. Regulation of α2-adrenoceptors in human vascular smooth muscle cells. American Journal of Physiology - Heart and Circulatory Physiology. 2004;286:H59–H67. doi: 10.1152/ajpheart.00268.2003. [DOI] [PubMed] [Google Scholar]
- Colquhoun EQ, Hettiarachchi M, Ye J-M, Rattigan S, Clark MG. Inhibition by vasodilators of noradrenaline and vasoconstrictor-mediated, but not skeletal muscle contraction-induced oxygen uptake in the perfused rat hindlimb; implications for non-shivering thermogenesis in muscle tissue. General Pharmacology: The Vascular System. 1990;21:141–148. doi: 10.1016/0306-3623(90)90610-x. [DOI] [PubMed] [Google Scholar]
- Cooke J, Shepherd J, Vanhoutte P. The effect of warming on adrenergic neurotransmission in canine cutaneous vein. Circ Res. 1984;54:547–553. doi: 10.1161/01.res.54.5.547. [DOI] [PubMed] [Google Scholar]
- Eid AH, Chotani MA, Mitra S, Miller TJ, Flavahan NA. Cyclic AMP acts through Rap1 and JNK signaling to increase expression of cutaneous smooth muscle α2C-adrenoceptors. American Journal of Physiology - Heart and Circulatory Physiology. 2008;295:H266–H272. doi: 10.1152/ajpheart.00084.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid AH, Maiti K, Mitra S, Chotani MA, Flavahan S, Bailey SR, Thompson-Torgerson CS, Flavahan NA. Estrogen increases smooth muscle expression of α2C-adrenoceptors and cold-induced constriction of cutaneous arteries. American Journal of Physiology - Heart and Circulatory Physiology. 2007;293:H1955–H1961. doi: 10.1152/ajpheart.00306.2007. [DOI] [PubMed] [Google Scholar]
- Faber JE. Effect of local tissue cooling on microvascular smooth muscle and postjunctional alpha 2-adrenoceptors. Am J Physiol Heart Circ Physiol. 1988;255:H121–H130. doi: 10.1152/ajpheart.1988.255.1.H121. [DOI] [PubMed] [Google Scholar]
- Fick A. V. On liquid diffusion. Philosophical Magazine Series 4. 1855;10:30–39. [Google Scholar]
- Flavahan NA, Lindblad LE, Verbeuren TJ, Shepherd JT, Vanhoutte PM. Cooling and alpha 1- and alpha 2-adrenergic responses in cutaneous veins: role of receptor reserve. Am J Physiol Heart Circ Physiol. 1985;249:H950–H955. doi: 10.1152/ajpheart.1985.249.5.H950. [DOI] [PubMed] [Google Scholar]
- Flouris AD, Cheung SS. Influence of thermal balance on cold-induced vasodilation. J Appl Physiol. 2009;106:1264–1271. doi: 10.1152/japplphysiol.91426.2008. [DOI] [PubMed] [Google Scholar]
- Keller DM, Sander M, Stallknecht B, Crandall CG. α-Adrenergic vasoconstrictor responsiveness is preserved in the heated human leg. The Journal of Physiology. 2010;588:3799–3808. doi: 10.1113/jphysiol.2010.194506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluess HA, Buckwalter JB, Hamann JJ, Clifford PS. Elevated temperature decreases sensitivity of P2X purifiergic receptors in skeletal muscle arteries. Journal of Applied Physiology. 2005;99:995–998. doi: 10.1152/japplphysiol.00319.2005. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Gisolfi CV. Circulatory responses to vasoconstrictor agents during passive heating in the rat. Journal of Applied Physiology. 1990;68:1220–1227. doi: 10.1152/jappl.1990.68.3.1220. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Kenney MJ, Massett MP, Morgan DA, Lewis SJ. Role of nitrosyl factors in the hemodynamic adjustments to heat stress in the rat. Am J Physiol Heart Circ Physiol. 1997;273:H1537–H1543. doi: 10.1152/ajpheart.1997.273.3.H1537. [DOI] [PubMed] [Google Scholar]
- Krogh A. The rate of diffusion of gases through animal tissues, with some remarks on the coefficient of invasion. The Journal of Physiology. 1919;52:391. doi: 10.1113/jphysiol.1919.sp001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Ferguson RA, Kjær M, Bangsbo J. ATP and heat production in human skeletal muscle during dynamic exercise: higher efficiency of anaerobic than aerobic ATP resynthesis. The Journal of Physiology. 2003;549:255–269. doi: 10.1113/jphysiol.2002.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson S, Aagaard P, Larsson B, Kjaer M. Passive energy absorption by human muscle-tendon unit is unaffected by increase in intramuscular temperature. Journal of Applied Physiology. 2000;88:1215. doi: 10.1152/jappl.2000.88.4.1215. [DOI] [PubMed] [Google Scholar]
- Massett MP, Lewis SJ, Bates JN, Kregel KC. Effect of heating on vascular reactivity in rat mesenteric arteries. Journal of Applied Physiology. 1998a;85:701–708. doi: 10.1152/jappl.1998.85.2.701. [DOI] [PubMed] [Google Scholar]
- Massett MP, Lewis SJ, Bates JN, Kregel KC. Modulation of temperature-induced tone by vasoconstrictor agents. Journal of Applied Physiology. 1999;86:963–969. doi: 10.1152/jappl.1999.86.3.963. [DOI] [PubMed] [Google Scholar]
- Massett MP, Lewis SJ, Kregel KC. Effect of heating on the hemodynamic responses to vasoactive agents. Am J Physiol Regul Integr Comp Physiol. 1998b;275:R844–R853. doi: 10.1152/ajpregu.1998.275.3.R844. [DOI] [PubMed] [Google Scholar]
- Massett MP, Lewis SJ, Stauss HM, Kregel KC. Vascular reactivity and baroreflex function during hyperthermia in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1282–R1289. doi: 10.1152/ajpregu.2000.279.4.R1282. [DOI] [PubMed] [Google Scholar]
- Mustafa S, Thulesius O, Ismael H. Hyperthermia-induced vasoconstriction of the carotid artery, a possible causative factor of heatstroke. Journal of Applied Physiology. 2004;96:1875. doi: 10.1152/japplphysiol.01106.2003. [DOI] [PubMed] [Google Scholar]
- Myrer JW, Measom G, Fellingham GW. Temperature changes in the human leg during and after two methods of cryotherapy. J Athl Train. 1998;33:25–29. [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Garcia-Villalon AL, Fernandez N, Monge L, Gomez B, Dieguez G. Effects of hyperthermia on contraction and dilatation of rabbit femoral arteries. Journal of Applied Physiology. 1998;85:2205–2212. doi: 10.1152/jappl.1998.85.6.2205. [DOI] [PubMed] [Google Scholar]
- Raven PB, Niki I, Dahms TE, Horvath SM. Compensatory cardiovascular responses during an environmental cold stress, 5 degrees C. J Appl Physiol. 1970;29:417–421. doi: 10.1152/jappl.1970.29.4.417. [DOI] [PubMed] [Google Scholar]
- Roberts MF, Chilgren JD, Zygmunt AC. Effect of Temperature on Alpha-Adrenoceptor Affinity and Contractility of Rabbit Ear Blood Vessels. Journal of Vascular Research. 1989;26:185–196. doi: 10.1159/000158767. [DOI] [PubMed] [Google Scholar]
- Ryan AJ, Gisolfi CV. Responses of rat mesenteric arteries to norepinephrine during exposure to heat stress and acidosis. Journal of Applied Physiology. 1995;78:38–45. doi: 10.1152/jappl.1995.78.1.38. [DOI] [PubMed] [Google Scholar]
- Saltin B, Hermansen L. Esophageal, rectal, and muscle temperature during exercise. Journal of Applied Physiology. 1966;21:1757. doi: 10.1152/jappl.1966.21.6.1757. [DOI] [PubMed] [Google Scholar]
- Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiologica Scandinavica. 2000;168:511–518. doi: 10.1046/j.1365-201x.2000.00703.x. [DOI] [PubMed] [Google Scholar]
- Siddegowda YKB, Leo MDM, Kumar D, Hooda OK, Prakash VR, Mishra SK. Influence of heat stress on the reactivity of isolated chicken carotid artery to vasoactive agents. Experimental Physiology. 2007;92:1077–1086. doi: 10.1113/expphysiol.2007.038844. [DOI] [PubMed] [Google Scholar]
- Symons J, Rendig S, Stebbins C, Longhurst J. Microvascular and myocardial contractile responses to ischemia: influence of exercise training. Journal of Applied Physiology. 2000;88:433. doi: 10.1152/jappl.2000.88.2.433. [DOI] [PubMed] [Google Scholar]
- Vanamoorthy P, Pandia M, Bithal P, Valiaveedan S. Refractory hypotension due to intraoperative hypothermia during spinal instrumentation. 2010:56–58. doi: 10.4103/0019-5049.60500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte P, Lorenz R. Effect of temperature on reactivity of saphenous, mesenteric, and femoral veins of the dog. The American journal of physiology. 1970;218:1746. doi: 10.1152/ajplegacy.1970.218.6.1746. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, Shepherd J. Effect of temperature on reactivity of isolated cutaneous veins of the dog. American Journal of Physiology. 1970;218:187. doi: 10.1152/ajplegacy.1970.218.1.187. [DOI] [PubMed] [Google Scholar]
- Williams DA, Segal SS. Feed artery role in blood flow control to rat hindlimb skeletal muscles. The Journal of Physiology. 1993;463:631–646. doi: 10.1113/jphysiol.1993.sp019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Crandall CG. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Auton Neurosci. 2002;97:122–128. doi: 10.1016/s1566-0702(02)00046-2. [DOI] [PubMed] [Google Scholar]
- Wray DW, Nishiyama SK, Donato AJ, Richardson RS. Human Vascular Aging: Limb-Specific Lessons. Exercise and Sport Sciences Reviews. 2010;38:177–185. doi: 10.1097/JES.0b013e3181f45413. 110.1097/JES.1090b1013e3181f45413. [DOI] [PubMed] [Google Scholar]