Abstract
The fundamental properties and pH-sensitivity of chitosan/gelating hydrogels were investigated using spectroscopic and microelectro mechanical (MEMS) measurement approaches. Turbidimetric titration revealed that there were electrostatic attractive interactions between tripolyphosphate (TPP), chitosan, and gelatin in the acidic pH range, depending on their degree of ionization. The pH-sensitive swelling behavior of the hydrogels was investigated by monitoring the deflection of hydrogel-coated microcantilevers, which exhibited a sensitive and repeatable response to solution pH. The deflection of the microcantilever increased as the pH decreased, and the response speed of the system exhibited a nearly linear relationship with pH. The effects of the pH and concentration of TPP solution, as well as the ratio of chitosan to gelatin in gel precursor solutions, on the pH sensitivity of the hydrogels were also investigated. It was found that the swelling of the hydrogel is mainly a result of chain relaxation of chitosan–TPP complexes caused by protonation of free amino groups in chitosan, which depends on the crosslinking density set during the formation of the network. An increase in initial crosslink density induced a decrease in swelling and pH sensitivity. It can be concluded from this study that pH-sensitive chitosan gel properties can be tuned by preparatory conditions and inclusion of gelatin. Furthermore, microcantilevers can be used as a platform for gaining increased understanding of environmentally sensitive polymers.
Keywords: chitosan, pH-sensitivity, hydrogels, smart gels, microcantilever
INTRODUCTION
Hydrogels are capable of providing a variety of useful properties in the field of biomedical engineering, such as ophthalmologic devices, biosensors, biomembranes, and carriers for controlled delivery of drugs or proteins (Peppas et al., 2000). Hydrogels are water-swollen polymeric networks containing chemical or physical crosslinks, which can undergo volume transitions in response to minute changes in environmental stimuli such as pH (Karadag et al., 2005; Kim et al., 2003a,b), ionic strength (Sakiyama et al., 2003), temperature (Aoki et al., 1994), and electric fields (Kim et al., 2003a,b). Such polymeric systems are often called “intelligent” or “smart” materials because of their response to external signals. Among various environmental stimuli of intelligent hydrogels, pH-responsive mechanisms have been considerably investigated because pH is a relatively convenient and effective stimulus in many applications, such as used as drug delivery and biosensor systems. The pH sensitivity of a hydrogel is a change in volume of the hydrogel in response to pH changes in the surrounding medium, caused by the presence of weakly acidic or basic functional groups on the polymer backbone (Soppimath, 2001).
Among the commercially available polymers for intelligent hydrogels, chitosan is currently receiving a great deal of interest for its interesting intrinsic properties, such as biocompatibility, biodegradability, promotion of wound healing, and anti-bacteriostatics (Agnihotri et al., 2004; Krajewska, 2001). Chitosan is a copolymer of β-(1→4)-linked-2-acetamido-2-deoxy-d-glucopyranose and 2-amino-2-deoxy-d-glucopyranose. This polycationic biopolymer is generally obtained by alkaline deacetylation from chitin, which is the main component of the exoskeleton of crustaceans such as shrimp and crawfish.
Due to the presence of ionizable amino groups, chitosan is a cationic polyelectrolyte with a pKa of 6.5, and is one of a few naturally occurring materials that can form hydrogels by complexation with anionic polyelectrolytes. For example, gelatin type B (isoionic point, pI, around 5.0) can form polyelectrolyte complexes (PECs) with chitosan. Gelatin is the partially denatured product of collagen, and gelatins of different pI can be prepared by proper choice of preconditioning of the gelatin stock. To improve the mechanical properties of PEC hydrogels, crosslinking is commonly performed. Because many crosslinking agents used to perform covalent crosslinking may induce toxicity if present even in trace quantity before in vivo administration, ionically crosslinked chitosan hydrogels are preferred; these are generally believed to be well-tolerated and their potential medical and pharmaceutical applications are numerous since small molecule ionic crosslinkers are typically biocompatible (Berger et al., 2004).
Many studies have been performed to elucidate the swelling behavior of various pH-sensitive hydrogels. The most common method employed to determine the swelling behavior involves weighing of gel slabs, placing them in different pH solutions for a defined time, extraction and blotting to remove surface water, and finally weighing (Kim and Peppas, 2002). Disadvantages of this method include difficulty in controlling how much water is removed from the gel and the poor mechanical integrity of the soft swollen gel, which can easily break apart during repeated handling. Other typical methods include calculating the volume change by measuring the diameter of gel discs (Sakiyama et al., 1999) or measuring drug release from the hydrogel matrix during exposure to different pH solutions (Shu et al., 2001). It has been reported that the swelling of ionic-crosslinked chitosan hydrogels under acidic conditions (below pH 4) is pronounced and measurable using the methods described above, while under near-neutral conditions the swelling of the gels is less pronounced and difficult to quantify (Berger et al., 2004; Shu et al., 2001). Therefore, the swelling behavior of chitosan hydrogels in the physiological pH range, which is critical for in vivo applications, is still unclear.
Microcantilevers provide a sensitive platform for chemical and biological sensors (Fritz et al., 2000) and can provide excellent dynamic response, greatly reduced size, high precision, and increased reliability. These systems can be integrated onto micromechanical components with on-chip electronic circuitry. Since pH-sensitive hydrogels swell in response to pH and the gel volume is a function of external pH, it is intuitive that the swelling of a hydrogel film immobilized on a microcantilever will cause the cantilever to bend; this phenomenon has been experimentally observed for various polymer types with extremely high sensitivity (Bashir et al., 2002; Zhang et al., 2004). Such systems offer not only an approach to precise pH measurement, but also an attractive tool for screening and evaluating environmentally sensitive gels.
The objective of this study was to investigate the swelling mechanism of chitosan/gelatin hydrogels with and without tripolyphosphate (TPP)-induced ionic crosslinking in the physiological pH range. The turbidimetric titration method was used to investigate the interactions among TPP, chitosan, and gelatin molecules in solution. The swelling response was then evaluated by coating the responsive hydrogels on microcantilevers and exposing the modified cantilevers to solutions with pH ranging from 6 to 7.45.
MATERIALS AND METHODS
Materials
Chitosan (low molecular weight, MW ~50,000 Da), gelatin (type B, Bloom 225), sodium TPP, phosphate buffered saline (PBS tablets), and 1H,2H,2H-perfluorodecanethiol (PFDT) were purchased from Sigma.
Instruments
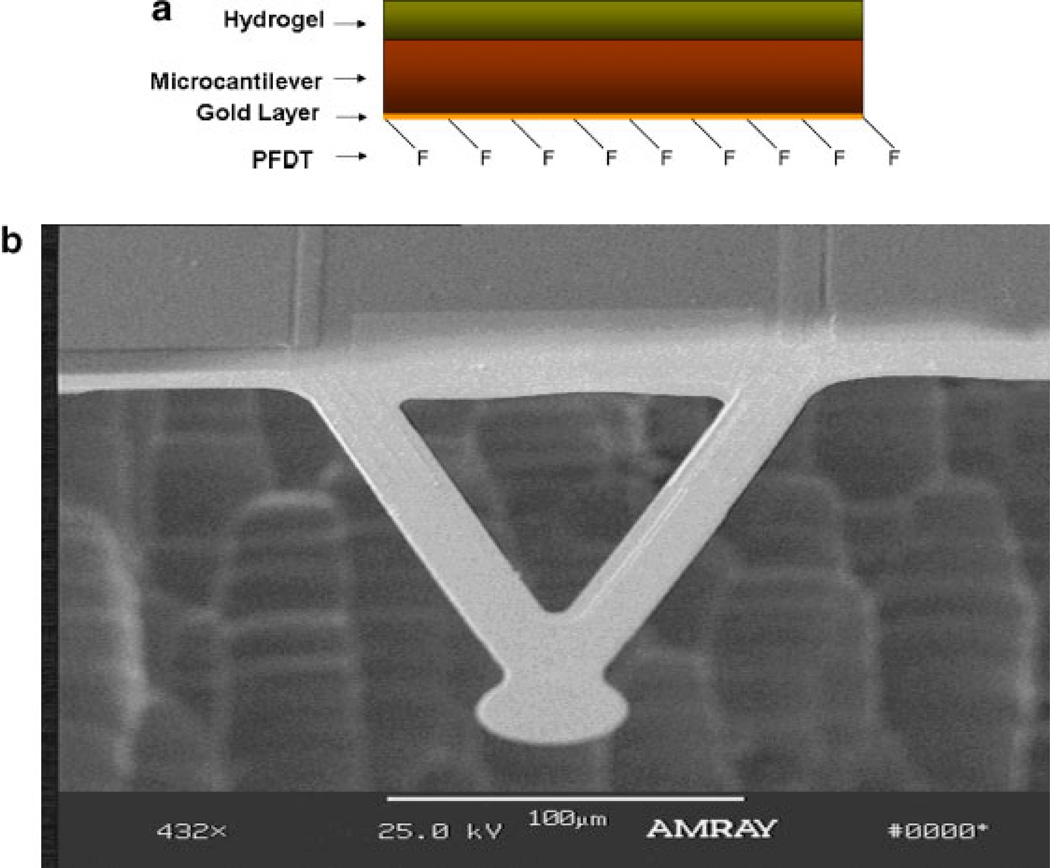
Turbidity was measured by monitoring transmittance with a Perkin-Elmer Lambda 45 UV-Vis spectrometer. The cantilevers used experimentally were V-shaped silicon structures of 200 µm in length, 50 µm width, and 2 µm thickness micromachined by dry plasma (ICP) etching. One side of the cantilever had a thin film of chromium (3 nm), followed by a 20 nm layer of gold, both deposited by electron beam evaporation. The other side of the cantilever was a naturally grown oxide layer. Details of the devices and fabrication procedures are given elsewhere (Tang et al., 2004).
Turbidimetric Titration
The interactions of chitosan, gelatin, and TPP molecules were investigated by turbidimetric titration. The dependence of the polymer solution turbidity on pH was obtained according to reported methods (Mattison et al., 1995). Briefly, 0.1M NaOH was added into the solution at constant ionic strength and at constant concentrations. Gelatin, chitosan, and TPP solutions were prepared independently and filtered with 20 µm nylon membranes (MAGNA) prior to mixing. Upon addition of base, the solution was gently stirred with a magnetic bar until a stable transmission reading (%T) was obtained. Adigital pHmeter was used to monitor the solution pH. Changes in transmittance were monitored at 420 nm, and the turbidity was calculated as 100-%T.
Preparation of Hydrogel-Coated Microcantilevers
To selectively attach the hydrogels on one surface of a microcantilever, PFDT was self-assembled onto the goldcoated surface to block the attachment of the hydrogel (Yan et al., 2004). Cantilevers were coated with PFDT by placing the cantilevers in 5 × 10−3M PFDT ethanol solution for 24 h, and then rinsing with ethanol three times. The microcantilevers were then placed on a quartz slide, separated from the quartz surface by a 15 µm parafilm spacer so that there was a 15 µm distance between the microcantilever tip and the quartz surface. Chitosan (2% w/w) and gelatin (2% w/w) were mixed together, and the slide was dipped in the mixture and cooled to 4°C for 3 h. Then TPP solution was added in and left overnight for gelation. The coated microcantilevers were then stored in 0.01M PBS solution pH 7.45 for 24 h. Figure 1a contains a side-view schematic of the hydrogelcoated cantilever, and a scanning electron micrograph of a typical cantilever as used in the experiments is shown in Figure 1b.
Figure 1.
a: Architecture of chitosan/gelatin-coated microcantilever; (b) electron micrograph of custom-fabricated microcantilever device. The cantilever extends 200 µm from the support structure. [Color figure can be seen in the online version of this article, available at www.interscience.wiley.com.]
Deflection Measurements
The procedures used for measurement of cantilever deflection were similar to those reported previously (Bashir et al., 2002) All solutions were adjusted to have the same buffer concentration and ionic strength with different pH. The microcantilever response was measured in a flow-through glass cell (Veeco Metrology, Santa Barbara, CA) arranged in an atomic force microscope. Initially, the microcantilevers were exposed to 0.01M, pH 7.45 PBS solution by pumping it through the cell at a flow rate of 40 mL/h with the aid of a syringe pump. After a baseline reading was established, 2mL of sample solution (0.01M PBS at a different pH) was pumped through the sample cell at the same flow rate. Then, after 3 min of exposure to the sample solution, fresh baseline PBS solution (pH, 7.45) was circulated back into the fluid cell. Bending was measured as a change in the position of a laser beam reflected from the microcantilever onto to a fourquadrant diode.
RESULTS AND DISCUSSION
Turbidimetric Titration
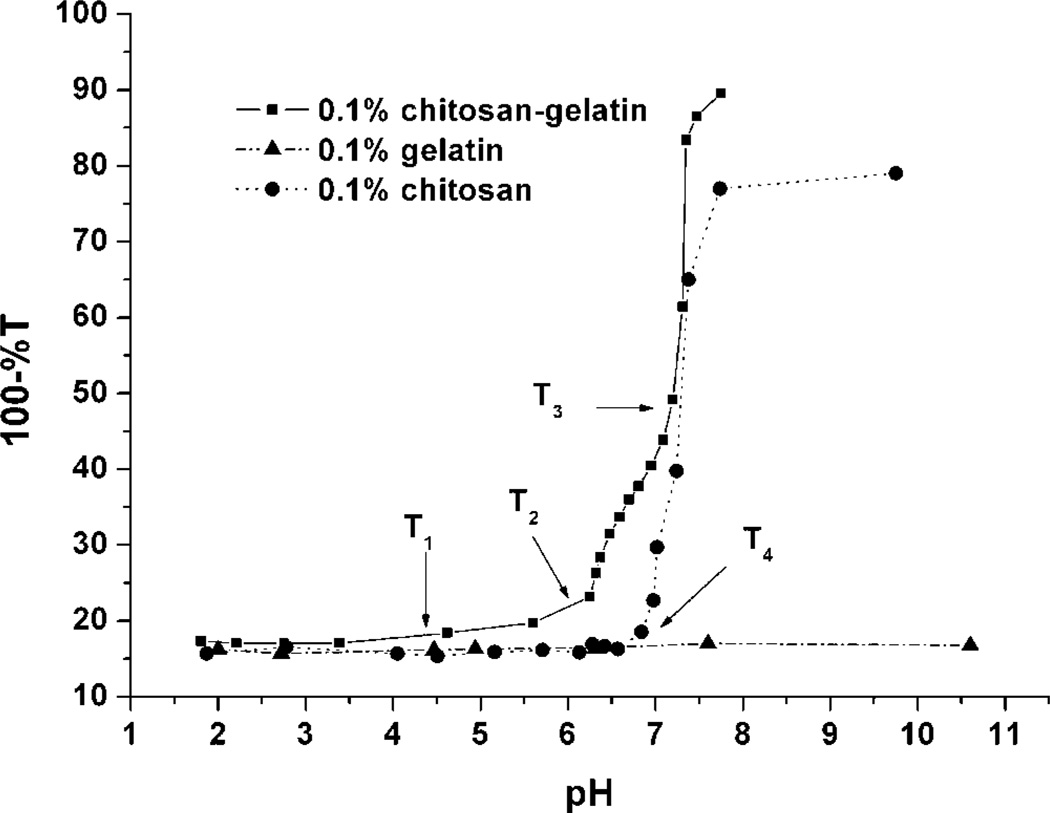
The results of the turbidity titration curves of gelatin, chitosan, and gelatin/chitosan mixture solutions are shown in Figure 2. Three turbidity change regions (T1, T2, and T3) were revealed by the curve for the gelatin/chitosan mixture. The first point, T1 (between pH 4 and 5), was not as pronounced as the other two points, though it can be observed that the turbidity of the solution was approximately constant (and the solution was visibly clear) at pH below T1, and the turbidity of the solution was higher and increased slowly with pH above T1. Above T2, from pH 6 to 7.2, the turbidity of the solution increased quickly. As the pH of the solution increased above T3, the substantial increase in turbidity indicated the presence of a coacervate.
Figure 2.
Turbidity titration curves of gelatin, chitosan, and gelatin/chitosan mixture solutions (%T measured at 420 nm).
This behavior is likely due to the presence of gelatin, which has a pI of 4.5–5.0, with chitosan, which possesses a pKa around 6.5. At pH values lower than gelatin’s pI, both gelatin and chitosan molecules have an overall positive charge. The repulsive forces between the positively charged gelatin and positively charged chitosan prevent the formation of complexes, and the two kinds of molecules coexist separately within the solution. When the pH is above the gelatin pI, but below the chitosan pKa (between 4 and 6), the gelatin molecules have negative charge, which can react with the positively charged chitosan to form complexes. From previous reports, it is known that the net charge density of gelatin is rather low, approximately 15 ionized groups per 105 g of gelatin at pH 6.5 (Veis and Aranyi, 1960). Therefore, even as gelatin reacts with chitosan, the complex formed still has high overall positive charge like the free polyelectrolyte and displays a pH-dependent mobility that decreases to zero at the point of coacervation. The positive charge between the complexes and the thermodynamic mobility of the complexes keeps the solution stable. Thus, the region of small change surrounding T1 is a result of the pI of gelatin influenced the mixture solution.
At pH values near the pKa of chitosan (pKa around 6.5), the positive charge density of chitosan decreases dramatically. Some complexes conjugate together to form larger particles in order to reach a stable balance in the solution, due to the decrease of charge density of the complex surface, and the turbidity of the solution increases. Thus, the appearance of T2 is the primary effect of the chitosan pKa on the mixture solution.
Compared with the curve of chitosan/gelatin mixture, the curve of pure chitosan showed only a single inflection point, T4, where the chitosan molecules lost positive charge and began to coacervate. In contrast, the gelatin curve exhibited no obvious change, which suggests that the pH change in this range does not influence gelatin solution behavior, as expected. Gelatin is one of a few proteins that possess a random coil configuration, and gelatin behavior in solution follows Flory–Huggins lattice solution theory (Mattison et al., 1995). In addition, the charge density of gelatin molecule is relatively low, so minimal differences in ionization are expected. It is noteworthy that the behavior of these materials in response to titration is consistent and reversible, with minimal observed hysteresis, regardless of the initial pH conditions.
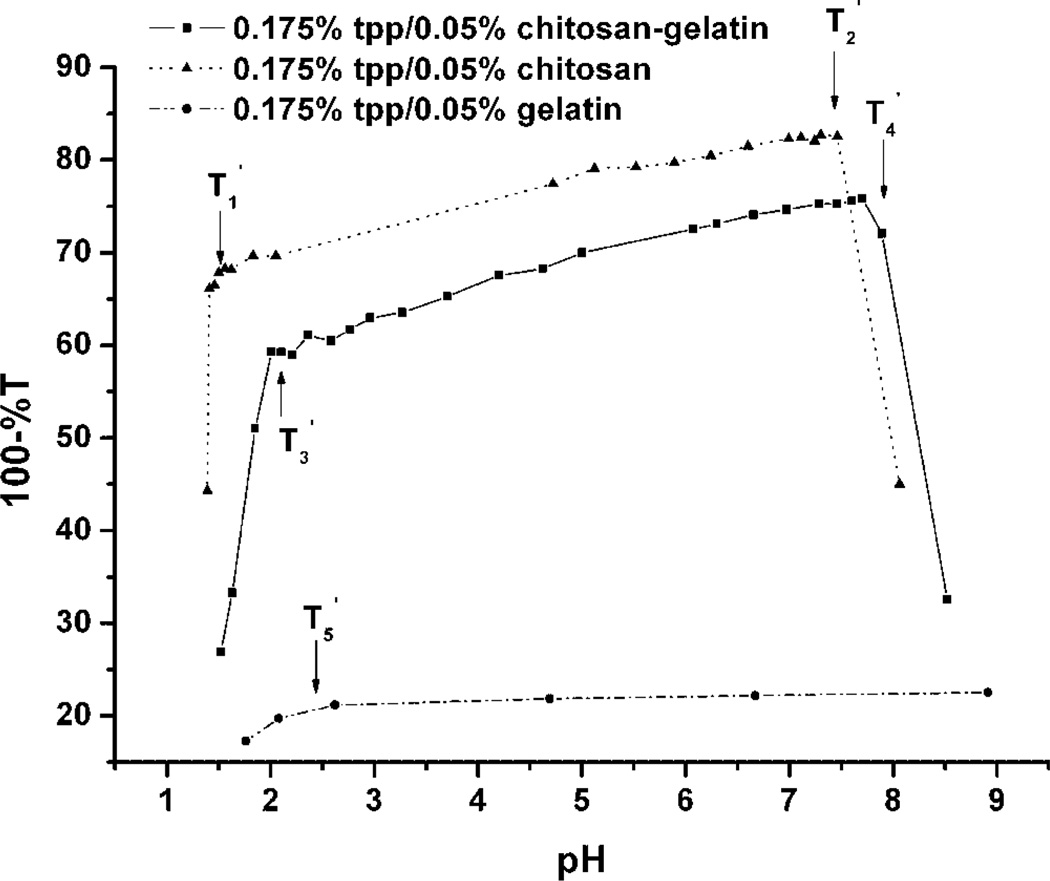
Similar experiments were conducted to compare the solution-phase behavior of chitosan/gelatin hydrogels in the presence of TPP, a common ionic crosslinker for polycationic gels. The results of turbidity titration of TPP/polymer systems are shown in Figure 3. It is clear that the curves of TPP/chitosan and TPP/chitosan/gelatin exhibit a similar overall trend. Both systems had two points of change at , and , At pH values below , the turbidity increased dramatically with increasing pH, and at pH above but below , the turbidity increased slowly with pH. At pH above , the turbidity decreased due to precipitation.
Figure 3.
Turbidity titration curves of TPP/gelatin, TPP/chitosan, and TPP/gelatin/chitosan mixture solutions (%T measured at 420 nm).
From previous reports (Shu and Zhu, 2001), it is believed that the negative charge of TPP is strongly pH-dependent at values below pH 2. The charge density of chitosan does not change much in this pH range, so the effect of acidic titration on turbidity is due only to TPP ionization. In this region, as pH decreases, fewer ionic groups of TPP react with the positively charged amine groups of chitosan to form complexes, which results in decreased turbidity with decreasing pH. At pH values above 2, the ionic density of TPP increases slowly, which is reflected in the proportional change in turbidity. In the range of pH 2–7, the turbidity of the solution increases slowly, and the complexes maintain a positive charge. Therefore, is due to the strong dependence of charge density on pH near the pKa of TPP.
At the point of , precipitation was observed in the solution. At this time, most of the amine groups on chitosan chains reacted with TPP ions, and the free positive charge of the complexes decreased to a very low level, since the solution pH was above the pKa of chitosan. Thus, the stability of the solution was destroyed and precipitation occurred. Since gelatin has a very low charge density, the degree of TPP reacted with gelatin is low, as shown in Figure 3. A small increase in gelatin solution turbidity can be observed from the curve for pH values below , which again is the result of the increase in charge density of TPP. In this pH range, cationic gelatin molecules can react with TPP ions, but the overall effect is small.
Taken together, the turbidity titration test results suggest that there are electrostatic attractive interactions between chitosan/gelatin in the acidic pH range, depending on their degree of ionization. Gelatin molecules can form PECs with chitosan molecules, such that they become entrapped in the chitosan gel via electrostatic bonds. The key point is that chitosan and gelatin can form uniform hydrogels at low temperature due to the gelation character of gelatin, without any need for other crosslinkers (Yan et al., 2004). In the TPP/gelatin/chitosan system, the crosslinking structure is mainly formed by the reaction between chitosan and TPP, which can help the hydrogel keep its shape at room temperature. Meanwhile, the findings also prove that chitosan cannot form uniform gels with only TPP because precipitation occurs if these two molecules complex in solution at high concentration.
Based on the turbidity test results, the hydrogel coatings for microcantilevers were prepared using both gelatin and TPP, as described above. First, the chitosan and gelatin were mixed at room temperature (25°C), which is above the gelatin gelation point, and the solution was cooled to 4°C to form a uniform gel. Ionic crosslinking was then initiated by addition of TPP under gel conditions to avoid the precipitation that would result from the direct reaction between TPP and chitosan.
Microcantilever Deflection Measurements
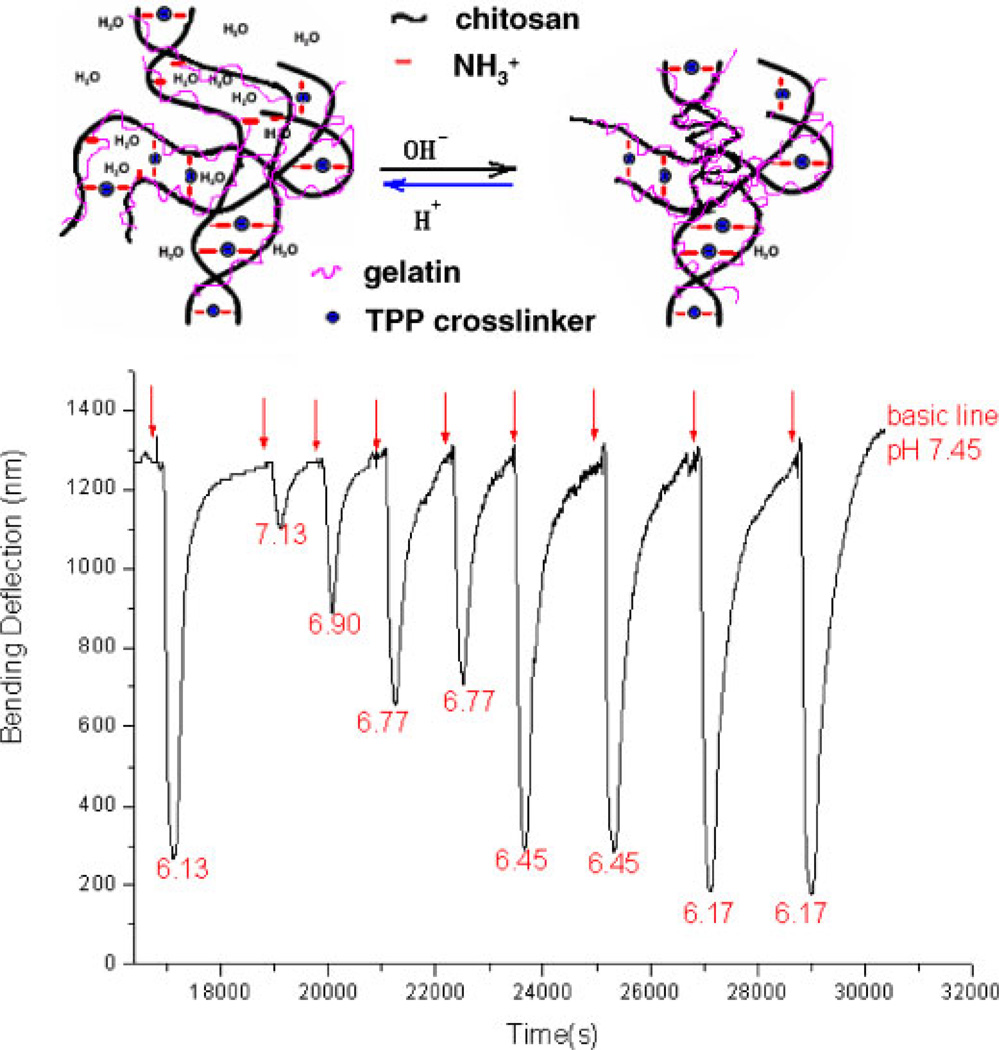
Microcantilevers were used to provide a sensitive test of the pH-induced swelling behavior of chitosan/gelatin hydrogels in the physiological pH range from 6 to 7.45. A 15 µm thick TPP-crosslinked chitosan/gelatin hydrogelcoated microcantilever was initially exposed to a constant flow (40 mL/h) of PBS (pH, 7.45) for a baseline measurement. When solutions with pH other than 7.45 were injected into the fluid cell, the microcantilever exhibited bending due to the swelling of the hydrogel immobilized on one size of the beam. Following reversion to the initial PBS buffer, the microcantilever gradually returned to its original baseline position. Both the total deflection and the speed of the bending response were dependent on the pH change. The bending rate (−dB/dt) was calculated from the slope of the bending curve; since the same dwell time was used for each pH measurement in this study, the bending rate was used to indicate pH changes in cases where steady-state deflection was not reached during the 3-min sample period. The bending response of the gel cured in pH 6, 3.5% (m/v) TPP solution is shown in Figures 4 and 5. These results suggest that the response of a gel cured at pH 6 had a transient section, where the response was not repeatable, early in the experiment (Fig. 4) and a steady-state response was reached after sufficient preconditioning by cyclic exposure to different pH (Fig. 5).
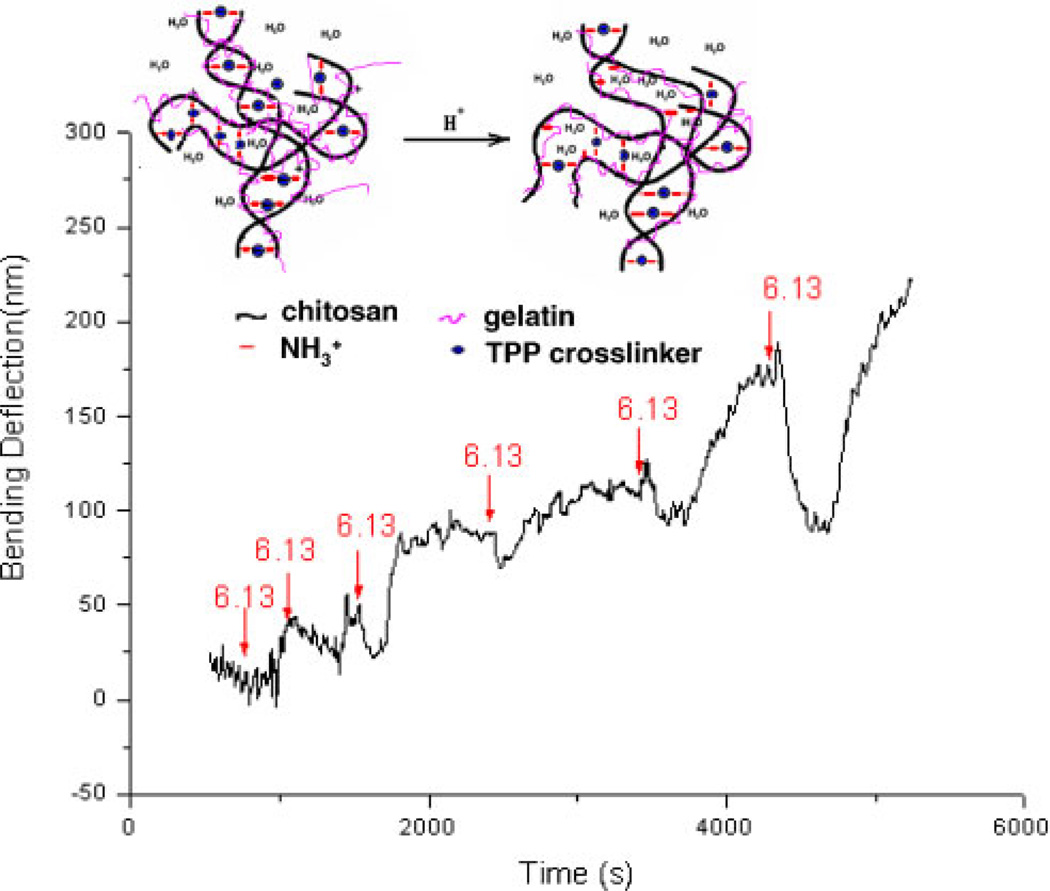
Figure 4.
The transient bending response as a function of time for chitosan/gelatin (C:G = 1:1, 3.5% TPP at pH = 6.0) gel-coated microcantilever, upon injection of a 0.01MPBS at pH 6.13. Mediumfor baseline readings was 0.01M PBS, pH 7.45. Injection times are indicated with arrows. [Color figure can be seen in the online version of this article, available at www.interscience.wiley.com.]
Figure 5.
Steady-state (preconditioned) bending response versus time for chitosan/gelatin (C:G = 1:1, 3.5% TPP at pH = 6.0) gel-coated microcantilever, upon injection of a 0.1M PBS at various pH. Injection times are indicated with arrows. [Color figure can be seen in the online version of this article, available at www.interscience.wiley.com.]
As shown in Figure 4, the gel was exposed to pH 7.45 PBS to establish the baseline value. Then, pH 6.13 PBS was injected into the cell, and a correspondingly small negative deflection was observed. Next, pH 7.45 PBS solution was introduced and the cantilever moved toward the original position. However, rather than settling at the initial point, a net positive deflection was observed. Next, the fresh PBS solutions of the same pH 6.13 and pH 7.45 were injected into the cell alternatively, and the microcantilever responded in each case. However, but an upward drift in the deflection profile was observed during the experiment, and the magnitude of the response was increasing with the number of cycles. These observations indicate that the hydrogel swelled a small amount while the low pH solution was injected and then shrank when exposed to higher pH solution, but in this early stage some irreversible changes are occurring in the system.
The transient pH-response of the gel indicates that a structural change occurs inside the hydrogel, which can be explained as follows. A structure with high crosslink density is formed after the gel is prepared in pH 6 TPP solution. As the gel is exposed to acidic buffer, a small number of bonds between the TPP molecules and the amino groups of chitosan are broken, due to the competitive reaction between H+ and with TPP ions. As a result, more free groups are left, meaning a gel with more ionizable groups that will respond to pH has been produced. The gel swells because of increased electrostatic repulsion between the cationic chains; at the same time, the polymer chains become more hydrophilic, owing to the increased charge, thus leading to increased hydration of the polymer chain. Then, when a basic solution is introduced, the groups become neutralized by OH− to form NH2, which decreases the repulsion force between the chitosan chains. In addition, the hydrophobicity of the gel also increases because more NH2 groups are presented on the chitosan chains. The hydrophobic effect causes the molecular chains to aggregate and water molecules are extruded. Therefore, the hydrogel shrinks when the external pH increases because there are more groups available after one pH-stimuli response, the hydrogel shrinks more, which caused decreased volume. Thus, the chitosan/gelatin/TPP gels can be “reconditioned” by repeated exposure to different pH values to reach a steady-state distribution of and readily titratable to arrive at a consistent pH-sensitive behavior.
After repeated swelling and shrinking, the swelling of the gel reached a consistent behavior. In this state, the microcantilever coated with the hydrogel showed a sensitive and repeatable response to different pH (Fig. 5). It is important to note that the 3-min sample time was not enough for the cantilever to settle at a final deflection in every case, so the peak deflection observed in the graph is only the deflection reached at the point when the baseline rinse buffer began recirculating. However, it is clear from the bending profiles that the response to changing pH is repeatable and proportional to pH. In the steady state, the microcantilever deflection increased as the pH decreased from 7.45 to 6.1, a sensitivity of approximately 1,000 nm total deflection/pH unit. These results indicate that the chemical structure formed by the interaction between chitosan molecules and TPP ions reaches an equilibrium after preconditioning, and the gel arrives at a steady-state composition that responds reversibly to ambient pH.
The results of turbidity tests suggest that the crosslinking structure of the TPP/gelatin/chitosan system is mainly formed by the reaction between chitosan and TPP. Since the gel maintains a stable and consistent, fully reversible response, the swelling of the gel is mainly attributed to chain relaxation of chitosan–TPP complexes by the protonation of the unbound −NH2 groups, but not by the dissociation of ion bridges. At lower pH, the entering H+ ions protonate the free amino groups on the chitosan molecule chains instead of competitively reacting with TPP ions. The protonation and deprotonation of the free amino groups changes the repulsion between the same charged groups on the chitosan molecule, which results in a volume change of the hydrogel, and the volume change is reflected by the bending of the microcantilever. Since the gel crosslinking structure is stable in this stage, the bending of the microcantilever is reversible and reproducible.
In this study, the external pH change was controlled in a tight range between 6.14 and 7.45, which is close to the pKa of chitosan. In this pH range, the positive charge density of chitosan molecule increases dramatically when external pH decreases, as proven by the turbidity results. Therefore, the swelling of the gel increased as external pH decreased, and the deflection of gel-coated cantilevers correspondingly increased.
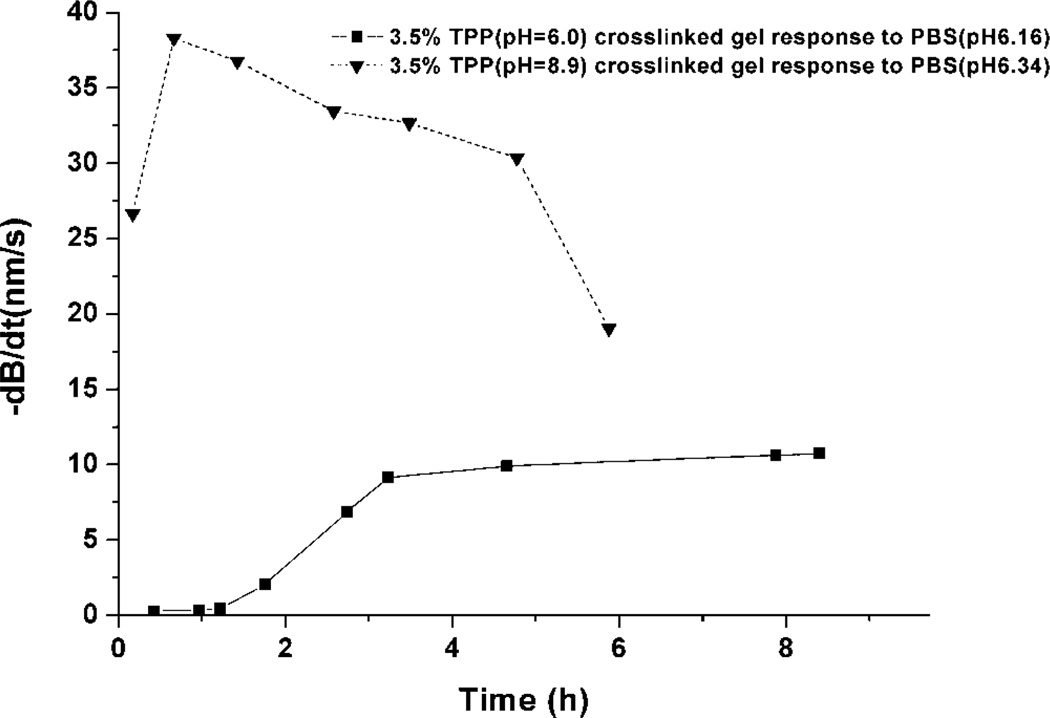
As noted above, a transient response was observed during the acid-induced swelling of ionic-crosslinked chitosan-based system; such behavior that is generally not observable by other methods used to quantify swelling. This phenomenon is shown clearly in Figure 6. The response rates of two gel-cantilever systems, cured at different pH values, were measured for repeat exposures to acidic stimuli. These data, shown in Figure 6, were collected by repeated measurement of deflection speed in response to pH 6 PBS after equilibration in pH 7.4 PBS at several time points. The dB/dt given in the graph is the highest deflection rate, which occurs shortly after introduction of the sample solution. The measurements presented here are limited by the experimental conditions, as the pH 7.45 rinse buffer automatically enters the cell 3 min after the low-pH sample solution injection. Thus, there is generally not enough time for the hydrogel to reach an equilibrium swelling state, and therefore the maximum deflection rate was used to describe the swelling response rather than instead of peak deflection.
Figure 6.
Maximum bending rate (−dB/dt) of cantilevers modified with chitosan/gelatin hydrogel crosslinked by 3.5% TPP at pH 6.0 and 8.9. Each data point is a separate deflection rate measurement in response to addition of pH 6 PBS to a cantilever (baseline of pH 7.4 PBS).
From the data in Figure 6, it appears that after six cycles of pH change from 7.45 to 6.16, the swelling response of the gel prepared at pH 6 became stable. The exact nature of the transient behavior depends on the preparatory conditions; for example, the same transient behavior is not observed in the plot of gel prepared at pH 8.9. Since it has been reported that the ionic interaction of chitosan with TPP is pH-dependent (Mi et al., 1999), it is not surprising that the response rate plots of the hydrogel prepared at different pH are not the same. The values of pH 6 and 8.9 for preparation were selected based on the results of Figure 3, which also suggests that the interaction of chitosan with TPP is pH-dependent. At pH lower than the pKa of chitosan, the interaction between chitosan and TPP is ionic-crosslinking controlled, whereas at pH 8.9, the interactions are believed to be precipitate-controlled, accompanied with slight ionic-crosslinking dependence. The absolute deflection and rate of response were much larger for GEL 8.9 compared to GEL 6.0. This behavior is attributed to the different internal structure obtained by the curing conditions. GEL 8.9 has a relatively low crosslinking density, the initial structure of the hydrogel is loose, and there are relatively more free amino groups on the chitosan molecules. Thus, the response to pH is very large at the beginning. However, this kind of crosslinking structure is not durable; the swelling decreases after the initial rapid change and, after a slow reverse response period, the response speed of GEL 8.9 drops quickly. The crosslinking density decreases, due to dissociation of TPP, and the hydrogel is partially dissolved. In contrast, the gel cured at pH 6.0, while exhibiting a very small response prior to conditioning and an overall slower response, produced a very consistent deflection rate behavior after the initial conditioning period.
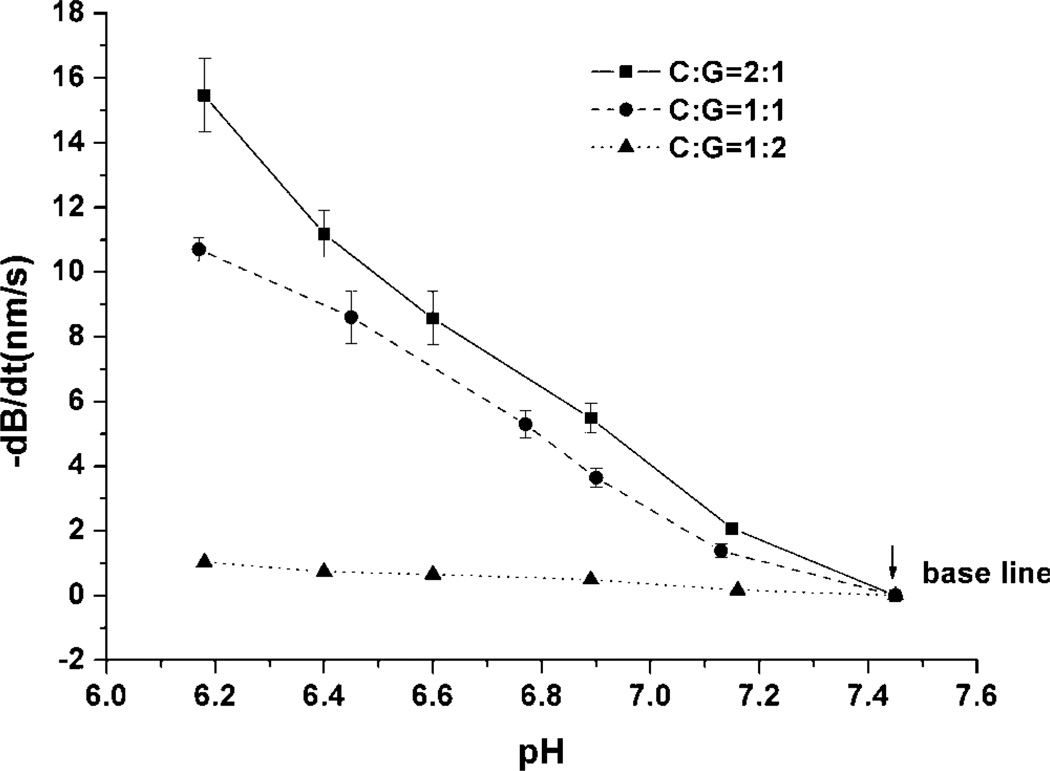
In contrast to the initial response behavior, the response speed as a function of pH for preconditioned gels was nearly linear, as shown in Figure 7. The ratio of chitosan to gelatin (C:G) in the precursor mixture also influenced the swelling behavior of the hydrogels and the deflection of the coated microcantilevers. The three different batches of hydrogel had nearly linear bending response speed as a function of pH, but the gels with higher C:G ratio exhibited higher pH sensitivity. This result was anticipated, as higher C:G means there are relatively more chitosan molecules per unit volume; thus, there are more free amino groups provided in the gel if the other experimental conditions are kept same. As noted before, the swelling of gel is dependent on the protonation of amino groups in the structure. Therefore, the hydrogel with a higher molar ratio of amino groups induces faster bending response speed of the coated microcantilever, compared to that of a gel with lower amino group concentration.
Figure 7.
Maximum response rate (−dB/dt) as a function of pH for cantilevers modified with gels comprising different chitosan:gelatin ratios (crosslinked with 3.5% TPP pH, 6.0).
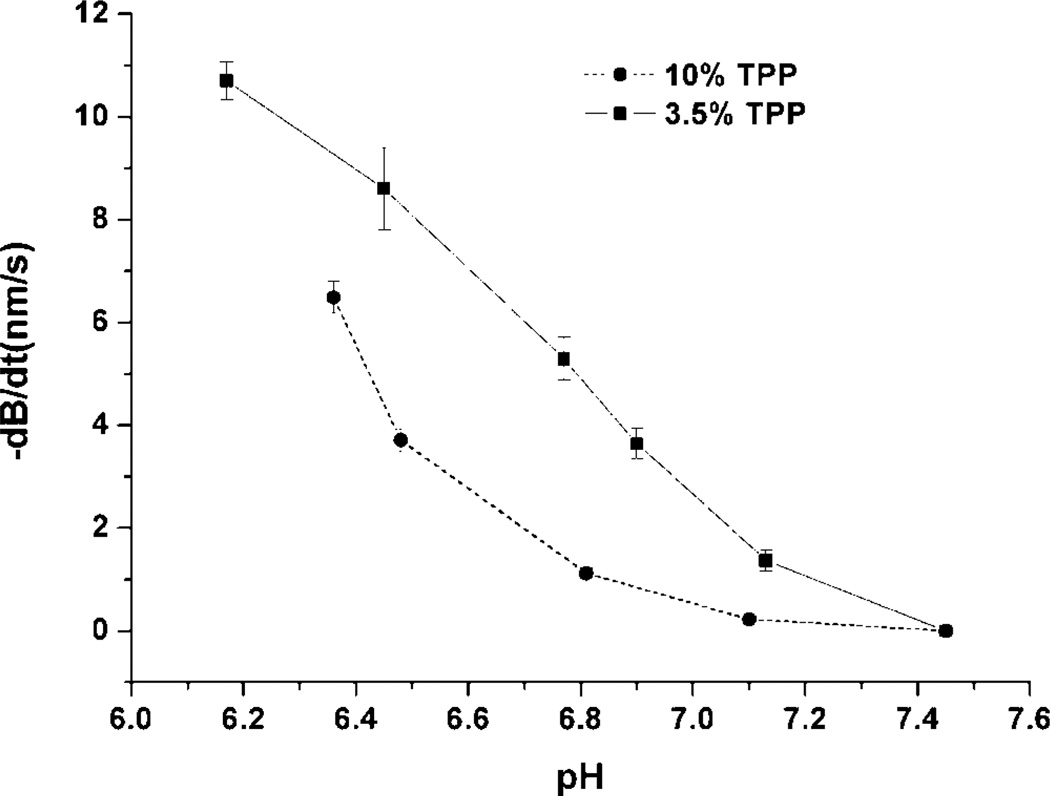
The effect of initial TPP concentration on the hydrogel swelling characteristics was also studied using the microcantilever approach. The steady-state pH response rate of chitosan/gelatin gel crosslinked by 10% (GEL 10) or 3.5% TPP solution (GEL 3.5) at pH 6.0 is shown in Figure 8. GEL 3.5 exhibited higher pHsensitivity than GEL10, and the pH-dependent profile was more linear than GEL10. As noted above, swelling of the gel is mainly influenced by ionic interactions between chitosan chains, which depend on the crosslinking density as set during the formation of the network (curing). An increase in crosslinking density results in a decrease in swelling and pH sensitivity by improving the stability of the network. Since GEL 10 has higher crosslinking density than GEL 3.5, there are relatively fewer free groups available on the chitosan chain for GEL 10, and the volume change of the hydrogel caused by the protonation of the amino group is decreased.
Figure 8.
Maximum response speed (−dB/dt) as a function of pH for microcantilevers coated with chitosan/gelatin, crosslinked with different amounts of TPP at pH 6.0.
CONCLUSIONS
In this study, the properties of chitosan-based hydrogels were studied to determine their potential as pH-sensitive gels in sensor and drug release applications. Using turbidity titration and microcantilever deflection analysis, the interactions between chitosan, gelatin, and the ionic-crosslinker TPP at different pH levels were explored. The results suggest that the crosslinking structure of the TPP/gelatin/chitosan system is mainly determined by the reaction between the amino groups of chitosan and TPP ions, and this reaction depends strongly on the pH of association. Swelling of this kind of hydrogel is believed to be influenced most by the chain relaxation of chitosan–TPP complex caused by the protonation of free amino groups in chitosan, which depended on the crosslinking density set during the formation of the network. An increase in crosslinking density induced a decrease in swelling and pH sensitivity.
A prototype “sensitive and stable” hydrogel (G:C = 1:1, 3.5% TPP solution pH 6.0) was then used as a typical hydrogel in further experiments. The gels exhibited an initial transient, non-reproducible response during early exposure to solutions of varying pH, but eventually reached a very consistent steady-state pH-dependent behavior. At its steady state, the microcantilever coated with the hydrogel showed a sensitive bending response (1,000 nm/∆pH) over the near-neutral pH range from 6.1 to 7.45; the deflection of the microcantilever increased as the pH decreased; and the response speed as a function of pH was approximately linear.
It can be concluded from this study that ionically crosslinked chitosan/gelatin hydrogels are attractive for environmentally responsive systems with applications such as sensors and controlled-release devices. A key property of these smart materials is the ability to “tune” the swelling and stability properties by the selecting composition and curing conditions. Thus, there is a practical tradeoff between mechanical integrity and pH-responsivity, and both of these qualities are affected by the amount of chitosan, gelatin, and TPP as well as the pH at which curing is performed. By controlling the gel composition and curing conditions, the properties of the gel can be tuned for a desired application.
The results also demonstrate the potential of microcantilevers as a platform for testing environmentally sensitive polymers that have relatively smaller volume changes, which are difficult to determine using other techniques. This may be particularly useful in applications where less pronounced pH-dependent swelling is desired. Furthermore, while not a focus of this study, the results support the concept that hydrogel-coated microcantilevers could be further investigated as candidates for biological sensors by introduction of molecular recognition agents such as enzymes into the gel.
Acknowledgments
Contract grant sponsor: US Army
Contract grant number: W81XWH-04-1-0596
References
- Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J Control Release. 2004;100:5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Aoki T, Kawashima M, Katono H, Sanui K, Ogata N, Okano T, Sakurai Y. Temperature-responsive interpenetrating polymer networks constructed with poly(acrylic acid) and poly(N,N-dimethylacrylamide) Macromol. 1994;27:947–952. [Google Scholar]
- Bashir R, Hilt JZ, Elibol O, Gupta A, Peppas NA. Micromechanical cantilever as an ultrasensitive pH microsensor. Appl Phys Lett. 2002;81:3091–3093. [Google Scholar]
- Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Europ J Pharm Biopharm. 2004;57:19–34. doi: 10.1016/s0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- Fritz J, Baller MK, Lang HP, Rothuizen H, Vettiger P, Meyer E, Güntherodt HJ, Gerber C, Gimzewski JK. Translating biomolecular recognition into nanomechanics. Science. 2000;288:316–318. doi: 10.1126/science.288.5464.316. [DOI] [PubMed] [Google Scholar]
- Karadag E, Uzum OB, Saraydin D. Water uptake in chemically crosslinked poly(acrylamide-co-crotonic acid) hydrogels. Mater Des. 2005;26:265–270. [Google Scholar]
- Kim B, Peppas NA. Synthesis and characterization of pH-sensitive glycopolymers for oral drug delivery system. J Biomater Sci Polymer Edn. 2002;13(11):1271–1281. doi: 10.1163/156856202320893000. [DOI] [PubMed] [Google Scholar]
- Kim B, Flamme KL, Peppas NA. Dynamic swelling behavior of pH-sensitive anionic hydrogels used for protein delivery. J Appl Polym Sci. 2003a;89:1606–1613. [Google Scholar]
- Kim SJ, Lee CK, Lee YM, Kim IY, Kim SI. Electrical/pH-sensitive swelling behavior of polyelectrolyte hydrogels prepared with hyaluronic acid-poly(vinyl alcohol) interpenetrating polymer networks. Reactive Funct Polym. 2003b;55:291–298. [Google Scholar]
- Krajewska B. Diffusional properties of chitosan hydrogel membranes. J Chem Technol Biotechnol. 2001;76:636–642. [Google Scholar]
- Mattison KW, Brittain IJ, Dubin PL. Protein-polyelectrolyte phase boundaries. Biotechnol Prog. 1995;11:632–637. [Google Scholar]
- Mi FL, Shyu SS, Lee ST, Wong TB. Kinetic study of chitosan-tripolyphosphate complex reaction and acid-resistive properties of the chitosan-tripolyphosphate gel beads prepared by in-liquid curing method. J Polym Sci B: Polym Phys. 1999;37:1551–1564. [Google Scholar]
- Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physicochemical foundations and structural design of hydrogels in medicine and biology. Ann Rev Biomed Eng. 2000;2:9–29. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- Sakiyama T, Takata H, Kikuchi M, Nakanishi K. Polyelectrolyte complex gel with high pH sensitivity prepared from dextran sulfate and chitosan. J Appl Poly Sci. 1999;73:2227–2233. [Google Scholar]
- Sakiyama T, Tsutsui T, Masuda E, Imamura K, Nakanishi K. Ionization characteristics of polyelectrolyte complex gels: Analysis based on their swelling behaviors. Macromol. 2003;36:5039–5042. [Google Scholar]
- Shu XZ, Zhu KJ. Chitosan/gelatin microspheres prepared by modified emulsification and ionotropic gelation. J Microencapsulation. 2001;18:237–245. doi: 10.1080/02652040010000415. [DOI] [PubMed] [Google Scholar]
- Shu XZ, Zhu KJ, Song WH. Novel pH-sensitive citrate cross-linked chitosan film for drug controlled release. Int J Pharm. 2001;212:19–28. doi: 10.1016/s0378-5173(00)00582-2. [DOI] [PubMed] [Google Scholar]
- Soppimath KS. Chemically modified polyacrylamide-g-guar gum based crosslinked anionic microgels as pH sensitive drug delivery systems: Preparation and characterization. J Control Release. 2001;75:331–345. doi: 10.1016/s0168-3659(01)00404-7. [DOI] [PubMed] [Google Scholar]
- Tang Y, Fang J, Yan X, Ji H-F. Fabrication and characterization of SiO2 microcantilever for microsensor application. Sens Act B. 2004;97:109–113. [Google Scholar]
- Veis A, Aranyi C. Phase separation in polyelectolyte systems. I. Complex coacervates of gelatin. J Phy Chem. 1960;64:1203. [Google Scholar]
- Yan X, Ji H, Lvov Y. Modification of microcantilevers using layer-by-layer nanoassembly film for glucose measurement, chemical physics letters. Chem Phys Lett. 2004;396:34–37. [Google Scholar]
- Zhang Y, Brown GM, Snow D, Sterling R, Ji HF. ApHsensor based on a microcantilever coated with intelligent hydrogel. Instrum Sci Technol. 2004;32:361–369. [Google Scholar]