Abstract
Objective
Systematically identify preoperative clinical risk factors for incident postoperative delirium in individuals undergoing hip fracture repair in order to guide clinicians in identifying high risk patients at admission.
Methods
Systematic review of prospective observational studies with estimation of association between preoperative risk factors and incident postoperative delirium in multivariate models. Electronic searches were conducted in PubMed, Embase, PsycINFO, CINAHL, Cochrane Library, Proquest Dissertations and Theses, and WorldCatDissertations. Hand searches were conducted in selected journals and their supplements.
Results
Search yielded 6,380 titles and abstracts from electronic databases and 72 titles from hand searches, and 10 studies met inclusion criteria. The following risk factors were significant in bivariate models: cognitive impairment, age, gender, institutionalization, functional impairment, BMI, albumin, comorbidities, ASA classification, acute medical conditions, polypharmacy and vision impairment. Among all of these risk factors, cognitive impairment most consistently remained statistically significant after adjusting for other risk factors in multivariate models, followed by BMI/albumin and multiple co-morbidities.
Conclusion
In our systematic review, cognitive impairment was one of the strongest preoperative risk factors for postoperative delirium after hip fracture surgery. Preoperative cognitive assessment may be one of the most useful methods of identifying those who are at high risk for postoperative delirium, and prioritizing delivery of delirium prevention measures.
Keywords: Risk factors, Postoperative, Delirium, Hip fracture
Introduction
As the populations in the United States (U.S.) and other countries age, the number of older adults with hip fractures will continue to rise. It is estimated that the annual hip fracture rate in the U.S. alone is close to 300,000, and is expected to exceed 6 million world-wide by 2050 (Cooper, Campion, Melton, 1992; Stevens and Rudd, 2013). Hip fracture has the highest incidence and associated costs of all fractures that occur among adults 65 years and older, with estimated 2.9 billion dollars in Medicare costs (Centers for Disease Control and Prevention, 1996). It is also associated with multitude of complications including prolonged rehabilitation, loss of independence, and mortality (Bentler et al., 2009), with a recent study showing a 36 % mortality at 6 months among nursing home residents (Neuman et al., 2014).
One of the major complications of hip fracture is delirium, with an incidence of up to 54% in this population (Bruce et al., 2007). It is a complication which costs upwards of 6.5 billion dollars in Medicare hospital expenditures (Rubin et al., 2011), and is associated with poor functional recovery (Marcantonio et al., 2000) and institutionalization (Krogseth et al., 2014). However, delirium is a preventable condition with available effective hospital-based delirium prevention approaches (Inouye et al., 1999; Marcantonio et al., 2001). Therefore, prioritizing and targeting patients who are at higher risk of delirium will help clinicians to identify high risk patients for close monitoring and implementation of delirium preventive strategies including proactive geriatrics co-management at all stages of perioperative care. In addition, stratification of high risk patients would enable efficient design of future intervention studies as well as cost effective delivery of these interventions (Inouye et al., 2000; Rubin et al., 2011).
In order to identify those who are at high risk of postoperative delirium, several systematic reviews have examined preoperative delirium risk factors in non-cardiac surgery (Dasgupta and Dumbrell, 2006), or in mixed orthopedic surgeries including elective knee and hip surgeries (Bruce et al., 2007). However, the incidence of delirium is usually higher after hip fracture surgery (5–53.3%) compared to elective hip surgery (3.6–28.3%) (Bruce et al., 2007). This suggests that the type of surgery and underlying condition may result in different magnitudes of delirium risk associated with a risk factor (Dasgupta and Dumbrell, 2006). Nevertheless, systematic reviews focusing on hip fracture are lacking, despite its frequency, costs, and clinical relevance. In addition, many of the existing studies have been limited by including patients with prevalent delirium and focusing solely on preoperative risk factors from bivariate analysis, which make it difficult to identify independent risk factors for incident delirium following surgery.
This systematic review examined potential preoperative risk factors for delirium after hip fracture surgery, focusing on studies that examined incident delirium after surgery and investigated independent association of risk factors with postoperative delirium in multivariate models. The overall goal of the study was to find common risk factors across studies that will be most informative in guiding clinicians in identifying high risk patients.
Methods
Literature Search Strategy
We searched PubMed, EMBASE, PsycINFO, CINAHL, and Cochrane Library from inception of database to April 15, 2013 without any language restrictions. We also searched for unpublished dissertations using Proquest Dissertations and Theses, and WorldCatDissertations. We hand-searched 15 journals and supplemental sections of five journals limited to issues published in 1990 or later (Bruce et al., 2007). Complete list of hand-searched journals, journal supplements and search terms for electronic and hand-searches can be found in Appendix 1 (on-line).
Study Selection
The title and abstract of each article were independently reviewed by two reviewers. Studies were included for the following criteria: i) prospective observational study; ii) adult patients (≥18 age) who underwent hip fracture surgery; iii) provided data on incident postoperative delirium by excluding individuals who had delirium before surgery; iv) included data on a concurrent, non-historical group of patients who underwent hip fracture surgery, but did not develop postoperative delirium; v) delirium assessed using Diagnostic and Statistical Manual of Mental Disorders (DSM) III or IV edition, or DSM derived criteria such as Confusion Assessment Method (CAM), Delirium Symptom Interview, Delirium Rating Scale, or Neelon and Champagne (NEECHAM) Confusion Scale; vi) risk factors examined in multivariate model.
Studies were excluded for the following criteria: i) randomized controlled trials (clustered and cross-over), retrospective studies (cohort and case-control), cross-sectional studies, or review articles; ii) Mixed study population: data on patients who underwent hip fracture surgery could not be separated from other patients (e.g. other surgical/medical patients or elective hip surgery patients); iii) data on incident delirium could not be separated from prevalent (present on admission) delirium; iv) did not examine at least one preoperative risk factor for postoperative delirium; v) no estimate of association between risk factors and postoperative delirium; vi) animal study; vii) foreign language (non-English). Studies with only bivariate results were excluded, since our goal was to examine studies with adequate control for confounding. Included and excluded studies are reported using the PRISMA systematic review protocol (Moher et al., 2009).
Data Extraction and Management
Reviewers divided into two groups (EO and ML, EO and TF), and double data extraction and double data entry were conducted. Any discrepancies were resolved through discussion and consensus agreement. Quality assessment was completed by two independent reviewers based on pre-defined criteria. Data from Lee et al. (Lee et al., 2011) encompassed some of the data presented by Zakriya et al. (Zakriya et al., 2002) and Sieber et al. (Sieber et al., 2011). The latter two studies were not incorporated into the data presented in Table 2 and 3. However, they were included in data extraction for other information in this review (Table 1). A variable was categorized as significant (S) in bivariate models if it met the cut-off p-value as determined in each study (Table 2). P-value of 0.05 was chosen for significance in multivariate models (Table 3).
Table 2.
Analysis of risk factors by significance in bivariate models. Studies by Sieber et al (Sieber et al., 2011) and Zakriya et al. (Zakriya et al., 2002) from Table 1 were not included in Table 2 because they are subgroup analysis from the same study cohort as the study by Lee et al. (Lee et al., 2011). Lee et al. was chosen for this analysis as the study includes the largest number of subjects from the same cohort. P-values are not stated if they were not reported or could not be calculated from other data available.
| Risk Factors (p-value) |
Cognitive Impair- ment |
Age | Gender | Institu- tionalization |
Functional Impairment a |
BMI and Albuminb |
Multiple Co- morbidities |
ASA c Classi- fication |
Acute Medical Condi- tions d |
Poly- pharmacy e |
Vision Impair- ment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | |||||||||||
| Andersson (2001) | S (<0.0001) | S (<0.0001) | S (<0.002) | S (<0.02) | S (<0.01) | S (<0.0001) | |||||
|
| |||||||||||
| Bjoro (2008) | S (0.000) | S (0.009) | S (0.002) | S (0.004) |
S
f (0.003) NS (0.26) |
NS (0.67) | S (0.009) | NS (0.19) | |||
|
| |||||||||||
| Goldenberg (2006)g | S | S | S | S | S | S | S | ||||
|
| |||||||||||
| Juliebo (2009) | S (<0.001) | S (0.005) | NS (0.41) | S (0.005) | S (0.001) |
S
h (0.003) NS (0.64) |
NS (0.56) | S (0.004) | S (0.002) | ||
|
| |||||||||||
| Lee (2011)i | S (0.000) | S (0.002) | S (0.004) | NS (0.84) | S (0.000) | S (0.000) | |||||
|
| |||||||||||
| Morrison (2003)j | S (<0.001) | S (0.02) | S (0.11) | S (0.08) | S (0.001) | S k | |||||
|
| |||||||||||
| Nie (2011) | NS l (0.61) | NS (0.54) | NS (0.39) | ||||||||
|
| |||||||||||
| Santos (2005) | S (0.03) | NS (0.62) | NS (0.36) | S (0.013) | NS (0.34) | ||||||
Functional impairment was defined differently in studies: “help from others before admission”(Andersson, Gustafson, Hallberg, 2001), ADL score ≥ 2 (GOLDENBERG et al., 2006), Barthel index 19 or 20 (Juliebo et al., 2009), and FIM score (Morrison et al., 2003).
BMI in quartiles (Lee et al., 2011), BMI ≤ 20kg/m2(Bjoro, 2008; Juliebo et al., 2009), albumin <3.4g/dL (Bjoro, 2008), albumin (median value) (Juliebo et al., 2009), or albumin < 3.5 g/dL (GOLDENBERG et al., 2006).
American Society of Anesthesiologist (ASA). ASA group III, IV, or V (Juliebo et al., 2009), ASA ≥ 4 (Lee et al., 2011), ASA II, III, or IV (Santana Santos et al., 2005), ASA I–II or III–V (Bjoro, 2008),
Acute medical conditions included “preoperative medical treatment for cardiovascular or pulmonary problems” (Andersson, Gustafson, Hallberg, 2001), “CHF on admission” and “abnormal BP on admission” (Morrison et al., 2003).
Polypharmacy was defined as “>5” (Bjoro, 2008), “≥ 5” (Juliebo et al., 2009), and “>3 medications” (GOLDENBERG et al., 2006).
BMI (p=0.003), albumin (p=0.26).
The goal of this study was to develop a prediction model for the risk of postoperative delirium (POD). These variables were identified as predictors of POD in the bivariate model, but cut off p-value was not reported in the article.
BMI (p=0.003), albumin (p=0.64).
P-values for the stratified bivariate analysis by dementia status were published by Lee et.al.. The p-values presented in this table were calculated by the author for the entire cohort from available data.
In this study, p <0.15 in the bivariate analysis was the cut-off value for incorporation into the multivariate model, and thus categorized as “significant.”
“abnormal BP” (p=0.12), “abnormal heart rhythm” (p=0.01),”substernal chest pain” (p=0.001), “CHF” (p<0.001), “respiratory compromise” (p=0.59).
Nie et al. reported that different cut-off scores were used to identify cognitive dysfunction depending on educational levels (MMSE <25 for middle school or higher, <21 for elementary school, and <18 for no schooling.). However, baseline bivariate statistics are only reported with comparison of group mean MMSE (p=0.61), and not as a dichotomous variable.
Table 3.
Analysis of risk factors by significance in multivariate models. P-values and effect sizes are not stated if they were not reported or could not be calculated from other data available.
| Risk Factors (p-value) |
Cognitive Impair- ment |
Age | Gender | Institu- tionalization |
Functional Impairment |
BMI and Albumin a |
Multiple Co- morbidities |
ASA Classifica- tion b |
Acute Medical Condi- tions c |
Poly- pharmacy |
Vision Impair- ment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | |||||||||||
| Andersson (2001) |
S (<0.009) HR 1.30 |
S (<0.003) HR 1.09 |
NS | NS |
S (<0.0001) HR 12.24 |
S (<0.01) HR 2.84 |
S (<0.0009) HR 3.92 |
||||
|
| |||||||||||
| Bjoro (2008) |
S (0.001) OR 3.90 |
NS (0.10) OR 1.9 |
NS (0.19) OR 2.1 |
NS (0.55) OR 0.8 |
S (0.01) OR 2.3 |
NS (0.06) d OR 1.8 |
|||||
|
| |||||||||||
| Goldenberg (2006)e |
S
f (0.006) OR 13.1 (0.03) OR 6.9 |
NS g (0.06) OR 5.1 |
NS | NS |
S (0.03) OR 1.8 |
NS |
S (0.02) OR 33.6 |
||||
|
| |||||||||||
| Juliebo (2009) |
S (0.005) OR 2.93 |
NS | NS | NS |
S (0.01) OR 2.92 |
NS | NS | ||||
|
| |||||||||||
| Lee (2011) |
S (<0.001) OR 2.74 |
S (0.004) OR 1.05 |
S (0.003) OR 2.10 |
S (0.005) OR 1.14 |
|||||||
|
| |||||||||||
| Morrison (2003) h |
S (<0.001) RR 3.6 |
NS (0.8) RR1.0 |
NS (0.08) RR 0.6 |
NS (0.5) RR1.3 |
NS (0.98) RR 1.0 |
S i | |||||
|
| |||||||||||
| Nie (2011) | NS j (0.28) OR 3.88 |
||||||||||
|
| |||||||||||
| Santos (2005) | NS (0.32) OR 1.21 |
NS (0.37) OR 1.17 |
NS (0.17) OR 1.30 |
||||||||
Hazard Ratio (HR), Odds Ratio (OR), Relative Risk (RR).
(BMI ≤ 20kg/m2) (Bjoro, 2008; Juliebo et al., 2009)or albumin < 3.5 (GOLDENBERG et al., 2006).
Lee et al did not put ASA in the multivariate model even though they were significant in the bivariate model due to collinearity with other variables (Lee et al., 2011).
Acute medical conditions included “preoperative medical treatment for cardiovascular or pulmonary problems” (Andersson, Gustafson, Hallberg, 2001), “CHF on admission” and “abnormal BP on admission” (Morrison et al., 2003).
ASA or “severity of illness” was treated as a “precipitating factor” in this analysis and was incorporated into multivariate analysis with other precipitating factors.
P-values were calculated from OR with 95% CI reported in the article.
Set Test (ST) cut off score <20 (p=0.06), MMSE <24 (p=0.03).
Age is noted as one of the independent predictors of POD in multivariate logistic regression analysis with OR of 5.1 (95% CI 0.9–26.8). Calculated p-value is 0.0598 or 0.06. Therefore, it was categorized as not significant (NS) in this systematic review.
In this study, the highest RR was 5.4 for parenteral morphine sulfate equivalent of < 10 mg per day.
“abnormal BP” (RR 2.3; p=0.01), “abnormal heart rhythm” (RR 1.7; p=0.3),”substernal chest pain” (RR1.9; p=0.4), “CHF” (RR 2.9; p<0.001),
Nie et al. did not report a p-value, but reported OR 3.88 (95% CI 0.45–33.19) which calculates to p-value of 0.28.
Table 1.
Baseline characteristics of selected studies
| Study | Country | Setting | Study Period | Sample Size, (% female) | Age Mean (SD) [Range] a | Delirium Assessment Tools b | Postoperative Assessment Duration | Delirium Incidence in all [Incidence in cognitively impaired] (%) |
|---|---|---|---|---|---|---|---|---|
| Andersson (2001) | Sweden | Central county hospital | 02/1994 05/1996 |
208c | no delirium: 78.9 (7.3) [65–95] delirium: 86.0 (6.6) [67–96] |
DSM-IV OBS |
Daily until delirium resolution | 20.2 |
| Bjoro (2008) | Norway | University & community hospital | 09/2005 12/2006 |
204 (77.9) | 83 (6.7) [66–100] | CAM MDAS |
Daily up to postoperative day 4 | 34.3 [56.5] |
| Goldenberg (2006) | USA | Community hospital | 11/2000 03/2002 |
77 (64.9) | 81.9 (7.5) [66–98] | CAM | Daily until discharge | 48.1 |
| Juliebo (2009) | Norway | University hospital | 09/2005 12/2006 |
187 (77.5) | 84 (78–88) d | CAM | Daily (weekdays) | 36.4 [60.3] |
| Lee (2011) | USA | University hospital | 1999 2008 |
425 (73.2) | 80.2 (6.8) [64–101] | CAM | Daily until discharge | 35 [54] |
| Morrison (2003) | USA | 4 city hospitals | 07/1997 08/1998 |
541 (81.7) | age <70, N= 49 e age 70–79, N = 141 age 80+, N= 351 |
CAM | Daily (weekdays) | 16 |
| Nie (2011) | China | NR f | NR | 123 (69.1) | no delirium: 75.3 (0.8) delirium: 75 (2) |
CAM DRS-R-98 |
Daily up to 6 days | 13 |
| Santos (2005) | Sweden | University hospital | 06/2003 07/2003 |
34 (73.5) | no delirium: 81.5 (10.2) delirium: 82.9 (6.3) |
CAM DSM IV |
Daily up to 5 days; until discharge if delirium | 55.9 |
| Sieber (2011) | USA | University hospital | 04/2005 07/2009 |
236 (71.6) | 81.5 (7.1) | CAM | Daily until discharge | 25.4 |
| Zakriya (2002) | USA | University hospital | 03/1999 12/2000 |
168 (81.5) | no delirium: 77 (1) delirium: 79 (1) |
CAM | Daily until discharge | 28 |
Age was categorized by those with no delirium or delirium in four studies. Age range was not reported in some of the studies.
Diagnostic and Statistical Manual (DSM)-IV; Organic Brain Syndrome (OBS) scale; Confusion Assessment Method (CAM); Memorial Delirium Assessment Scale (MDAS); Delirium Rating Scale-Revised (DRS-R-98).
Gender information was not available for the “emergency surgery” subgroup.
Median 84, Interquartile range (IQR) 78–88.
Age categorized in three groups with number of individuals in each category, but no mean, standard deviation (SD) or age range stated.
NR = not reported.
Results
Study Characteristics
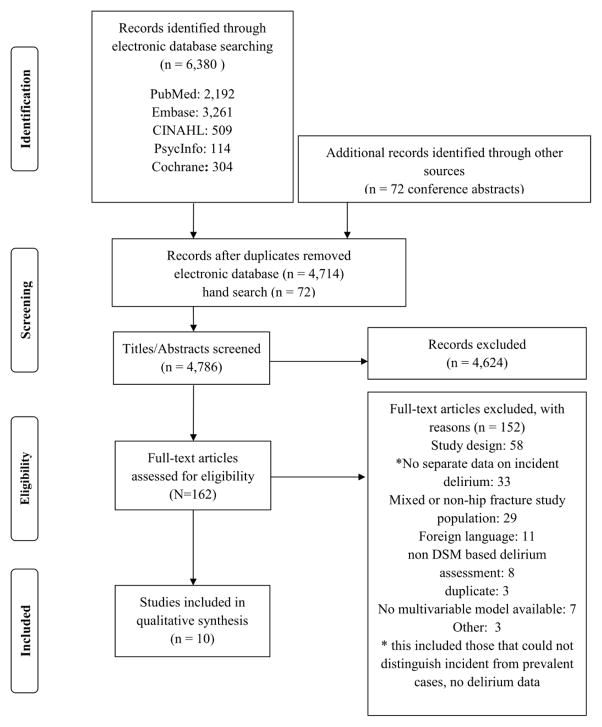
Initial search yielded 6,380 titles and abstracts from the electronic databases and 72 titles from hand searches. After duplicates were removed, 4,786 abstracts were reviewed for initial screening, and 162 for the next stage of review. After inclusion and exclusion criteria were applied, 10 full text articles, including one dissertation, were chosen for detailed analysis (Figure 1). Reasons for exclusion are listed on the flow diagram.
Figure 1.
Flow diagram of search strategy
Postoperative Delirium Assessment
The incidence of postoperative delirium ranged widely from 13% (Nie et al., 2012) to 55.9 % (Santana Santos et al., 2005), and were identified by DSM-IV or DSM derived instrument. Among studies that specifically stratified preoperative risk factors by baseline cognitive function, postoperative delirium incidence was consistently higher in those who had cognitive impairment compared to those who did not (Table 1). Some studies specifically excluded those with diagnosis of dementia (Zakriya et al., 2002; Nie et al., 2012).
In terms of delirium frequency, most studies reported daily assessment (Zakriya et al., 2002; GOLDENBERG et al., 2006; Pedersen et al., 2006; Lee et al., 2011; Nie et al., 2012), and some reported twice daily assessments (Andersson, Gustafson, Hallberg, 2001; Santana Santos et al., 2005). Others reported daily assessments limited to weekdays (Morrison et al., 2003; Juliebo et al., 2009).
Delirium Severity and Duration of Delirium
Delirium severity was assessed in only two studies: one (Bjoro, 2008) used the MDAS, and the other (Nie et al., 2012) used the Delirium Rating Scale–Revised-98 (DRS-R-98) (Trzepacz et al., 2001). Four studies reported delirium duration, with two reporting delirium of one day duration in 39% (Bjoro, 2008) to 75% (Nie et al., 2012) of cases. One study (Santana Santos et al., 2005) reported an overall mean of 2.9 days for delirium duration, and another (Andersson, Gustafson, Hallberg, 2001) reported duration ranging from one to nine days, with overall mean of 3 days.
Preoperative Risk Factors of Postoperative Delirium
Cognitive Impairment
Mini-Mental Status Examination (MMSE) (Folstein, Folstein, McHugh, 1975) was the most widely used tool for cognitive evaluation. Additional approaches to quantify preoperative cognitive state were the short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE-SF) (Jorm, 1994) or the Set Test (Isaacs and Kennie, 1973). One study used an unvalidated simple four item screen to define preoperative cognitive status (Morrison et al., 2003). Methods of classifying cognitive impairment status varied among studies, ranging from those that relied on cognitive testing tools (Zakriya et al., 2002; Santana Santos et al., 2005; Bjoro, 2008; Juliebo et al., 2009; Lee et al., 2011; Sieber et al., 2011; Nie et al., 2012) to those that also used additional information such as Diagnostic and Statistical Manual-IV (DSM-IV) criteria (GOLDENBERG et al., 2006), incorporated evaluation by clinicians (Juliebo et al., 2009; Lee et al., 2011; Sieber et al., 2011), or information from medical records for additional history of dementia diagnosis (Morrison et al., 2003; Lee et al., 2011; Sieber et al., 2011).
In seven studies, cognitive impairment was a significant risk factor of postoperative delirium in the bivariate models. It remained statistically significant in six out of the eight studies in the multivariate models. Among those six studies, three studies reported cognitive impairment to have the largest effect sizes among all the risk factors in the multivariate models, and two studies reported it to have the second largest effect sizes (Table 3).
Several studies further stratified the patients by cognitive status, and examined preoperative risk factors separately. In the cognitively impaired, female gender, femoral neck fracture, abnormal blood pressure, heart failure on admission, meperidine use, and low doses of opioid analgesia (parenteral morphine sulfate equivalents of < 10 mg/day) were significant preoperative risk factors in one study (Morrison et al., 2003), and BMI and laboratory WBC values in another (Bjoro, 2008). A third study showed that only time between emergency department (ED) and surgery was a significant preoperative risk factor (Lee et al., 2011).
In the cognitively intact, Functional Independence Measure (FIM) score, severe pain at rest and low doses of opioid analgesia (parenteral morphine sulfate equivalents of < 10 mg/day) were significant preoperative risk factors (Morrison et al., 2003), in addition to American Society of Anesthesiologist (ASA) classification (Bjoro, 2008). One study showed that age, male gender, Body Mass Index (BMI), and number of medical comorbidities were significant preoperative risk factors (Lee et al., 2011). As the risk factors examined in these studies did not overlap, it was difficult to draw conclusions from aggregated data. These studies also did not report whether interactions were examined in the statistical models.
Age and Gender
Among eight studies that examined age as a risk factor, six studies showed a significant association with postoperative delirium in the bivariate models (Table 2). However, age remained significant in only two studies after adjusting for other risk factors in multivariate models, and the effect sizes were small in both studies (Table 3). Only two out of five studies showed a significant association between gender and postoperative delirium in the bivariate models (Table 2), and only one remained significant in the final multivariate models (Table 3).
Institutionalization and Functional Impairment
Among studies examining institutionalization as a risk factor for postoperative delirium, all showed significant associations in the bivariate models (Table 2). However, none remained significant in multivariate models (Table 3). Similarly, none of the studies that showed significant associations between functional impairment and postoperative delirium in the bivariate models (Table 2) remained significant in the multivariate models (Table 3). Methods of classifying functional impairment varied widely among the studies, ranging from a simple categorization as needing “help from others before admission”(Andersson, Gustafson, Hallberg, 2001), to using more standardized tools such as activities of daily living (ADL) (GOLDENBERG et al., 2006; Juliebo et al., 2009; Lee et al., 2011) to Functional Independence Measure (FIM) (Morrison et al., 2003).
BMI and Albumin
Three studies examined body mass index (BMI) (Bjoro, 2008; Juliebo et al., 2009; Lee et al., 2011) and three studies examined albumin (GOLDENBERG et al., 2006; Bjoro, 2008; Juliebo et al., 2009). Two that used BMI below 20kg/m2 as impaired showed significant association with postoperative delirium in the bivariate models (Table 2), and remained significant in the multivariate models (Table 3). One study that examined albumin < 3.5 g/dL also showed significant association with postoperative delirium in both the bivariate and multivariate models (Tables 2 and 3).
Multiple Comorbidities, Acute Medical Conditions and American Society of Anesthesiologists (ASA) physical status classifications
Three out of five studies that examined multiple comorbidities found them to be significantly associated with postoperative delirium in the bivariate models (Table 2). In two of the three this association was significant in the multivariate models as well (Table 3).
Two studies examined acute medical conditions that required treatment upon admission including cardiovascular or pulmonary problems (Andersson, Gustafson, Hallberg, 2001) as well as congestive heart failure or abnormal blood pressure (Morrison et al., 2003). These were found to be significantly associated with postoperative delirium in the multivariate models (Table 3).
Three out of four studies that examined American Society of Anesthesiologist (ASA) physical classification system found it to be significant in the bivariate models (Table 2). However, one study did not incorporate ASA classification in the multivariate model due to collinearity (Lee et al., 2011), and the other two were not significant in the multivariate models (Table 3).
Polypharmacy
Similar to the categorization of multiple comorbidities, different studies used different criteria to define polypharmacy. One used > 3 medications (GOLDENBERG et al., 2006), and others used > 5 medications (Bjoro, 2008; Juliebo et al., 2009) to define polypharmacy. Two showed significance in the bivariate models (Table 2), but only one retained significance in the multivariate model (Table 3).
Vision and Hearing Impairment
Only one study examined vision and hearing impairment (Andersson, Gustafson, Hallberg, 2001). Self- reported vision impairment was associated with delirium in both the bivariate and multivariate models (Tables 2 and 3). Hearing impairment, which also relied on patient self-report was not associated with post-op delirium (Andersson, Gustafson, Hallberg, 2001).
Quality of Studies
Selection bias: 6 out of 10 studies specifically noted that consecutive patients were enrolled into the studies (Zakriya et al., 2002; Santana Santos et al., 2005; GOLDENBERG et al., 2006; Bjoro, 2008; Juliebo et al., 2009; Lee et al., 2011). However, only 2 out of 10 compared baseline differences between those who were included in the study and those who were excluded (GOLDENBERG et al., 2006; Juliebo et al., 2009). There were no significant differences age (GOLDENBERG et al., 2006; Juliebo et al., 2009), gender (Juliebo et al., 2009), morbidity (GOLDENBERG et al., 2006), or lab values (GOLDENBERG et al., 2006) in these studies.
Measurement error: there was variability in ascertainment of baseline characteristics. One study collected information from medical records (Santana Santos et al., 2005). Others collected information from medical records and from a combination of patient, proxy interviews, and staff interviews (Morrison et al., 2003; Bjoro, 2008; Lee et al., 2011; Nie et al., 2012). The study that reported on vision and hearing impairment relied on self-report (Andersson, Gustafson, Hallberg, 2001). None of the studies clearly stated whether outcomes were assessed by raters masked to baseline exposure/risk factors.
Although all the studies examined clinical variables in adjusted models, only two studies specifically stated that effect modifications were explored by examining interactions between variables (Juliebo et al., 2009) or that collinearity was checked between risk factors. (Lee et al., 2011).
Discussion
This systematic review demonstrates that cognitive impairment is the most consistently significant preoperative risk factor for postoperative delirium after hip fracture surgery, followed by BMI or albumin levels and multiple comorbidities, all of which had at least two studies that were significant in multivariate models with effect sizes greater than 1.1. Although the exact underlying pathophysiology of delirium is not known, some of the leading hypotheses are similar to those proposed for neurodegenerative processes such as Alzheimer’s disease and other types of dementia. One is central cholinergic deficiency representing an underlying vulnerability that predisposes individuals to delirium (Hshieh et al., 2008). Another is inflammation, which may play an important role both as a predisposing factor in the form of CNS inflammation as well as a precipitating factor from systemic inflammation such as infection (Maclullich et al., 2008). Therefore, it makes sense that in a majority of studies the most consistent risk for postoperative delirium is pre-existing cognitive impairment, and this finding is similar to previously published studies (Bitsch et al., 2004; Dasgupta and Dumbrell, 2006).
Although baseline cognitive impairment is an important risk factor for postoperative delirium, cognitive assessment is often not conducted prior to emergency hip fracture repair. One reason is the perception that there is not enough time to perform cognitive testing in the preoperative setting. However, many studies, including those in this systematic review, demonstrate the feasibility of preoperative cognitive testing in emergency settings. Most studies reviewed (Zakriya et al., 2002; Santana Santos et al., 2005; GOLDENBERG et al., 2006; Bjoro, 2008; Lee et al., 2011; Sieber et al., 2011; Nie et al., 2012) used MMSE, but one demonstrated that even a four-item screening questionnaire was sensitive enough to detect cognitive impairment predictive of postoperative delirium (Morrison et al., 2003). Therefore, cognitive testing should become part of the standard of care for preoperative assessment for hip fracture surgery.
Another important risk factor was BMI and blood albumin levels. While low BMI and albumin levels are thought to represent poor nutritional status, they may also be reflective of inflammatory states associated with chronic disease (Kaysen, 2009). In four studies that examined either factor, postoperative delirium was associated with low BMI in two studies and low albumin in one study. As inflammation likely plays a large role in delirium pathogenesis (Maclullich et al., 2008), BMI and albumin may be indirect measures of systemic inflammation related to general medical conditions. In addition, individuals with low albumin may be susceptible to greater bioavailability of drugs highly bound to albumin, and therefore at greater risk of side effects including delirium (Alagiakrishnan and Wiens, 2004).
In this review, while several studies reported age to be a significant risk for delirium in bivariate models, this association lost significance in all but two studies. While loss of statistical significance in multivariate models may be due to the narrow age range of subjects in these studies (Francis, Martin, Kapoor, 1990), the association between age and delirium is likely mediated by other risk factors, such as cognitive impairment.
Several studies assessed preoperative physical condition as a risk factor of delirium by examining number of medical comorbidities, acute medical conditions, and the ASA classification. Although disease burden is an important factor in delirium risk assessment, the studies reviewed had different ways of defining multiple comorbidities as well as acute medical conditions. It might be more informative if specific conditions thought to increase postoperative delirium (e.g. vascular risk factors) are examined separately (Noimark, 2009).
An interesting finding is that although five studies showed a significant association between institutionalization and postoperative delirium in bivariate models, in none was this association significant in multivariate models. We see similar results with functional impairment, associated with postoperative delirium in bivariate models in five of six studies, but not in any multivariate models. One explanation is that these variables may be collinear, since most residents of skilled nursing facilities have functional impairments. However, this also demonstrates the robustness of the association between cognitive impairment and postoperative delirium, as this factor remained significant in multivariate models even though it is likely highly correlated with the aforementioned variables.
The strengths of this systematic review include the rigorous synthesis of evidence across studies, involving patients across hospitals. Important methodological advances over prior reviews are exclusion of prevalent delirium that allowed us to elucidate preoperative risk factors of incident delirium, and examination of studies that that had multivariate models enabling us to identify independent risk factors. This work will allow development of prediction models to identify high risk groups who will benefit from preventive interventions for delirium.
The limitations of this review include the small number of studies available, the different risk factors examined across each study, and the variability in measures and outcomes used. Meta-analysis of the risk factors was not performed due to the limitations of the studies, as there were not enough significant risk factors in multivariate models among the selected studies. Although examination of preoperative clinical risk factors is important in understanding underlying risk, they do not explain all the variances in the outcome of postoperative delirium. As preoperative risk factors represent predisposing vulnerability, other factors such as intraoperative and postoperative risk factors which are precipitating risk factors are also important determinants of the outcome (Inouye and Charpentier, 1996).
Conclusion
In summary, cognitive impairment was one of the most important preoperative risk factors for delirium after hip fracture surgery, followed by BMI/albumin levels and multiple comorbidities. Given the strength of this association, a careful preoperative assessment of cognitive function would be most important in identifying those who are at the highest risk of postoperative delirium. Furthermore, future delirium intervention studies may be designed based on stratification of subjects by preoperative cognitive function.
In addition, baseline measures of BMI and blood albumin levels may be helpful in identifying those with underlying inflammatory status. While age is important, age alone should not be used to risk stratify patients, and careful assessments of cognitive function and other medical conditions should be taken into account.
Key points.
Hip fracture repair is associated with high incidence of postoperative delirium
Preoperative cognitive impairment is most consistently associated with postoperative delirium even adjusted for other risk factors, followed by BMI/albumin and multiple co-morbidities.
Preoperative cognitive assessment is one of the most important methods of identifying those who are at high risk of postoperative delirium.
Acknowledgments
Funding Source: Dr. Oh was supported by 5KL2RR025006 [Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1 TR 001079 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH)], 1K23AG043504-01 (NIA/NIH), R21AG0337695 (NIA/NIH), P50 AG005146, UB4HP19193-03 (HRSA), The Rosalinde and Arthur Gilbert Foundation/AFAR New Investigator Award in Alzheimer’s disease, the Roberts Gift Fund, and the Hobson Gift Fund. Dr. Inouye was supported in part by K07AG041835 (NIA/NIH) and the Milton and Shirley F. Levy Family Chair.
Appendix 1
Hand-searched journals
Acta Anaesthesiologica Scandinavica, Age and Ageing, Anaesthesia Anesthesia and Analgesia, Anesthesiology, Canadian Journal of Anaesthesia, Clinical Orthopaedics and Related Research, Dementia and Geriatric Cognitive Disorders, Gerontology, International Journal of Geriatric Psychiatry, International Psychogeriatrics, Journal of Bone and Joint Surgery, Journal of the American Geriatrics Society (JAGS), Journals of Gerontology, New England Journal of Medicine, and the Journal of the American Medical Association
Hand-searched journal supplements
Anesthesiology, Acta Anaesthesiologica Scandinavica, Canadian Journal of Anaesthesia, JAGS, and Psychogeriatrics
PubMed Search Strategy
| #1 | “hip fractures”[mh] OR hip fracture* [All Fields] OR “hip” [mh] OR “hip” [All Fields] OR Subtrochanteric Fracture* [All Fields] OR Trochanteric Fracture* [All Fields] OR Intertrochanteric Fracture* [All Fields] OR “Femoral Neck Fractures” [mh] OR femoral neck fracture* [All Fields] OR Femur Neck Fracture* [All Fields] |
| #2 | “delirium” [mh] OR “delirium” [All Fields] OR “delirium, dementia, amnestic, cognitive disorders” [mh] OR organic mental disorder* [All Fields] OR “traumatic psychosis” [All Fields] OR “nonpsychotic organic brain syndrome” [All Fields] OR (“mental disorders”[mh] AND (“1969/01/01”[PDAT] : “1974/12/31”[PDAT])) OR “Confusion” [mh] OR cognitive defect* OR cognitive disorder* OR cognitive deficit* OR “cognitive dysfunction” OR cognitive impairment* OR organic brain syndrome* OR organic brain disease* OR “disorientation” [All Fields] OR organic brain disorder* [All Fields] OR confus* [All Fields] OR delir* OR “psychosis” [All Fields] OR “Intraoperative complications” [mh] OR Intraoperative complication* [All Fields] OR intra-operative complication* [All Fields] OR perioperative complication* [All Fields] OR peri-operative complication* [All Fields] |
| #3 | “Orthopedics” [mh] OR orthopedic* [All Fields] OR orthopaedic* [All Fields] OR “Surgical Procedures, Operative” [mh] OR operative procedure* [All Fields] OR “surgery” [All Fields] OR “surgery” [subheading] OR “surgeries” [All Fields] OR “surgical” [All Fields] OR surgi* [All Fields] OR “monitoring, intraoperative” [mh] OR “intraoperative monitoring” [All Fields] OR “intra-operative monitoring” [All Fields] OR “intraoperative period” [mh] OR intraoperative period* [All Fields] OR intra-operative period* [All Fields] OR “intraoperative care” [mh] OR “intraoperative care” [All Fields] OR “intra-operative care” [All Fields] OR “perioperative care” [All Fields] OR “peri-operative care” [All Fields] |
| #4 | #1 AND #2 AND #3 |
Embase Search Strategy
| #1 | ‘hip fracture’/exp OR ‘hip fracture’ OR ‘hip fractures’ OR ‘femur subtrochanteric fracture’/exp OR subtrochanteric NEAR/2 fracture* OR ‘femur intertrochanteric fracture’/exp OR intertrochanteric NEAR/2 fracture* OR ‘femur fracture’/exp OR femoral NEAR/2 fracture* OR ‘trochanteric fracture’ OR ‘trochanteric fractures’ OR ‘hip’/exp OR ‘hip’:ab,ti |
| #2 | ‘delirium’/exp OR delirium OR ‘cognitive defect’/exp OR ‘cognitive defect’ OR ‘cognitive defects’ OR ‘cognitive disorder’ OR ‘cognitive disorders’ OR ‘cognitive deficit’ OR ‘cognitive deficits’ OR ‘cognitive dysfunction’ OR ‘cognitive impairment’ OR ‘cognitive impairments’ OR ‘organic mental disorder’ OR ‘organic mental disorders’ OR ‘traumatic psychosis’ OR ‘nonpsychotic organic brain syndrome’ OR ‘organic brain syndrome’/exp OR ‘oragnic brain syndrome’ OR ‘organic brain disease’ OR ‘confusion’/exp OR confusion OR ‘disorientation’ OR ‘organic brain disorder’ OR ‘organic brain disorders’ OR confus* OR delir* OR ‘psychosis’/exp OR ‘psychosis’ OR intraoperative AND (‘complication’/exp OR complication) OR ‘intraoperative complication’ OR ‘intraoperative complications’ OR ‘intra-operative complication’ OR ‘intra-operative complications’ OR ‘perioperative complication’ OR ‘perioperative complications’ OR ‘peri-operative complication’ OR ‘peri-operative complications’ |
| #3 | ‘orthopedics’/exp OR ‘orthopedic’ OR ‘orthopedics’ OR ‘orthopaedic’ OR ‘orthopaedics’ OR ‘orthopedic surgery’/exp OR ‘surgery’/exp OR ‘surgery’ OR surgi* OR ‘operative procedure’ OR ‘operative procedures’ OR ‘intraoperative period’/exp OR ‘intraoperative period’ OR ‘intraoperative periods’ OR ‘intra-operative complication’ OR ‘intra-operative complications’ OR ‘perioperative period’/exp OR ‘perioperative period’ OR ‘perioperative periods’ OR ‘perioperative complication’/exp OR ‘perioperative complication’ OR ‘perioperative complications’ OR ‘peri-operative complication’ OR ‘peri-operative complications’ |
| #4 | #1 AND #2 AND #3 |
PsycINFO Search Strategy
| S1 | DE “Hips” OR hip fracture* OR femoral neck fracture* OR hip OR hips |
| S2 | DE “Delirium” OR DE “Organic Brain Syndromes” OR organic brain syndrome* OR organic mental disorder* OR DE “mental confusion” OR DE “cognitive impairment” OR cognitive impairment* OR cognitive defect* OR cognitive disorder* OR cognitive deficit* OR “cognitive dysfunction” OR organic brain disease* OR disorientation OR organic brain disorder* OR confus* OR delir* OR DE “psychosis” OR psychosis |
| S3 | Orthopedics OR orthopedic OR operative procedure* OR DE “surgery” OR surgery OR surgeries OR surgi* |
| S4 | S1 AND S2 AND S3 |
CINAHL Search Strategy
| S1 | (MM “Hip”) OR hip* OR (MM “Hip Fractures, Stress”) OR (MM “Hip Fractures”) OR (MM “Hip Surgery”) OR subtrochanteric fracture* OR trochanteric fracture* OR femoral neck fracture* OR (MM “femur neck”) OR femur neck fracture* |
| S2 | (MM “Delirium”) OR (MM “Delirium, Dementia, Amnestic, Cognitive Disorders”) OR (MM “Organic Mental Disorders”) OR (MM “Confusion”) OR (MM “Acute Confusion (NANDA)”) OR (MM “Cognition Disorders”) OR (MM “Intraoperative Complications”) OR (MM “Intraoperative Care”) OR confus* OR delir* OR organic mental disorder* OR cognitive defect* OR cognitive disorder* OR cognitive deficit* OR “cognitive dysfunction” OR cognitive impairment* OR organic brain syndrome* OR organic brain disease* OR disorientation OR organic brain disorder* OR intraoperative complication* OR intraoperative care OR intra-operative complication* OR intra-operative care OR perioperative complication* OR peri-operative complication* |
| S3 | (MM “Orthopedics”) OR orthopedic* OR orthopaedic* OR (MM “Orthopedic Surgery”) OR (MM “Hip Surgery”) OR (MM “Surgery, Operative”) OR surgi* OR operative procedure* OR “surgery” OR “surgeries” OR “intraoperative monitoring” OR “intraoperative period” OR “intraoperative care” |
| S4 | S1 AND S2 AND S3 |
Cochrane Library Search Strategy
| #1 | MeSH descriptor: [Hip Fractures] explode all trees |
| #2 | MeSH descriptor: [Arthroplasty, Replacement, Hip] explode all trees |
| #3 | intra-articular fracture* or periprosthtic fracture* or hip* or femoral neck fractures or subtrochanteric fracture* or trochanteric fracture* or intertrochanteric fracture* or femur neck fracture*:ti,ab,kw |
| #4 | #1 or #2 or #3 |
| #5 | MeSH descriptor: [Delirium] explode all trees |
| #6 | MeSH descriptor: [Delirium, Dementia, Amnestic, Cognitive Disorders] explode all trees |
| #7 | MeSH descriptor: [Confusion] explode all trees |
| #8 | organic mental disorder* or confus* or disorientation or “organic brain disorder” or “organic brain disorders” or delir* or “psychosis” or “intraoperative complications” or “intraoperative complication” or “intra-operative complication” or “intra-operative complications” or “perioperative complication” or “perioperative complication” or “peri-operative complications” or “peri-operative complications”:ti,ab,kw |
| #9 | MeSH descriptor: [Intraoperative Complications] explode all trees |
| #10 | #5 or #6 or #7 or #8 or #9 |
| #11 | MeSH descriptor: [Orthopedics] explode all trees |
| #12 | MeSH descriptor: [Surgical Procedures, Operative] explode all trees |
| #13 | MeSH descriptor: [Intraoperative Period] explode all trees |
| #14 | MeSH descriptor: [Intraoperative Care] explode all trees |
| #15 | MeSH descriptor: [Perioperative Period] explode all trees |
| #16 | MeSH descriptor: [Perioperative Care] explode all trees |
| #17 | MeSH descriptor: [Monitoring, Intraoperative] explode all trees |
| #18 | Orthopedic* or orthopaedic* or “operative procedure” or “operative procedures” or surgery or surgeries or surgi* or “intraoperative monitoring” or “intra-operative monitoring” or “intraoperative period” or “intra-operative period” or “intraoperative care” or “intra-operative care” or “intraoperative period” or “intra-operative period” or “perioperative period” or “peri-operative period” or “perioperative care” or “peri-operative care”:ti,ab,kw |
| #19 | #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 |
| #20 | #4 and #10 and #19 |
Hand Search Strategy
“Subtrochanteric Fracture,” “Trochanteric Fracture,” “Intertrochanteric Fracture,” “Femoral Neck fracture”
“confusion,” “disorientation”
“surgery,” “operation,” “ intraoperative,” “perioperative,” “preoperative”
References
- Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80:388–393. doi: 10.1136/pgmj.2003.017236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson EM, Gustafson L, Hallberg IR. Acute confusional state in elderly orthopaedic patients: factors of importance for detection in nursing care. Int J Geriatr Psychiatry. 2001;16:7–17. doi: 10.1002/1099-1166(200101)16:1<7::aid-gps261>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Bentler SE, Liu L, Obrizan M, Cook EA, Wright KB, Geweke JF, Chrischilles EA, Pavlik CE, Wallace RB, Ohsfeldt RL, Jones MP, Rosenthal GE, Wolinsky FD. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170:1290–1299. doi: 10.1093/aje/kwp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsch M, Foss N, Kristensen B, Kehlet H. Pathogenesis of and management strategies for postoperative delirium after hip fracture: a review. Acta Orthop Scand. 2004;75:378–389. doi: 10.1080/00016470410001123. [DOI] [PubMed] [Google Scholar]
- Bjoro K. Pain Treatment: A Risk Factor for Delirium in Older Adult with HIp Fracture 2008 [Google Scholar]
- Bruce AJ, Ritchie CW, Blizard R, Lai R, Raven P. The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int Psychogeriatr. 2007;19:197–214. doi: 10.1017/S104161020600425X. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Incidence and costs to Medicare of fractures among Medicare beneficiaries aged >65 years-United States, July 1991–June 1992. 1996;45:877–83. [PubMed] [Google Scholar]
- Cooper C, Campion G, Melton LJ., 3rd Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2:285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54:1578–1589. doi: 10.1111/j.1532-5415.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263:1097–1101. [PubMed] [Google Scholar]
- GOLDENBERG G, KISELEV P, BHARATHAN T, BACCASH E, GILL L, MADHAV V, DHILLON P, THAKUR C. Predicting post-operative delirium in elderly patients undergoing surgery for hip fracture. Psychogeriatrics. 2006;6:43–48. doi: 10.1111/j.1479-8301.2006.00146.x. [DOI] [Google Scholar]
- Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008;63:764–772. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852–857. [PubMed] [Google Scholar]
- Inouye SK, Bogardus ST, Jr, Baker DI, Leo-Summers L, Cooney LM., Jr The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc. 2000;48:1697–1706. doi: 10.1111/j.1532-5415.2000.tb03885.x. [DOI] [PubMed] [Google Scholar]
- Inouye SK, Bogardus ST, Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LM., Jr A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- Isaacs B, Kennie AT. The Set test as an aid to the detection of dementia in old people. Br J Psychiatry. 1973;123:467–470. doi: 10.1192/bjp.123.4.467. [DOI] [PubMed] [Google Scholar]
- Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- Juliebo V, Bjoro K, Krogseth M, Skovlund E, Ranhoff AH, Wyller TB. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57:1354–1361. doi: 10.1111/j.1532-5415.2009.02377.x. [DOI] [PubMed] [Google Scholar]
- Kaysen GA. Biochemistry and biomarkers of inflamed patients: why look, what to assess. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S56–63. doi: 10.2215/CJN.03090509. [DOI] [PubMed] [Google Scholar]
- Krogseth M, Wyller TB, Engedal K, Juliebo V. Delirium is a risk factor for institutionalization and functional decline in older hip fracture patients. J Psychosom Res. 2014;76:68–74. doi: 10.1016/j.jpsychores.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Lee HB, Mears SC, Rosenberg PB, Leoutsakos JM, Gottschalk A, Sieber FE. Predisposing Factors for Postoperative Delirium After Hip Fracture Repair in Individuals with and without Dementia. J Am Geriatr Soc. 2011;59:2306–2313. doi: 10.1111/j.1532-5415.2011.03725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65:229–238. doi: 10.1016/j.jpsychores.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618–624. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Morrison RS, Magaziner J, Gilbert M, Koval KJ, McLaughlin MA, Orosz G, Strauss E, Siu AL. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76–81. doi: 10.1093/gerona/58.1.m76. [DOI] [PubMed] [Google Scholar]
- Neuman MD, Silber JH, Magaziner JS, Passarella MA, Mehta S, Werner RM. Survival and Functional Outcomes After Hip Fracture Among Nursing Home Residents. JAMA Intern Med. 2014 doi: 10.1001/jamainternmed.2014.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Zhao B, Zhang YQ, Jiang YH, Yang YX. Pain and cognitive dysfunction are the risk factors of delirium in elderly hip fracture Chinese patients. Arch Gerontol Geriatr. 2012;54:e172–4. doi: 10.1016/j.archger.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Noimark D. Predicting the onset of delirium in the post-operative patient. Age Ageing. 2009;38:368–373. doi: 10.1093/ageing/afp024. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006;199:265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Rubin FH, Neal K, Fenlon K, Hassan S, Inouye SK. Sustainability and scalability of the hospital elder life program at a community hospital. J Am Geriatr Soc. 2011;59:359–365. doi: 10.1111/j.1532-5415.2010.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana Santos F, Wahlund LO, Varli F, Tadeu Velasco I, Eriksdotter Jonhagen M. Incidence, clinical features and subtypes of delirium in elderly patients treated for hip fractures. Dement Geriatr Cogn Disord. 2005;20:231–237. doi: 10.1159/000087311. [DOI] [PubMed] [Google Scholar]
- Sieber FE, Mears S, Lee H, Gottschalk A. Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J Am Geriatr Soc. 2011;59:2256–2262. doi: 10.1111/j.1532-5415.2011.03729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JA, Rudd RA. The impact of decreasing U.S. hip fracture rates on future hip fracture estimates. Osteoporos Int. 2013;24:2725–2728. doi: 10.1007/s00198-013-2375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- Zakriya KJ, Christmas C, Wenz JFS, Franckowiak S, Anderson R, Sieber FE. Preoperative factors associated with postoperative change in confusion assessment method score in hip fracture patients. Anesth Analg. 2002;94:1628–32. doi: 10.1097/00000539-200206000-00050. table of contents. [DOI] [PubMed] [Google Scholar]