Abstract
Objectives
To characterize glucose levels and variability in young children with T1D.
Methods
144 children ages 4–10 diagnosed with T1D prior to age 8 were recruited at 5 DirecNet centers. Participants used a continuous glucose monitor (CGM) every 3 months during an 18-month study. Among the 144 participants, 135 (mean age 7.0 years, 47% female) had a minimum of 48 hours of CGM data at >5 out of 7 visits and were included in analyses. CGM metrics for different times of day were analyzed.
Results
Mean HbA1c at the beginning and end of the study was 7.9% (63 mmol/mol). Fifty percent of participants had glucose levels >180mg/dL (10.0 mmol/L) for >12 hours/day and >250 mg/dL (13.9 mmol/L) for >6 hours/day. Median time <70 mg/dL (3.9 mmol/L) was 66 minutes/day and <60 mg/dL (3.3 mmol/L) was 39 minutes/day. Mean amplitude of glycemic excursions (MAGE) was lowest overnight (12AM–6AM). The percent of CGM values 71–180 mg/dL (3.9–10.0 mmol/L) and the overall mean glucose correlated with HbA1c at all visits. There were no differences in CGM mean glucose or coefficient of variation between the age groups of 4–<6, 6–<8 and 8–<10.
Conclusions
Suboptimal glycemic control is common in young children with T1D as reflected by glucose levels in the hyperglycemic range for much of the day. New approaches to reduce postprandial glycemic excursions and increase time in the normal range for glucose in young children with T1D are critically needed. Glycemic targets in this age range should be revisited.
Keywords: Continuous glucose monitoring, Pediatric, Type 1 diabetes
Introduction
Parents of young children with type 1 diabetes (T1D) face many challenges to achieve optimal glycemic control. There are often irregular eating patterns and activity is highly variable leading to frequent hyperglycemia and hypoglycemia. Young children are often very sensitive to insulin and not always able to recognize hypoglycemia (1) due, in part, to blunted counter-regulatory responses (2) that exacerbate the problem. In view of the potential adverse effects of hypoglycemia on the developing brain, until recently the American Diabetes Association (ADA) has recommended higher A1c and blood glucose targets for pre-teens than for adolescents (3). Prior to June 2014, ADA A1c goals were <8.5% (69 mmol/mol) for children less than 6 years of age, <8.0% (64 mmol/mol) for ages 6–12 and <7.5% (58 mmol/mol) for teenagers (4). Similarly, target pre-prandial blood glucose levels of 100–180 mg/dL (5.6–10.0 mmol/L) and overnight level of 110–200 mg/dL (6.1–11.1 mmol/L) were recommended for children <6 compared with pre-prandial blood glucose values of 90–180 mg/dL (5.0–10.0 mmol/L) and overnight levels of 100–180 mg/dL (5.6–10.0 mmol/L) in 6–12 year olds. On the other hand, there is increasing evidence that hyperglycemia also has adverse effects on the developing brain of young children, suggesting the need for limiting exposure to hyperglycemia as well as hypoglycemia in young children with T1D (5, 6).
Fingerstick glucose monitoring provides a limited view of true glycemic patterns, and often fails to recognize the magnitude of glycemic excursions and may underestimate time spent in hypoglycemic and hyperglycemic ranges (7, 8). As part of the Diabetes Research in Children Network (DirecNet) study of the effect of glycemic control on cognitive function and neuro-anatomic development in young children with T1D, continuous glucose monitoring (CGM) data were collected every 3 months over 18 months. In this paper we present the results of detailed analyses of these serial CGM assessments, which have allowed us to more fully characterize the limitations of current treatment methods to effectively limit the exposure of young children with T1D to hyperglycemia and hypoglycemia.
Methods
The study was conducted by the five DirecNet clinical centers and the coordinating center. Data for this study were obtained through a DirecNet study conducted to compare neuroanatomical findings with targeted measures of neurocognitive function at baseline and at 18 months in very young children with T1D. The protocol and consent forms were approved by the Institutional Review Board at each center. Written informed consent was obtained from the parent or guardian prior to enrollment. Major eligibility criteria for the study included 1) clinical diagnosis of type 1 diabetes and using daily insulin therapy for at least one month, 2) age 4.0 to <10.0 years, (if age 8.0 to <10.0 years, then diagnosis of diabetes must have been prior to 8.0 years of age and prepubertal by physical examination), and 3) positive antibody testing if onset of T1D < 1 year of age.
CGM data were collected every 3 months for the 18 months of the study. At least 48 hours of CGM data at a 3-month time point was required for inclusion in the analyses. Participants who had 4 or fewer time points with at least 48 hours of CGM data were not included in the analyses; 9 participants were not included due to insufficient CGM data, leaving a cohort of 135 participants for the analyses.
For participants using unblinded CGM as part of their diabetes care, data from their personal CGM was collected every 3 months for the 18 months of the study. Otherwise a blinded CGM (iPro2®, Medtronic MiniMed, Northridge, CA or DexCom SEVEN Plus®, DexCom, San Diego, CA) was worn every 3 months. Among the 135 participants included in the analyses, 73 used a blinded iPro2 only, 3 used blinded DexCom and iPro2, 21 used a personal unblinded CGM device, and 38 had a combination of blinded CGM for some time points and unblinded CGM for others. Among the 135 participants included in the analyses, CGM data were available for a median of 943 hours during the 18-month period of the study, with 27% of participants having 463–<800 hours, 33% having 800–<1000 hours, 15% having 1000–<1500 hours and 25% having 1500–7411 hours of use. Participants using the blinded CGM had CGM data for a median of 750 hours, and those using an unblinded CGM had a median of 1,346 hours of data.
HbA1c was measured every 3 months by a local point of care device or local laboratory and quality assurance performed per manufacturer specifications.
Statistical Methods
CGM indices were calculated from data downloads at enrollment and every 3 months through 18 months. The average CGM indices across all 7 visits for each participant were calculated giving equal weight to each time point. Median (interquartile range) for each CGM index was reported for different time periods of the day. Association of CGM indices with HbA1c at each visit was assessed by Spearman correlation. Differences in averaged CGM indices among age groups were assessed by Kruskal Wallis test. Sensitivity analysis was conducted by including every T1D participant (N=144) in the original cohort. The mean amplitude of glycemic excursions was calculated as originally defined (9).
Results
Mean age (±SD) of the 135 participants was 7.0 ±1.7 years; 47% were female (Table 1). Mean age of onset of T1D was 4.1 ± 1.9 years (range 0.9–8 years). At study baseline, 75 (56%) participants were on insulin pump therapy and the remainder used multiple daily injections of insulin for treatment of T1D. Mean HbA1c for all subjects was 7.9±0.9% [63±9 mmol/mol] (range 6.3–10.2% [45–88 mmol/mol]) at study baseline and 7.9±0.9% [63±10 mmol/mol] (range 6.0–10.8% [42–95 mmol/mol]) at 18 months. ADA criteria for HbA1c in effect at the time of the study were met at baseline by 86 (64%): 26 (63%) of the 41 4–5 year olds (ADA HbA1c target of 8.5% [69 mmol/mol]) and 60 (64%) of the 94 6–9 year olds (target of 8.0% [64 mmol/mol]), and HbA1c was <7.5% [58 mmol/mol] (current ADA target) in 48 (36%). Median of each participant’s mean sensor glucose values was 191 mg/dL (10.6 mmol/L) (Table 2). HbA1c was lower for the unblinded group compared with the blinded group at enrollment (7.6±0.8 % [59±9 mmol/mol] vs 8.0±0.9 % [64±9 mmol/mol], p=0.008) and at 18 months (7.4±0.7 % [58±8 mmol/mol] vs 8.1±1.0 % [65±11 mmol/mol], p<0.001). HbA1c also was lower for insulin pump users compared with multiple daily injections at enrollment (7.7±0.8 % [61±9 mmol/mol] vs 8.1±0.9 % [65±10 mmol/mol], p=0.02) and at 18 months (7.8±0.8 % [61±8 mmol/mol] vs 8.2±1.2 % [66±13 mmol/mol], p=0.03). As some participants were using an unblinded CGM device on a regular basis, CGM use was higher in the unblinded group (table 3) compared with the blinded group (table 4). Compared with the blinded CGM participants, participants using unblinded CGM had less hypoglycemia (3.3% vs 5.1% of values less than 70 mg/dL [3.9 mmol/L]), more euglycemia (53% vs 43% of values 71–180 mg/dL [3.9–10.0 mmol/L]) and less hyperglycemia (17% vs 26% of values >250 mg/dL [13.9 mmol/L]).
Table 1.
Demographics and Clinical Characteristics at Study Enrollment
| N=135 | |
|---|---|
| Age (years) | |
| 4–<6 | 41 (30%) |
| 6–<8 | 63 (47%) |
| 8–<10 | 31 (23%) |
| mean ± SD (range) | 7.0 ± 1.7 (4.0 to 9.99) |
| Female | 63 (47%) |
| Race/Ethnicity a | 81%W, 7%H, 4%AA, 1%A, 7%O |
| BMI percentile median (25th, 75th percentile) | 72% (59%, 89%) |
| Diabetes duration (years) | |
| median (25th, 75th percentile) | 2.3 (1.1, 4.4) |
| Range | 0.1 to 7.9 |
| Age at onset (years) | |
| mean ± SD | 4.1 ± 1.9 |
| Range | 0.9 to 8.0 |
| Severe Hypoglycemia and Diabetic Ketoacidosis | |
| Prior to Enrollment | |
| One or more Severe Hypoglycemia Event b N (%) | 22 (16%) |
| DKA at Diagnosis c N (%) | 43 (32%) |
| DKA Between Diagnosis and Enrollment c N (%) | 5 (4%) |
| Between Enrollment and 18 Months | |
| Severe Hypoglycemia Event N (%) | 6 (4%) |
| DKA N (%) | 4 (3%) |
| HbA1c (%) (mmol/mol) mean ± SD [range] | |
| Enrollment | 7.9 ± 0.9 (63 ± 9) |
| [6.3 to 10.2 (45 to 88)] | |
| 18 Months | 7.9 ± 0.9 (63 ± 10) |
| [6.0 to 10.8 (42 to 95)] | |
W: White, H: Hispanic or Latino, AA: African American, A: Asian, O: other
Includes 17 participants with one episode, 3 with two, 1 with three and 1 with five episodes.
Excluded 2 participants with unknown DKA history
Table 2.
CGM Indices in T1D Participants (N=135)
| Overall | Daytime (6am–8pm) | Late Evening (8pm–12am) | Overnight (12am–6am) | |
|---|---|---|---|---|
| Hours of CGM values per participant | 943 (789–1554) | 541 (446–902) | 159 (137–260) | 244 (208–394) |
| % ≤60 mg/dL (3.3 mmol/L) | 2.7% (1.6%–4.3%) | 2.0% (1.3%–3.4%) | 2.2% (0.9%–3.7%) | 3.7% (1.7%–6.6%) |
| Minutes per day | 38 (24–62) | 29 (18–49) | 32 (13–53) | 54 (25–96) |
| % ≤70 mg/dL (3.9 mmol/L) | 4.6% (3.2%–7.1%) | 4.2% (2.5%–6.2%) | 4.1% (2.4%–6.0%) | 6.0% (3.6%–10%) |
| Minutes per day | 66 (47–102) | 61 (36–89) | 59 (34–87) | 86 (52–147) |
| % 71–180 mg/dL (3.9–10.0 mmol/L) | 45% (38%–52%) | 44% (37%–52%) | 46% (37%–56%) | 43% (34%–54%) |
| Minutes per day | 644 (541–749) | 628 (534–755) | 661 (533–811) | 622 (491–778) |
| % >180 mg/dL (10.0 mmol/L) | 50% (41%–57%) | 51% (42%–58%) | 48% (36%–60%) | 48% (36%–61%) |
| Minutes per day | 718 (592–825) | 731 (599–841) | 693 (521–862) | 686 (525–871) |
| % >250 mg/dL (13.9 mmol/L) | 25% (18%–33%) | 26% (18%–33%) | 25% (15%–33%) | 21% (14%–33%) |
| Minutes per day | 363 (257–470) | 377 (257–473) | 354 (215–479) | 302 (195–471) |
| Mean glucose (mg/dL) (mmol/L) | 191 (175–209) | 196 (177–209) | 192 (166–213) | 184 (165–208) |
| [10.6 (9.7–11.6)] | [10.9 (9.8–11.6)] | [10.7 (9.2–11.8)] | [10.2 (9.2–11.6)] | |
| Glucose Coefficient of Variation (SD/Mean) | 43% (40%–46%) | 42% (39%–45%) | 40% (37%–44%) | 38% (35%–43%) |
| MAGE (mg/dL) (mmol/L) | 159 (141–171) | 152 (137–170) | 103 (89–118) | 80 (70–93) |
| [8.8 (7.8–9.5)] | [8.4 (7.6–9.4)] | [5.7 (4.9–6.6)] | [4.4 (3.9–5.2)] |
Data are shown as median (interquartile range)
Table 3.
Unblinded CGM Indices in T1D Participants (N=59a)
| Overall | Daytime (6am–8pm) | Late Evening (8pm–12am) | Overnight (12am–6am) | |
|---|---|---|---|---|
| Hours of CGM values per participant | 1346 (670–3157) | 775 (397–1793) | 226 (111–550) | 334 (180–828) |
| % ≤60 mg/dL (3.3 mmol/L) | 1.3% (0.4%–2.6%) | 1.3% (0.3%–2.3%) | 1.5% (0.4%–3.0%) | 1.3% (0.3%–3.2%) |
| Minutes per day | 19 (6–37) | 18 (4–34) | 22 (6–43) | 18 (4–47) |
| % ≤70 mg/dL (3.9 mmol/L) | 3.3% (1.6%–5.0%) | 3.0% (1.2%–4.7%) | 3.2% (1.7%–5.4%) | 3.6% (1.1%–5.6%) |
| Minutes per day | 47 (23–72) | 43 (18–67) | 46 (24–78) | 52 (16–80) |
| % 71–180 mg/dL (3.9–10.0 mmol/L) | 53% (45%–60%) | 56% (42%–63%) | 50% (41%–63%) | 48% (43%–60%) |
| Minutes per day | 768 (642–871) | 804 (609–903) | 727 (596–901) | 692 (616–866) |
| % >180 mg/dL (10.0 mmol/L) | 43% (35%–52%) | 40% (32%–54%) | 44% (33%–55%) | 47% (34%–54%) |
| Minutes per day | 620 (507–755) | 570 (466–783) | 631 (476–791) | 670 (490–777) |
| % >250 mg/dL (13.9 mmol/L) | 17% (11%–25%) | 17% (10%–28%) | 17% (9%–29%) | 16% (8%–25%) |
| Minutes per day | 244 (163–364) | 238 (142–400) | 251 (131–412) | 235 (117–362) |
| Mean glucose (mg/dL) (mmol/L) | 175 (165–198) | 173 (161–200) | 178 (159–203) | 183 (163–195) |
| [9.7 (9.2–11.0)] | [9.6 (8.9–11.1)] | [9.9 (8.8–11.3)] | [10.2 (9.1–10.8)] | |
| Glucose Coefficient of Variation (SD/Mean) | 40% (37%–44%) | 41% (37%–44%) | 39% (35%–43%) | 36% (32%–41%) |
| MAGE (mg/dL) (mmol/L) | 136 (124–155) | 131 (116–149) | 93 (81–107) | 76 (67–87) |
| [7.6 (6.9–8.6)] | [7.3 (6.4–8.3)] | [5.2 (4.5–5.9)] | [4.2 (3.7–4.8)] |
Table 4.
Blinded CGM Indices in T1D Participants (N=114a)
| Overall | Daytime (6am–8pm) | Late Evening (8pm–12am) | Overnight (12am–6am) | |
|---|---|---|---|---|
| Hours of CGM values per participant | 750 (554–916) | 427 (319–530) | 128 (93–155) | 193 (143–234) |
| % ≤60 mg/dL (3.3 mmol/L) | 2.9% (1.8%–4.8%) | 2.3% (1.3%–3.7%) | 2.2% (0.8%–3.7%) | 4.5% (2.2%–7.8%) |
| Minutes per day | 42 (25–69) | 34 (18–54) | 32 (12–53) | 64 (31–112) |
| % ≤70 mg/dL (3.9 mmol/L) | 5.1% (3.3%–7.4%) | 4.5% (2.7%–6.5%) | 4.3% (2.1%–6.2%) | 7.2% (4.2%–11%) |
| Minutes per day | 73 (47–106) | 65 (39–94) | 61 (30–90) | 103 (60–158) |
| % 71–180 mg/dL (3.9–10.0 mmol/L) | 43% (36%–50%) | 43% (36%–51%) | 43% (34%–56%) | 42% (31%–51%) |
| Minutes per day | 613 (512–713) | 614 (515–730) | 620 (483–801) | 598 (443–728) |
| % >180 mg/dL (10.0 mmol/L) | 51% (43%–57%) | 52% (46%–59%) | 50% (39%–61%) | 48% (37%–62%) |
| Minutes per day | 739 (623–825) | 743 (660–852) | 723 (557–874) | 698 (526–892) |
| % >250 mg/dL (13.9 mmol/L) | 26% (19%–34%) | 27% (22%–34%) | 26% (18%–36%) | 21% (15%–34%) |
| Minutes per day | 380 (280–493) | 389 (314–492) | 376 (260–523) | 301 (214–485) |
| Mean glucose (mg/dL) (mmol/L) | 193 (179–212) | 197 (183–212) | 194 (174–218) | 185 (164–210) |
| [10.7 (9.9–11.8)] | [10.9 (10.2–11.8)] | [10.8 (9.7–12.1)] | [10.3 (9.1–11.7)] | |
| Glucose Coefficient of Variation (SD/Mean) | 44% (40%–47%) | 43% (40%–46%) | 41% (37%–45%) | 39% (35%–43%) |
| MAGE (mg/dL) (mmol/L) | 167 (150–182) | 163 (142–176) | 106 (91–124) | 82 (70–96) |
| [9.3 (8.3–10.1)] | [9.1 (7.9–9.8)] | [5.9 (5.1–6.9)] | [4.6 (3.9–5.3)] |
Over the 18 months, overall CGM metrics showed that significant time was spent in the hyperglycemic range, with 50% of overall participants having CGM values >180mg/dL (10.0 mmol/L) for more than 12 hours a day and >250 mg/dL (13.9 mmol/L) for more than 6 hours a day (Table 2). In contrast, hypoglycemia was much less frequent, although the median time <70 mg/dL (3.9 mmol/L) was still 66 minutes per day and <60 mg/dL (3.3 mmol/L) was 38 minutes per day. There were no appreciable differences in CGM glucose metrics between the age groups of 4–<6, 6–<8 and 8–<10 (Supplemental Table 1). At 18 months, the current ADA target of <7.5% (58 mmol/mol) was met by 45 (33%) of the children.
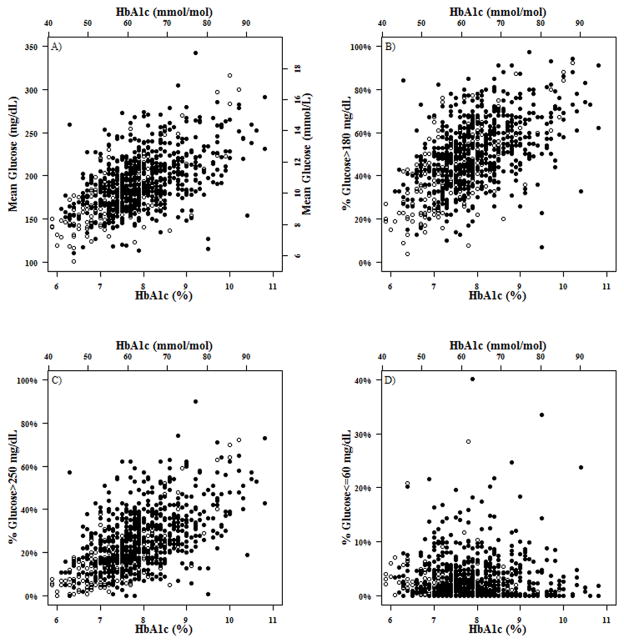
Not surprisingly, for all subjects glucose variability was less overnight than during the daytime (median MAGE 80 mg/dL [4.4 mmol/L] versus 152 mg/dL [8.4 mmol/L], p<0.001) while hypoglycemia was more frequent overnight (median time <70 mg/dL [3.9 mmol/L] 6.0% versus 4.2%, p<0.001). As shown in the Figure 1A, mean glucose correlated with HbA1c at all visits (r = 0.53 to 0.61), as did the percentage of values between 71–180 mg/dL (3.9–10.0 mmol/L) (r=−0.53 to −0.58). As shown in Figure 1D, there was not a relationship between the frequency of glucose values <60 mg/dL (3.3 mmol/L) and A1c (r = −0.17 to 0.08). Sensitivity analysis demonstrated that results were similar when including all the 144 T1D participants in the analyses (results not shown).
Figure 1. A) Mean Glucose vs. A1c; B) %Glucose >180 mg/dL (10.0 mmol/L) vs. A1c; C) %Glucose >250 mg/dL (13.9 mmol/L) vs. A1c; D) %Glucose ≤60 mg/dL (3.3 mmol/L) vs. A1c.
Each point on the scatterplot denotes one study visit. There are multiple points from the same subject. Black dots represent blinded CGM and hollow circles represent unblinded CGM.
Discussion
Hyperglycemia is the leading cause of diabetes complications, micro- and macrovascular disease, as well as kidney, retina and peripheral nerve tissue damage, effects that are well-known to be cumulative (10). In addition, the negative cognitive impact of hyperglycemia is increasingly being recognized. Young children with T1D in our study had significant exposure to hyperglycemia, with half of the children having CGM glucose values over 180 mg/dL (10.0 mmol/L) for 12 or more hours a day and over 250 mg/dL (13.9 mmol/L) for 6 or more hours a day. Significant hyperglycemia was documented both during the day as well as overnight. These observations may be due to the fact that many parents of young children with T1D have concerns about the risks of hypoglycemia, and feel more comfortable with running blood glucose levels higher. A recent study suggests that mothers who worry most about hypoglycemia compensate by maintaining their child’s blood glucose levels above recommended levels (11).
Young children are undergoing rapid neurocognitive development and in our study 51% of CGM readings were >180 mg/dL (10.0 mmol/L) from 6AM–8PM (mean glucose during this time was 196 mg/dL [10.9 mmol/L]), which is when children are actively learning and in school. In the neuro-imaging and neuro-cognitive portions of our study, subtle cognitive differences were present in the children with T1D compared with age-matched healthy controls despite relatively short T1D duration (5). Significant differences in brain gray matter volumes (3) and white matter microstructure (12) in widespread brain regions also were observed; these differences were primarily associated with hyperglycemia. These data collectively suggest that chronic hyperglycemia is detrimental to the developing brain. A study of adults by Cox et al. demonstrated that during hyperglycemia (>270 mg/dL [15.0 mmol/L]) adults with diabetes had a significant slowing of cognitive performance tests and an increased number of mental subtraction errors (13). Limiting hyperglycemia in young children with T1D may be particularly important during times of active learning.
Despite finding that many of the children had hyperglycemia for more than half the day, 64% of the children met the ADA HbA1c age-specific target at the time the study was conducted (ADA goal A1c < 8.5% [69 mmol/mol] for age 0–6, and <8% [64 mmol/mol] for ages 6–12) at study baseline (mean A1c 7.9% [63 mmol/mol]) and 60% at 18 months (mean A1c 7.9% [63 mmol/mol]). Although participants using unblinded CGM had a lower HbA1c and more time in the glucose target range of 71–180 mg/dL (3.9–10.0 mmol/L) than those not using CGM as part of daily diabetes management, they still had glucose concentrations above 180 mg/dL (10.0 mmol/L) for almost half the day. It is appealing to credit CGM for the better glycemic control seen in regular CGM users than nonusers; however, there may have been other factors that contributed to this difference. The Diabetes Patienten Verlaufsdokumenation (DPV) registry (14) in Germany and Austria has reported substantially lower HbA1c levels in young children (15) with T1D than has been reported by the T1D Exchange registry in the U.S (16). One explanation for this could be that the target A1c for children with diabetes in Europe is 7.5% (58 mmol/mol), the standard set by the International Society for Pediatric and Adolescent Diabetes (ISPAD) (17) rather than the prior ADA target of 8.0–8.5% (64–69 mmol/mol). There are other factors that could contribute to these differences including overall use of CGM as well as details regarding insurance coverage of CGM between these populations. Some countries in Europe are recommending increased use of CGM in young children (18). Our finding that many young children with T1D are spending the majority of the day in the hyperglycemic range plus the fact that hyperglycemia has been shown to have deleterious effects on the brains of these children (6) provide support for the recent lowering of the ADA HbA1c target for this age group to 7.5% (58 mmol/mol) (3). The wide range of mean glucose levels and percentages of time above 250 mg/dL (13.9 mmol/L) for a given HbA1c level found in this study as well as in prior studies (19–21) indicate that HbA1c may not be the sole best indicator of glucose control in children with T1D. While HbA1c can be useful for monitoring changes in glycemic control over time, substantial hyperglycemia and glycemic variability are occurring even when HbA1c levels are at target.
Young children with T1D often spend the majority of the day in the hyperglycemic range, with relatively low amounts of hypoglycemia. New approaches to examine the timing and action of insulin could help to reduce glycemic excursions and increase time in the normal range for glucose in young children with T1D, particularly as there may be risk of cognitive development with significant hyperglycemia. Given the glycemic excursions observed in our study, there should be consideration to re-evaluate glycemic targets ranges for young children, with respect for the risks of hyperglycemia and hypoglycemia. Other variables that could be examined which might influence glycemic variability include timing if insulin related to meals, activity patterns insulin sensitivity. Future long term studies examining these variables using continuous glucose monitoring and closely examining postprandial glycemic variability in relation to HbA1c can further elucidate methods to achieve improved glycemic control.
Supplementary Material
Acknowledgments
The authors thank the children and their families as well as the clinical and imaging staff at all of the investigator sites. We also thank our external collaborators for use of their imaging facilities, including University of California at San Francisco, El Camino Hospital, and University of Florida & Shands Jacksonville. This research was supported by funding from the NIH (DIRECNET U01 HD41890, HD41906, HD41908, HD41915, HD41918, HD56526) and UL1 RR024992. N.H. White reports receiving payment for consultancy from Novo Nordisk and Daiichi Sankyo and payments to his institution from Bristol-Myers Squibb for a research grant. S. Weinzimer reports receiving payment to his institution from a Medtronic grant. He reports receiving payment from Animas for consultancy, payment from Eli Lilly for lectures including service on speaker bureaus, and payment from Insuline Medical for stock/stock options. Also reports receiving “honoraria for consultancy” for Medtronic and Tandem. M. Tansey, R. Beck, K. Ruedy, C. Kollman, W. Tamborlane, P. Cheng, L. Fox, N. Mauras, E. Tsalikian report no conflict of interest.
Abbreviations
- MAGE
mean amplitude of glycemic excursions
The DirecNet Study Group
The DirecNet Study Group: Clinical Centers: (Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator, (C) for Coordinators and (PM) for Psychometrician.) Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA: Eva Tsalikian, MD (PI); Michael J. Tansey, MD (I); Julie Coffey, MSN (C); Joanne Cabbage (C); Sara Salamati (C); Amy Conrad, PhD (PM); Nemours Children’s Clinic, Jacksonville, FL: Nelly Mauras, MD (PI); Larry A. Fox, MD (I); Allison Cato, PhD (I); Kim Englert, RN, BSN, CDE (C); Kaitlin Sikes, ARNP, MSN (C); Tina Ewen (C); Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA: Bruce A. Buckingham, MD (PI); Darrell M. Wilson, MD (I); Tandy Aye, MD (I); Kimberly Caswell, ARNP (C); Kristin Schleifer (PM); Christian Ambler (PM); Department of Pediatrics, Yale University School of Medicine, New Haven, CT: Stuart A. Weinzimer, MD (PI); William V. Tamborlane, MD (I); Amy Steffen, BS (C); Kate Weyman, MSN (C); Melinda Zgorski, BSN (C); Jodie Ambrosino, PhD (I); Washington University in St. Louis, St. Louis, MO: Neil H. White, MD, CDE (PI); Ana Maria Arbelaez, MD, (I); Lucy Levandoski, PA-C (C); Angie Starnes, RN, BSN, CDE (C), Tamara Hershey, PhD (I); Sarah June Grafeman, PhD (PM); Coordinating Center: Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD; Katrina J. Ruedy, MSPH; Craig Kollman, PhD; Peiyao Cheng, MPH; Beth Stevens; Nelly Njeru; Ryan Chapman Image Coordinating Center: Allan L. Reiss, MD; Naama Barnea-Goraly, MD; Matthew J. Marzelli, BS; Paul M. Mazaika, PhD; Daniel X. Peng, BS; Cognitive Core: Tamara Hershey, PhD; Allison Cato, PhD; Emily Bihun, MA; Amal Al-Lozi, BA; Allison Bischoff, BA; Michaela Cuneo, BA; Aiden Bondurant, BA. Data and Safety Monitoring Board: Mark Sperling, MD; Dorothy M. Becker, MBBCh; Patricia Cleary, MS; Carla Greenbaum, MD; Antoinette Moran, MD.
Footnotes
A full listing of the members of the study group is included in the acknowledgements
References
- 1.Bober E, Buyukgebiz A, Verrotti A, Chiarelli F. Hypoglycemia, hypoglycemia unawareness and counterregulation in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2005;18:831–41. doi: 10.1515/jpem.2005.18.9.831. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Research in Children Network (DirecNet) Study Group. Blunted Counterregulatory Hormone Responses to Hypoglycemia in Young Children and Adolescents With Well-Controlled Type 1 Diabetes. Diabetes Care. 2009;32:1954–9. doi: 10.2337/dc08-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang JL, Kirkman MS, Laffel LMB, Peters AL on behalf of the Type 1 Diabetes Sourcebook A. Type 1 Diabetes Through the Life Span: A Position Statement of the American Diabetes Association. Diabetes Care. 2014 doi: 10.2337/dc14-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes A. Standards of Medical Care in Diabetes 2014. Diabetes Care. 2014;37:S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 5.Cato MA, Mauras N, Ambrosino J, Bondurant A, Conrad AL, Kollman C, et al. Cognitive Functioning in Young Children with Type 1 Diabetes. Journal of the International Neuropsychological Society. 2014;20:238–47. doi: 10.1017/S1355617713001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marzelli MJ, Mazaika PK, Barnea-Goraly N, Hershey T, Tsalikian E, Tamborlane W, et al. Neuroanatomical Correlates of Dysglycemia in Young Children With Type 1 Diabetes. Diabetes. 2014;63:343–53. doi: 10.2337/db13-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care. 2001;24:1858–62. doi: 10.2337/diacare.24.11.1858. [DOI] [PubMed] [Google Scholar]
- 8.Diabetes Research in Children Network (DirecNet) Study Group. Eight-point glucose testing versus the continuous glucose monitoring system in evaluation of glycemic control in type 1 diabetes. J Clin Endocrinol Metab. 2005;90:3387–91. doi: 10.1210/jc.2004-2510. [DOI] [PubMed] [Google Scholar]
- 9.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean Amplitude of Glycemic Excursions, a Measure of Diabetic Instability. Diabetes. 1970;19:644–55. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 10.The Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 11.Freckleton E, Sharpe L, Mullan B. The Relationship Between Maternal Fear of Hypoglycaemia and Adherence in Children with Type-1 Diabetes. International Journal of Behavioral Medicine. 2013:1–7. doi: 10.1007/s12529-013-9360-8. [DOI] [PubMed] [Google Scholar]
- 12.Barnea-Goraly N, Raman M, Mazaika P, Marzelli M, Hershey T, Weinzimer SA, et al. Alterations in White Matter Structure in Young Children With Type 1 Diabetes. Diabetes Care. 2014;37:332–40. doi: 10.2337/dc13-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox DJ, Kovatchev BP, Gonder-Frederick LA, Summers KH, McCall A, Grimm KJ, et al. Relationships Between Hyperglycemia and Cognitive Performance Among Adults With Type 1 and Type 2 Diabetes. Diabetes Care. 2005;28:71–7. doi: 10.2337/diacare.28.1.71. [DOI] [PubMed] [Google Scholar]
- 14.Gerstl EM, Rabl W, Rosenbauer J, Grobe H, Hofer SE, Krause U, et al. Metabolic control as reflected by HbA1c in children, adolescents and young adults with type-1 diabetes mellitus: combined longitudinal analysis including 27,035 patients from 207 centers in Germany and Austria during the last decade. European Journal of Pediatrics. 2008;167:447–53. doi: 10.1007/s00431-007-0586-9. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig-Seibold CU, Holder M, Rami B, Raile K, Heidtmann B, Holl RW, et al. Continuous glucose monitoring in children, adolescents, and adults with type 1 diabetes mellitus: analysis from the prospective DPV diabetes documentation and quality management system from Germany and Austria. Pediatric Diabetes. 2012;13:12–4. doi: 10.1111/j.1399-5448.2011.00835.x. [DOI] [PubMed] [Google Scholar]
- 16.Wood JR, Miller KM, Maahs DM, Beck RW, DiMeglio LA, Libman IM, et al. Most Youth With Type 1 Diabetes in the T1D Exchange Clinic Registry Do Not Meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes Clinical Guidelines. Diabetes Care. 2013;36:2035–7. doi: 10.2337/dc12-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rewers MJ, Pillay K, de Beaufort C, Craig ME, Hanas R, Acerini CL, et al. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatric Diabetes. 2014;15:102–14. doi: 10.1111/pedi.12190. [DOI] [PubMed] [Google Scholar]
- 18.Scaramuzza A, Cherubini V, Tumini S, Bonfanti R, Buono P, Cardella F, et al. Recommendations for self-monitoring in pediatric diabetes: a consensus statement by the ISPED. Acta Diabetologica. 2014;51:173–84. doi: 10.1007/s00592-013-0521-7. [DOI] [PubMed] [Google Scholar]
- 19.Mauras N, Beck R, Xing D, Ruedy K, Buckingham B, Tansey M, et al. A Randomized Clinical Trial to Assess the Efficacy and Safety of Real-Time Continuous Glucose Monitoring in the Management of Type 1 Diabetes in Young Children Aged 4 to <10 Years. Diabetes Care. 2012;35:204–10. doi: 10.2337/dc11-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tansey M, Weinzimer S, Beck R, Ruedy K, Cheng P, Tamborlane W, et al. Extended 6-Month Follow-Up of A Randomized Clinical Trial to Assess the Efficacy and Safety of Real-Time Continuous Glucose Monitoring in the Management of Type 1 Diabetes in Young Children Aged 4 to <10 Years. Diabetes Care. 2013;36:e63-e. doi: 10.2337/dc12-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsalikian E, Fox L, Weinzimer S, Buckingham B, White NH, Beck R, et al. Feasibility of prolonged continuous glucose monitoring in toddlers with type 1 diabetes. Pediatric Diabetes. 2012;13:301–7. doi: 10.1111/j.1399-5448.2011.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.