Abstract
The major metabolite of the estrogenic pesticide methoxychlor (MXC) HPTE is a stronger ESR1 agonist than MXC and acts also as an ESR2 antagonist. In granulosa cells (GCs), FSH stimulates estradiol via the second messenger cAMP. HPTE inhibits estradiol biosynthesis, and this effect is greater in FSH-treated GCs than in cAMP-treated GCs. Therefore; we examined the effect of MXC/HPTE on FSH-stimulated cAMP production in cultured GCs. To test involvement of ESR-signaling, we used the ESR1 and ESR2 antagonist ICI 182,780, ESR2 selective antagonist PHTPP, and ESR2 selective agonist DPN. ESR1 and ESR2 mRNA and protein levels were quantified. Both HPTE and MXC inhibited the FSH-induced cAMP production. ICI 182,780 and PHTPP mimicked the inhibitory action of HPTE. MXC/HPTE reduced FSH-stimulated Esr2 mRNA and protein to basal levels. MXC/HPTE also inhibited FSH-stimulated Esr1. The greater inhibition on FSH-stimulated GCs is likely due to reduced cAMP level that involves ESR-signaling, through ESR2.
Keywords: ovary, endocrine disruptors, estrogen receptors, follicle-stimulating hormone, cyclic AMP, methoxychlor, HPTE
1. Introduction
Methoxychlor (MXC), an organochlorine pesticide previously thought to be innocuous due to its low bioaccumulation in mammals [1], was subsequently shown to have unintended side effects on reproductive function in multiple animal species including mice, rats, and primates [2-4]. MXC is a weak estrogenic compound [5, 6]. The effect of MXC on reproduction is suspected to be a function of its more active metabolites, especially 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE), which acts as estrogen receptor α (ERα; ESR1) agonist and ERβ (ESR2) antagonist [5]. The specificity of receptor interaction may explain the observed tissue-specific effects of MXC in vivo. For example, MXC acts as an ESR1 agonist in the mouse uterus where ESR1 is the primary ESR subtype, inducing tissue growth [7]. In contrast, MXC acts in an inhibitory manner in the ovary [8, 9], an organ where ESR2 is the more dominant ESR subtype [10]. However, it is likely that actions through ESR1 are also involved in ovarian effects as it is also expressed in the ovary [11].
In the ovary, the granulosa cells directly interact with and support the oocyte to maturity throughout folliculogenesis and are the primary source of estradiol-17β (E2). Immature rat granulosa cell culture has been extensively used as an in vitro model to study ovarian biology [7]. Our lab has shown that HPTE inhibits FSH-induced E2 production in a dose-dependent manner in rat granulosa cells in vitro [12, 13]. We have also shown that HPTE reduces the expression of luteinizing hormone receptor (Lhcgr) and P450 cholesterol side-chain (Cyp11a1) in the granulosa cell [12, 13]. In adult female rats, fetal and neonatal exposure to MXC resulted in multiple reproductive dysfunctions such as early onset of puberty and of first estrus, irregular cyclicity, reduced litter size, and decreased number of corpora lutea in ovaries [9]. That study also demonstrated that ovarian markers such as ESR2 and LHCGR proteins were reduced. In addition, superovulation studies with exogenous gonadotropins showed a reduction in ovulation rates, suggesting that the effects of MXC were (at least in part) directly in the ovary.
ESR signaling is required for normal development and function of the ovary [14]. HPTE binds to ESRs [5] and disrupts the synthesis of E2 in granulosa cells [13]. The biosynthesis of E2 is primarily stimulated by FSH binding to its receptor and the activation of protein kinase A (PKA) through cAMP production [15, 16]. Recently, we observed that HPTE had a greater inhibitory effect on FSH-induced steroidogenesis and global gene expression than on cAMP analog dibutyryl cAMP (dbcAMP)-induced steroidogenesis and global gene expression in the granulosa cell [12, 13]. These observations suggest that cAMP production is a target of MXC and HPTE in the granulosa cell. It is known that ESR- and PKA-signaling pathways closely interact with each other in the uterus and breast [17, 18] as well as in the ovary [19, 20]. Interestingly, a recent report suggested that ESR2 also modulates cAMP production and Lhcgr expression in ovarian granulosa cells [21], leading to our hypothesis that regulation of granulosa cell cAMP by MXC/HPTE involves ESRs, especially ESR2.
The objective of this study was to examine the effects of HPTE and MXC on FSH-induced intracellular cAMP production in rat granulosa cells in vitro. In order to investigate potential involvement of ESR-signaling, especially ESR2, we examined the effects of ICI 182,780 (ICI), an ESR1 and ESR2 antagonist, and PHTPP, a selective ESR2 antagonist, and whether they mimicked the effects of HPTE and MXC on cAMP production.
2. Materials and Methods
2.1. Animals
Ovaries from 21- to 27-day-old Sprague-Dawley rats were used to prepare granulosa cell cultures as previously described [12]. Animals were maintained in accordance with the Rutgers University Institutional Animal Care and Use Committee guidelines. Animals were allowed ad libitum feed and water in a room with a 14:10 h light:dark cycle at a constant temperature (~22°C).
2.2. Chemicals
The chemical agents PHTPP (4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol), DPN (2,3-bis(4-hydroxyphenyl)-propionitrile), and ICI (ICI 182,780), all of which have > 99% purity, were purchased from Tocris Bioscience (Ellisville, MO). FSH (oFSH-20; 4453 IU/mg) was purchased from the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases (Dr. A. F. Parlow, Harbor-UCLA, Torrance, CA). HPTE was synthesized from MXC using a method similar to a previously reported method [22], and was confirmed to be > 99% pure by HPLC and thin-layer chromatography (TLC). TLC was conducted during the conversion reaction, post-reaction, during column separation and on a series of fractions collected to monitor the stage of the separation. Fractions were matched with expected Rf values and coupled with HPLC results; these tests support the high purity of HPTE.
2.3. Granulosa Cell Culture
Cells were plated at a density of 1 – 1.5 × 106 cells in 3 ml of medium per well in a 6-well plate for RNA, protein, and cAMP analysis or at a density of 3 – 4 × 105 in 1 ml of medium per well in a 24-well plate for cell viability and cAMP analysis. Cells were cultured in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12) containing 5% fetal bovine serum with 100 IU/ml of penicillin and 100 μg/ml of streptomycin. The incubator was set to an atmosphere of 5% CO2 in air at 37°C, and cultures were allowed to acclimate for at least 24 h. The culture medium was then aspirated and replaced with serum-free DMEM/F12 containing 0.1 μM androstenedione.
Cultures were treated with 10 μM of MXC or HPTE, or left untreated, for a 48-h period in the presence or absence of 3 ng/ml of FSH. Doses were selected based on previous work that showed efficacy (FSH) and no overt cytotoxicity (MXC and HPTE) in the cell culture [13]. In additional experiments, cells were cultured with ICI (5 μM), DPN (1 μM), or PHTPP (1 μM), using ethanol (0.1%) as the vehicle. These concentrations were chosen based on previously reported doses [23]. The cells were collected for intracellular cAMP, RNA, and protein isolation and to test cell viability. The medium was collected for E2 radioimmunoassay (RIA) analysis.
2.4. Cell Viability Assay
To measure the effect of the treatments on cell viability, a colorimetric assay based on the cleavage of the tetrazolium salt WST-1 (WST-1 assay; Roche Diagnostics GmbH, Mannheim, Germany) by mitochondrial dehydrogenase was used as prescribed by the manufacturer’s directions [24]. The cultures were incubated at 37°C for 3 h following the 48-h treatment period. Absorbance was measured at 450 nm after a 1-min shaking period.
2.5. Radioimmunoassay
Estradiol-17β was measured using a COAT-A-COUNT assay kit (Siemens Healthcare Diagnostics, Deerfield, IL) according to the manufacturer’s instructions. The assay sensitivity was 8 pg/ml and intra- and inter-assay coefficients of variance were 5.5 and 6.4%, respectively.
2.6. cAMP Measurement
To measure intracellular cAMP levels, granulosa cells were lysed and assayed according to the manufacturer’s instructions for the cAMP Biotrak Enzymeimmunoassay (EIA) system (RPN225 Protocol 3, GE Healthcare Life Sciences, Piscataway, NJ). Briefly, samples were treated with lysis buffer and incubated for 10 min with gentle shaking. Samples were then transferred into a donkey anti-rabbit IgG coated 96-well plate and incubated for 2 h in the presence of rabbit anti-cAMP antiserum. Next, cAMP-horseradish peroxidase conjugate was added for 1-h treatment followed by decanting and 4 consecutive washes. The enzyme substrate 3,3′,4,4′-tetramethylbenzidine / 1% hydrogen peroxide (TMB) was used to initiate color change in the wells. Sulfuric acid was added after a 30-min incubation with TMB to stop the reaction, and absorbance was then measured at a wavelength of 450 nm.
Concentrations of cAMP (fmol/well) were calculated from a standard curve prepared by plotting percent bound as a function of the log of cAMP values. In order to compare cAMP concentrations that are obtained from 6-well and 24-well plates (larger vs. smaller surface area), the values were normalized to protein concentration (mg/ml). The cAMP values for five treatment groups (basal, FSH, FSH+HPTE, ICI, and ICI+HPTE) were compared between results from 6-well and 24-well plates, and no significant difference was found (data not shown).
2.7. RNA Isolation and Real-Time Quantitative PCR (qPCR)
RNA isolation was performed according to the guidelines for TRIzol Reagent (Invitrogen, Carlsbad, CA). RNA purity and concentration were measured by spectrophotometry. Integrity of RNA was confirmed using electrophoresis (data not shown).
High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) was used to synthesize cDNA from 2 μg of RNA for qPCR. All transcript levels were quantitated by qPCR using Power Sybr Green PCR Master Mix (Applied Biosystems, Foster City, CA) and a StepOnePlus™ Real-Time PCR system (Applied Biosystems). Primers for Esr1, Esr2, and hypoxanthine-guanine phosphoribosyltransferase (Hprt) were designed using Primer-Blast (NCBI, Bethesda, MD) (Supplementary Table 1). The housekeeping gene Hprt was used to normalize the mRNA level for each treatment group.
All qPCR quality-control checks were done in accordance with the Minimum Information for Publication of Real-Time Quantitative PCR Experiments [25]. Briefly, analysis of the slope of the calibration curves indicated efficient amplification (> 90%). Dissociation curves showed no evidence of primer-dimer formation. One-way ANOVA confirmed that no treatment effect was observed on Hprt (data not shown). Controls including no RT product and no template control were used to ensure specificity of amplification.
2.8. Protein Extraction and Western Blot Analysis
Granulosa cells in culture wells were lysed with cOmplete Lysis-M kit (Roche Applied Science, Manheim, Germany). Protein concentrations in the cell lysates were measured using DC Protein Assay Kit II (Bio-Rad Life Sciences, Hercules, CA). Protein (20 μg) mixed with sample-loading buffer was heated at 70°C for 10 min. Proteins were run on 12% Bis-Tris-HCl-buffered denaturing polyacrylamide gels (Nu-Page, Invitrogen, Carlsbad, CA) under reducing conditions and transferred to nitrocellulose membranes. Ponceau S stain (Sigma) was used to verify the even and complete transfer of proteins onto the membrane. ESR1 (mouse anti-human ESR1; Thermoscience, Fremont, CA; Cat#MS-1071-S) and ESR2 (rabbit anti-rat ESR2; Pierce Antibodies, Rockford, IL; Cat#PA1310B) antibodies and goat anti-mouse HRP-conjugated IgG (Santa Cruz Biotechnology, Santa Cruz, CA; Cat#sc-2302) or goat anti-rabbit HRP-conjugated IgG (H+L) (Invitrogen, Cat#65-6120) were prepared (1:2,000) in 10% powdered milk and Tris-Buffered Saline Tween 20 (TBST) buffer and incubated overnight for primary antibodies, and 1 h for secondary antibodies. Blots were washed with TBST before and after each incubation period. Visualization of the target proteins was performed using an electrochemiluminescence detection system (Perkin-Elmer, Waltham, MA; Cat#NEL100001EA) with β-actin (ACTB) used to monitor consistent protein loading. ImageJ (NIH, http://rsb.info.nih.gov/ij/) was used to quantify band intensity.
2.9. Statistical Analysis
Protein, EIA, RIA, and qPCR data were analyzed statistically by one-way ANOVA (Prism 4.0 GraphPad, San Diego, CA) followed by Dunnet’s or Newman-Keuls multiple comparison tests. Values were presented as mean ± standard error of the mean (SEM). Calculations of correlation coefficient between cAMP production and ESR gene expression were performed using the Pearson’s Correlation test in Microsoft Excel with p-values calculated using an online p-Value Calculator for Correlation Coefficients (DanielSoper.com on December 16, 2013). Levels of significance were determined with α set at p < 0.05.
3. Results
3.1. MXC and HPTE inhibited FSH-stimulated cAMP production
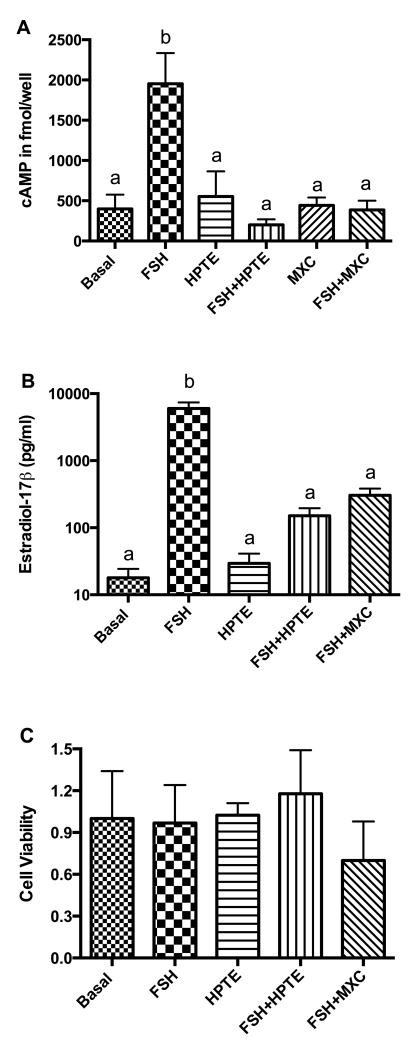
Treatment of granulosa cells with FSH for 48 h stimulated intracellular cAMP (fmol/well) approximately 5-fold as compared to unstimulated (basal) granulosa cells (1953 ± 381.1 vs 399±175.7; p < 0.01). In the presence of FSH, HPTE (201.9 ± 69.16) and its parent compound, MXC (387.1 ± 114.4), inhibited production of cAMP (p < 0.01; Fig. 1A). HPTE treatment did not have any effect on basal cAMP levels (Fig. 1A) and neither did MXC.
Figure 1. Effect of MXC and HPTE on FSH-stimulated intracellular cAMP and estradiol-17β (E2) production and cell viability.
Granulosa cells were incubated in the absence (basal) or presence of FSH (3ng/ml), HPTE (10 μM), a combination of HPTE and FSH, or MXC (10 μM) alone or in combination with FSH for 48 h. Media were collected for E2 radioimmunoassay, and cells were harvested for intracellular cAMP determination or cell viability analysis; these techniques were performed as described in sections 2.4 through 2.6. (A) cAMP was expressed as mean fmol/well ± SEM (n ≥ 3). (B) E2 was expressed as pg/ml ± SEM of culture medium (n = 5). (C) Cell viability measured with the WST-1 assay showed no difference between control and treatments (n = 3). b = significantly different (p < 0.01) from control as determined by one-way ANOVA followed by Dunnett’s multiple comparison post-hoc analysis.
The effect on E2 secretion (pg/ml) was consistent with cAMP results. FSH stimulated E2 over 300-fold (p < 0.01). In combination with FSH, HPTE or MXC reduced E2 to its basal levels (Fig. 1B). Treatment with HPTE alone did not affect basal E2 production. In order to test if HPTE and MXC treatments were affecting viability, the numbers of cells were estimated using the WST-1 assay. The results showed no statistically significant differences between treatment groups and controls (Fig. 1C). Albeit not statistically significant, the MXC reduced the cell viability in the presence of FSH, which can be attributed to the growth-inhibitory or cytotoxic effects of MXC [26].
3.2. Effects of the ESR2 antagonists and agonist on FSH-induced cAMP
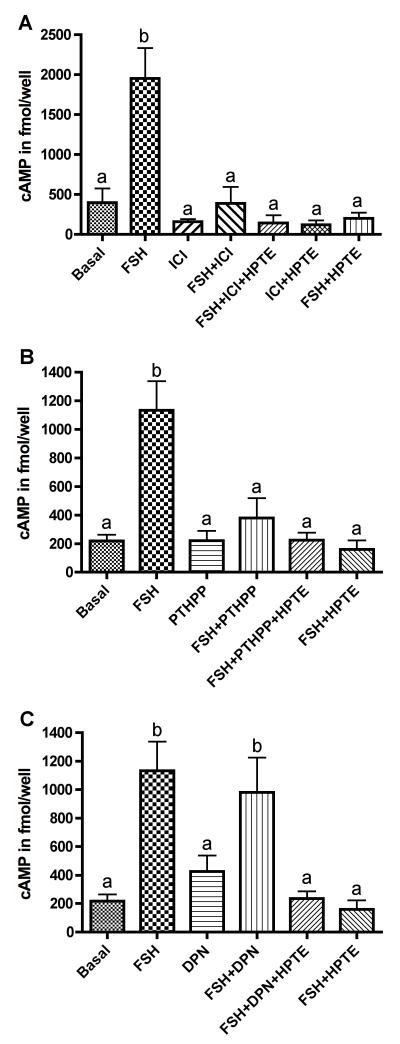
Since the ESR- and PKA-signaling systems interact with each other in various cell types [17, 18], including granulosa cells [20], we tested whether ESR antagonism would lead to inhibition of cAMP production. Granulosa cells were exposed to ICI, an ESR1 and ESR2 antagonist, in the presence or absence of FSH. We choose to use only HPTE in these assays because it has been shown to be as effective as MXC, if not more so (see Fig. 1). FSH-stimulated cAMP production was inhibited by ICI (p < 0.01; Fig. 2A), at a level comparable to the inhibition by HPTE. In combination with HPTE, inhibitory effects of ICI on cAMP production were not different from that of ICI alone. These results suggest that ESR signaling is involved in regulating cAMP production in the granulosa cell.
Figure 2. Effect of ICI 182,780, PHTPP, or DPN on FSH-mediated cAMP production.
(A) Granulosa cells were treated with 5 μM ICI 182,780 (ICI) in the presence or absence of 3 ng/ml FSH. Treatment with ICI inhibited FSH-mediated cAMP production. Co-treatment of ICI with HPTE (10 μM), in the absence or presence of FSH, did not affect cAMP any more than either compound alone did. (B) Granulosa cells were treated with 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (1 μM, PHTPP) in the presence or absence of FSH. Treatment with PHTPP inhibited FSH-mediated cAMP production. (C) Granulosa cells were treated with 2,3-bis(4-hydroxyphenyl)-propionitrile (1 μM, DPN) in the presence or absence of FSH. DPN was co-administered with HPTE for 48 h in the presence of FSH and did not reverse the inhibitory effect of HPTE on cAMP production. Data was expressed as normalized mean fmol/well (n ≥ 4). Analysis of cAMP was determined by enzyme immunoassay and normalized to protein concentration as described in section 2.6. Different letters indicate significant difference between treatment groups were as determined by one-way ANOVA (p < 0.01) followed by Dunnett’s multiple comparison post-hoc analysis.
We tested the effects of an ESR2 antagonist, PHTPP, on cAMP production. PHTPP reduced FSH-mediated cAMP production by 80% (p < 0.01) while it had no effect on basal cAMP (Fig. 2B). In addition, PHTPP and HPTE co-treatment did not cause any significant inhibition in FSH-mediated cAMP production as compared to PHTPP treatment alone. We also tested if the use of an ESR2 agonist could restore cAMP production in the granulosa cell. We selected DPN based on its high affinity for ESR2 (EC50 = 0.85 nM) [27]. The presence of DPN did not significantly alter the inhibitory effect of HPTE on the FSH-induced cAMP level (Fig. 2C).
3.3. MXC and HPTE inhibit ESR2 mRNA and protein levels
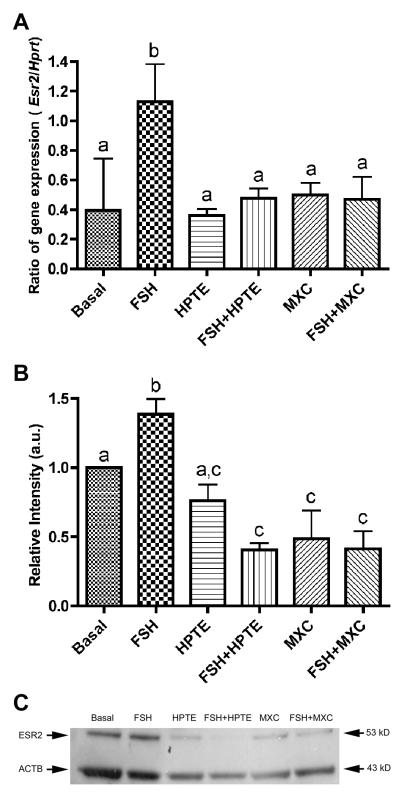
We then examined the effect of MXC and HPTE on Esr2 expression in granulosa cells. MXC and HPTE did not alter basal levels of Esr2 expression (Fig. 3A). FSH induced more than a 2-fold increase in Esr2 expression (p < 0.01), which was reversed by MXC and HPTE treatment to the basal level. We next examined the effect of MXC and HPTE on ESR2 protein levels using Western blotting, which confirmed the qPCR results. MXC and HPTE inhibited FSH-stimulated ESR2 protein levels (p < 0.01) and reduced ESR2 protein to 31% of FSH alone (Fig. 3B and C).
Figure 3. Effect of HPTE and MXC on estrogen receptor β (Esr2) mRNA and protein levels in granulosa cells.
(A) Esr2 mRNA was induced by 3 ng/ml FSH-treatment at least 2-fold over basal levels. HPTE (10 μM) or MXC (10 μM) inhibited FSH-induced Esr2 expression. Total RNA was extracted from granulosa cells and analyzed by qPCR for Esr2 mRNA expression as described in section 2.7. All expression values were normalized with Hprt as a reference gene (n = 4). (B) Western blots supported the qPCR result and showed that FSH stimulated the protein levels of ESR2, which were inhibited by HPTE and MXC. ImageJ (NIH) was used to quantify protein band intensity. β-actin (ACTB) was used to normalize ESR2 levels (n = 3) and samples were compared to basal. Different letter indicates significant difference between treatments groups as determined by one-way ANOVA (p < 0.01) followed by Newman-Keul multiple comparison post-hoc analysis. (C) A representative Western blot is shown.
3.4. MXC and HPTE reduce ESR1 mRNA levels
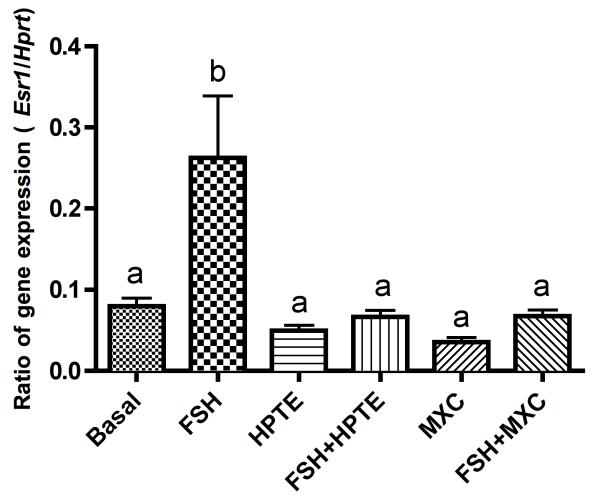
Similar to Esr2, expression of Esr1 mRNA was stimulated by FSH, which was suppressed by MXC and HPTE (p < 0.01; Fig. 4). Again, no changes due to MXC or HPTE were observed in Esr1 mRNA in the absence of FSH. The relative Esr1 expression was much lower than the expression of Esr2 (Fig. 3A). However, ESR1 protein was undetectable in granulosa cells because no visible band was present in the Western blot analysis (not shown). Because a dual-band (67 and 58 kDa) was detected when MCF-7 cells were used as a positive control (not shown), we believe that the lack of and ESR 1 band was due to the low expression levels of ESR1 in granulosa cells and not necessarily due to failure of the antibody to bind to ESR1 protein or failure of Western blot procedures.
Figure 4. HPTE and MXC reduce estrogen receptor α (Esr1) mRNA in granulosa cells.
Gene expression of Esr1 was induced by 3 ng/ml FSH alone 3- to 4-fold over basal. MXC (10 μM) or HPTE (10 μM) inhibited FSH-induced Esr1 expression. Total RNA was extracted from granulosa cells and analyzed by qPCR for Esr1 mRNA expression as described in section 2.8. All expression values were normalized with Hprt as a reference gene n = 4). Different letters indicate significant difference between treatment groups as determined by one-way ANOVA (p < 0.01) followed by Dunnett’s multiple comparison post-hoc analysis.
3.5. Correlation analysis of cAMP and estrogen receptor mRNA expression
Analysis of the relationship between cAMP, Esr1, and Esr2 was conducted to examine the correlation between gene expression of ESRs and FSH-induced cAMP production in granulosa cells. Esr1 showed a significant positive correlation with intracellular cAMP (r2 = 0.745 and p = 0.00025). The correlation of Esr2 with cAMP was less robust but significant (r2 = 0.513 and p = 0.025). In addition, a strong positive correlation between Esr1 and Esr2 mRNA levels (r2 = 0.835 and p = 0.000008) was observed.
4. Discussion
This study investigated the effect of MXC and its metabolite HPTE on FSH-mediated cAMP production in granulosa cells and the potential role of ESRs, especially ESR2, in this effect. Treatment with HPTE or MXC reduced cAMP production, which was mimicked by ESR antagonists. MXC/HPTE also affected Esr1 and Esr2 gene expression and ESR2 protein levels. Correlation analysis suggested an association between a reduction in cAMP production and Esr1 and Esr2 levels in HPTE/MXC-treated granulosa cells.
MXC and HPTE inhibited FSH-induced cAMP production in the granulosa cells. This result supports our previous observations that HPTE has a stronger inhibitory action towards FSH-induced steroid production in cultured rat granulosa cells as compared to that induced by cAMP [12, 13]. In contrast to results presented here, others have shown no effect of MXC on cAMP production in primary porcine granulosa cell culture [28]. This difference may be due to differences in experimental conditions, such as the combined use of IBMX and E2 in the study by Chedrese and Feyles. An additional reason could be due to species-specific effects of MXC or HPTE. Inhibitory effects of other EDCs, such as phthalate esters, on FSH-induced intracellular cAMP levels in reproductive tissues, including rat granulosa cells, have previously been shown [29], and species-specific effects of EDCs have been suggested [30].
The results showing inhibitory effects of MXC and HPTE on FSH-induced production of cAMP suggest that one potential site of inhibition on FSH-induced E2 production in the granulosa cell is upstream of cAMP generation as previously proposed [13]. Using a mouse antral follicle culture system, others have reported that MXC reduces E2 levels and inhibits expression of genes encoding steroidogenic enzymes and reduces expression of genes encoding Cyp1b1, an estrogenmetabolizing enzyme [31]. These results do not exclude effects on cAMP production, which was not measured in previous studies. In addition, FSH-induced steroidogenic enzymes and ovarian differentiation markers, Cyp11a1 and Lhcgr, were down-regulated by HPTE in cultured rat granulosa cells [12], suggesting that granulosa cell differentiation is inhibited. This in vitro effect on Cyp11a1 and Lhcgr was mirrored in the protein levels of CYP11A1 and LHCGR from MXC-treated ovaries in vivo [9]. Thus, these results suggest that the inhibitory actions of MXC on granulosa differentiation are mediated, at least in part, by a reduction in intracellular cAMP levels.
The inhibitory actions of MXC and HPTE on cAMP production in granulosa cells are likely to involve ESR signaling, especially ESR2. The ESR1 and ESR2 antagonist ICI and selective ESR2-antagonist PHTPP mimicked the inhibitory actions of MXC and HPTE on FSH-induced cAMP production. In addition, HPTE and MXC down-regulated Esr2 mRNA and protein and Esr1 mRNA levels in granulosa cells. Moreover, correlation analyses showed that significant associations exist between reductions in cAMP and Esr1 and Esr2 mRNA levels. A close interaction between ESR- and cAMP-signaling pathways has previously been shown in various cell types, including breast and uterine cells [18, 32]. Synergistic actions of E2 and cAMP on ovarian follicular maturation in vivo [19] and on granulosa cell differentiation in vitro [20] have been documented. More direct evidence for the synergism between ESR-2 and cAMP-signaling pathways has recently been obtained from a mouse strain that lacks functional Esr2 (Esr2−/−) [21]. Granulosa cells from Esr2−/− mice fail to fully differentiate [33], which is at least in part due to their inability to produce adequate levels of cAMP in response to FSH [21]. We have observed that female rats treated with MXC during development have reduced levels of ovarian ESR2 protein along with impaired follicular maturation and ovulation in adulthood [9, 34]. Taken together, these results suggest that some of the inhibitory actions of MXC and HPTE in granulosa cells involve signaling through ESR2.
Surprisingly, co-treatment of HPTE with DPN did not reverse the inhibitory effect of HPTE on cAMP production even though DPN has a greater affinity for ESR2 than HPTE or MXC has [27]. The down-regulation of ESR2 protein in response to incubation with MXC and HPTE (shown by Western blotting) may explain the lack of effect of DPN on cAMP production since a reduction in the binding sites for DPN would reduce its activity. Inhibitory effects of MXC on ESR2 protein were shown in the medial preoptic area of the hypothalamus in ewes [35] and, as mentioned above, in rat ovaries [9, 34]. Alternatively, the agonistic activity of DPN may be altered, similar to the case for another ‘selective estrogen receptor modulator’, trans-hydroxytamoxifen (THT), as previously reported by Fujimoto and Katzenellenbogen [36]. In their study, when cAMP levels were elevated in MCF-7 breast cancer cells, the antagonistic action of THT shifted towards an agonistic direction. In a similar but opposite manner to THT, in this study DPN’s agonistic action may be lost or shifted toward an antagonistic direction when cAMP levels are lower as shown in the granulosa cells following HPTE treatment.
The inhibitory actions of MXC and HPTE in the granulosa cell may also involve ESR1. MXC and HPTE inhibited Esr1 mRNA levels. Additionally, the inhibition of Esr1 exhibited a higher association with cAMP production than Esr2. ESR1 is primarily localized in the theca and interstitial cells but has been shown to be localized in rat granulosa cells, albeit less abundantly [23]. Previous studies by Flaws and colleagues using a mouse strain that overexpresses ESR1 showed an increased sensitivity to MXC and its metabolites, suggesting that ESR1 is involved in the inhibitory actions of MXC in the ovary [11, 37, 38]. However, these results do not rule out the involvement of ESR2. One way to obtain a definitive answer for the involvement of ESR2 is to use GC cells from Esr2-null mouse strains, which has not been reported so far.
Our results show that estrogen receptor expression decreases along with cAMP and E2 production in granulosa cells when challenged by MXC or HPTE. In addition, MXC stimulates the gene encoding CYP1B1, an enzyme that metabolizes E2. Based on the results from this and previous studies, we can suggest that MXC and HPTE can affect PKA- and ESR-signaling pathways at multiple points (Fig. 5). MXC and HPTE reduce E2 levels by inhibition of its biosynthesis through down-regulation of steroidogenic enzymes (e.g., CYP19A1), which can result from down-regulation of cAMP levels. MXC and/or HPTE can also reduce E2 levels through up-regulation of the E2-metabolizing enzyme CYP1B1 (Supplementary Figure 1). In addition, MXC and HPTE can also cause down-regulation of ESR and lead to ESR antagonism.
Figure 5. Working hypothesis for the effect of MXC/HPTE on estrogen receptor (ESR) signaling in granulosa cells in vitro.
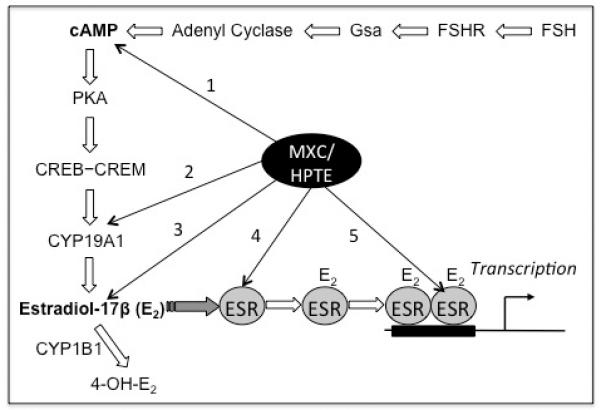
Multiple ESR-signaling-related processes are affected by MXC or HPTE, which include (1) production of cAMP, (2) expression of steroidogenic enzymes CYP11A1 (not depicted in the figure) and CYP19A1, (3) biosynthesis and metabolism of estradiol-17β (E2), (4) ESR expression and (5) actions of ESR. These effects suggest that ESR- and cAMP-signaling pathways are targets of MXC/HPTE (see Discussion for further information).
5. Conclusions
These results suggest that cAMP- and ESR-signaling pathways are affected by MXC and HPTE. Although these results do not show causation, they do suggest a strong association between down-regulation of cAMP production and inhibition of the ESR-signaling pathway. Coupled with previously reported inhibitory effects of MXC/HPTE on E2 production and steroidogenic enzymes (e.g., Cyp19a1) and Lhcgr, these results suggest an involvement of ESR2-mediated mechanisms in the inhibitory effects of MXC on FSH-mediated cAMP production in granulosa cells and shed light on MXC-induced ovarian dysfunction.
Supplementary Material
Highlights.
Methoxcychlor (MXC) and HPTE inhibit FSH-induced cAMP levels in granulosa cells.
ESR antagonists mimic the inhibitory actions of MXC and HPTE.
MXC and HPTE inhibit FSH-induced expression of ESR1 and ESR2 in granulosa cells.
The inhibitory actions of MXC and HPTE may involve ESR-regulated pathways.
Acknowledgements
Special thanks to Dr. Theodore van Es for his generous support in the synthesis of HPTE and Dr. Margaret James for her kind supply of technical support and standards. Additionally, we would like to thank Dr. Jason Richardson for the use of laboratory space and equipment, Dr. Kathy Manger for editing and Dr. Aparna Zama for the critical reading of the manuscript. This work was supported in part by National Institute of Environmental Health Sciences (NIEHS) grants [ES013854, ES017059, and ES017847 to M.U.]; Bristol Myers-Squibb Fellowship [C.N.H.]; and NIEHS Center grant [ES005022].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- 1.Kapoor IP, et al. Comparative metabolism of methoxychlor, methiochlor, and DDT in mouse, insects, and in a model ecosystem. J Agric Food Chem. 1970;18(6):1145–52. doi: 10.1021/jf60172a017. [DOI] [PubMed] [Google Scholar]
- 2.Hall DL, et al. Effect of methoxychlor on implantation and embryo development in the mouse. Reprod Toxicol. 1997;11(5):703–8. doi: 10.1016/s0890-6238(97)00026-9. [DOI] [PubMed] [Google Scholar]
- 3.Gray LE, Jr., et al. A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fundam Appl Toxicol. 1989;12(1):92–108. doi: 10.1016/0272-0590(89)90065-1. [DOI] [PubMed] [Google Scholar]
- 4.Golub MS, et al. Effects of exogenous estrogenic agents on pubertal growth and reproductive system maturation in female rhesus monkeys. Toxicol Sci. 2003;74(1):103–13. doi: 10.1093/toxsci/kfg090. [DOI] [PubMed] [Google Scholar]
- 5.Gaido KW, et al. Differential interaction of the methoxychlor metabolite 2,2-bis-(phydroxyphenyl)-1,1,1-trichloroethane with estrogen receptors alpha and beta. Endocrinology. 1999;140(12):5746–53. doi: 10.1210/endo.140.12.7191. [DOI] [PubMed] [Google Scholar]
- 6.Schlenk D, et al. Biotransformation and estrogenic activity of Methoxychlor and its metabolites in channel catfish (Ictalurus punctatus) Marine Environmental Research. 1998;46(1-5):159–162. [Google Scholar]
- 7.Tiemann U. In vivo and in vitro effects of the organochlorine pesticides DDT, TCPM, methoxychlor, and lindane on the female reproductive tract of mammals: a review. Reprod Toxicol. 2008;25(3):316–26. doi: 10.1016/j.reprotox.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RK, et al. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci. 2006;93(2):382–9. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- 9.Armenti AE, et al. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol. 2008;233(2):286–96. doi: 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond A, Fuller P. The importance of ER{beta} signalling in ovarian function. J Endocrinol. 2010;205(1):15–23. doi: 10.1677/JOE-09-0379. [DOI] [PubMed] [Google Scholar]
- 11.Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol Sci. 2006;93(1):180–8. doi: 10.1093/toxsci/kfl034. [DOI] [PubMed] [Google Scholar]
- 12.Harvey CN, et al. Effect of the methoxychlor metabolite HPTE on the rat ovarian granulosa cell transcriptome in vitro. Toxicol Sci. 2009;110(1):95–106. doi: 10.1093/toxsci/kfp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zachow R, Uzumcu M. The methoxychlor metabolite, 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane, inhibits steroidogenesis in rat ovarian granulosa cells in vitro. Reprod Toxicol. 2006;22(4):659–65. doi: 10.1016/j.reprotox.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Britt KL, Findlay JK. Regulation of the phenotype of ovarian somatic cells by estrogen. Mol Cell Endocrinol. 2003;202(1-2):11–7. doi: 10.1016/s0303-7207(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 15.Hunzicker-Dunn M, Maizels ET. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal. 2006;18(9):1351–9. doi: 10.1016/j.cellsig.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma SC, et al. Expression and function of estrogen receptor subtypes in granulosa cells: regulation by estradiol and forskolin. Endocrinology. 1999;140(9):4320–34. doi: 10.1210/endo.140.9.6965. [DOI] [PubMed] [Google Scholar]
- 17.Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci U S A. 1994;91(18):8517–21. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aronica SM, Katzenellenbogen BS. Progesterone receptor regulation in uterine cells: stimulation by estrogen, cyclic adenosine 3′,5′-monophosphate, and insulin-like growth factor I and suppression by antiestrogens and protein kinase inhibitors. Endocrinology. 1991;128(4):2045–52. doi: 10.1210/endo-128-4-2045. [DOI] [PubMed] [Google Scholar]
- 19.Richards JS, et al. Ovarian follicular development in the rat: hormone receptor regulation by estradiol, follicle stimulating hormone and luteinizing hormone. Endocrinology. 1976;99(6):1562–70. doi: 10.1210/endo-99-6-1562. [DOI] [PubMed] [Google Scholar]
- 20.Welsh TH, Jr., Zhuang LZ, Hsueh AJ. Estrogen augmentation of gonadotropin-stimulated progestin biosynthesis in cultured rat granulosa cells. Endocrinology. 1983;112(6):1916–24. doi: 10.1210/endo-112-6-1916. [DOI] [PubMed] [Google Scholar]
- 21.Deroo BJ, et al. Estrogen receptor beta is required for optimal cAMP production in mouse granulosa cells. Mol Endocrinol. 2009;23(7):955–965. doi: 10.1210/me.2008-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuchal LD, et al. Demethylation of the pesticide methoxychlor in liver and intestine from untreated, methoxychlor-treated, and 3-methylcholanthrene-treated channel catfish (Ictalurus punctatus): evidence for roles of CYP1 and CYP3A family isozymes. Drug Metab Dispos. 2006;34(6):932–8. doi: 10.1124/dmd.105.009068. [DOI] [PubMed] [Google Scholar]
- 23.Chen YJ, et al. Crucial role of estrogen receptor-alpha interaction with transcription coregulators in follicle-stimulating hormone and transforming growth factor beta1 up-regulation of steroidogenesis in rat ovarian granulosa cells. Endocrinology. 2008;149(9):4658–68. doi: 10.1210/en.2008-0063. [DOI] [PubMed] [Google Scholar]
- 24.Peskin AV, Winterbourn CC. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1) Clin Chim Acta. 2000;293(1-2):157–66. doi: 10.1016/s0009-8981(99)00246-6. [DOI] [PubMed] [Google Scholar]
- 25.Bustin SA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–22. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 26.Tiemann U, Pohland R, Schneider F. Influence of organochlorine pesticides on physiological potency of cultured granulosa cells from bovine preovulatory follicles. Theriogenology. 1996;46(2):253–65. doi: 10.1016/0093-691x(96)00182-3. [DOI] [PubMed] [Google Scholar]
- 27.Meyers MJ, et al. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44(24):4230–51. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 28.Chedrese PJ, Feyles F. The diverse mechanism of action of dichlorodiphenyldichloroethylene (DDE) and methoxychlor in ovarian cells in vitro. Reprod Toxicol. 2001;15(6):693–8. doi: 10.1016/s0890-6238(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 29.Treinen KA, Dodson WC, Heindel JJ. Inhibition of FSH-stimulated cAMP accumulation and progesterone production by mono(2-ethylhexyl) phthalate in rat granulosa cell cultures. Toxicol Appl Pharmacol. 1990;106(2):334–40. doi: 10.1016/0041-008x(90)90252-p. [DOI] [PubMed] [Google Scholar]
- 30.Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect. 2003;111(2):139–45. doi: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basavarajappa MS, et al. Methoxychlor reduces estradiol levels by altering steroidogenesis and metabolism in mouse antral follicles in vitro. Toxicol Appl Pharmacol. 2011;253(3):161–9. doi: 10.1016/j.taap.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.el-Tanani MK, Green CD. Interaction between estradiol and cAMP in the regulation of specific gene expression. Mol Cell Endocrinol. 1996;124(1-2):71–7. doi: 10.1016/s0303-7207(96)03930-5. [DOI] [PubMed] [Google Scholar]
- 33.Couse JF, et al. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology. 2005;146(8):3247–62. doi: 10.1210/en.2005-0213. [DOI] [PubMed] [Google Scholar]
- 34.Zama AM, Uzumcu M. Fetal and neonatal exposure to the endocrine disruptor methoxychlor causes epigenetic alterations in adult ovarian genes. Endocrinology. 2009;150(10):4681–91. doi: 10.1210/en.2009-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahoney MM, Padmanabhan V. Developmental programming: impact of fetal exposure to endocrine-disrupting chemicals on gonadotropin-releasing hormone and estrogen receptor mRNA in sheep hypothalamus. Toxicol Appl Pharmacol. 2010;247(2):98–104. doi: 10.1016/j.taap.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujimoto N, Katzenellenbogen BS. Alteration in the agonist/antagonist balance of antiestrogens by activation of protein kinase A signaling pathways in breast cancer cells: antiestrogen selectivity and promoter dependence. Mol Endocrinol. 1994;8(3):296–304. doi: 10.1210/mend.8.3.7517003. [DOI] [PubMed] [Google Scholar]
- 37.Paulose T, et al. Increased sensitivity of estrogen receptor alpha overexpressing antral follicles to methoxychlor and its metabolites. Toxicol Sci. 2011;120(2):447–59. doi: 10.1093/toxsci/kfr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulose T, et al. Estrogen receptor alpha overexpressing mouse antral follicles are sensitive to atresia induced by methoxychlor and its metabolites. Reprod Toxicol. 2012;33(3):353–60. doi: 10.1016/j.reprotox.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.