Abstract
Purpose
To examine predictors of ammonia exposure and hyperammonemic crises (HAC) in patients with urea cycle disorders (UCDs).
Methods
The relationships between fasting ammonia, daily ammonia exposure, and HACs were analyzed in >100 UCD patients.
Results
Fasting ammonia correlated strongly with daily ammonia exposure (r=0.764, p<0.001). For patients with fasting ammonia levels <0.5 ULN, 0.5 to <1.0 ULN, and ≥1.0 ULN, the probability of a normal average daily ammonia value was 87%, 60%, and 39%, respectively, and 10.3%, 14.1%, and 37.0% of these patients experienced ≥1 HAC over 12 months. Time to first HAC was shorter (p=0.008) and relative risk (4.5×; p=0.011) and rate (~5×, p=0.006) of HACs higher in patients with fasting ammonia ≥1.0 ULN vs. <0.5ULN; relative risk was even greater (20×; p=0.009) in patients ≥6 years. A 10 or 25 μmol/L increase in ammonia exposure increased the relative risk of a HAC by 50% and >200% (p<0.0001), respectively. The relationship between ammonia and HAC risk appeared independent of treatment, age, UCD subtype, dietary protein intake, or blood urea nitrogen. Fasting glutamine correlated weakly with AUC0-24 and was not a significant predictor of HACs.
Conclusions
Fasting ammonia correlates strongly and positively with daily ammonia exposure and with the risk and rate of HACs, suggesting that UCD patients may benefit from tight ammonia control.
Keywords: glycerol phenylbutyrate, hyperammonemia, RAVICTI, sodium phenylbutyrate
INTRODUCTION
Urea cycle disorders (UCDs) are inborn errors of metabolism involving deficiencies of enzymes required for ureagenesis. They are manifested by acute and chronic hyperammonemia and medical management is aimed at reducing ammonia levels through the restriction of protein intake and the use of alternate pathway drugs to enhance waste nitrogen excretion. Current UCD treatment guidelines indicate that ammonia should be kept within normal limits, but otherwise provide limited guidance on the specific timing of blood draws or target levels.1
Glycerol phenylbutyrate (GPB, HPN-100, RAVICTI®; Hyperion Therapeutics, Inc., Brisbane, CA) is a sodium- and sugar-free ammonia-lowering agent that was approved in the United States in 2013 for UCD patients ≥ 2 years of age who cannot be managed by dietary protein restriction and/or amino acid supplementation alone. The GPB clinical trials compared 24-hour ammonia levels during short-term steady-state dosing with GPB or sodium phenylbutyrate (NaPBA) among 80 UCD patients ages 2 months to over 70 years and studied longer-term outcomes, including hyperammonemic crises (HACs), among 100 UCD patients, including 49 pediatric patients, treated with GPB for up to 1 year.
The present report has taken advantage of over 1000 prospectively collected timed blood samples to analyze correlates of daily ammonia exposure, assessed as 24-hour area under the curve, as well as the utility of ammonia and glutamine as predictors of HACs.
METHODS
Clinical Studies
Data from four short-term switchover (SO) studies and three 12-month safety extension (SE) studies in adult and pediatric UCD patients were included in the analyses; all have been previously described. Protocols UP1204-003, HPN-100-005SO, and HPN-100-012SO were short-term, open-label, fixed sequence SO studies that evaluated ammonia control during equivalent dosing of GPB versus NaPBA.2-4 Study HPN-100-006 was a pivotal, randomized, double-blind, active-controlled, crossover study that established non-inferiority of GPB to NaPBA as assessed by venous ammonia.5 Most patients completing these protocols plus additional patients (a total of 100 of whom 49 were pediatric) were enrolled into safety extension studies and received GPB for 12 months.5-7
All protocols and informed consents were reviewed and approved by the Investigational Review Board of each participating institution prior to initiation. Eligible subjects had a confirmed or clinically suspected UCD and had been receiving NaPBA prior to enrollment. In all studies, patients received GPB three times daily (or more frequently in small children to match their prior NaPBA schedule) at a daily dose equivalent to their previously prescribed NaPBA dose.
Ammonia and Glutamine Measurements
During the crossover studies, serial venous blood samples for ammonia analyses were collected over 24 hours after the patient had received 1-2 weeks of dosing (steady state) with either NaPBA or GPB. Samples for glutamine and ammonia were collected after overnight [≥ 4-6 hours] fasting and prior to breakfast and the first daily drug dose; additional ammonia samples were collected at 8, 12, and 24 hours post-dose for pediatric patients; and approximately every 4 hours for adults. During the 12-month studies, blood samples for ammonia and glutamine analyses were collected monthly and information on HACs was recorded.
Baseline ammonia was defined as the screening or month 0 value when the patient was on NaPBA prior to receiving GPB. An HAC was defined as compatible clinical symptoms associated with one or more ammonia levels ≥ 100 μmol/L. Ammonia and HAC data were also collected retrospectively for up to 12 months prior to enrollment in each study while patients were receiving NaPBA. Ammonia and glutamine concentrations were measured by an accredited hospital laboratory at each study site and ammonia values were normalized to a standard range of 9 to 35 μmol/L.
Statistical Analyses
Short-term Studies
Fasting ammonia levels were determined from the pre-dose and 24-hour time points for steady-state measurements and maximum levels (Cmax) represented the highest levels determined during the 24 hour period. Ammonia 24-hour area under the concentration versus time curve from time 0 (pre-dose) to 24 hours (AUC0-24) was calculated using the linear trapezoid rule and average daily ammonia was calculated as AUC0-24 divided by time. The relationship between fasting ammonia and AUC0-24 was analyzed using logistic regression. Potential covariates examined included age, weight, drug type, and total daily dietary protein intake. Prediction intervals were calculated using standard least-squares methods and bootstrap methods.8 Bootstrap intervals are less dependent on the observed data than standard intervals, and therefore provide a more credible predication interval.
Fasting ammonia values were also divided into categories (< 0.5, 0.5 - < 1.0, and ≥ 1.0) relative to the upper limit of normal (ULN). The probability that a patient whose fasting ammonia falls within a given category would have normal total daily ammonia exposure was modeled using Generalized Estimating Equations (GEE) assuming an exchangeable correlation structure and a logit link function. Confidence limits around the outcome probabilities were computed using the bootstrap method.8
12-Month Studies
The time to first HAC was determined by Kaplan-Meier analysis9 for the entire population, by age group, dichotomous age group, gender and baseline ammonia ULN categories. Survival distributions between covariate values were computed using a log-rank test.10 Cox regression models were used to quantify the relative risk of time to first HAC.
The relationship between total ammonia exposure over time and the risk of HAC occurrence was explored by calculating time normalized area under the curve (TNAUC12months) for ammonia for patients with and without an HAC during the 12-month treatment with GPB. The AUC for ammonia, measured monthly, was calculated for each patient using the trapezoid rule over the course of the 12 -month study. TNAUCxmonth was calculated by dividing AUC by the final study month of an ammonia assessment.
For the cumulative rate of HAC over 12 months, a negative binomial regression was performed with a flexible scale parameter to account for overdispersion of the HAC count data11 with an offset to account for varying time in the study between patients. A multivariable negative binomial regression of HAC rate was also developed to adjust for potential confounders.
A linear mixed regression model was used for the analysis of admission, peak, and discharge ammonia levels by patient characteristics. Log-transformed values were used for peak and discharge ammonia levels values and the model controlled for clustering of outcomes for patients who had more than one HAC.12 A GEE logistic regression model was used to assess the association between the duration of HAC and baseline ammonia values.13 Spearman correlations were used to assess the relationship between baseline ammonia and glutamine levels.
RESULTS
The analyses included over 1000 ammonia results from 114 UCD patients, including 100 (51 adult, 49 pediatric) in the 12-month dosing protocols. Except for fasting ammonia, baseline characteristics (age, gender, UCD subtype) were similar across baseline ammonia ULN categories, although there was a slightly higher proportion of patients aged 6 to 11 years in the 0.5 to < 1.0 ULN group than in the other two groups and mean baseline glutamine values were slightly higher in the ≥ 1.0 ULN group than in the other ULN groups (Table 1).
Table 1.
Patient Characteristics In Relation to Baseline Ammonia Values
| Variable | Baseline Ammonia | Total N = 100 | |||
|---|---|---|---|---|---|
| < 0.5 ULN N = 39 | 0.5 - < 1.0 ULN N = 34 | ≥ 1.0 ULN N = 27 | |||
| Age (years): mean (SD) | 22.9 (17.7) | 17.4 (15.0) | 18.6 (12.8) | 19.6 (15.9) | |
| Age Group: N (%) | Adult: ≥ 18 | 22 (56.4) | 14 (41.2) | 15 (55.6) | 51 (51.0) |
| Pediatric: < 18 | 17 (43.6) | 20 (58.8) | 12 (44.4) | 49 (49.0) | |
| < 2 | 3 (7.7) | 2 (5.9) | 2 (7.4) | 7 (7.0) | |
| 2 - 5 | 7 (17.9) | 4 (11.8) | 5 (18.5) | 16 (16.0) | |
| 6 - 11 | 5 (12.8) | 10 (29.4) | 2 (7.4) | 17 (17.0) | |
| 12 - 17 | 2 (5.1) | 4 (11.8) | 3 (11.1) | 9 (9.0) | |
| Gender: N (%) | Male | 15 (38.5) | 9 (26.5) | 9 (33.3) | 33 (33.0) |
| Female | 24 (61.5) | 25 (73.5) | 18 (66.7) | 67 (67.0) | |
| Fasting Laboratory Values: mean (SD) | |||||
| Ammonia (μmol/L) | 11 (3.7) | 30.8 (7.7) | 51.9 (19.5) | 28.80 (22.43) | |
| Glutamine (μmol/L) | 694 (199.0) | 710 (183.3) | 857 (306.0) | 743.4 (238.23) | |
| BUN (mmol/L) | 3.4 (1.5) | 2.5 (1.0) | 2.5 (1.7) | 2.8 (1.4) | |
| UCD Subtype: n (%) | |||||
| ARG | 1 (2.6) | 0 (0.0) | 1 (3.7) | 2 (2.0) | |
| ASL | 7 (18.0) | 5 (14.7) | 1 (3.7) | 13 (13.0) | |
| ASS | 4 (10.3) | 3 (8.8) | 5 (18.5) | 12 (12.0) | |
| CPS | 1 (2.6) | 0 (0.0) | 0 (0.0) | 1 (1.0) | |
| HHH | 2 (5.1) | 1 (2.9) | 0 (0.0) | 3 (3.0) | |
| OTC | 24 (61.5) | 25 (73.5) | 20 (74.1) | 69 (69.0) | |
| 12-Month Incidence Rate of HAC | |||||
| Pre-enrollment on NaPB A | 0.433 | 0.389 | 1.071 | 0.581 | |
| During study on GPB | 0.135 | 0.150 | 0.711 | 0.288 | |
ARG: arginase; ASL arginosuccinate lyase; ASS: arginosuccinate synthase; BUN: blood urea nitrogen; CPS: carbonyl phosphate synthetase; HHH: hyperornithinemia–hyperammonemia–homocitrullinuria; OTC: ornithine transcarbamylase; UCD: urea cycle disorder; ULN: upper limit of normal; HAC: hyperammonemic crisis.
Short-Term Studies
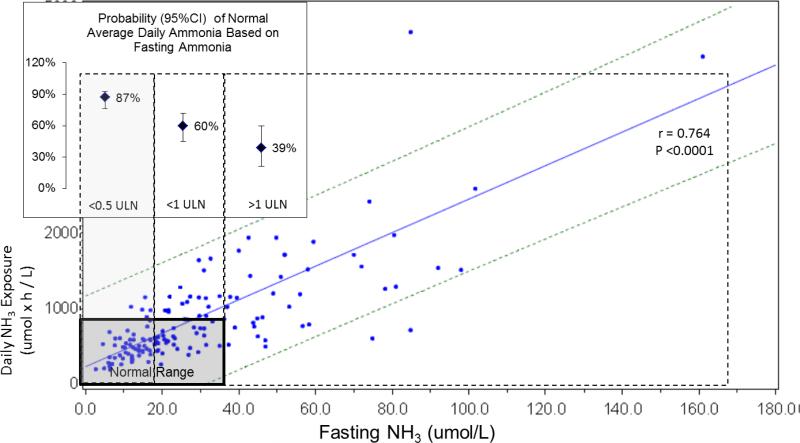
Blood ammonia levels exhibited considerable fluctuation over 24 hours; fasting ammonia values from morning blood draws exhibited the least variability (Figure S1). There was a strongly positive and statistically significant relationship between fasting ammonia and AUC0-24 (r = 0.764; p < 0.0001) (Figure 1). There was no discernable difference in the relationships observed with NaPBA and GPB treatment, so data for both drugs were combined. None of the potential covariates examined (e.g. age, weight, gender and dietary protein) had a clinically or statistically significant impact on the model and these covariates were not included in the final model.
Figure 1.
Correlation of fasting ammonia and daily ammonia exposure in short term crossover studies (r = 0.764; p < 0.0001; N = 80). Bolded box indicates normal range. Dashed lines represent 95% confidence intervals. Dotted boxes indicate fasting ammonia categories. Inset Figure: Probability of average daily ammonia being within normal limits by fasting ammonia ULN categories.
When AUC and Cmax outcomes were modeled using ordered ULN categories, patients with a fasting ammonia < 0.5 ULN had a greater likelihood of having a normal average daily ammonia value than patients with a fasting ammonia in other categories (insert in Figure 1).
12-Month Studies
Ammonia
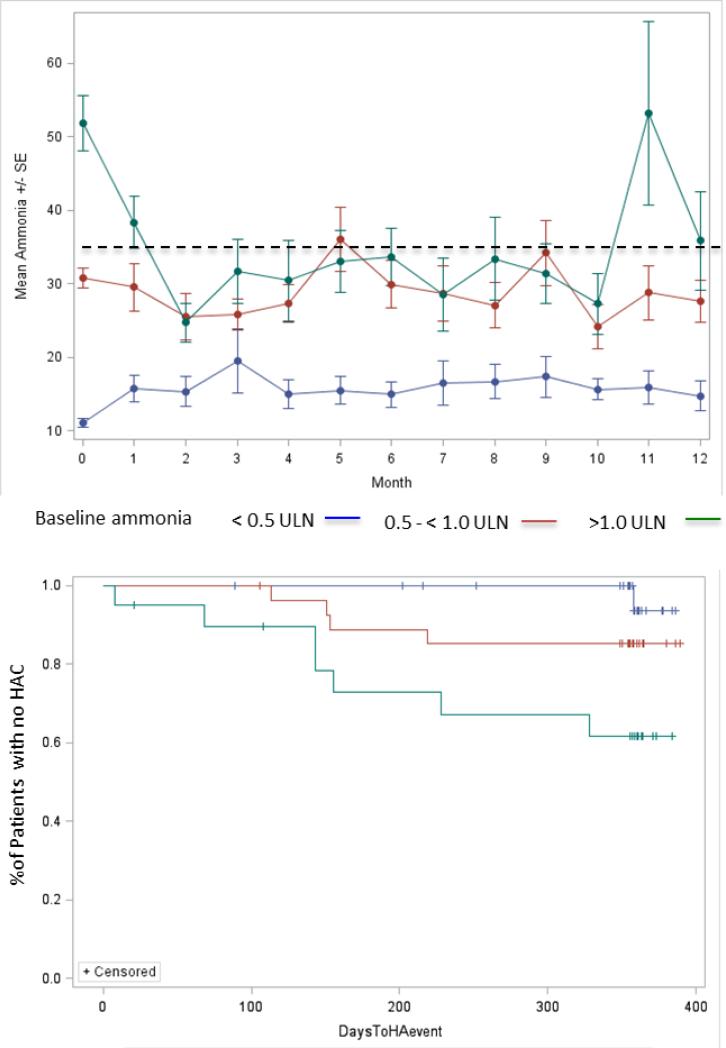
Ammonia levels during up to 12 months of dosing were strongly related to baseline levels as categorized in relation to the ULN. Patients whose baseline ammonia was < 0.5 ULN had mean monthly ammonia levels < 35 μmol/L throughout the study and their ammonia values showed less variability than did those among patients in the higher baseline ammonia categories (Figure 2, top panel). In the ammonia ≥ 1.0 ULN category, mean fasting ammonia decreased towards the normal range after approximately 3 months of GPB treatment (Figure 2, top panel).
Figure 2.
Ammonia and HAC rate. Top Panel: Mean (± SE) ammonia values during 12 months of treatment with GPB by baseline fasting ammonia ULN category. Dotted line indicates upper limit of ammonia normal range (35 μmol/L). Bottom panel: Time to first HAC by baseline ammonia ULN category for patients at least 6 years old at baseline (P = 0.0077 by log-rank test).
Thirty patients (30%) experienced a total of 54 HACs in the 12 months prior to starting GPB treatment and 19 patients (19%) experienced a total of 27 HACs during 12 months of GPB treatment (Table 1). The proportion of patients experiencing an HAC increased in relation to baseline ammonia ULN categories (p = 0.0236) and was slightly higher for pediatric than for adult patients. Gender and UCD subtype had no effect on the rate of HAC. Mean (SD) baseline fasting ammonia in patients with a HAC was 41.8 (23.7) μmol/L as compared to 25.7 (17.8) μmol/L in patients with no HAC (p = 0.0023).
The time to first HAC was significantly shorter (p = 0.0077) and the risk of HAC higher (4.5× p = 0.011) in patients whose baseline ammonia was ≥ 1.0 ULN as compared with patients whose baseline ammonia was < 0.5 ULN (Figure 2). After adjusting for age, gender, and race, patients with one or more baseline ammonia values ≥ 1.0 ULN had approximately 5.5x the risk of having at least one HAC than patients with baseline ammonia < 0.5 ULN (p = 0.005). When patients < 6 years old at baseline (who eat more frequently and for whom measurement of fasting ammonia is problematic) were excluded from the analysis, those with baseline ammonia values ≥ 1.0 ULN had an approximately 20x increase in risk of having an HAC than patients with baseline ammonia < 0.5 ULN (p = 0.009).
The median time in the study was 361 days. The overall rate of HACs was 0.288 per patient per year and the rate was significantly higher among patients with baseline ammonia ≥ 1.0 ULN than for those with baseline ammonia < 0.5 ULN (0.711 vs. 0.135/patient/year; p = 0.0057). Total ammonia exposure calculated as time normalized area under the curve during 12 months of GPB dosing (TNAUC12month) was significantly lower in patients who did not experience an HAC (23.6 vs 35.0 μmol/L; p=0.0006). The relative risk of experiencing an HAC increased by about 50% and 270%, respectively, for every 10 and 25 μmol/L increase in ammonia exposure (p<0.0001) (Figure S3).
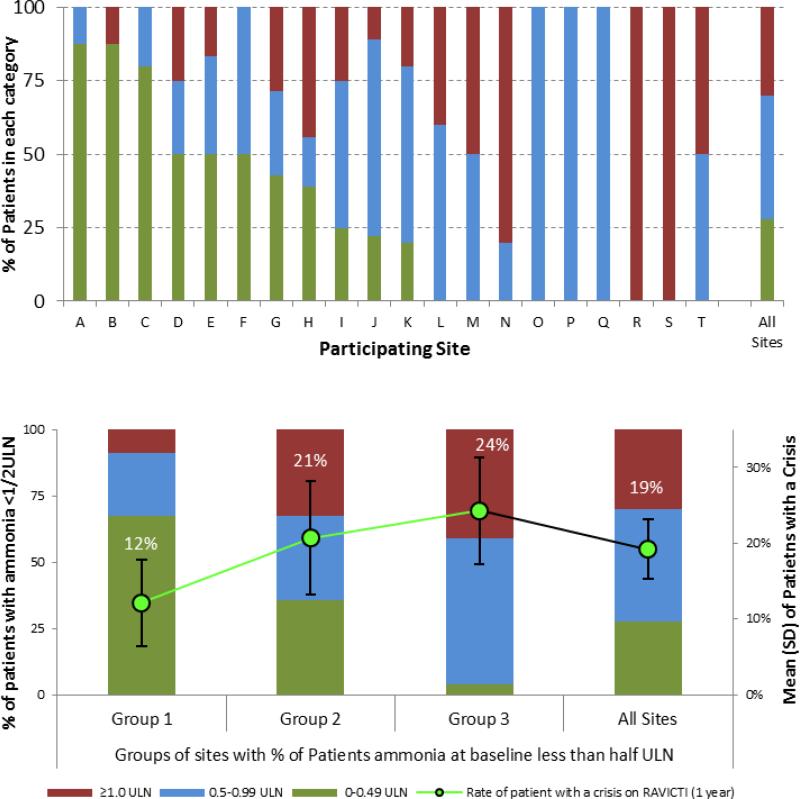
The proportion of patients with baseline ammonia levels within each ULN category varied considerably among study sites (Figure 3, top panel), which were therefore grouped into three categories based on the percentage of patients enrolled whose baseline fasting ammonia was < 0.5 of the ULN at baseline: Group 1 (6 study sites; 33 patients, of whom >50% had baseline ammonia levels < 0.5 ULN); Group 2 (3 study sites; 29 patients, of whom 25%-50% had baseline ammonia levels < 0.5 ULN); and Group 3 (11 sites; 38 patients, of whom < 25% had baseline ammonia < 0.5 ULN) (Figure 3, bottom panel and Table S1). The proportion of patients who experienced an HAC was lowest (12%) among Group 1 sites and increased progressively to 21% and 24% in Group 2 and 3 sites (Figure 3, bottom panel). Although the mean age of patients in group 3 was lower compared to group 1 (14.7 vs 24.5 yrs), other markers of disease severity including the proportion of females with OTC deficiency and restriction of dietary protein were similar among the groups.
Figure 3.
Ammonia levels and HAC rate by study site. Top Panel: Percentage of patients at each study site with baseline ammonia <0.5 ULN, 0.5 – 1 ULN, and ≥ 1.0 ULN. Bottom Panel: Percentage of patients in each ammonia ULN category by sites grouped in relation to ammonia levels among patients enrolled at the site. Group 1 (6 study sites; 33 patients, of whom >50% had baseline ammonia levels < 0.5 ULN); Group 2 (3 study sites; 29 patients, of whom 25%-50% had baseline ammonia levels < 0.5 ULN); and Group 3 (11 sites; 38 patients, of whom < 25% had baseline ammonia < 0.5 ULN)
During the 12 month GPB dosing protocols, HACs were generally of short duration; 48% resolved within 1 day and 64% in less than 2 days. Most HACs were triggered by intercurrent illness and infection and the most commonly reported clinical symptoms reported were vomiting (63%) and lethargy (37%) and (48%) (Table S2). Patients with baseline ammonia < 1.0 ULN tended to have a higher likelihood of experiencing an HAC of less than 2 days duration than patients with baseline ammonia ≥ 1.0 ULN (odds ratio 3.06), but this difference was not statistically significant.
Glutamine
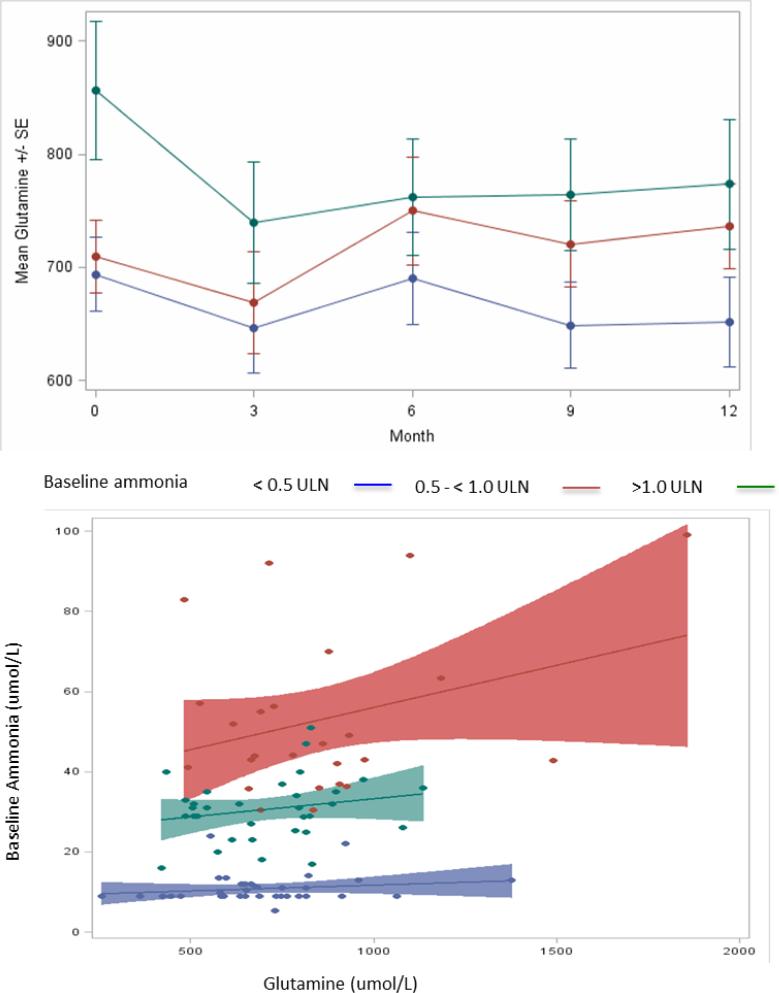
Monthly glutamine levels were generally higher in patients in the higher baseline ammonia ULN groups and, by three months of treatment, had decreased in the patients with the highest baseline glutamine at baseline (Figure 4, top panel). However, there was no statistically significant difference between glutamine in patients with or without an HAC assessed either at baseline (782.4 vs. 732.0 μmol/L; p = 0.150), during the long term follow up or as total glutamine exposure (TNAUC12month of 720.9 vs. 699.4 μmol/L*m; p=0.686).
Figure 4.
Relationship between glutamine and ammonia levels by baseline ammonia categories. Top panel: Glutamine levels during 12 months of GBP treatment; mean (± SE) monthly glutamine levels by baseline ammonia ULN category. Bottom panel: Scatterplot of baseline glutamine by ammonia ULN categories. The overall correlation between glutamine and ammonia was significant (r = 0.27; p = 0.008), whereas none of the correlations within ammonia ULN categories was significant (r=0.12; 0.18 and 0.12 respectively; p=NS for all)
There were statistically significant but comparatively weak correlations between baseline ammonia and glutamine levels at the time of enrollment (r = 0.27; p = 0.008) as well as between fasting glutamine levels and daily ammonia exposure on GPB or NaPBA during the switchover studies (r = 0.292, p<0.001). However, when the relationship between glutamine and ammonia categories with respect to the ULN was analyzed, glutamine values varied substantially for patients in each category and the overall correlation appeared to be driven by patients whose baseline ammonia exceeded 1.0x ULN (Figure 4). Patients with proximal deficiencies (OTC and CPS) showed a stronger correlation between glutamine and ammonia (r=0.56; p=0.020; Figure S2). There was no correlation between baseline ammonia and UCD subtype or levels of either citrulline (r=0.04; p=0.414) or argininosuccinic acid (r=0.14; p=0.158).
DISCUSSION
Twenty-four hour blood sampling in UCD patients demonstrated that blood ammonia levels fluctuated considerably over 24 hours, even among the comparatively stable and well-controlled UCD patients enrolled in these trials, making interpretation of random blood ammonia levels difficult. In order to identify the optimal timing of blood draws and a target level for ammonia in UCD patients, post-hoc statistical analyses of clinical trial data were conducted to assess the short-term relationship between fasting ammonia levels and daily ammonia exposure and the long-term relationship between fasting ammonia levels and HACs in patients with UCDs.
Analysis of pooled data from the short-term studies showed a strong positive relationship between fasting ammonia and ammonia AUC0-24. Because the wide prediction intervals observed with this analysis compromised the utility of individual levels for predicting daily ammonia exposure, the relationship between fasting ammonia levels categorized in relation to the ULN and the likelihood that a patient would have an average or maximal blood ammonia within normal limits was modeled using Generalized Estimating Equations. These analyses demonstrated that fasting ammonia categorized in relation to the ULN (< 0.5 ULN, 0.5 to < 1.0 ULN; and ≥ 1.0 ULN) was strongly predictive of the likelihood that maximal and mean daily ammonia values would be within normal limits. This finding indicates that patients with fasting ammonia outside the normal range are likely being exposed to high levels of ammonia during the day, even if they do not exhibit any identifiable symptoms.
In order to further assess the importance of these correlations, data on ammonia, glutamine, and HACs collected during the 12-month studies were further analyzed using the patient groups by baseline ammonia ULN category. The relationship between baseline ammonia ULN category and long-term outcome was demonstrated using several approaches. First, monthly ammonia levels continued to be lowest in patients with baseline ammonia levels < 0.5 ULN and highest in patients with baseline ammonia ≥ 1.0 ULN. Second, the proportion of patients experiencing an HAC increased significantly with increasing baseline ammonia ULN category. Third, the time to first HAC was significantly shorter and the risk of HAC was significantly greater in patients with baseline ammonia ≥ 1.0 ULN than in those with baseline ammonia < 0.5 ULN. This association between baseline ammonia and HAC risk was even more apparent when patients less than 6 years of age at baseline (who feed more frequently and for whom true fasting ammonia levels are difficult to collect) were excluded from the analysis. Finally, the cumulative rate of HAC was more than 5 times higher for patients with baseline ammonia ≥ 1.0 ULN than for patients with baseline ammonia < 0.5 ULN and was 20 times higher when patients less than 6 years of age were excluded.
Just as is true for the relationship between fasting ammonia and daily ammonia exposure, the relationship between fasting ammonia and HAC appears to be independent of treatment. While fewer crises were experienced by patients during treatment with GPB as compared with the prior year on NaPBA, the relationship between ammonia levels and the incidence of HACs was similar both prior to enrollment on NaPBA and subsequently during treatment with GPB.
The duration of HACs during GPB treatment was generally short, with more than 64% of crises lasting less than 2 days. Although ammonia values were generally higher in pediatric patients, there were no significant predictors for the admission, peak, or discharge ammonia levels during an HAC or for the duration of an HAC.
As compared with ammonia, glutamine appears a weaker predictor of HAC. While glutamine levels were higher at baseline in patients who experienced an HAC, there was no significant difference in glutamine between patients who did or did not experience an HAC during the study and no significant association between the rate of HAC and glutamine levels during the 12-months of dosing. Glutamine correlated modestly with fasting ammonia at baseline and decreased during GPB treatment in patients with the highest baseline glutamine levels. Of interest, the correlation between fasting ammonia and glutamine seemed to be primarily driven by patients with the highest ammonia values, a finding similar to the report by Maestri14 based on multiple samples in a single OTC patient. In addition, the relationship between ammonia and glutamine appeared strongest in patients with the proximal UCD subtypes, OTC and CPS (Figure S2), a finding which corroborates an earlier report by Wilson.15
Certain limitations of the current analyses deserve emphasis. First, while these timed ammonia and glutamine samples were collected prospectively, the analyses are post hoc. Second, the fact that lower fasting ammonia levels correlate strongly with lower average daily ammonia exposure as well as lower risk and frequency of HAC could reflect intrinsic differences in disease severity; i.e., patients with more severely deficient urea synthesis may exhibit both higher ammonia levels and increased susceptibility to crises with even minor triggering events. Two considerations suggest an alternative explanation. First, baseline characteristics including age, gender, and UCD subtype were similar across these ULN groups. Second, data pertaining to ammonia levels and the frequency of HAC among patients enrolled at 20 sites in North America, even allowing for possible differences in compliance among patients at different sites, appear better explained by different responses to management or differing management approaches rather than regional differences in severity of illness. In any case, the findings should be interpreted with caution pending further study and prospective validation.
The findings suggest that fasting ammonia is a reliable surrogate for assessing adequacy of disease control, as it correlates strongly with total daily ammonia exposure as well as HACs. They further suggest that even mildly elevated ammonia over long periods of time may put patients at risk of HAC and that UCD patients, especially those ages 6 years and above, may benefit from maintaining fasting ammonia levels less than 0.5 ULN. More frequent monitoring of fasting ammonia levels may require identifying certified clinical laboratories conveniently located for patients and/or further development of ‘portable testing’ technology analogous to that in use for diabetes.
Supplementary Material
Figure S1: Variability in blood ammonia over 24 hours during steady state dosing with NaPBA or GPB in UCD patients (n = 80; left panel) and in individual patients (right panel).
Figure S2: Relationship between glutamine and ammonia levels by UCD subtype. Strong correlation between ammonia and glutamine with proximal defects (OTC, CPS) and not distal defects (ASL, ASS).
Figure S3: Relative risk (RR) of HAC per incremental increase in ammonia exposure. Time normalized ammonia area under the curve (TNAUC) over 12-months correlated directly with the risk of experiencing an HAC at any time during that period. A RR of 1.50 indicates a 50% increased risk of an HAC with each 10 units ammonia increase relative to patients with no increase in ammonia
ACKNOWLEDGMENTS
The authors gratefully acknowledge Richard Rowell (Hyperion) for his statistical review and thank the efforts of the study who made this study possible, including N. Schrager (Icahn School of Medicine at Mount Sinai), A. Donovan, J. Crawford, Pediatric TRU Staff, K. Defouw, J. Balliet (The Medical College of Wisconsin), M. Keuth, N. O'Donnell (Long Beach Memorial Hospital), M. Hussain, E. Bailey, A. Orton, M. Ambreen (The Hospital for Sick Children, University of Toronto, ON, Canada), C. Bailey, M.J. Dunkley(The University of Utah), J. Perry, V. de Leon, A. Niemi, K. Cusmano (Stanford University), T. Carlson, J. Parker (University of Minnesota), S. Burr (Children's Hospital Colorado), K. Simpson (Children's National Medical Center), K. Regis (Nationwide Children's Hospital), A. Behrend, T. Marrone (Oregon Health & Sciences University), N. Dorrani (University of California, Los Angeles), C. Heggie (Case Western Reserve University), S. Mortenson (Maine Medical Center), S. Deward (Children's Hospital of Pittsburgh), K. Bart, C. Duggan (SNBL), K. Murray, C. Dedomenico (Tufts Medical Center), C. Gross (University of Florida), L. Brody (Seattle Children's Hospital), M. Mullins, S. Carter, A. Tran, J. Stuff, TCH General Clinical Research Center nursing staff (Baylor), Kathy Lisam (Hyperion), and UCD Consortium members including Mark L. Batshaw, Mendel Tuchman, Matthias R. Baumgartner, Georg Hoffmann, Douglas S. Kerr, Margretta R. Seashore, Tamar Stricker, Susan Waisbren, Mark Yudoff, as well as the Clinical and Translational Science Awards/General Clinical Research Center Grants (Baylor College of Medicine, M01RR00188; Case Western Reserve University, NIDDK 1K08DK074573; Clinical and Translational Science Institute at Children's National Medical Center NIH/NCRR, UL1RR31988; Medical College of Wisconsin, UL1RR31973; Mount Sinai School of Medicine, UL1RR29887; Oregon Health & Science University, UL1RR24140; Stanford University, UL1RR25744; Tufts University, UL1RR25752; University of California, Los Angeles, UL1RR33176; University of Colorado, UL1RR25780; University of Florida, UL1RR29890; University of Minnesota, UL1RR33183; University of Pittsburgh, NIH UL1TR000005; University of Utah, UL1RR25764; University of Washington,UL1TR000423), the Urea Cycle Disorders Consortium (NIH Grant U54HD061221) and grants from the O'Malley Foundation, Kettering Fund, and Rotenberg Fund which provided support. SCS Nagamani is an awardee of the National Urea Cycle Disorders Foundation Research Fellowship and is supported by the Clinical Scientist Development Award by the Doris Duke Charitable Foundation.
List of Abbreviations
- AUC0-24
daily ammonia exposure assessed as 24 hour area under the curve
- Cmax
maximal daily ammonia concentration
- GPB
glycerol phenylbutyrate
- HAC(s)
hyperammonemic crisis (crises)
- NaPBA
sodium phenylbutyrate
- AUC 0-24
time normalized area under the curve over 24 hours
- TNAUC12months
time normalized area under the curve for up to 12 months
- UCD
urea cycle disorder
- ULN
upper limit of normal
Footnotes
ClinicalTrials.gov Identifier: NCT00551200, NCT00947544, NCT00947297, NCT00992459, NCT01347073
Compliance with Ethics Guidelines
Conflict of Interest Statement: M. Mokhtarani, D.F. Coakley and B.F. Scharschmidt are employees of Hyperion. None of the other authors have a financial interest in Hyperion, although payments were made by Hyperion to each of the study sites for services provided in the conduct of the clinical studies upon which these analyses were based, as well as to Accudata Solutions (DM) and Miguel Marino for their assistance with the biostatistical analyses. A. Schulze is a paid consultant to Hyperion.
Informed Consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Contributions of Individual Authors
B. Lee: Conduct of study, interpretation of data and guarantor.
G.A. Diaz, W. Rhead, U. Lichter-Konecki, A. Feigenbaum, S.A. Berry, C. Le Mons, J. Bartley, N. Longo, S.C. Nagamani, W. Berquist, R. Gallagher, D. Bartholomew, C.O. Harding, M.S. Korson, S.E. McCandless, W. Smith, S. Cederbaum, D. Wong, J.L. Merritt, J. Vockley, D. Kronn, R. Zori, M. Summar, D.F. Coakley, M. Mokhtarani, The UCD Consortium and B.F. Scharschmidt: Conduct of study and interpretation of data.
A. Schulze: Conduct of study, interpretation of data and a paid consultant to Hyperion. D.A. Milikien and M. Marino: Statistical analysis.
Contributor Information
Brendan Lee, Baylor College of Medicine, Houston, TX, USA and Howard Hughes Medical Institute, Chevy Chase, MD, USA.
George A. Diaz, Icahn School of Medicine at Mount Sinai, New York, NY, USA
William Rhead, The Medical College of Wisconsin, Milwaukee, WI, USA.
U. Lichter-Konecki, The UCD Consortium - Children's National Medical Center, Washington, DC, USA
Annette Feigenbaum, The Hospital for Sick Children, University of Toronto, Toronto, Canada
Susan A. Berry, University of Minnesota, Minneapolis, MN, USA
C. Le Mons, National Urea Cycle Disorders Foundation, Pasadena, CA, USA
James A Bartley, Miller Children's Hospital, Long Beach, CA, USA
Nicola Longo, University of Utah, Salt Lake City, UT, USA
Sandesh C. Nagamani, Baylor College of Medicine, Houston, TX, USA
William Berquist, Stanford University, Palo Alto, CA, USA
Renata Gallagher, Children's Hospital Colorado, Aurora, CO, USA
Dennis Bartholomew, Nationwide Children's Hospital, Columbus, OH, USA
Cary O. Harding, Oregon Health & Sciences University, Portland, OR, USA
Mark S. Korson, Tufts Medical Center, Boston, MA, USA
Shawn E. McCandless, University Hospitals Case Medical Center and Case Western Reserve University, Cleveland, OH, USA
Wendy Smith, Maine Medical Center, Portland, ME, USA
Stephen Cederbaum, University of California, Los Angeles, CA, USA
Derek Wong, University of California, Los Angeles, CA, USA
J. Lawrence Merritt, II, University of Washington, Seattle, WA, USA
A. Schulze, The Hospital for Sick Children, University of Toronto, Toronto, Canada
Gerard. Vockley, University of Pittsburgh, Pittsburgh, PA, USA
David Kronn, Westchester Medical Center, Westchester, NY, USA
Roberto Zori, University of Florida, Gainesville, FL, USA
Marshall Summar, The UCD Consortium - Children's National Medical Center, Washington, DC, USA
D.A. Milikien, Accudata Solutions, Inc., Lafayette, CA, USA
M. Marino, Oregon Health & Sciences University, Portland, OR, USA
D.F. Coakley, Hyperion Therapeutics, Brisbane, CA, USA
M. Mokhtarani, Hyperion Therapeutics, Brisbane, CA, USA.
B.F. Scharschmidt, Hyperion Therapeutics, Brisbane, CA, USA
REFERENCES
- 1.Berry GT, Steiner RD. Long-term management of patients with urea cycle disorders. J Pediatr. 2001;138(1 Suppl):S56–60. doi: 10.1067/mpd.2001.111837. discussion S60-51. [DOI] [PubMed] [Google Scholar]
- 2.Lee B, Rhead W, Diaz GA, et al. Phase 2 comparison of a novel ammonia scavenging agent with sodium phenylbutyrate in patients with urea cycle disorders: safety, pharmacokinetics and ammonia control. Mol Genet Metab. 2010;100(3):221–228. doi: 10.1016/j.ymgme.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichter-Konecki U, Diaz GA, Merritt JL, 2nd, et al. Ammonia control in children with urea cycle disorders (UCDs); phase 2 comparison of sodium phenylbutyrate and glycerol phenylbutyrate. Mol Genet Metab. 2011;103(4):323–329. doi: 10.1016/j.ymgme.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith W, Diaz GA, Lichter-Konecki U, et al. Ammonia control in children ages 2 months through 5 years with urea cycle disorders: comparison of sodium phenylbutyrate and glycerol phenylbutyrate. J Pediatr. 2013;162(6):1228–1234. 1234, e1221. doi: 10.1016/j.jpeds.2012.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz GA, Krivitzky LS, Mokhtarani M, et al. Ammonia control and neurocognitive outcome among urea cycle disorder patients treated with glycerol phenylbutyrate. Hepatology. 2013;57(6):2171–2179. doi: 10.1002/hep.26058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mokhtarani M, Diaz GA, Rhead W, et al. Elevated phenylacetic acid levels do not correlate with adverse events in patients with urea cycle disorders or hepatic encephalopathy and can be predicted based on the plasma PAA to PAGN ratio. Mol Genet Metab. 2013;110(4):446–453. doi: 10.1016/j.ymgme.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry SA, Lichter-Konecki U, Diaz GA, et al. Glycerol phenylbutyrate treatment in children with urea cycle disorders: pooled analysis of short and long-term ammonia control and outcomes. Mol Genet Metab. 2014;112(1):17–24. doi: 10.1016/j.ymgme.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davison A, Hinkley D. Bootstrap Methods and their Application. Cambridge Univeresity Press; London: 1997. [Google Scholar]
- 9.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53(282):457–481. [Google Scholar]
- 10.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J Royal Statist Soc Series A (General) 1972:185–207. [Google Scholar]
- 11.Breslow N. Tests of hypotheses in overdispersed Poisson regression and other quasi-likelihood models. J Am Statist Assoc. 1990;85(410):565–571. [Google Scholar]
- 12.Dean CB, Nielsen JD. Generalized linear mixed models: a review and some extensions. Lifetime Data Analysis. 2007 Dec;13(4):497–512. doi: 10.1007/s10985-007-9065-x. [DOI] [PubMed] [Google Scholar]
- 13.Hardin JW. Generalized Estimating Equations (GEE) John Wiley & Sons, Ltd.; 2005. [Google Scholar]
- 14.Maestri NE, McGowan KD, Brusilow SW. Plasma glutamine concentration: a guide in the management of urea cycle disorders. J Pediatr. 1992;121(2):259–261. doi: 10.1016/s0022-3476(05)81200-4. [DOI] [PubMed] [Google Scholar]
- 15.Wilson CJ, Lee PJ, Leonard JV. Plasma glutamine and ammonia concentrations in ornithine carbamoyltransferase deficiency and citrullinaemia. J Inherit Metab Dis. 2001;24(7):691–695. doi: 10.1023/a:1012995701589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Variability in blood ammonia over 24 hours during steady state dosing with NaPBA or GPB in UCD patients (n = 80; left panel) and in individual patients (right panel).
Figure S2: Relationship between glutamine and ammonia levels by UCD subtype. Strong correlation between ammonia and glutamine with proximal defects (OTC, CPS) and not distal defects (ASL, ASS).
Figure S3: Relative risk (RR) of HAC per incremental increase in ammonia exposure. Time normalized ammonia area under the curve (TNAUC) over 12-months correlated directly with the risk of experiencing an HAC at any time during that period. A RR of 1.50 indicates a 50% increased risk of an HAC with each 10 units ammonia increase relative to patients with no increase in ammonia