Abstract
Efforts to identify host determinants for malaria have been hindered by the absence of a nucleus in erythrocytes, precluding genetic manipulation in the cell where the parasite replicates. We used cultured red blood cells derived from hematopoietic stem cells to carry out a forward genetic screen for Plasmodium falciparum host determinants. We found that CD55 is an essential host factor for P. falciparum invasion. CD55-null erythrocytes were refractory to invasion by all isolates of P. falciparum because parasites failed to attach properly to the erythrocyte surface. Thus, CD55 is an attractive target for the development of malaria therapeutics. Hematopoietic stem cell-based forward genetic screens may be valuable for the identification of additional host determinants of malaria pathogenesis.
Severe malaria is caused by Plasmodium falciparum and is one of the leading causes of mortality among children globally (1). During infection, parasites invade and replicate within human erythrocytes (2). Host erythrocyte polymorphisms that confer resistance to severe malaria have been identified by epidemiologic approaches (3, 4). Genome-wide association studies have searched for host determinants of malaria, but functional validation in the erythrocyte remains challenging due to the absence of a nucleus (5–7). Recent advances in the ex vivo production of erythrocytes now enable generation of genetically altered cells that support P. falciparum infection (8–11). Here, we used ex vivo-cultured red blood cells (cRBCs) in a forward genetic screen to identify host determinants of malaria infection.
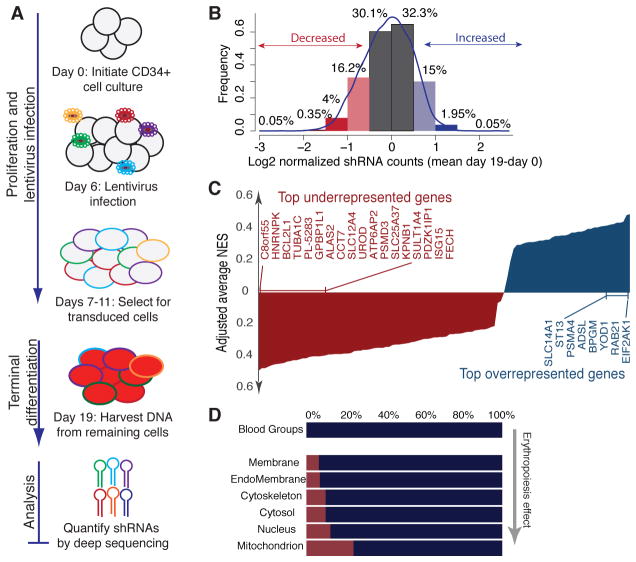
The human erythrocyte is a terminally differentiated minimal cell that lacks organelles and a nucleus (12). To identify erythrocyte proteins that influence P. falciparum infection, we designed a screening strategy involving RNAi-based knockdown of gene expression in hematopoietic progenitor cells, induction of ex vivo erythropoiesis, and finally infection of terminally differentiated erythroblasts with P. falciparum. Because gene knockdowns that impact erythroid development could have potent yet nonspecific effects on parasites in this approach, we first screened the erythrocyte proteome to identify genes that influence terminal differentiation of erythrocytes (Fig. 1A, Fig. S1A, Dataset S1). CD34+ hematopoietic stem cells (HSCs) were transduced with our pooled erythrocyte proteome shRNA library and induced to proliferate and differentiate to the orthochromatic erythroblast stage (when parasite infection can occur) (13). After 19 days, we quantified each shRNA in the surviving orthochromatic erythroblasts relative to the original library, with the prediction that shRNAs underrepresented in the differentiated erythroblasts would target genes important for erythropoiesis.
Fig. 1.
Pooled shRNA screen for genes that regulate terminal erythroid differentiation. (A) Schematic of pooled shRNA screen. CD34+ HSC were induced toward erythroid development, transduced with shRNA library on Day 6 and selected with puromycin. On day 19, shRNA proviruses in orthochromatic erythroblasts were quantified by Illumina sequencing. (B) Change in relative abundance of 5530 shRNAs in erythrocyte proteome library after 19 days of differentiation. (C) RNAi Gene Enrichment Ranking (RIGER) analysis of 116 candidate genes based on magnitude of erythropoiesis phenotype. NES= Normalized Enrichment Score. (D) Predicted localization of library components, with percent of genes that influence erythropoiesis in red.
The erythropoiesis screen yielded sufficient cells for full coverage of the library (>1500 cells per shRNA) (Fig. S1B) (14), and deep sequencing of shRNA proviruses isolated from the terminally differentiated erythroid cells revealed a normal distribution (Fig. S1C). 4.4% of shRNAs were depleted more than 2-fold in day-19 cRBCs relative to the original library pool, whereas 2% were significantly enriched (Fig. 1B), indicating these shRNAs can influence erythropoiesis. We ranked genes based on the depletion or enrichment of multiple shRNAs (15), and identified 116 candidates that grouped into categories relevant to terminal erythroid differentiation, including heme metabolism, protein turnover, and apoptosis (Fig. 1C, Fig. S1D, Fig. S2, Datasets S2–S3). We validated four top hits (Fig. S3, Dataset S4). This functional analysis of erythropoiesis provides a framework to study host determinants of malaria infection.
To identify factors that influence host susceptibility to P. falciparum infection, we chose to focus on a small subset of the erythrocyte proteome: 42 genes encoding human blood groups. All known P. falciparum receptors fall within this group, and the shRNAs targeting these genes did not significantly impact erythroid development (Fig. 1D). Also, focusing on a small gene set increased the sensitivity to a level required for the inherently complex parasite screen (16).
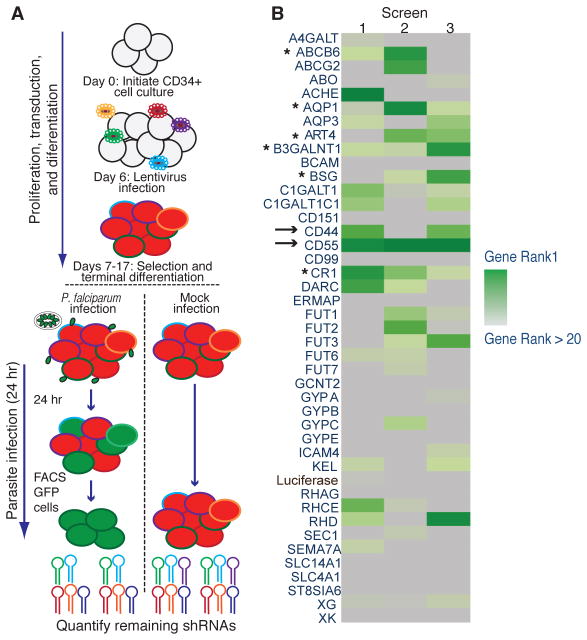
We transduced hematopoietic progenitor cells (HPCs) with a pooled lentivirus shRNA library targeting the blood group genes (Fig. 2A). At the late orthochromatic erythroblast stage, we infected the knockdown cells with a Green Fluorescent Protein (GFP)-expressing line of P. falciparum strain 3D7. We isolated the parasitized cells and quantified the relative abundance of each shRNA in the population by deep sequencing (Fig. S4). In parallel, we quantified the abundance of each shRNA in a control population of knockdown cRBCs not exposed to parasites. Hairpins underrepresented in infected cells compared to control cells were considered hits, because they may target host genes required for efficient parasite infection.
Fig. 2.
Pooled shRNA screen to identify host determinants of P. falciparum infection. (A) Schematic of blood group shRNA screen. HPCs were transduced with pooled lentivirus library expressing 308 shRNAs targeting 42 blood group genes. Knockdown cRBCs infected with P. falciparum were sorted, and shRNAs were quantified by deep sequencing. (B) RIGER analysis ranking results for three independent experiments. Genes were ranked based on NES scores (green heat map). →, top hits; *, additional candidates.
We ranked candidates from three replicates (Fig. 2B, Dataset S5) (15). Among the highly ranked genes were basigin (BSG) and CR1, both of which have described roles in P. falciparum invasion (17–19). The top-ranked candidate was CD55, alias Decay-Accelerating Factor (DAF), which carries the Cromer blood group antigens. CD55 is a GPI-linked complement-regulatory protein that protects cells from lysis by complement (20). On epithelial cells, CD55 is a receptor for bacterial and viral pathogens (21–23). Another hit, CD44, defines the Indian blood group and facilitates keratinocyte invasion by Group A Streptococcus (24, 25).
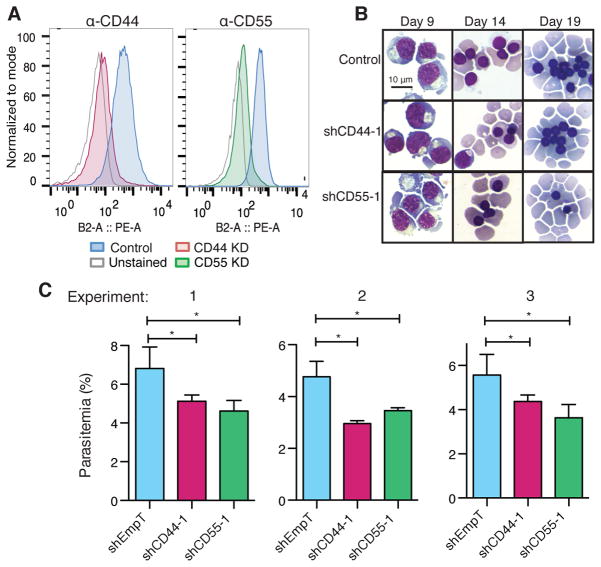
For validation, we expressed individual shRNAs targeting CD44 and CD55 in HPCs to generate mature red blood cells deficient in CD44 or CD55 (Fig. 3A). Morphological development of the knockdown cells was indistinguishable from control cRBCs (Fig. 3B), as were levels of Band 3, CD49d, and CD71 (Fig. S5) (26). To determine whether the amount of CD44 or CD55 on red blood cells influences P. falciparum infection, we assessed parasite invasion into the knockdown cRBCs compared to control cRBCs. We observed ~30% reduction in parasitemia in cells deficient for either CD44 or CD55 relative to control cRBCs (Fig. 3C). The same trend was observed using alternative shRNAs for CD44 and CD55, suggesting that the results were not because of off-target effects of the shRNAs (Fig. S6). Levels of known P. falciparum receptors were unchanged in CD44- and CD55-deficient cRBCs, suggesting that the observed effects on invasion were directly attributable to CD44 and CD55 (Fig. S7).
Fig. 3.
Validation of CD44 and CD55 as host factors required for P. falciparum invasion. (A) CD44 and CD55 levels on day 19/20 cRBCs expressing CD44, CD55, or control shRNAs (EmpT). Detection was by antibody staining and flow cytometry. (B) Morphology of differentiating cRBCs depleted for CD44 and CD55, detected by May-Grünwald and Giemsa staining. (C) P. falciparum strain 3D7 invasion assays in control, CD44 knockdown, and CD55 knockdown cRBCs. Three independent biological replicates from two distinct bone marrow donors are shown. Mean ± SD, n=2 or 3. *, p<0.05, one-tailed t test.
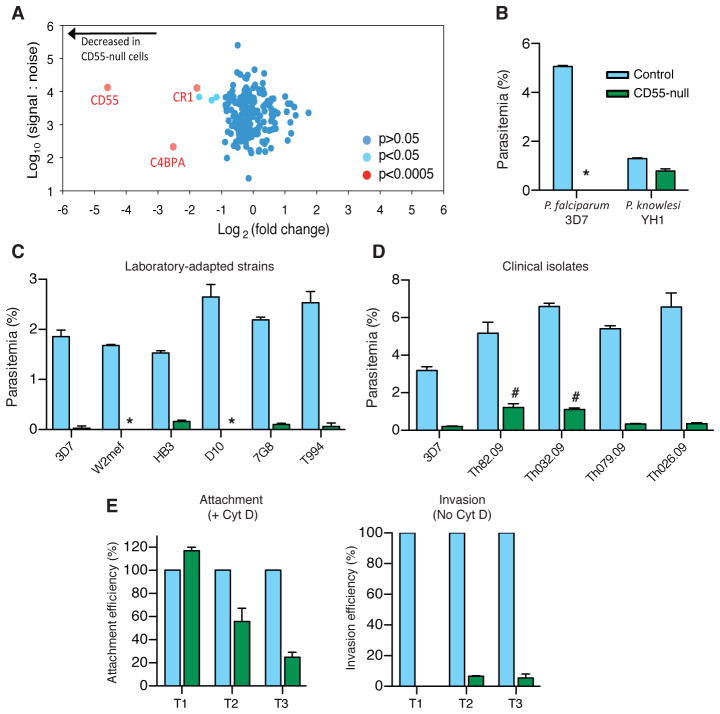
Complete absence of Cromer blood group antigens is rare and has been described in nine individuals with the Inab phenotype, most of whom have stop codons in the CD55 coding sequence (20). Cells from Inab individuals have normal osmotic fragility, do not undergo hemolysis in vivo, and are not particularly susceptible to complement-mediated lysis (27, 28). We used mature erythrocytes from a Japanese patient with the Inab phenotype (termed CD55-null cells) (29) to test if CD55 has an essential role for P. falciparum invasion. To first characterize any inherent differences between the CD55-null and wild type (wt) control samples, we adapted plasma membrane profiling (PMP) using tandem mass tag-based mass spectrometry for use in erythrocytes (30). CD55 was absent from the Inab erythrocytes (Fig. 4A). Only two other notable differences were found between the CD55-null and wt cells, including a 4-fold reduction in CR1, well within the range of natural variation (19), and a similar reduction in C4BPA, a serum protein that binds to complement regulatory proteins. Only a weak association has been observed between parasite invasion efficiency and natural variation in CR1 levels, and only when all sialylated proteins have been removed from the cell surface (18, 19).
Fig. 4.
CD55 is a critical host receptor for P. falciparum. (A) Scatter plot of plasma membrane proteins identified by PMP of CD55-null erythrocytes relative to controls from two unrelated individuals, and quantified by > 2 peptides. (B) Invasion of control erythrocytes (blue) or CD55-null erythrocytes (green) by P. falciparum strain 3D7 or P. knowlesi strain YH1. *, below detection. (C) Invasion by laboratory-adapted P. falciparum strains. (D) Invasion by P. falciparum clinical isolates. #, thin smears showed 0.6–1% gametocytes. For B-D: Mean ± SD, N=3. 10,000 cells scored per well by flow cytometry. (E) Efficiency of P. falciparum 3D7 merozoite attachment to the surface of CD55-null (green) versus control (blue) RBCs using cytochalasin D (Cyt D). Invasion was measured in the absence of Cyt D. T1=30 min, T2=60 min, T3=180 min after addition of schizonts (Fig. S10). Attachment to controls at T1 was 3.6–5.3%. Mean ± SD, N=2. 20,000 cells scored per well by flow cytometry.
To determine the contribution of CD55 to P. falciparum infection in mature erythrocytes, we used the CD55-null cells in invasion assays. The CD55-null erythrocytes were refractory to invasion by P. falciparum strain 3D7, revealing an essential requirement for host CD55 in parasite infection (Fig. 4B). This finding suggests that the reduced but substantial parasitemia observed in the CD55-knockdown cells depended on residual CD55. The CD55-null cells were similarly non-susceptible to invasion by additional laboratory-adapted P. falciparum strains, including the CR1-independent W2mef line (Fig. 4C). Parasite invasion was also significantly impaired in erythrocytes from a second, unrelated Inab patient with a different genetic basis for the condition (Fig. S8A) (31). Proliferation of parasites was also diminished in these Inab cells (Fig. S8B), suggesting that CD55 would be required to support a productive malaria infection.
Besides laboratory-adapted strains, several clinical isolates of P. falciparum from Senegalese patients were also dependent on CD55 for invasion (Fig. 4D), providing additional evidence that CD55 is likely a critical host factor for all P. falciparum strains. In contrast to P. falciparum, the zoonotic human malaria parasite P. knowlesi invaded CD55-null and wt erythrocytes with similar efficiency (Fig. 4B). Thus, CD55 on human erythrocytes may interact with a parasite ligand specific to P. falciparum that is not expressed by other malaria parasites. Deletion of known invasion ligands (EBA140, EBA175, EBA181, RH1, RH2a or RH2b) did not enable parasite invasion in the absence of CD55 (Fig. S9).
To determine if CD55 is involved in attachment of P. falciparum to the erythrocyte or at a later stage of the invasion process, we used cytochalasin D to enable isolation of cells with parasites adhered to the outer surface (32). Initially, attachment of P. falciparum merozoites was similar for the CD55-null and wt cells, but over time parasites selectively detached from the cells lacking CD55, mirroring the invasion defect (Fig. 4E). Thus, CD55 may not be required for the primary interaction of merozoites with the RBC surface, but instead be critical for the stage of committed, irreversible attachment seen during formation of the tight junction (33).
Our results reveal an essential role for CD55 in P. falciparum invasion of human red blood cells. Levels of CD55 have been found to vary dynamically during clinical malaria and may influence the course of infection (34). Moreover, we identified two CD55 polymorphisms significantly enriched in persons with ancestral exposure to malaria (Table 1), both of which have been previously described almost exclusively in individuals of African descent (20).
Table 1.
Geographic distribution of CD55* coding variants as percent of population, from the 1000 genomes Project, Phase I.
| ΔAA† ISBT‡ |
R52L CROM3 |
L82R CROM8 |
Y133H | A227P CROM1 |
I231V | V333I | G354A | G372E | |
|---|---|---|---|---|---|---|---|---|---|
| Pops (1092)§ | Exp|| | ||||||||
| Aggr(688) | low | 0 | 0 | 0 | 00 | 0 | 0 | 0 | 0 |
| TSI(98) | low | 0 | 0 | 0 | 00 | 1.02 | 0 | 0 | 0 |
| ASW(61) | high | 4.92 | 0 | 1.64 | 01.64 | 0 | 0 | 0 | 0 |
| CLM(60) | high | 0 | 0 | 0 | 03.33 | 0 | 0 | 0 | 1.67 |
| LWK(97) | high | 0 | 0 | 0 | 14.43 | 0 | 0 | 1.03 | 0 |
| YRI(88) | high | 5.68 | 1.14 | 0 | 01.14 | 0 | 1.14 | 0 | 0 |
|
| |||||||||
| p-value¶ | 3.56 × 10−5 | 0.28 | 0.28 | 7.85 × 10−11 | 1 | 0.28 | 0.28 | 0.28 | |
CD55 isoform ENST00000367064 (DAF-2).
Amino acid change and location: rs numbers are given in Methods.
International Society of Blood Transfusion type.
Populations: Aggr, aggregated populations with no variant in sample (CHS, Southern Han Chinese, China; MXL, Mexican Ancestry in Los Angeles, CA; PUR, Puerto Rican in Puerto Rico; CEU, Utah residents with North and West European ancestry; CHB, Han Chinese in Bejing; FIN, Finnish in Finland; GBR, British in UK; IBS, Iberian populations in Spain; JPT, Japanese in Tokyo); TSI, Toscani in Italy; ASW, African Ancestry in Southwest US; CLM, Colombian in Medellin, Colombia; LWK, Luhya in Webuye, Kenya; YRI, Yoruba in Ibadan, Nigeria. The numbers of individuals sampled are shown in parentheses.
Exposure of current or ancestral population to malaria based on WHO DALY (Disability-Adjusted Life Year per 100,000 population) malaria data: high, DALY ≥ 10; low, DALY < 10.
Fisher’s exact test comparing pops with high and low exposure.
The existence of hematologically normal individuals completely lacking CD55 suggests that targeting CD55 on erythrocytes would not elicit significant toxicity. In light of its critical role in parasite infection, CD55 could serve as an attractive target for the development of malaria therapeutics (35). Aside from CD55, the only other known strain-transcendent receptor for P. falciparum, basigin, binds to RH5, which is a leading malaria vaccine candidate (17, 36–37). Investigations into the molecular details of how CD55 and basigin interact with parasites will fuel new approaches to prevention and treatment of malaria. Our studies also establish the feasibility of forward genetic cellular screening using cultured erythrocytes derived from HSCs to identify critical host determinants of P. falciparum malaria biology and pathogenesis.
Supplementary Material
One Sentence Summary.
RNAi screening of blood group proteins found CD55 is a strain-transcendent host factor for erythrocyte invasion by Plasmodium falciparum.
Acknowledgments
We thank D. Wirth, M. Ganter, A. Nicholson-Weller, M. Waldor, S. Lux, R. Husson, B. Burleigh, and members of the Duraisingh Lab for helpful discussions and/or reading of the manuscript. We thank C. Westhoff and C. Lomas-Francis of the New York Blood Center for gift of the second Inab sample. We thank U. Kanjee for technical assistance and D. Ndiaye, S. Mboup, and S. Volkman for P. falciparum clinical isolates from Senegal. This work was supported by a Gates Foundation Grand Challenges Exploration Award OPP1035276 (M.T.D.), NIH R01AI091787 (M.T.D.), a Pediatric Scientist Development Program Fellowship from the Eunice Kennedy Shriver National Institute of Child Health and Human Development K12-HD000850 (E.S.E), NIH K08 1K08AI103034-01A1 (E.S.E.), Boston Children’s Hospital Faculty Development Award (E.S.E.), and the Cambridge Biomedical Research Center, UK (M.P.W. and L.V.N.). Additional data can be found in the Supplementary Material.
Footnotes
“This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at https://urldefense.proofpoint.com/v2/url?u=http-3A__www.sciencemag.org_&d=BQICaQ&c=qS4goWBT7poplM69zy_3xhKwEW14JZMSdioCoppxeFU&r=-6Xus2u89wY5MG4Oxo_nmADnnbMO18TBEJ1Df9qI9g5EKyNAWhsCvP51aS-kFMGB&m=Q387SMCi0a8QicDEbuH_i7bGA4g4xL6riZEFMy3Kbyk&s=toRglvKhabE9BgZVm0jI78Qb6j7BlV-Ej02GoUwxKro&e=. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.”
Materials and Methods
References and Notes
- 1.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.Fairhurst RM, Wellems TE. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Mandell GL, Bennett JE, Dolin R, editors. Vol. 2. Livingstone Churchill Elsevier; Philadelphia, PA: 2010. pp. 3437–3462. chap. 275. [Google Scholar]
- 3.Allison AC. Protection afforded by sickle-cell trait against subtertian malareal infection. British medical journal. 1954;1:290–294. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor SM, Fairhurst RM. Malaria parasites and red cell variants: when a house is not a home. Current opinion in hematology. 2014;21:193–200. doi: 10.1097/MOH.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timmann C, Thye T, Vens M, vans JE, May J, et al. Genome-wide association study indicates two novel resistance loci for severe malaria. Nature. 2012;489:443–446. doi: 10.1038/nature11334. [DOI] [PubMed] [Google Scholar]
- 6.Jallow M, Teo YY, Small KS, Rockett KA, Deloukas P, et al. Genome-wide and fine-resolution association analysis of malaria in West Africa. Nature genetics. 2009;41:657–665. doi: 10.1038/ng.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rockett KA, Clarke GM, Fitzpatrick K, Hubbart C, et al. Reappraisal of known malaria resistance loci in a large multicenter study. Nature genetics. 2014;11:1197–204. doi: 10.1038/ng.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giarratana MC, Kobari L, Lapillonne H, Chalmers D, Kiger L, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nature biotechnology. 2005;23:69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 9.Giarratana MC, Rouard H, Dumont A, Kiger L, Safeukui I, et al. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118:5071–5079. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamez PA, Liu H, Fernandez-Pol S, Haldar K, Wickrema A. Stage-specific susceptibility of human erythroblasts to Plasmodium falciparum malaria infection. Blood. 2009;114:3652–3655. doi: 10.1182/blood-2009-07-231894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bei AK, Brugnara C, Duraisingh MT. In vitro genetic analysis of an erythrocyte determinant of malaria infection. J Infect Dis. 2010;202:1722–1727. doi: 10.1086/657157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasini EM, Kirkegaard M, Mortensen P, Lutz HU, Thomas AW, Mann M. In-depth analysis of the membrane and cytosolic proteome of red blood cells. Blood. 2006;108:791–801. doi: 10.1182/blood-2005-11-007799. [DOI] [PubMed] [Google Scholar]
- 13.Materials and methods can be found online in the Supplementary Material
- 14.Strezoska Z, Licon A, Haimes J, Spayd KJ, Patel KM, Sullivan K, Jastrzebski K, Simpson KJ, Leake D, van Brabant Smith A, Vermeulen A. Optimized PCR conditions and increased shRNA fold representation improve reproducibility of pooled shRNA screens. PloS One. 2012;7:e42341. doi: 10.1371/journal.pone.0042341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo B, Cheung HW, Subramanian A, Sharifnia T, Okamoto M, et al. Highly parallel identification of essential genes in cancer cells. Proc Natl Acad Sci U S A. 2008;105:20380–20385. doi: 10.1073/pnas.0810485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.See Supplemental Text online in the Supplementary Material
- 17.Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2012;480:534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tham WH, Wilson DW, Lopaticki S, Schmidt CQ, Tetteh-Quarcoo PB, et al. Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand. Proc Natl Acad Sci U S A. 2010;107:17327–17332. doi: 10.1073/pnas.1008151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spadafora C, Awandare GA, Kopydlowski KM, Czege J, Moch JK, et al. Complement receptor 1 is a sialic acid-independent erythrocyte receptor of Plasmodium falciparum. PLoS Pathog. 2010;6:e1000968. doi: 10.1371/journal.ppat.1000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storry JR, Reid ME, Yazer MH. The Cromer blood group system: a review. Immunohematology. 2010;26:109–118. [PubMed] [Google Scholar]
- 21.Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–131. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 22.Nowicki B, Truong L, Moulds J, Hull R. Presence of the Dr receptor in normal human tissues and its possible role in the pathogenesis of ascending urinary tract infection. The American journal of pathology. 1988;133:1–4. [PMC free article] [PubMed] [Google Scholar]
- 23.O’Brien DP, Romero-Gallo J, Schneider BG, Chaturvedi R, Delgado A, et al. Regulation of the Helicobacter pylori cellular receptor decay-accelerating factor. The Journal of biological chemistry. 2008;283:23922–23930. doi: 10.1074/jbc.M801144200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrager HM, Alberti S, Cywes C, Dougherty GJ, Wessels MR. Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A Streptococcus to CD44 on human keratinocytes. The Journal of clinical investigation. 1998;101:1708–1716. doi: 10.1172/JCI2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cywes C, Wessels MR. Group A Streptococcus tissue invasion by CD44-mediated cell signalling. Nature. 2001;414:648–652. doi: 10.1038/414648a. [DOI] [PubMed] [Google Scholar]
- 26.Hu J, Liu J, Xue F, Halverson G, Reid M, et al. Isolation and functional characterization of human erythroblasts at distinct stages: implications for understanding of normal and disordered erythropoiesis in vivo. Blood. 2013;121:3246–3253. doi: 10.1182/blood-2013-01-476390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid ME, Mallinson G, Sim RB, Poole J, Pausch V, et al. Biochemical studies on red blood cells from a patient with the Inab phenotype (decay-accelerating factor deficiency) Blood. 1991;78:3291–3297. [PubMed] [Google Scholar]
- 28.Merry AH, Rawlinson VI, Uchikawa M, Daha MR, Sim RB. Studies on the sensitivity to complement-mediated lysis of erythrocytes (Inab phenotype) with a deficiency of DAF (decay accelerating factor) British journal of haematology. 1989;73:248–253. doi: 10.1111/j.1365-2141.1989.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi J, et al. A case of Inab phenotype (IFC-) with anti-IFC. Jpn J Transfusion and Cell Therapy. 2008;54:258. (in Japanese) [Google Scholar]
- 30.Weekes MP, Tan SY, Poole E, Talbot S, Antrobus R, et al. Latency-associated degradation of the MRP1 drug transporter during latent human cytomegalovirus infection. Science. 2013;340:199–202. doi: 10.1126/science.1235047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hue-Roye K, Powell VI, Patel G, Lane D, Maguire M, Chung A, Reid ME. Novel molecular basis of an Inab phenotype. Immunohematology. 2005;21:53–55. [PubMed] [Google Scholar]
- 32.Miller LH, Aikawa M, Johnson JG, Shiroishi T. Interaction between cytochalasin B-treated malarial parasites and erythrocytes. Attachment and junction formation. The Journal of experimental medicine. 1979;149:172–184. doi: 10.1084/jem.149.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowman AF, Berry D, Baum J. The cellular and molecular basis for malaria parasite invasion of the human red blood cell. The Journal of cell biology. 2012;198:961–971. doi: 10.1083/jcb.201206112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gwamaka M, Fried M, Domingo G, Duffy PE. Early and extensive CD55 loss from red blood cells supports a causal role in malarial anaemia. Malaria journal. 2011;10:386. doi: 10.1186/1475-2875-10-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardner MR, Kattenhorn LM, Kondur HR, von Schaewen M, Dorfman T, Chiang JJ, Haworth KG, Decker JM, Alpert MD, Bailey CC, Neale ES, Fellinger CH, Joshi VR, Fuchs SP, Martinez-Navio JM, Quinlan BD, Yao AY, Mouquet H, Gorman J, Zhang B, Poignard P, Nussenzweig MC, Burton DR, Kwong PD, Piatak M, Lifson JD, Gao G, Desrosiers RC, vans DTE, Hahn BH, Ploss A, Cannon PM, Seaman MS, Farzan M. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature. 2015 doi: 10.1038/nature14264. published online EpubFeb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright KE, Hjerrild KA, Bartlett J, Douglas AD, Jin J, et al. Structure of malaria invasion protein RH5 with erythrocyte basigin and blocking antibodies. Nature. 2014;515:427–430. doi: 10.1038/nature13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss GE, Gilson PR, Taechalertpaisarn T, Tham WH, de Jong NW, Harvey KL, Fowkes FJ, Barlow PN, Rayner JC, Wright GJ, Cowman AF, Crabb BS. Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Plasmodium falciparum Invasion of Erythrocytes. PLoS pathogens. 2015;11:e1004670. doi: 10.1371/journal.ppat.1004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goh SH, Joselyn M, Lee YT, Danner RL, et al. The human reticulocyte transcriptome. Physiol Genomics. 2007;30:172–178. doi: 10.1152/physiolgenomics.00247.2006. [DOI] [PubMed] [Google Scholar]
- 39.Moffat J, Grueneberg DA, Yang X, Kim SY, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 40.Nkrumah LJ, Muhle RA, Moura PA, Ghosh P, et al. Efficient site-specific integration in Plasmodium falciparum chromosomes mediated by mycobacteriophage Bxb1 integrase. Nat Methods. 2006;3:615–621. doi: 10.1038/nmeth904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merryweather AT, Atzberger A, Soneji S, Gray N, et al. Global gene expression analysis of human erythroid progenitors. Blood. 2011;117:e96–108. doi: 10.1182/blood-2010-07-290825. [DOI] [PubMed] [Google Scholar]
- 42.Collins CR, Hackett F, Strath M, Penzo M, Withers-Martinez C, Baker DA, Blackman MJ. Malaria parasite cGMP-dependent protein kinase regulates blood stage merozoite secretory organelle discharge and egress. PLoS pathogens. 2013;9:e1003344. doi: 10.1371/journal.ppat.1003344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.