Abstract
Natalizumab, a monoclonal antibody directed against α4 integrins, has to date been associated with 377 cases of progressive multifocal leukoencephalopathy (PML) worldwide in patients receiving treatment for multiple sclerosis (MS). Due to the limited number of histological studies, the interplay between MS and PML lesions has not been investigated.
We report the clinical, radiological and histological findings of a MS patient who developed PML after 32 months of natalizumab monotherapy. Following withdrawal of natalizumab, she received plasma exchange, mefloquine and mirtazapine, but passed away soon thereafter. Post mortem studies were restricted to examination of the brain and spinal cord.
Extensive PML lesions, characterized by the presence of JCV DNA were found in the cerebral white matter and neocortex. Sharply demarcated areas of active PML lesions contained prominent inflammatory infiltrates composed of approximately equal numbers of CD4+ and CD8+ T cells, consistent with an immune reconstitution inflammatory syndrome (IRIS). Conversely, all MS lesions identified were hypocellular, long-standing inactive plaques characterized by myelin loss, relative axonal preservation, and gliosis, and importantly, were devoid of JCV-DNA and active inflammation.
Chronic inactive MS lesions were separate and distinct from nearby PML lesions. Furthermore, IRIS greatly affected the shape and appearance of PML lesions but did not involve MS lesions.
Keywords: Multiple sclerosis, JC virus, Progressive Multifocal Leukoencephalopathy, natalizumab, immune reconstitution inflammatory syndrome
Introduction
Progressive multifocal leukoencephalopathy (PML) (1) is a fulminant inflammatory demyelinating disease caused by the reactivation of the polyomavirus JC (JCV) in the setting of immunosuppression. As of July 2, 2013, 377 cases of PML have been diagnosed in multiple sclerosis (MS) patients who received immunomodulatory treatment with natalizumab (Tysabri ®) (2). Natalizumab is a humanized monoclonal antibody against α4 integrins, administered monthly, which prevents egress of all leukocytes from the bloodstream into brain parenchyma, thereby decreasing immunosurveillance. T-lymphocytes are therefore sequestered in the blood vessels, and cannot prevent reactivation and spread of JCV in the CNS. Since the biological activity of natalizumab can extend to up to three months, MS patients who develop PML usually are treated with plasma exchange. This decreases blood levels of natalizumab and hastens the return of lymphocytes into the CNS (3). This phenomenon usually is associated with an immune reconstitution inflammatory syndrome (IRIS), which by itself can be damaging to the brain. IRIS is characterized clinically by paradoxical worsening of the neurological symptoms at the time of immune recovery, and MRI may show enlargement of PML lesions, contrast enhancement and swelling (3, 4).
Of 377 MS-PML patients, 88 (23 %) have died (2), but histological postmortem examination of brain has been limited. In the initial report of natalizumab-associated PML occurring in a MS patient (5), no residual MS lesions were identified in the CNS. More recently, a few reports have described limited results of stereotactic biopsies (6-9) . An additional detailed postmortem examination of a natalizumab-associated PML patient failed to demonstrate JCV infection in brain lesions (10). The coexistence of lesions of JCV and MS, and their possible interactions have not been described. We present detailed clinical, radiological and pathological findings in a MS patient who developed PML during natalizumab therapy, and who at autopsy demonstrated lesion of both.
Materials and Methods
Histopathological Methods
Formalin-fixed, paraffin-embedded sections were studied by routine neuropathological stains (i.e. H/E; LFB/PAS; Bielschowsky), as well as single and double-labeled immunohistochemistry and immunofluorescence using antibodies listed in Table 1, as previously described (11).
Table 1. Antibodies used for IHC and IF staining.
| Antigen; product name | Host | Isot. | Target | Source |
|---|---|---|---|---|
|
| ||||
| PAB597 | m | NA | JCV VP1 | |
| SV40 T Ag (v-300); sc-20800 | r | IgG | JCV T Ag | Santa Cruz Biotec., Santa Cruz, CA |
| MAP-2; HM-2; M4403 | m | IgG1 | Neurons | Sigma, St-Louis, MO |
| MAP-2; ab5622 | r | IgG | Neurons | Chemicon, Temecula, CA |
| MAP-2; LS-B290 | ch | IgY | Neurons | Lifespan Bioscience, Seattle, WA |
| GFAP ; M0761; 6F2 | m | IgG1 | Astrocytes | Dako, Carpinteria, CA |
| GFAP ; Z0334 | r | IgG | Astrocytes | Dako, Carpinteria, CA |
| GFAP; ab4674 | ch | IgY | Astrocytes | Abcam, Cambridge, MA |
| CNPase; C5922; 11-5B | m | IgG1 | Myelin/Oligo | Sigma, St-Louis, MO |
| CNP; HPA023266 | r | IgG | Myelin/Oligo | Sigma, St-Louis, MO |
| MOG; EP4281; ab109746 | r | IgG | Myelin/Oligo | Abcam, Cambridge, MA |
| MAG; ab89780 | m | IgG1 | Myelin/Oligo | Abcam, Cambridge, MA |
| CD8; 1A5; VP-C325 | m | IgG1 | CD8 | Vector laboratories, Burlingame, CA |
| CD3; CD3-12; OASA11552 | rat | IgG | CD3 | Aviva Systems Biology, San Diego, CA |
| PLP | m | PLP | Serotec, USA | |
| NF | m | NF | Dako, Denmark | |
| KiM1P | m | KiM1P | Dr. Radzun, Goettingen, Germany | |
Isot: isotype, m: mouse, ch; chicken, r: rabbit, oligo: oligodendrocytes
For double staining, primary antibodies from different species (mouse, rat, rabbit or chicken) were used with appropriately matched (species and isotypes) secondary Abs. These antibodies were conjugated either to alkaline phosphatase (AP) or horseradish peroxidase (HRP) for IHC or to Alexa Fluor 350, 488 & 568 (Invitrogen), according to the manufacturer's instructions for IF. Single and double IHC were performed with the Vectastain Elite ABC Kits (Vector, Burlingame, CA), according to the manufacturer's instructions. Negative controls, for IHC and IFA, included omission of the primary Abs, use of isotype matched controls and use of sections from individuals known to be free of JCV-infected cells.
Characterization of the PML lesions
Viral protein immunoreactivity (IHC for JCV T Ag (v-300) or VP1 (PAB597) was correlated to the extent and pattern of demyelination using double-labeling for myelin proteins, CNPase, MAG, and MOG. JCV-infected cells were identified by double-labeling for specific cells (i.e., neurons and glia). In situ hybridization for JCV DNA was performed using a slightly modified technique (12), in which streptavidin-AP was replaced by streptavidin-HRP (DAB detection) and a tyramide amplification step added. PCR amplification of JCV DNA extracted from brain samples was performed using T Ag-specific primers as previously described (13).
Characterization of the MS lesions
Demyelinating activity in MS lesions based on myelin degradation product in macrophages were classified according to previously published criteria (14).
Characterization of inflammatory infiltrates in the MS and PML lesions
CD3+ and CD8+ T lymphocytes in both PML & MS lesions were examined relative to JCV VP1 (PAB597) expression. CD4+ T-cells were inferred based on double labeling for CD3 and CD8 (double positive consistent with a CD8+ T-cells; CD3+/CD8- cells considered CD4+ T-cells).
Results
Case report
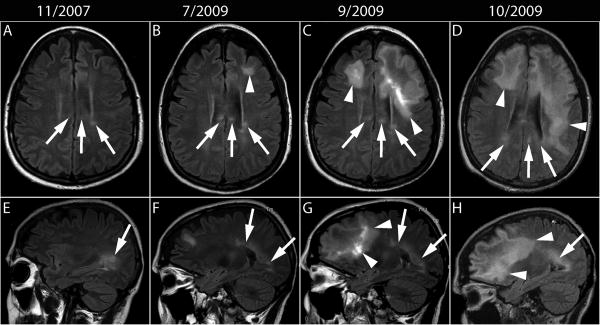
A 52-year-old woman diagnosed with relapsing remitting MS of 14 years duration had been treated with several immunomodulatory and immunosuppressive drugs, including interferon beta, glatiramer acetate, methotrexate and cyclophosphamide. In November 2006, she began natalizumab monotherapy, 300 mg IV every four weeks for refractory exacerbations. One year later, MRI showed stable MS lesions in the left periventricular area and the splenium of the corpus callosum (Fig 1. A and E). By June 2008, she was tetraplegic due to accumulating disability and required admission to a skilled nursing facility. She missed two injections of natalizumab in January and February 2009. In June 2009, she presented with cognitive decline. An MRI performed in July 2009, 32 months after onset of natalizumab therapy, showed a new left frontal subcortical non-enhancing white matter lesion in addition to stable chronic MS lesions (Fig 1.B and F). A few weeks later, she developed a speech deficit and worsening cognitive difficulties. She was treated with a short course of dexamethasone, and received her last injection of natalizumab in early September 2009. Repeat MRI performed in September 2009 showed marked progression of the left frontal lesion, extending across the genu of the corpus callosum into the right frontal lobe, associated with mild mass effect on the left lateral ventricle (Fig 1.C and G) and enhancement. A lumbar puncture performed in October 2009 showed 36 RBC, 7 WBC, glucose of 67 and protein of 77 mg/dl. JCV CSF PCR was positive, showing 9,900 JCV DNA copies/ml, thereby confirming a diagnosis of PML. She had a detectable cellular immune response against JCV, mediated by both CD4+ and CD8+ T cells, and was treated with 5 alternate day courses of plasma exchange, mirtazapine 30 mg qd and mefloquine 250 mg weekly. Later that month, CSF showed undetectable JCV DNA and MRI showed progression of the PML lesions in the right frontal, left frontal, and parietal lobes (Fig 1. D and H) with minimal associated enhancement. Despite these interventions, there was no clinical improvement. She developed aspiration pneumonia and died in November 2009, four months after PML onset, two months after the last natalizumab injection, three and half weeks after onset of plasma exchange and 12 days after the last MRI.
Fig 1.
MRI evolution of MS and PML lesions. (A-D) Axial and (E-H) corresponding sagittal FLAIR images. (A, E) After one year of natalizumab monotherapy, MS lesions are present in the left periventricular area and the splenium of the corpus callosum (arrows). (B, F) Twenty months later a new left subcortical lesion (arrowhead) can be seen in the left frontal lobe, remote from the old MS lesions (arrows). (C, G) Two and a half month later, the left frontal lobe lesion has extended posteriorly and contralaterally through the genu of the corpus callosum to the right frontal lobe white matter (arrowheads), while the MS lesions stable appearance (arrows). (D, H) One month later the PML lesions have spread further in the right frontal lobe and the left frontal and parietal lobes, close, but separate from the MS lesions (arrows).
Postmortem examination was restricted to the brain and spinal cord. The formalin-fixed brain showed mild generalized swelling and was sectioned in the axial plane to correlate with recent MRI. Corresponding to the axial FLAIR images, the gross slabs showed the MS lesions were well demarcated somewhat retracted firm areas of brown discoloration, whereas the PML lesions were softened, granular and “moth-eaten” in appearance.
Several MS lesions were identified in the white matter, including five periventricular lesions (three periventricular, one in the midbrain tectum around the cerebral aqueduct and one in the medulla around the fourth ventricle), two in the cerebellar peduncles, one in the basis pontis subpially, one in the medullary pyramid, two in the white matter of the spinal cord and two in the optic tract. Also identified were five lesions in deep gray matter structures: one each in the amygdala, the tail of the caudate, putamen, claustrum and inferior olivary nucleus. Microscopically, all white and deep gray matter MS lesions were chronic, inactive, demyelinated and hypocellular plaques, characterized by mild axonal loss, gliosis, and largely devoid of inflammatory T cell infiltrates. All lesions showed mild microglial activation without macrophages, except for one spinal cord lesion that contained PAS-positive macrophages, consistent with more recent demyelination. Cortical MS lesions included 16 intracortical and subpial chronic lesions.
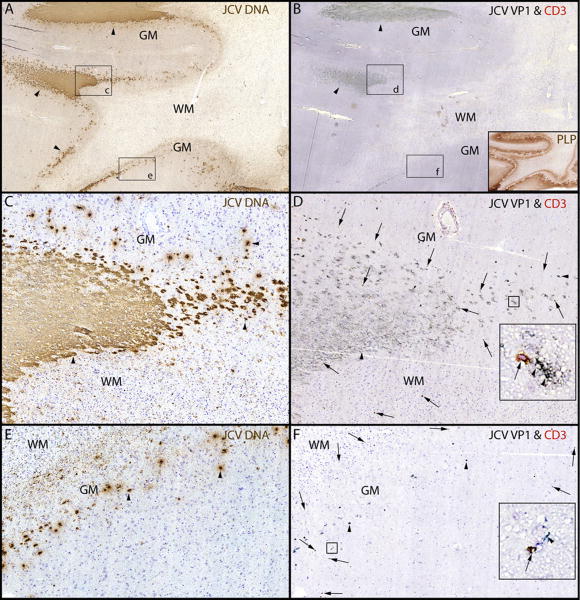
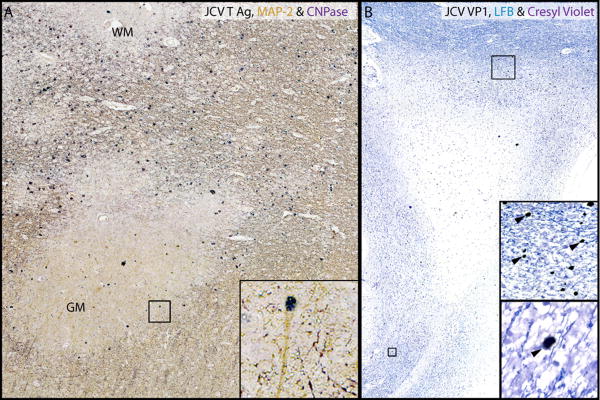
Extensive PML lesions were present in the subcortical and deep white matter of both cerebral hemispheres, as well as the corpus callosum and the brain stem, and characterized at the active edge of the lesion by relative myelin preservation and a high density of infected oligodendrocytes. Myelin loss was more apparent towards the center of the PML lesion, which contained few infected cells. ISH showed abundance of JCV DNA, localized mostly to sharply demarcated areas at the periphery of large lesions, and involving the gray-white matter junction and the gray matter (Fig 2. A, C, E, arrowheads). IHC staining with an antibody against SV40 that cross reacts against JCV major capsid protein VP1 of sequential sections showed a similar staining pattern at the gray-white junction, indicating the presence of a productive, lytic infection of glial cells (Fig 2. B, D, arrowheads). The extent of PML-associated demyelination in the white matter and the cortex is illustrated in Fig 2. B inset. Only a few infected cells were present in gray matter (Fig 2. F). A particular feature of these lesions was the well-demarcated aspect of JCV infected areas. CD3+ T cells were present at the edge of actively infected areas (Figure 2B, D, F, arrows), and often in close contact with JCV-infected cells (Fig 2 D, F. insets).
Fig 2.
PML lesions are sharply demarcated and harbor CD3+ T cells.
(A) ISH for JCV DNA (Cresyl violet counterstaining and (B) IHC for JCV VP1 & CD3 show that JCV-infected cells (arrowheads) are mostly restricted to the junction between white matter (WM) and gray matter (GM) and the lower layers of the cortex. Conversely, the deep WM is almost free of JCV-infected cells and totally demyelinated as shown by proteolipid protein staining (PLP, brown (B inset)), but not cavitary. (C-F). Magnifications of rectangles c and e from A show a sharp demarcation of the areas containing JCV-infected cells (arrowheads) at the gray-white junction (C). The edge of JCV infection spreads in the lower layer of the cortex (E, arrowheads). Magnification of rectangles d and f from B, show that cells expressing JCV VP1 protein (arrowheads) are surrounded by CD3+ T cells (marked by arrows in D and F). The majority of these T cells (D and F insets, arrow) are located in close proximity to the JCV-infected cells (D and F insets, arrowheads). Overall, the T cells are mostly found in areas where the cell nuclei contain JCV DNA (C) and express JCV VP1 (D), .
Topographical relationships between PML and MS lesions we studied by comparing ISH and IHC for JCV DNA. PML lesions were more obvious and extensive as demonstrated by ISH than by IHC and luxol fast blue. Infected cells within PML lesions contained JCV T Ag (typically expressed early in the viral replication cycle as well as JCV VP1 capsid protein (indicating the presence of mature viral particles). Conversely, chronic inactive MS lesions showed absence of JCV DNA or JCV proteins.
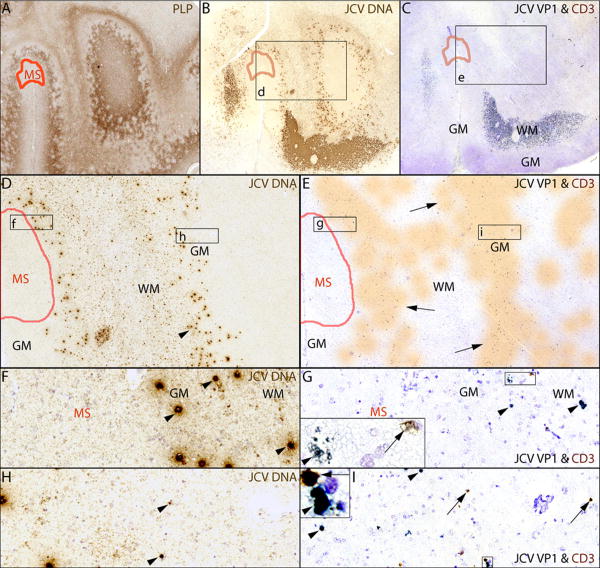
The juxtaposition between PML and MS lesions is illustrated in an area of the left occipital lobe containing PML lesions adjacent to a cortical MS plaque (Fig 3). A chronic subpial MS plaque (A) is adjacent to PML lesions, located in the subcortical white matter and the lower layers of the cortex. The boxed areas, d in (B) and e in (C) are magnified respectively in (D) and (E) and show JCV-infected cells (D, arrowheads) located immediately adjacent to the MS lesion, and a significant inflammatory infiltrate of CD3+ T cells (E, orange shaded areas, arrows) is present in the PML lesions but absent in the MS lesion. Higher magnification of boxes F-I (from D and E) show the MS lesion is entirely devoid of JCV infection or CD3+ T cell infiltrates, whereas CD3+ T cells (G, I arrows) are located in close proximity of JCV-infected cells (F-I, arrowheads).
Fig 3.
Proximity of MS and PML lesions in the left occipital lobe
(A-C) IHC staining for PLP (A) shows a subpial MS lesion (delineated in red) and demyelination lesions of PML involving the subcortical white matter (WM) and lower layers of the cortical gray matter (GM), which contain numerous cells harboring JCV DNA (B, JCV ISH & Cresyl violet counterstaining) and expressing JCV VP1 protein in C (LFB counterstaining). (D-E) The magnifications of the boxes s d & e from B & C show that cells harboring JCV DNA (D, arrowheads), those expressing JCV VP1 and CD3+ T cells (E) are not present in the MS lesion. (E) The distribution of the CD3+ T cells is shaded in orange (arrows). (F-I) Higher magnifications of the MS-PML interface (boxes f, g) and the lower GM side of the PML lesion (boxes h, i) show that cells harboring JCV DNA (F, arrowhead), those expressing JCV VP1 (G, arrowheads) and CD3+ T cells (G, arrows) are present at the border of the MS lesion. JCV infection of the gray matter is shown in (H, I arrowheads). Insets in G & I at higher magnification show CD3+ T cells in close proximity of cells expressing JCV VP1 protein.
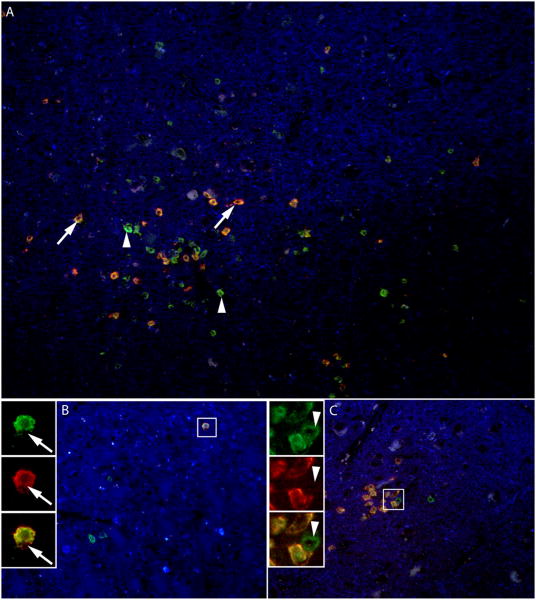
To determine the nature of the inflammatory T cell infiltrate (Fig 4), double IF staining for CD3 (green) and CD8 (red) in the PML lesions was performed. As shown in Fig 4. A, an approximately equal number of cells are CD3+/ CD8- (green, arrowheads) or CD3+/ CD8+ (yellow, arrows). No CD3-/CD8+ (red) cells are present. Higher magnification shows a CD3+/CD8+ T cell (B, arrow) and a CD3+/CD8- T cell (C, arrowhead), the latter corresponding to a CD4+ T cell. The magnitude of the inflammatory infiltrate, consisting of both CD4+ and CD8+ T cells in the PML lesions, was consistent with IRIS.
Fig 4.
CD8+ and CD4+ T cells are present in PML lesions
(A-C) Double IF staining for CD3 (Alexa Fluor 488, green) and CD8 (Alexa Fluor 568, red) and autofluorescence (blue). (A) Similar number of CD8+ T cells (CD3+/CD8+, yellow-red, arrows) and CD4+ T cells (CD3+/CD8-, green, arrowheads) can be seen at the border of this PML lesion. (B) Higher magnification with insets show a CD8+ T cells (arrows) in both red (CD8) and green (CD3) channels. Conversely in (C), a CD4+ T cell (arrowhead) is characterized by absence of staining of in the red channel (CD8) but staining in the green channel (CD3).
In addition, occasional JCV-infected neurons expressing JCV T Ag, but not JCV VP1, were present in lesions at the gray-white junction and in gray matter (Fig 5. A), findings consistent with non-productive infection of cortical neurons by JCV, as in other PML cases (11). Extensive PML lesions were found in the corpus callosum, some extending into the septum pellucidum, (Fig 5. B). A small PML focus also involved hippocampal white matter (not illustrated).
Fig 5.
Non-productive infection of hemispheric cortical neurons and productive infection of glial cells in the septum pellucidum.(A). Triple IHC for JCV T Ag (blue), MAP-2 (orange) & CNPase (brown). Rare JCV-infected cortical neurons expressing T Ag are present at the edge of a PML lesion in the GM (inset). (B) IHC for JCV VP1 (blue). Frequent glial cells expressing JCV VP1 can be seen in the corpus callosum (upper inset) and in the white matter of the septum pellucidum (lower inset).
Discussion
Coexistence of active PML and chronic inactive MS lesions
To our knowledge, this is the first histopathologically confirmed case report showing coexistent MS and PML lesions in a natalizumab-treated patient. It provides a unique opportunity to compare radiological and histological findings in natalizumab-associated PML as well as examine the early manifestations of superimposed IRIS.
PML lesions were extensive, corresponding to the MRI findings, and harbored ongoing productive JCV infection. Conversely, MS lesions were chronic inactive plaques devoid of active inflammatory infiltrates. While PML lesions displayed a large number of cells containing JCV DNA or proteins, MS lesions were entirely devoid of JCV infection, even when abutting PML lesions. This may be explained by the relative absence of remaining glial cells that could potentially get infected by JCV in chronic MS lesions. As such, the MS plaques and PML lesions were distinct and pathologically independent.
IRIS affects PML, but spares MS lesions
IRIS was originally described in HIV-infected patients treated with combination antiretroviral therapy, in which the recovering immune system was able to mount a cellular response. PML- IRIS is a common consequence of plasma exchange (PLEX) immunomodulatory treatment, and thought due to the return of lymphocytes to the brain parenchyma. The influence of IRIS may be heightened when natalizumab is withdrawn and the action of the drug diminished, leading to catastrophic inflammation, with swelling, mass effect and herniation, often requiring high doses of corticosteroids.
In this case, the clinical manifestations of IRIS were limited to mild enhancement of PML lesions on MRI. Histological examination of PML lesions revealed prominent T-cell infiltrates within and at the active edge of lesions, characterized by equal numbers of CD4+ and CD8+ T cells. This differs from PML-IRIS lesions in AIDS patients, who typically have low CD4+ T-cell counts in peripheral blood and a predominance of CD8+ T cells in brain parenchyma (15). Since JCV-specific CD4+ and CD8+ T-cells were detected in blood 4 weeks prior to death, it is possible that T cells in the brain were actively involved in lysing JCV-infected cells. In contrast, inflammatory infiltrates spared chronic MS lesions. Since the patient did not develop an MS exacerbation during her brief course off natalizumab, it appears that the inflammatory reaction associated with IRIS did not lead to an MS-like autoimmune-mediated response directed against myelin. These data suggests that in MS patients with PML-IRIS, T cells are driven by JCV antigen specificity, and do not cause an exuberant response as in HIV-infected patients (15).
PML lesions involve both white and gray matter
PML lesions were extensive in white matter, including involvement of the septum pellucidum, an area that is not classically regarded as a target of JCV infection. This involvement of the septum could result in dissemination of infection to the fornix and hippocampus. Numerous leucocortical and intracortical PML lesions were present, occasionally in close proximity to subpial MS lesions. No subpial lesions of PML, however, were identified, consistent with previous reports (16, 17), suggesting that this location may be unique to MS. In addition, some cortical neurons sustained non-productive infection by JCV, similarly to previous observation in other PML cases (11).
This case exemplifies the risks of natalizumab therapy and provides further insights in PML pathogenesis in the context of MS. This patient's aggressive relapsing form of MS, required multiple immunomodulatory and immunosuppressive therapies before switching to natalizumab, which she received as monotherapy for 32 months. If she was JCV seropositive, like 98% of natalizumab-associated PML cases (2), the risk of her developing PML in the setting of prior immunosuppression use and natalizumab treatment for more than 24 months is estimated to be 1/90, according to a recent algorithm (18). This is significant and second only to the risk in patients with advanced AIDS not receiving antiretroviral treatment, which is 1/20 (19).
Her clinical presentation with progressive cognitive decline and speech difficulties superimposed to her neurological deficit attributed to MS was commensurate with the location of the PML lesions, as reported in other series (3, 4).
Lack of concomitant demonstration of JCV infection and MS lesions in other cases
Our study expands previous biopsy and autopsy findings of natalizumab-associated PML. Earlier biopsy studies found no evidence of JCV infection (6, 7, 9), or only rare single infected cells in 4/5 cases (8). In the original autopsy case report by Kleinschmidt-Demasters et al. (5), no residual MS lesions were identified histologically amongst widespread PML lesions containing numerous cells harboring JCV DNA, suggesting the possibility that PML may have obscured entirely the MS lesions. This also may have occurred in some of the MS lesions in our case, given the extent of PML that was observed. Another reported case (10), in contrast to our patient, demonstrated no detectable JCV-infected cells by IHC for JCV T Ag within extensive demyelinated lesions attributed to PML-IRIS. Failure to demonstrate JCV T Ag by IHC may have been due to technical factors related to the suboptimal sensitivity of PAb416 (DP02), for detection of JCV in formalin-fixed paraffin-embedded tissue (20, 21). Alternatively, it is possible that PML lesions were “burned out” in their case, since the postmortem exam was performed 9 months after the onset of PML (10).
Conclusion
This case demonstrates the coexistence of chronic inactive MS and PML lesions harboring active JCV infection, characterized by the presence of JCV DNA, T Ag and VP1 protein, as well as an inflammatory infiltrates of CD4+ and CD8+ T cells. In addition, restrictive JCV infection of rare cortical pyramidal neurons was demonstrated, consistent with previous observations (11). IRIS greatly altered the characteristics of PML lesions, while sparing MS lesions. Whether PML may alter the nature of active MS lesions remains to be determined.
Acknowledgments
Funding: This study was supported in part by grants from the National Multiple Sclerosis Society and from Biogen Idec.
This study was supported by NMSS grant RG 4523-A-1 and NIH grants R56 NS 041198, R01 NS 047029 and 074995, and K24 NS 060950 (to Dr Koralnik), and by grants from the Saskatchewan Health Research Foundation (2633, to Dr. Popescu), the Canada Research Chairs program (to Dr. Popescu), the National Multiple Sclerosis Society (NMSS RG3185-B-3, to Dr. Lucchinetti), and the National Institutes of Health (1R01NS049577, to Dr. Lucchinetti). The funding agencies had no input in the investigations or analysis of the results.
Footnotes
Authors contributions: Christian Wüthrich was involved in the characterization of the PML lesions with single and double IHC and IFA stainings, preparing and reviewing the manuscript and figures.
Bogdan F.Gh. Popescu was involved in the characterization of the MS lesions with IHC stainings, preparing and reviewing the manuscript.
Sarah Gheuens was involved in reviewing the clinical informations, preparing and reviewing the manuscript.
Michael Marvi took care of patient, provided clinical informations and reviewed the manuscript.
Ronald Ziman took care of patient, provided clinical informations and reviewed the manuscript.
Stephen Pojen Denq took care of patient, provided clinical informations and reviewed the manuscript.
Mylyne Tham was involved with the IHC stainings and the characterization of the cortical MS lesions.
Elizabeth Norton was involved with the single and double IHC and IFA stainings.
Joseph E. Parisi was involved with characterization of the MS and PML lesions, the selection of the tissue samples during the brain autopsy and reviewing the manuscript.
Xin Dang did the molecular analysis of JCV DNA
Claudia F. Lucchinetti was involved in reviewing the clinical informations, the characterization of the MS lesions, preparing and reviewing the manuscript.
Igor J Koralnik was involved in reviewing the clinical informations, the characterization of the PML lesions, preparing and reviewing the manuscript and figures.
Conflict of Interest: Dr. Gheuens and Elizabeth Norton are currently employees of Biogen Idec. Dr. Koralnik receives royalties for chapters in the online textbook UpToDate. Dr. Lucchinetti is listed as author and receives royalties for patent re: Aquaporin-4 associated antibodies for diagnosis of neuromyelitis optica; receives royalties from the publication of Blue Books of Neurology: Multiple Sclerosis 3 (Saunders Elsevier, 2010). Dr. Parisi serves on scientific advisory boards for the US Government Defense Health Board and the FDA and receives royalties from the publication of Principles & Practice of Neuropathology, 2nd ed. (Oxford University Press, 2003).
Dr. Popescu served as a speaker for Teva Innovation Canada.
Ms. Tham, Mr. Marvi, Dr. Dang, Dr. Denq, Dr. Wüthrich and Dr. Ziman declare that they have no competing interests.
References
- 1.Gheuens S, Wuthrich C, Koralnik IJ. Progressive multifocal leukoencephalopathy: why gray and white matter. Annu Rev Pathol. 2013e;8:189–215. doi: 10.1146/annurev-pathol-020712-164018. [DOI] [PubMed] [Google Scholar]
- 2.PML Incidence in Patients Receiving TYSABRI® (natalizumab) [database on the Internet] [Accessed on 7/24/2013];BioGenIDEC. 2013 Available from: http://medinfo.biogenidec.com.
- 3.Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010e;9:438–46. doi: 10.1016/S1474-4422(10)70028-4. [DOI] [PubMed] [Google Scholar]
- 4.Dahlhaus S, Hoepner R, Chan A, Kleiter I, Adams O, Lukas C, Hellwig K, Gold R. Disease course and outcome of 15 monocentrically treated natalizumab-associated progressive multifocal leukoencephalopathy patients. J Neurol Neurosurg Psychiatry. 2013e doi: 10.1136/jnnp-2013-304897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005e;353:369–74. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 6.Kuhle J, Gosert R, Buhler R, Derfuss T, Sutter R, Yaldizli O, Radue EW, Ryschkewitsch C, Major EO, Kappos L, Frank S, Hirsch HH. Management and outcome of CSF-JC virus PCR-negative PML in a natalizumab-treated patient with MS. Neurology. 77:2010–6. doi: 10.1212/WNL.0b013e31823b9b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenhard T, Biller A, Mueller W, Metz I, Schonberger J, Wildemann B. Immune reconstitution inflammatory syndrome after withdrawal of natalizumab? Neurology. 75:831–3. doi: 10.1212/WNL.0b013e3181f07362. [DOI] [PubMed] [Google Scholar]
- 8.Metz I, Radue EW, Oterino A, Kumpfel T, Wiendl H, Schippling S, Kuhle J, Sahraian MA, Gray F, Jakl V, Hausler D, Bruck W. Pathology of immune reconstitution inflammatory syndrome in multiple sclerosis with natalizumab-associated progressive multifocal leukoencephalopathy. Acta Neuropathol. 2011e;123:235–45. doi: 10.1007/s00401-011-0900-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwab N, Hohn KG, Schneider-Hohendorf T, Metz I, Stenner MP, Jilek S, Du Pasquier RA, Gold R, Meuth SG, Ransohoff RM, Bruck W, Wiendl H. Immunological and clinical consequences of treating a patient with natalizumab. Mult Scler. 2012e;18:335–44. doi: 10.1177/1352458511421919. [DOI] [PubMed] [Google Scholar]
- 10.Kleinschmidt-DeMasters BK, Miravalle A, Schowinsky J, Corboy J, Vollmer T. Update on PML and PML-IRIS occurring in multiple sclerosis patients treated with natalizumab. J Neuropathol Exp Neurol. 2012e;71:604–17. doi: 10.1097/NEN.0b013e31825caf2c. [DOI] [PubMed] [Google Scholar]
- 11.Wuthrich C, Koralnik IJ. Frequent infection of cortical neurons by JC virus in patients with progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2012e;71:54–65. doi: 10.1097/NEN.0b013e31823ede59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wuthrich C, Kesari S, Kim WK, Williams K, Gelman R, Elmeric D, De Girolami U, Joseph JT, Hedley-Whyte T, Koralnik IJ. Characterization of lymphocytic infiltrates in progressive multifocal leukoencephalopathy: co-localization of CD8(+) T cells with JCV-infected glial cells. J Neurovirol. 2006e;12:116–28. doi: 10.1080/13550280600716604. [DOI] [PubMed] [Google Scholar]
- 13.Wuthrich C, Dang X, Westmoreland S, McKay J, Maheshwari A, Anderson MP, Ropper AH, Viscidi RP, Koralnik IJ. Fulminant JC virus encephalopathy with productive infection of cortical pyramidal neurons. Ann Neurol. 2009e;65:742–8. doi: 10.1002/ana.21619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucchinetti CF, Parisi J, Bruck W. The pathology of multiple sclerosis. Neurol Clin. 2005e;23:77–105. doi: 10.1016/j.ncl.2004.09.002. vi. [DOI] [PubMed] [Google Scholar]
- 15.Gray F, Bazille C, Adle-Biassette H, Mikol J, Moulignier A, Scaravilli F. Central nervous system immune reconstitution disease in acquired immunodeficiency syndrome patients receiving highly active antiretroviral treatment. J Neurovirol. 2005e;11(Suppl 3):16–22. doi: 10.1080/13550280500511741. [DOI] [PubMed] [Google Scholar]
- 16.Moll NM, Rietsch AM, Ransohoff AJ, Cossoy MB, Huang D, Eichler FS, Trapp BD, Ransohoff RM. Cortical demyelination in PML and MS: Similarities and differences. Neurology. 2008e;70:336–43. doi: 10.1212/01.WNL.0000284601.54436.e4. [DOI] [PubMed] [Google Scholar]
- 17.Lassmann H, Lucchinetti CF. Cortical demyelination in CNS inflammatory demyelinating diseases. Neurology. 2008e;70:332–3. doi: 10.1212/01.wnl.0000298724.89870.d1. [DOI] [PubMed] [Google Scholar]
- 18.Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012e;366:1870–80. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 19.Berger JR, Kaszovitz B, Post MJ, Dickinson G. Progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. A review of the literature with a report of sixteen cases. Ann Intern Med. 1987e;107:78–87. doi: 10.7326/0003-4819-107-1-78. [DOI] [PubMed] [Google Scholar]
- 20.Itoyama Y, Webster HD, Sternberger NH, Richardson EP, Jr, Walker DL, Quarles RH, Padgett BL. Distribution of papovavirus, myelin-associated glycoprotein, and myelin basic protein in progressive multifocal leukoencephalopathy lesions. Ann Neurol. 1982e;11:396–407. doi: 10.1002/ana.410110414. [DOI] [PubMed] [Google Scholar]
- 21.Munoz-Marmol AM, Mola G, Fernandez-Vasalo A, Vela E, Mate JL, Ariza A. JC virus early protein detection by immunohistochemistry in progressive multifocal leukoencephalopathy: a comparative study with in situ hybridization and polymerase chain reaction. J Neuropathol Exp Neurol. 2004e;63:1124–30. doi: 10.1093/jnen/63.11.1124. [DOI] [PubMed] [Google Scholar]