Abstract
Magnetic stimulation of the nervous system, e.g. transcranial magnetic stimulation (TMS), has been used both to unravel basic structure and function of the nervous system as well as to treat neurological diseases, i.e. clinical depression. Despite progress in both areas, ongoing advancements have been limited by a lack of understanding of the mechanism by which magnetic stimulation alters neural activity. Here, we report responses of cortical neurons to magnetic stimulation arising from a sub-millimeter coil. Cell attached patch clamp was used to record neural activity of layer 5/6 pyramidal neurons of the prefrontal cortex (PFC) in the in vitro mouse brain slice preparation. The fields arising from the small coil were quite different from those arising during clinical TMS but nevertheless allowed the responses of cortical neurons to magnetic stimulation to be probed. For example, the focal nature of induced fields allowed the sensitivity of different regions within targeted pyramidal neurons, e.g. apical dendrite, soma and axon hillock, to be compared. We found that PFC pyramidal neurons were not sensitive to single pulses of stimulation regardless of coil location. However, regions of the apical dendrite and proximal axon were both sensitive to repetitive stimulation as long as the orientation of the induced electric field was aligned with the long axis of the neuron. These results suggest that neurons of the PFC are sensitive to weak magnetic fields and further, that this type of approach may be useful for unraveling some of the mechanisms underlying TMS.
I. Introduction
Transcranial magnetic stimulation (TMS) is a method for non-invasively modulating neural activity of the brain [1]. Because it is also pain free and its effects are transient, TMS is an attractive tool for studying brain function and has been used to delineate cortical circuitry as well as to clarify the functional roles for specific cortical regions [2]. The effects of repetitive TMS (rTMS) persist for long periods of time and as such, it is used for the treatment of neurological disease; the best success to date has been for the treatment of depression [3].
Unfortunately however, ongoing improvements in either the quality or the consistency of TMS or rTMS for depression have been limited. Similarly, treatments for other neurological diseases have struggled to demonstrate consistency. The slow pace of progress is thought to occur, at least in part, from an inability to understand the mechanisms of TMS, e.g., a lack of understanding of the neural responses that arise from stimulation as well as an understanding of how to shape such responses by changes to the parameters of stimulation [4].
Attempts to experimentally measure the response of pyramidal and other cortical neurons to TMS have been hampered by methodological challenges. For example, in animal studies, large coil sizes (relative to the size of the brain) activate neurons from multiple regions (including the cortex and deeper regions), impeding the ability to accurately identify response origins [5]. Recently, we showed that neurons of the retina could be activated in vitro by the magnetic fields generated from a micro-coil (0.5 mm diameter × 1.0 mm length) [6]. The small size of these coils opens up a new line of research because it allows the interactions between magnetic stimulation and neurons from other regions of the CNS to be probed in precise detail. Here, we studied the response of L5 pyramidal neurons from mouse pre-frontal cortex (PFC) in the coronal slice to stimulation from such a coil. Our setup allowed translation of the coil so that stimulation could be isolated to different regions of the targeted neuron (e.g. apical dendrite, soma and axon hillock) thereby allowing sensitivity of the different regions to be compared. Rotation of the coil allowed the sensitivity to different field orientations to be compared as well.
II. Methods
A. Preparation of the µMS coil
Air-core multilayer inductors (ELJ-RFR10JFB, Panasonic Electronic Devices Corporation of America, Knoxville, TN) were assembled with copper wires (34-AWG, polyurethane inner coat and nylon over coat) (Belden, Richmond, IN), and then coated with 10 µm thick parylene-C (EIC Laboratories, Norwood, MA, USA). After completion of the coil assembly, total direct current (DC) resistance of the µMS coils was ~8 Ω (range 7.5–8.5 Ω). The coil assemblies were tested before and after each experiment to ensure that there was no leakage of current, e.g. to ensure that elicited responses did not arise from direct electric stimulation.
B. µMS Drive
The output of a function generator (AFG3021B, Tektronix Inc., Beaverton, OR) was connected to a 1,000 W audio amplifier (PB717X, Pyramid Inc., Brooklyn, NY) with a bandwidth of 70 kHz. The µMS coil input was set by the function generator and then amplified; the gain of the amplifier was 2.87 V/V. The shape of the pulsatile waveform output from the function generator was transformed by the amplifier into a multi-phasic waveform consisting of a short-duration, high-amplitude biphasic waveform immediately followed by a lower-amplitude and more prolonged damped sinusoid [6]. For a 1V pulse from the function generator, the amplitude of the biphasic component was 2.87 V and its duration was 20 µs while the peak amplitude of the damped sinusoid was ~1 V and its duration was ~4 ms. This was the amplifier output and therefore the input to the micro-coil. Peak input to the coil ranged from 0–28.7 V and stimulus durations ranged from 20 µs – 1 s. [6] Sinusoidal waveforms were not distorted by the amplifier; coil input amplitudes for sinusoids ranged from 0–6 V and frequencies ranged from 250–10,000 Hz. The audio amplifier was powered by a battery (LC-R1233P, Panasonic Corp., Newark, NJ), thereby uncoupling the stimulation and recording systems.
C. Animal preparation and Electrophysiology
Electrophysiological recordings were performed using brain slices prepared from 17–30 day old mice (C57BL/6J; Jackson Laboratory, Bar Harbor, ME). The 300–400 µm thick coronal slices containing the prefrontal cortex (PFC) were prepared and incubated for two hours at room temperature in an artificial cerebrospinal fluid (aCSF) solution containing (in mM) 125 NaCl, 1.25 NaH2PO4, 2.5 KCl, 25 NaHCO3, 1 MgCl2, 2 CaCl2, and 25 glucose, equilibrated with 95% O2-5% CO2 (pH 7.4). The PFC slices were transferred and mounted, caudal side down, to a plastic recording chamber (RC-27L, Warner Instruments, LLC, Hamden, CT) with plastic slice anchor (SHD-27LP/2, Warner Instruments). The chamber with slices was maintained at 30±2°C, and continuously superfused (3 ml/min) with oxygenated aCSF solution.
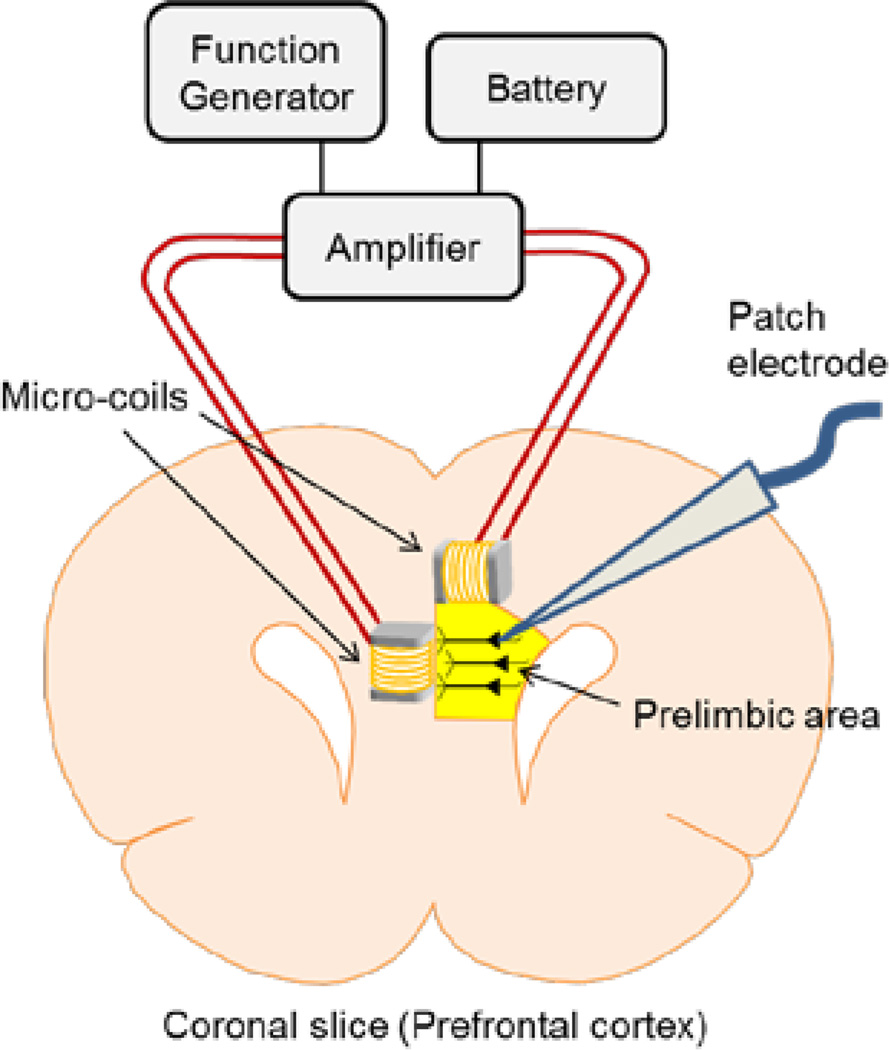
PFC layer 5 pyramidal neurons were targeted under visual control (Fig. 1). The neural signal was recorded with a patch electrode (4–8 MΩ) that was filled with superfusate and positioned onto the surface of a targeted cell soma (cell-attached mode). Two silver-chloride-coated wires served as the ground and were positioned at opposite edges of the recording chamber, each approximately 15 mm from the targeted cell. The micro-coil assembly was fixed in the micromanipulator such that the main axis of the coil was oriented parallel or perpendicular to the brain slice surface (Fig. 1). The coil assembly was lowered into the bath until the bottom edge of the coil was 100 µm above the brain slice surface.
Figure 1. Experimental setup of magnetic stimulation of prefrontal cortex (PFC) layer 5 pyramidal neurons.
Schematic diagram of magnetic stimulation with micro-coils. Function generator output was amplified by the battery-operated amplifier and input to micro-coils. Cell-attached patch clamp was used to record the pyramidal neurons’ responses. Although two different orientations of the coil are illustrated, only a single coil at a time was positioned close to the targeted cell and stimulation was always delivered from only a single coil.
D. Data Analysis
Raw waveforms were recorded at a sample rate of 100 kHz and processed with custom software written in MATLAB. Many elicited responses contained a series of action potentials (spikes); these were confirmed as spikes by comparing them to those spikes elicited spontaneously. The timing of individual spikes was determined with a ‘matched filter’ - the average spontaneous spike was cross-correlated with the response waveform; peaks in the cross correlation were used to assign timing of individual action potentials [6–8].
III. Results
Electrophysiological experiments were conducted using only those PFC layer 5 pyramidal neurons which had large somata (>30 µm in diameter). Consistent with much previous work with PFC neurons [9], all of the pyramidal neurons in our in vitro preparation did not show spontaneous activity. The results below are derived from recordings in 42 cells (25 different slices).
A. Single Pulse Stimulation of PFC pyramidal neurons
We first investigated whether single pulses of magnetic stimulation could be used to activate PFC layer 5 pyramidal neurons. Such neurons typically require strong depolarizing input to initiate spiking. Both monophasic rectangular pulses as well as sinusoidal waveforms (half-periods) with durations ranging from 20 to 1000 µs were tested but neither elicited action potentials, even at the highest amplitudes tested. Translation of the micro-coil across all layers of cortex (1–6) was similarly ineffective as was movement of the coil to regions above the axon. This finding is in contrast to previous studies in both retina [6] and STN [8] for which spikes could be elicited by single pulses from the same coil. There was no increase in spontaneous spiking after the completion of stimulation in PFC pyramidal neurons for any trials (n=25) as occurred for some neurons of the STN.
B. Repetitive Stimulation of PFC pyramidal neurons
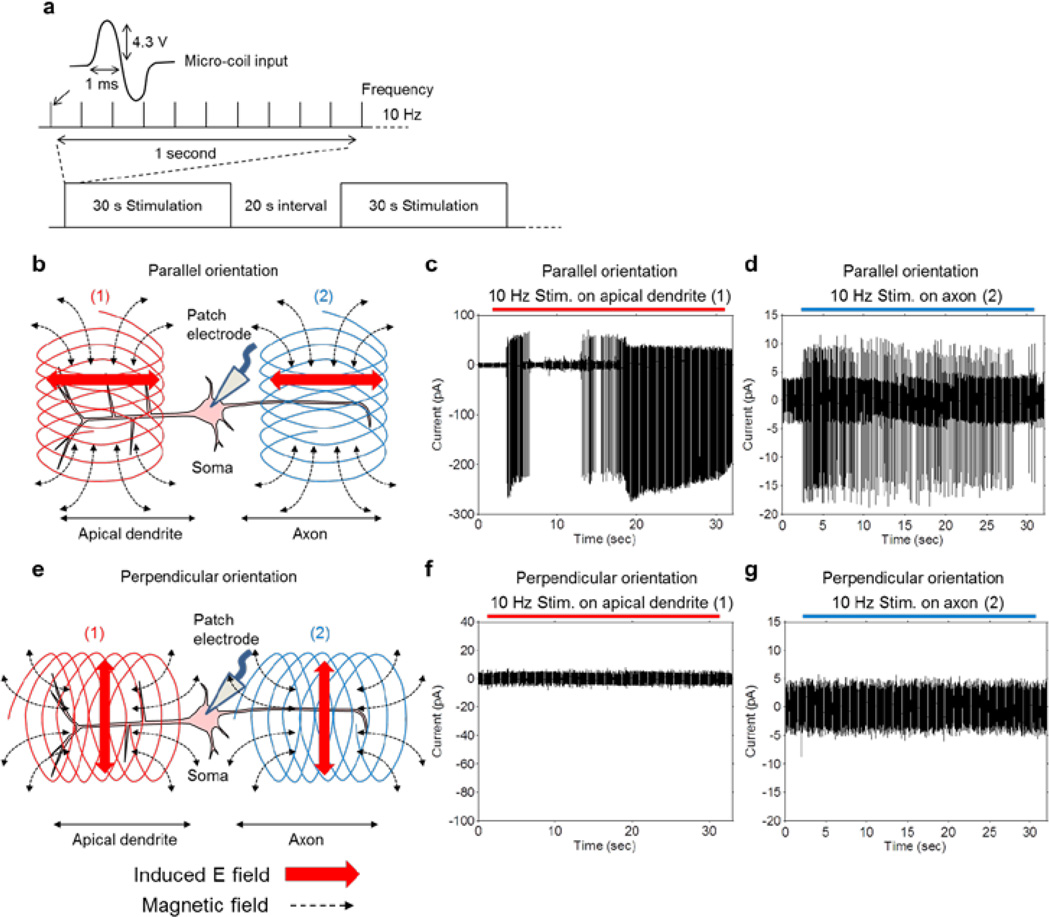
Because of the limited effectiveness of single pulse stimulation, we questioned whether repetitive stimulation might have a sub-threshold effect that would initiate activity over a longer period of time. In a previous study involving stimulation of neurons of the subthalamic nucleus (STN) [8] with the same micro-coil, we found that when half-period sinusoidal waveforms with 1 ms duration (corresponding to a frequency of 500 Hz) and an amplitude of 4.3 V were delivered to the micro-coil repetitively at 10 Hz, neuronal activity was strongly modulated. We therefore utilized this same pattern of stimulation (Fig. 2a), presented continuously for 30 seconds and followed by a ‘rest’ interval of 20 seconds during which no stimulation was delivered; the alternating pattern of stimulation and rest was repeated for 5 minutes.
Figure 2. Coil orientation and position alter PFC pyramidal neurons’ response.
(a) Coil input waveform with repetition rate of 10 Hz. The stimulation was presented for 30 seconds (300 pulses) and this pattern was repeated at every 50 seconds (20 seconds interval). (b) Parallel orientation: the induced electric field (red horizontal arrows) was aligned with the long axis of the pyramidal neurons. Coil was positioned either over the apical dendrite (1) or the axon (2). (c) Typical response from a pyramidal neuron with the coil in the parallel orientation and over the apical dendrite. Pyramidal neurons were strongly activated and generated full size action potentials. (d) Typical response from a pyramidal neuron when the coil was in the parallel orientation but over the axon. Responses consisted of small amplitude bi-phasic waveforms that were most likely antidromic action potentials. Note scale difference from c. (e) Perpendicular orientation of micro-coil: induced electric field (red vertical arrows) was orthogonal to the long axis of targeted pyramidal neurons. Coil was positioned either over the apical dendrite (1) or the axon (2). (f and g) Typical responses from perpendicularly oriented coils either over the apical dendrite (1) or the axon (2): The pyramidal neurons were not activated by the stimulation in either position.
We found that this pattern of stimulation could activate PFC pyramidal neurons but the effect of stimulation was highly dependent on the orientation of the coil. When the coil was oriented such that its central axis was parallel to the slice surface and perpendicular to the principal axis of the pyramidal neuron (referred to as ‘parallel orientation’, Fig. 2b), repetitive stimulation led to strong persistent spiking (Fig. 2c, n=35/35). When the coil was oriented such that its central axis was parallel to the slice surface and also parallel to the principal axis of the pyramidal neuron (referred to as ‘perpendicular orientation’, Fig. 2e), stimulation did not activate the same pyramidal neurons (n=9/9, Fig. 2f). The above experiments were all performed with the coil centered over the apical dendrite (location 1 in Figs. 2b and 2e).
When the coil was moved out over the proximal axon, ~200 µm from the soma (location 2 in Figs. 2b and 2e), stimulation in the parallel orientation elicited spiking (Fig. 2d). However, whereas stimulation over the apical dendrites elicited spiking at a rate >10 Hz with amplitudes of several hundred pA, (Fig. 2c), stimulation over the axon induced smaller amplitude action potentials that occurred at a lower frequency (n=6/6, Fig. 2d, note difference in y-axis scale). Presumably, the small action potentials elicited from axonal stimulation are antidromic spikes that back-propagate to the soma but do not generate full size action potentials [10].
IV. Discussion
The experiments conducted in this study demonstrate that PFC layer 5/6 pyramidal neurons can be activated by magnetic stimulation from a micro-coil. Further, we found that the level of modulation was affected by both the orientation as well as the location of the coil.
Single pulse stimulation is not effective to activate PFC pyramidal neurons
Single pulses of magnetic stimulation were not effective in eliciting spikes in PFC pyramidal neurons in the in vitro preparation. This is not too surprising given the relatively weak strength of the estimated induced electric field (0.2 ~ 0.3 V/m). The finding is also consistent with previous studies that have tried to activate pyramidal neurons with electric stimulation [9]. Pyramidal neurons in the brain slice are generally found to be inactive, probably because most of their excitatory synaptic input, arising from long distance connections, has been lost during preparation of the brain slice.
Repetitive stimulation is effective but the response is heterogeneous
Our results show that PFC layer 5 pyramidal neurons could be activated by repetitive 10 Hz magnetic stimulation arising from a micro-coil (Fig. 2c). However, activation occurred only when the coil axis was orthogonal to the long axis of the targeted neuron i.e. the induced electric field was aligned with the axis of the targeted neurons (red arrows, Fig. 2b). In contrast, spiking was never elicited when the coil axis was aligned with the long axis of the neuron i.e. the induced electric field was orthogonal to the axis of neurons (Fig. 2e), even after prolonged stimulation. Our findings are consistent with previous computational and physiological studies [11] which have shown that electric fields aligned along the axis of targeted neurons are more effective than electric fields oriented in a perpendicular direction. These findings are also consistent with studies in other regions of the CNS indicating that continuous sub-threshold stimulation can modulate neuronal activity. [12]
We found that pyramidal neurons could be strongly activated when the coil was positioned over the apical dendrite. This is somewhat surprising given that much previous work has largely focused on the sensitivity of the axon to magnetic stimulation [13, 14]. While we found that axons were also sensitive to magnetic stimulation, the responses elicited were different from the responses to stimulation over the apical dendrite. It will be interesting in future experiments to learn the downstream effects of these different types of responses.
Activation is mediated by magnetic stimulation
The contrast between the responses from the two different coil orientations provides an important control for demonstrating that the responses shown here do in fact arise from magnetic stimulation. Stimulation with the coil in the perpendicular orientation (Fig. 2e) was never effective while the parallel orientation (Fig. 2b) was always effective. This discrepancy greatly reduces the possibility that other, non-magnetic factors led to neuronal activation. For example, a transient temperature ‘shock’ would likely have been larger from the perpendicular orientation given the larger amount of overlap between coil and cell. Similarly, any capacitive current arising from perpendicular orientation of the coil would have had at least comparable strength to that from parallel orientation and might have been even stronger. Vibration of micro-coils was not observed during stimulation with the parameters used in this study greatly reducing the possibility that activation arises from some form of mechanical stress. Finally, the possibility that activation arose from inductances associated with the supply circuitry, e.g. the wires to and from the amplifier, is also eliminated from consideration because such inductances were identical for the two coil orientations. Thus, we conclude that micro-coils can be used to magnetically activate pyramidal neurons and that such activation can be studied with cell-attached patch clamp in the slice preparation.
Future avenues of research
Further study is underway to better understand the sensitivity of cortical neurons to a wider range of micro-coils and stimulation parameters. Fabrication of coils with different geometries may allow fields to be generated that better match those fields that arise during TMS – if so, this would be of great benefit as it might allow the response of single neurons to TMS to be elucidated.
Acknowledgments
Research supported by the Veterans Administration (MR 1I01RX000350) and by the National Eye Institute (R01-EY019967 and R01-EY023651).
References
- 1.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985 May.325(8437):1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 2.Wassermann EM, Zimmermann T. Transcranial magnetic brain stimulation: therapeutic promises and scientific gaps. Pharmacol Ther. 2012 Jan;133:98–107. doi: 10.1016/j.pharmthera.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, Hallett M, Post RM. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995 Oct 2;6:1853–1856. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- 4.Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007 Jul;8:559–567. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- 5.Salvador R, Miranda PC. Transcranial magnetic stimulation of small animals: a modeling study of the influence of coil geometry, size and orientation. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:674–677. doi: 10.1109/IEMBS.2009.5334070. [DOI] [PubMed] [Google Scholar]
- 6.Bonmassar G, Lee SW, Freeman DK, Polasek M, Fried SI, Gale JT. Microscopic Magnetic Stimulation of Neural Tissue. Nat. Commun. 2012 Jun.3:921. doi: 10.1038/ncomms1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SW, Eddington DK, Fried SI. Responses to pulsatile subretinal electric stimulation: effects of amplitude and duration. J. Neurophysiol. 2013 Apr.109(7):1954–1968. doi: 10.1152/jn.00293.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SW, Fried SI. Magnetic stimulation of subthalamic nucleus neurons using micro-coils for deep brain stimulation; Neural Engineering (NER:2013 6th International IEEE/EMBS Conference on; 2013. pp. 133–135. [Google Scholar]
- 9.Yang CR, Seamans JK, Gorelova N. Electrophysiological and morphological properties of layer V-VI principal pyramidal cells in rat prefrontal cortex in vitro. J. Neurosci. 1996 Mar.16(5):1904–1921. doi: 10.1523/JNEUROSCI.16-05-01904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheffield ME, Best TK, Mensh BD, Kath WL, Spruston N. Slow integration leads to persistent action potential firing in distal axons of coupled interneurons. Nat Neurosci. 2011 Feb.14(2):200–207. doi: 10.1038/nn.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2009 Oct.2(4):215–228. doi: 10.1016/j.brs.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gad P, Choe J, Shah P, Garcia-Alias G, Rath M, Gerasimenko Y, et al. Sub-threshold spinal cord stimulation facilitates spontaneous motor activity in spinal rats. J Neuroeng Rehabil. 2013;10:108. doi: 10.1186/1743-0003-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvador R, Silva S, Basser PJ, Miranda PC. Determining which mechanisms lead to activation in the motor cortex: a modeling study of transcranial magnetic stimulation using realistic stimulus waveforms and sulcal geometry. Clin Neurophysiol. 2011 Apr;122(4):748–758. doi: 10.1016/j.clinph.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maccabee PJ, Amassian VE, Eberle LP, Cracco RQ. Magnetic coil stimulation of straight and bent amphibian and mammalian peripheral nerve in vitro: locus of excitation. J Physiol. 1993 Jan;460:201–219. doi: 10.1113/jphysiol.1993.sp019467. [DOI] [PMC free article] [PubMed] [Google Scholar]