Abstract
Background. Effective tuberculosis control is compromised by a lack of clarity about the timeframe of viable Mycobacterium tuberculosis shedding after treatment initiation under programmatic conditions. This study quantifies time to conversion from smear and culture positivity to negativity in unselected tuberculosis patients receiving standardized therapy in a directly observed therapy short-course (DOTS) program.
Methods. Longitudinal cohort study following up 93 adults initiating tuberculosis therapy in Lima, Peru. Baseline culture and drug susceptibility tests (DSTs) were performed using the MBBacT, proportion, and microscopic observation drug susceptibility (MODS) methods. Smear microscopy and MODS liquid culture were performed at baseline and weekly for 4 weeks then every other week for 26 weeks.
Results. Median conversion time from culture positivity to culture negativity of 38.5 days was unaffected by baseline smear status. Patients with fully susceptible tuberculosis had a median time to culture conversion of 37 days; 10% remained culture positive at day 60. Delayed culture conversion was associated with multidrug resistance, regardless of DST method used; non-multidrug resistance as defined by the proportion method and MODS (but not MBBacT) was also associated with delay. Persistent day 60 smear positivity yielded positive and negative predictive values of 67% and 92%, respectively, for detecting multidrug resistance.
Conclusions. Smear and culture conversion in treated tuberculosis patients takes longer than is conventionally believed, even with fully susceptible disease, and must be accounted for in tuberculosis treatment and prevention programs. Persistent day 60 smear positivity is a poor predictor of multidrug resistance. The industrialized-world convention of universal baseline DST for tuberculosis patients should become the standard of care in multidrug resistance-affected resource-limited settings.
Mycobacterium tuberculosis causes disease in an estimated 9.1 million people annually and kills 1.5 million [1]. The directly observed therapy short-course (DOTS) strategy for global tuberculosis (TB) control depends on case detection by sputum smear and empirical treatment regimens to interrupt transmission [2]. Under DOTS, there has been an increase in case finding, an increase in treatment, and, in some settings, a decrease in the incidence of TB [3,4]. However, a rigorously applied DOTS program has not translated to success in all settings [5,6]. It is increasingly acknowledged that new infections originating from sputum smear—negative (but culture-positive) patients contribute significantly to the burden of TB transmission [7,8]. Patients with culture-positive but smear-negative TB account for 40%–60% of patients [9] but are not identified by sputum smear microscopy, the diagnostic method integral to DOTS programs. Drug resistance, an increasing problem globally [10], is also not detectable in this way. Multidrug-resistant (MDR) TB, estimated to affect 500,000 people worldwide, cannot be effectively treated with empirical DOTS therapy [11,12]. In response to these threats to global TB control, the World Health Organization recently recommended the wider use of sensitive liquid culture methods and drug susceptibility tests (DSTs) [13].
Treatment of TB is generally believed to rapidly reduce infectivity; many hospitals in the industrialized world consider 2 weeks of therapy to be sufficient to permit patients to leave isolation rooms. This dogma arises from observations made >30 years ago describing limited transmission from patients undergoing treatment to their contacts and from the known rapid bactericidal activity of isoniazid [14,15]. Conversion from smear positivity to negativity is often considered an indication of noninfectiousness. However, recent data indicate that variability in the infectiousness of treated hospitalized patients cannot be accounted for solely by smear status [16]. Treatment has been shown to diminish, but not eliminate, the infectiousness of TB shed by patients [15–18], and drug resistance ameliorates the effect of treatment on infectiousness [18]. In addition, untreated, smear-negative, TB culture-positive individuals may be responsible for almost 20% of transmission [7,8], indicating that smear negativity alone is not a good indication of a noninfectious state.
Few systematic data exist detailing the course of infectiousness of treated TB, particularly in developing country settings, even though such information is critical for successful infection control. There are few data regarding sputum smear conversion time, the effect of drug resistance on time to culture negativity, or the effect of drug resistance on time to culture conversion in smear-negative patients.
To address this knowledge gap, we performed a prospective cohort study in which the time for treated patients to become smear and culture negative and the factors that affected conversion were investigated. In addition, the impact of drug resistance was evaluated, and the ability of different DST assays to predict conversion based on susceptibility profiles was assessed.
Methods
Study design, participants, and setting. This prospective cohort study of patients with newly diagnosed pulmonary TB was performed in a well-functioning DOTS program at 15 government health clinics in Lima, Peru, during a period of 15 months. Participants in a diagnostic study investigating the performance of the microscopic observation drug susceptibility (MODS) assay for detection of TB and MDR TB [19] among patients suspected of having TB were invited to take part in this sub-study if they had been diagnosed as having a new episode of pulmonary TB, regardless of prior history of TB. Patients <18 years of age or unwilling or unable to provide written informed consent were excluded.
Sample collection and longitudinal detection of sputum positivity. Participants submitted 2 initial diagnostic sputum samples to the Peruvian national TB program for routine Ziehl-Neelsen staining at local health centers. The same samples were then retrieved and submitted to Universidad Peruana Cayetano Heredia for auramine stain smear microscopy and parallel mycobacterial culture by MODS, MBBacT (bioMérieux),and Lowenstein-Jensen culture [19]. On commencing TB therapy, participants were asked to provide a single sputum sample weekly for the first 4 weeks after the baseline sample then every 2 weeks from week 6 to week 26 after baseline. All samples were collected at health centers during scheduled DOTS visits. Only auramine smear and MODS culture were performed on longitudinal samples. Patients were only considered in the analysis if they contributed samples beyond baseline. All samples received, however scanty or salivary, were processed in the laboratory. If absolutely no sample could be produced the patient was considered as culture negative.
Laboratory methods. Sputum samples were decontaminated by the sodium hydroxide-N-acetyl-l-cysteine method [20]. An aliquot was removed for auramine smear microscopy; smears viewed under × 1000 magnification were graded as negative, 1+ (a total of ≥5 acid-fast bacilli [AFB] visualized in 300 high-power fields examined), or ≥2+ (≥1 AFB visualized in each of at least 35 of 100 high-power fields in slide length). The remaining decontaminate was split and for baseline samples used in parallel Lowenstein-Jensen, automated MBBacT, and MODS cultures as described previously [19] and for follow-up samples only in MODS. Indirect DSTs were performed in the automated MBBacT platform [21,22] and by the proportion method (performed by an external laboratory) for Lowenstein-Jensen isolates [20]. Direct DST was performed by the MODS assay as previously described [19,23,24], with resistance defined as growth in drug-free control wells and in wells containing drugs at the following concentrations: rifampicin, 1 μg/ mL; isoniazid, 0.4μg/mL; and ethambutol, 2.5 μg/mL. The microplate Alamar blue assay [25] was run in parallel for all Lowenstein-Jensen and MODS isolates for use in the event of discrepant susceptibility results. All assays were performed by laboratory personnel who were masked to other results. Pyrazinamide testing was not performed; streptomycin DSTs were performed, but because they were not part of the standard treatment regimen, analysis of the effect of streptomycin resistance on conversion was not undertaken.
Patient treatment. Criteria for commencing therapy were a positive sputum smear for AFB, positive sputum culture (Lowenstein-Jensen or MBBacT), or strong clinical suspicion and abnormal chest radiograph despite 4 negative sputum smears. New patients were started on the first-line regimen of rifampicin, isoniazid, ethambutol, and pyrazinamide for 2 months, followed by a 4-month continuation phase of rifampicin and isoniazid alone. MBBacT and proportion method DST results were given to health care providers.
Statistical analysis. Data were analyzed using Stata statistical software, version 9.1 (StataCorp); and Excel 2003 (Microsoft). The principal outcomes of interest were time to sputum smear and culture conversion and analysis of factors potentially influencing these end points: baseline auramine smear, baseline Ziehl-Neelsen smear, age, sex, and drug resis-tance as determined by MBBacT, proportion, and MODS DST results. Cumulative positive-to-negative conversion of aura-mine smear microscopy and MODS culture was assessed by Kaplan-Meier analysis. Auramine or culture conversion was defined by 2 consecutive negative results with the number of days to conversion calculated from the baseline diagnostic visit to the mean between the last positive and first negative sample. Patients who were lost to follow-up were censored at their terminal sample. Four patients with missing MBBacT, proportion, or MODS DST data were excluded from the DST Kaplan-Meier subanalysis. Variables with a P value >.10 were tested for compatibility with Cox proportional hazards assumption using log-log plots. Statistical differences between survival curves were determined using the log-rank test. Associations between conversion time and patient and disease characteristics were quantified in Cox regression analysis. Multivariate Cox regressions were performed to determine the interactions of MDR and non-MDR conditions. Fisher's exact 2-tailed test was used to calculate statistical differences between population characteristics, except for age, for which the Student t test for equality of means was used. The McNemar χ2 test for matched pairs was used to determine the concordance of DST methods.
Ethics review. The study protocol and consent forms were approved by the institutional review boards of Universidad Peruana Cayetano Heredia, Asociación Benéfica PRISMA, Dirección de Salud–III Lima Norte, Dirección de Salud–IV Lima Este, Imperial College London, and Johns Hopkins Bloomberg School of Public Health.
Results
Population characteristics. The baseline demographic and TB disease characteristics of the study population are outlined in Table 1. Of 1687 individuals with suspected TB enrolled in the parent study [19], 202 were found to be culture positive for pulmonary TB, of whom 93 (46.0%) participated in this study. All smear-positive patients were found to be culture positive. Recruited individuals were more likely to be sputum smear positive and less likely to have drug-resistant disease than those who were eligible but not recruited (P < .001 for each). After enrollment only 8 patients (8.6%) of the 93 dropped out before culture conversion was reached. For those who left the study, the mean follow-up period was 103 days. No significant demographic differences were found when patients were stratified on the basis of recruitment status, auramine positivity, or drug-resistant disease. Drug-resistant TB was frequent in the study cohort, although results varied between DST methods (Table 2). In general, MODS and the proportion method gave similar, more conservative estimates of isoniazid, ethambutol, and any resistance than MBBacT. MODS and the proportion method identified MDR disease in 6.6% and 7.5% of participants respectively, compared with 9.9% by MBBacT.
Table 1.
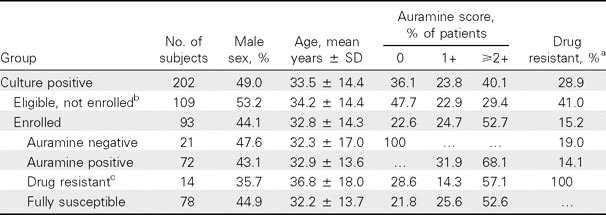
Study Population Characteristics
Table 2.
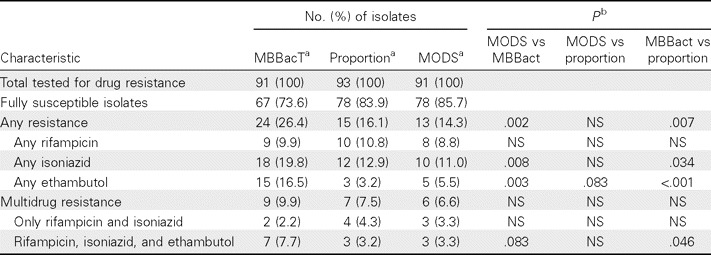
Comparison of Resistance Profiles, as Predicted by Different Susceptibility Tests
Overall time to auramine and culture conversion. Initially, Kaplan-Meier survival curves for auramine and culture conversion decreased steeply. However, neither auramine nor culture reached complete conversion by the end of the study (Figure 1). All patients were AFB smear negative at or before culture conversion. For patients who were auramine smear positive at baseline, smear negativity was reached in a median of 17.5 days (95% confidence interval [CI], 10–23.5 days) and culture negativity in 40.5 days (95% CI, 35.5–48 days); it took 47.5 days (95% CI, 36–64 days) for 90% to become auramine smear negative. Overall median time to culture conversion was 38.5 days (95% CI, 33–43.5 days), with no significant difference among patients based on baseline auramine status (P = .427). It took 93 days for 90% of participants to convert to culture negativity. Of patients still culture positive at day 60, only 18.8% remained smear positive. No patients reverted to culture positivity after conversion to culture negativity.
Figure 1.
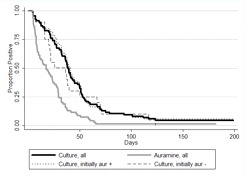
Kaplan-Meier survival estimate of time to auramine and culture conversion.
Risk factors for delayed sputum smear and culture conversion. A univariate Cox regression analysis was performed to determine factors that influence conversion times to smear and culture negativity (Table 3). Baseline positive or negative auramine smear status was not a significant predictor of culture conversion. Only the highest baseline auramine smear counts (≥2+) were predictive of longer times to smear and culture conversion (Figure 2). Regardless of the DST method used to classify resistance, MDR TB was highly significantly associated with longer conversion times for both smear and culture.
Table 3.
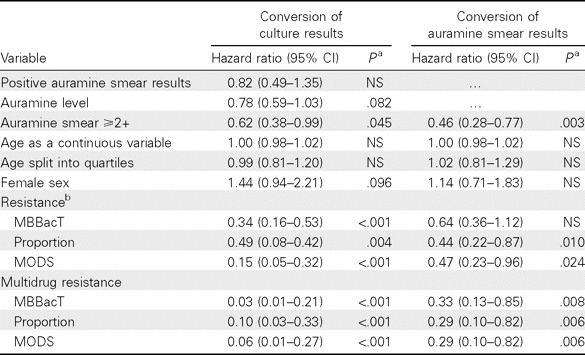
Univariate Cox Regression of Time to Auramine Smear and Culture Negativity
Figure 2.
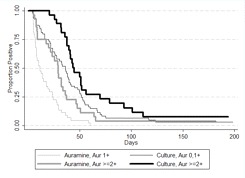
Kaplan-Meier survival estimate of time to auramine and culture conversion comparing high and low baseline auramine levels.
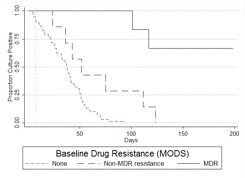
Culture conversion time as predicted by resistance profiles. Because the DST results from MBBacT, the proportion method, and MODS varied slightly, mutually exclusive survival curves were derived for patients with fully susceptible, MDR, and non-MDR disease to elucidate the relative importance of different degrees of resistance (Figure 3) and the comparative strength of effect according to the DST method used to define resistance. When all forms of resistance were excluded, the conversion curves decreased linearly, reaching 90% conversion by 60 days for participants with fully susceptible TB and median conversion between 36.5 and 37.5 days, regardless of the DST method. Of patients with MDR TB, 50%, 67%, and 67% as defined by the MBBacT, proportion, and MODS methods, respectively, remained culture positive at the end of the study. Compared with patients with fully susceptible disease, non-MDR disease, as defined by either MODS or the proportion method, was associated with a doubling of the time taken for 90% to achieve conversion, to 123.5 days (MODS, P = .010; proportion method, P = .029). Non-MDR disease defined by the MBBacT test was not found to be statistically significantly associated with delayed conversion time (P = .148). After controlling for the effect of MDR disease with multivariate Cox regression analysis, non-MDR disease as defined by the proportion method and MODS, but not by MBBacT, remained a significant predictor of delayed time to culture conversion (P = .007 and P = .002, respectively).
Figure 3.
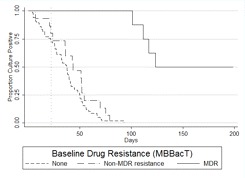
A, Kaplan-Meier survival estimate of time to culture conversion comparing resistance levels with baseline drug susceptibility tests (DSTs) by MBBacT. Log-rank test for equality of survivor function comparing non-multidrug-resistant (non-MDR) tuberculosis (TB) versus fully drug-susceptible TB (P = nonsignificant). Vertical line at 22 days indicates the median time to DST results using the MBBacT test after primary isolation by MBBacT. B, Kaplan-Meier survival estimate of time to culture conversion comparing resistance levels with baseline DSTs by proportion test. Log-rank test for equality of survivor function comparing non-MDR TB versus fully drug-susceptible TB (P = .029). Vertical line at 68 days indicates the mean time to DST results using the proportion test after primary isolation in Lowenstein-Jensen culture. C, Kaplan-Meier survival estimate of time to culture conversion comparing resistance levels with baseline DSTs by microscopic observation drug susceptibility (MODS). Log-rank test for equality of survivor function comparing non-MDR TB versus fully drug-susceptible TB (P = .010). Vertical line at 7 days indicates the median time to DST results using the MODS assay.
Utility of delayed smear conversion as surrogate of MDR TB. The association between delayed auramine smear conversion and MDR TB is examined in Table 4. At treatment initiation, 66.7% of patients with culture-positive MDR TB were sputum smear positive; by day 120, 80% of patients with persistently culture-positive MDR TB were smear negative. The positive predictive value of day 60 persistent smear positivity as a surrogate for MDR TB was 66.7%; the negative predictive value was 92.1%.
Table 4.
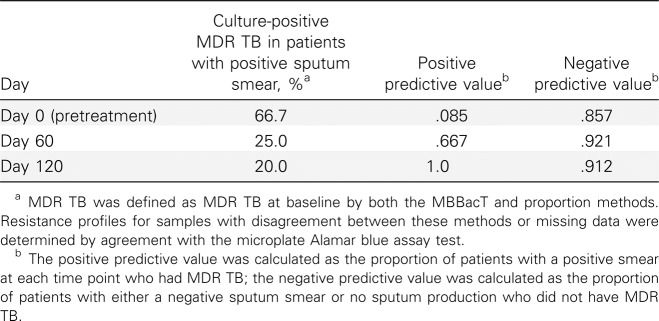
Predictive Value of Persistently Positive Smear Results for Multidrug-Resistant (MDR) Tuberculosis (TB)
Discussion
This study demonstrates that patients with TB who have fully susceptible disease remain sputum culture positive for much longer than is conventionally believed, regardless of baseline smear status. The influence of baseline sputum bacillary load on smear and culture conversion is relatively minor except at very high smear positivity. Conversion from smear or culture positivity to negativity is significantly delayed by both MDR and resistance that is not MDR, but the relative importance varies, depending on the DST method used to define resistance. Persistent smear positivity at day 60 is a poor predictor of MDR and thus not a good surrogate for DST.
The data presented here question the notion that patients with TB who are culture positive at baseline are noninfectious after 2 weeks of treatment or a negative smear. Most patients are both smear and culture positive at 2 weeks, and significant proportions remain positive for months. Even in patients with drug-susceptible disease receiving optimal therapy, median time to culture negativity is 37 days, whereas 10% remain culture positive at day 60. There is a growing body of evidence supporting these findings [26–29], but no studies have the intensity of participant follow-up achieved here, which, to the best of our knowledge, is the most comprehensive to date. By obtaining weekly cultures during the initial phase, we were able to generate data-rich survival curves capturing conversions close to the actual conversion day. A further strength was the use of MODS for more sensitive follow-up than conventional solid media culture.
Under most DOTS treatment regimens, patients step down therapy from intensive to consolidation-phase treatment after 60 days, although treatment is sometimes prolonged if the patient remains smear positive. Our data indicate frequent persistent culture positivity among smear-negative patients at this time point; this may partly explain high rates of apparent recurrence of MDR among patients considered “cured” by smear diagnosis [30]. The relative merit in resource-limited settings of using culture negativity rather than smear negativity to define cure and predict nonrecurrence warrants further evaluation.
Similarly, persistent smear positivity at specific time points in patients undergoing therapy is sometimes used as the principal trigger to perform DSTs. In Peru, day 120 smear positivity was previously used as an indication of likely MDR disease, although 60 days has also been recommended [31]. This study confirmed that only patients with MDR TB remain smear positive late (day 120) in treatment. However, the positive and negative predictive values of day 60 smear positivity as a surrogate for MDR TB were 66.7% and 92.1%, respectively, which is entirely unhelpful for the clinician evaluating an individual patient. Waiting 120 days to decide whether a DST is indicated facilitates ongoing nosocomial, domestic, and community transmission and compromises patient outcome.
MDR TB was the single best predictor of prolonged M. tuberculosis shedding, as detected by smear or culture; non-MDR TB was also important in delaying culture conversion [32]. All patients who were culture positive beyond 70 days were resistant to at least 1 drug, and a high proportion of patients with MDR TB shed viable mycobacteria beyond study completion termination. Similar results were seen in another, less follow-up intensive, hospital-based study in Peru [28], whereas a Canadian study demonstrated a comparable overall trend but with more rapid conversion rates [26]. Because delayed DSTs are strongly predictive of poor treatment response in patients with MDR TB [11], it is clear that only rapid, early DSTs can ensure that patients quickly receive effective treatments and minimize shedding of viable MDR mycobacteria.
Through repeated testing of multiple samples we were able to directly compare the predictive capabilities of the different DST methods. MDR TB as defined by the proportion and MODS tests showed slower culture conversion than that defined by MBBacT, indicating that the MODS and proportion tests more accurately identified strains that responded poorly to standard treatment. In addition, patients with non-MDR infections as detected by MODS or proportion tests, but not MBBacT, took significantly longer to convert than patients with fully susceptible strains. These data suggest that MBBacTdefined resistance was less clinically relevant than resistance as defined by MODS or proportion method DST.
Certain assumptions and limitations warrant discussion. Infectiousness is related not only to the presence of viable mycobacteria but also to the propagation of these organisms by cough, which may be reduced by TB therapy. Data on cough frequency were not collected, so we cannot describe this aspect of infectiousness. Next, it is possible that in vitro recovery of mycobacteria from expectorated sputum of treated patients may not be directly correlated with fitness to cause in vivo infection. Eligible study nonparticipants were more commonly smear negative and drug resistant; 100% participation might have altered the overall survival curves but would not have modified the subgroup analyses. The pyrazinamide DST was not routinely performed in this study, although pyrazinamide is a potent agent largely credited with the reduction in treatment duration achieved by short-course chemotherapy. Because of the inherently long laboratory delays, the proportion method DST results were available and acted on for only 4 identified patients with MDR TB during the study period; 2 received tailored regimens initiated more than 2 weeks before study completion, making the impact of specific treatment minimal. Although universally available within a few weeks, MODS results could not be used because MODS was an investigational test at this time. Human immunodeficiency virus (HIV) status was not established here, but HIV rates are low in Peru (∼2% patients with TB) [1], so these data cannot be extrapolated to populations with a high prevalence of HIV infection.
The need to expand rapid culture, early DST, and MDR-specific treatment regimens to developing countries is increasingly acknowledged [1,10–12,31–33]. Our data demonstrate that patients with TB undergoing treatment remain infectious for unexpectedly prolonged periods. It took 2 months of standard therapy to effect culture conversion in 90% of with patients TB with fully susceptible disease and at least twice as long to achieve the same with patients who have non-MDR TB, and <50% of patients with MDR TB became culture negative by the conclusion of the study. All extended shedders were drug resistant and most were smear negative, demonstrating that these issues are inextricably linked. Long-term smear-negative shedding and drug resistance must be considered jointly if TB transmission is to be controlled.
Figure 3.
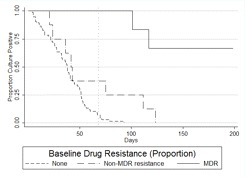
Figure 3.
Acknowledgments
We thank Fanny Garcia, Eleana Sanchez, Sonia Lopez, Rosmery Gutierrez, and Christian Solis for outstanding field work and Paula Maguina, Ana Rosa Contreras, and Teresa Rios for administrative support. We acknowledge the contributions of Juan Agapito (Hospital Nacional Cayetano Heredia) for the proportion method DST and Pilar Navarro for auramine smear microscopy. We express gratitude to Don Juanito and the study participants for their cooperation.
Financial support. This study was supported by the Wellcome Trust (grant 064672/Z/01/Z, D.A.J.M.), the National Institute for Health Research Biomedical Research Centre funding scheme at Imperial College (J.S.F. and D.A.J.M.), the National Institute of Allergy and Infectious Diseases (grant T35AI065385, S.P.F.), the National Institutes of Health/Fogarty Global Research Training (grant 3D43 TW006581-04S1, R.H.G.), and the National Institutes of Health/Fogarty International Clinical Research Scholar Awards (K.P.R.). Funders took no part in the design or interpretation of the study. The content of this article is solely the responsibility of the authors and does not necessarily represent the views of the funding sources.
Potential conflicts of interest. All authors: no conflicts.
References
- 1.World Health Organization. Report WHO/HTM/TB/ 2008.393. Geneva, Switzerland: World Health Organization; 2008. Global tuberculosis control: surveillance, planning, financing: WHO report 2008. [Google Scholar]
- 2.Stop TB Partnership World Health Organization. Report WHO/HTM/STB/2006.35. Geneva, Switzerland: World Health Organization; 2006. Global plan to stop TB 2006–2015. [Google Scholar]
- 3.The effect of tuberculosis control in China. Lancet. 2004;364:417–422. doi: 10.1016/S0140-6736(04)16764-0. [DOI] [PubMed] [Google Scholar]
- 4.Suarez PG, Watt CJ, Alarcon E, et al. The dynamics of tuberculosis in response to 10 years of intensive control effort in Peru. J Infect Dis. 2001;184:473–478. doi: 10.1086/322777. [DOI] [PubMed] [Google Scholar]
- 5.Huong NT, Duong BD, Co NV, et al. Tuberculosis epidemiology in six provinces of Vietnam after the introduction of the DOTS strategy. Int J Tuberc Lung Dis. 2006;10:963–969. [PubMed] [Google Scholar]
- 6.Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban com-munity in South Africa: the need for age-specific interventions. Clin Infect Dis. 2006;42:1040–1047. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- 7.Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Garduno E, Cook V, Kunimoto D, Elwood RK, Black WA, FitzGerald JM. Transmission of tuberculosis from smear negative patients: a molecular epidemiology study. Thorax. 2004;59:286–290. doi: 10.1136/thx.2003.011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mase SR, Ramsay A, Ng V, et al. Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis. 2007;11:485–495. [PubMed] [Google Scholar]
- 10.World Health Organization. Report WHO/HTM/TB/2007.387. Geneva, Switzerland: World Health Organization; 2007. The global MDR-TB & XDR-TB response plan 2007–2008. [Google Scholar]
- 11.Bonilla CA, Crossa A, Jave HO, et al. Management of extensively drug-resistant tuberculosis in Peru: cure is possible. PLoS ONE. 2008;3:e2957. doi: 10.1371/journal.pone.0002957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Report WHO/HTM/TB/2008.394. Geneva, Switzerland: World Health Organization; 2008. Anti-tuberculosis drug resistance in the world, fourth global report. [Google Scholar]
- 13.World Health Organization. Report on Conclusions and Recommendations of the Strategic and Technical Advisory Group for Tuberculosis, Seventh Meeting. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 14.Clinical diagnosis and management of tuberculosis and measures for its prevention and control. London, UK: National Institute for Health and Clinical Excellence; 2006. [PubMed] [Google Scholar]
- 15.Rouillon A, Perdrizet S, Parrot R. Transmission of tubercle bacilli: the effects of chemotherapy. Tubercle. 1976;57:275–299. doi: 10.1016/s0041-3879(76)80006-2. [DOI] [PubMed] [Google Scholar]
- 15.Escombe AR, Oeser C, Gilman RH, et al. The detection of airborne transmission of tuberculosis from HIV-infected patients, using an in vivo air sampling model. Clin Infect Dis. 2007;44:1349–1357. doi: 10.1086/515397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menzies D. Effect of treatment on contagiousness of patients with active pulmonary tuberculosis. Infect Control Hosp Epidemiol. 1997;18:582–586. doi: 10.1086/647678. [DOI] [PubMed] [Google Scholar]
- 18.Riley RL, Mills CC, O'Grady F, Sultan LU, Wittstadt F, Shivpuri DN. Infectiousness of air from a tuberculosis ward: ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis. 1962;85:511–525. doi: 10.1164/arrd.1962.85.4.511. [DOI] [PubMed] [Google Scholar]
- 19.Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355:1539–1550. doi: 10.1056/NEJMoa055524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Report WHO/tb/98.258. Geneva, Switzerland: World Health Organization; 1998. Laboratory services in TB control, parts I, II, and III. [Google Scholar]
- 21.Barreto AM, Araujo JB, de Melo Medeiros RF, de Souza Caldas PC. Evaluation of indirect susceptibility testing of Mycobacterium tuber culosis to the first-and second-line, and alternative drugs by the newer MB/BacT system. Mem Inst Oswaldo Cruz. 2003;98:827–830. doi: 10.1590/s0074-02762003000600020. [DOI] [PubMed] [Google Scholar]
- 22.Yew WW, Tong SC, Lui KS, Leung SK, Chau CH, Wang EP. Comparison of MB/BacT system and agar proportion method in drug susceptibility testing of Mycobacterium tuberculosis. Diagn Microbiol Infect Dis. 2001;39:229–232. doi: 10.1016/s0732-8893(01)00237-1. [DOI] [PubMed] [Google Scholar]
- 23.Caviedes L, Lee TS, Gilman RH, et al. The Tuberculosis Working Group in Peru. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. J Clin Microbiol. 2000;38:1203–1208. doi: 10.1128/jcm.38.3.1203-1208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore DA, Mendoza D, Gilman RH, et al. Microscopic observation drug susceptibility assay, a rapid, reliable diagnostic test for multidrugresistant tuberculosis suitable for use in resource-poor settings. J Clin Microbiol. 2004;42:4432–4437. doi: 10.1128/JCM.42.10.4432-4437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franzblau SG, Witzig RS, McLaughlin JC, et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol. 1998;36:362–366. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortun J, Martin-Davila P, Molina A, et al. Sputum conversion among patients with pulmonary tuberculosis: are there implications for removal of respiratory isolation? J Antimicrob Chemother. 2007;59:794–798. doi: 10.1093/jac/dkm025. [DOI] [PubMed] [Google Scholar]
- 27.Long R, Bochar K, Chomyc S, et al. Relative versus absolute noncontagiousness of respiratory tuberculosis on treatment. Infect Control Hosp Epidemiol. 2003;24:831–838. doi: 10.1086/502145. [DOI] [PubMed] [Google Scholar]
- 28.Kawai V, Soto G, Gilman RH, et al. Tuberculosis mortality, drug resistance, and infectiousness in patients with and without HIV infection in Peru. Am J Trop Med Hyg. 2006;75:1027–1033. [PMC free article] [PubMed] [Google Scholar]
- 29.Telzak EE, Fazal BA, Pollard CL, Turett GS, Justman JE, Blum S. Factors influencing time to sputum conversion among patients with smear-positive pulmonary tuberculosis. Clin Infect Dis. 1997;25:666–670. doi: 10.1086/513772. [DOI] [PubMed] [Google Scholar]
- 30.Migliori GB, Espinal M, Danilova ID, Punga VV, Grzemska M, Raviglione MC. Frequency of recurrence among MDR-tB cases ‘successfully’ treated with standardised short-course chemotherapy. Int J Tuberc Lung Dis. 2002;6:858–864. [PubMed] [Google Scholar]
- 31.Chavez Pachas AM, Blank R, Smith Fawzi MC, Bayona J, Becerra MC, Mitnick CD. Identifying early treatment failure on category I therapy for pulmonary tuberculosis in Lima Ciudad, Peru. Int J Tuberc Lung Dis. 2004;8:52–58. [PubMed] [Google Scholar]
- 32.Espinal MA, Kim SJ, Suarez PG, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283:2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 33.Suarez PG, Floyd K, Portocarrero J, et al. Feasibility and cost-effectiveness of standardised second-line drug treatment for chronic tuberculosis patients: a national cohort study in Peru. Lancet. 2002;359:1980–1989. doi: 10.1016/S0140-6736(02)08830-X. [DOI] [PubMed] [Google Scholar]


