Abstract
Aging in rodents and men is associated with reduced serum levels of testosterone and Leydig cell testosterone productions. To further investigate the mechanism by which Leydig cell testosterone production declines, the effect of knocking out Nrf2, a master regulator of phase 2 antioxidant genes, was examined. In wild-type mice, testosterone production and serum testosterone levels remained unchanged through middle age (8 months), but then were reduced significantly by old age (21–24 months). In contrast, serum testosterone levels and Leydig cell testosterone production were reduced significantly in the Nrf2−/− mice as early as middle age, and were reduced further in the aged mice. Reduced steroidogenesis in the knockout mice was associated with reduced antioxidant capacity, and increased expression of protein nitrotyrosine residues, a marker of ROS. These results support the hypothesis that, over time, increases in oxidative stress contribute to or cause the reduced testosterone production that characterizes Leydig cell aging.
Keywords: Leydig cells, Nrf2, aging, oxidative stress
1. INTRODUCTION
Aging in rodents and men is associated with reduced serum testosterone concentrations (Araujo et al., 2004; Chen et al., 1994; Harman et al., 2001). In Brown Norway rats, serum levels of luteinizing hormone (LH) do not change with age, but there is reduced responsiveness of Leydig cells to LH that results in reduced testosterone production and therefore in reduced serum testosterone levels (Chen et al., 2004, 2002, 1994). A number of deficits have been identified in the steroidogenic pathway of old Leydig cells that might account for the reduced LH-stimulated testosterone production, including reductions in cAMP production, steroidogenic acute regulatory protein (STAR), translocator protein (TSPO), cholesterol translocation from the cytosol into the mitochondria, cholesterol metabolism to pregnenolone in the mitochondria, and downstream steroidogenic enzymes of the mitochondria and smooth endoplasmic reticulum (Culty et al., 2002; Leers-Sucheta et al., 1999; Liao et al. 1993; Luo et al., 2005, 2001, 1996; Zirkin and Chen, 2000). Although the mechanism by which these changes occur remains uncertain, imbalance between the production of reactive oxygen species (ROS) and intracellular antioxidant defenses has been proposed (Cao et al., 2004; Chen et al., 2001, 2008; Chen and Zirkin, 1999; Lacombe et al., 2006; Luo et al., 2006).
It is well established that redox imbalance, over time, can cause damage to lipids, proteins, RNA, and DNA, and thus contribute to cellular functional changes (Ames et al., 1993; Beckman and Ames, 1998; Raha and Robinson, 2000; Rebrin and Sohal, 2008). Consistent with this, there is evidence, though largely correlative, that the redox imbalance that occurs with the aging of Leydig cells may lead to reductions in steroid formation. The antioxidant defense molecules superoxide dismutase-1 and -2, glutathione peroxidase and glutathione (GSH) are significantly reduced as Leydig cells age (Cao et al., 2004; Luo et al., 2006), while the superoxide content of the mitochondria (Chen et al., 2001) and lipid peroxidation (Cao et al., 2004) significantly increase.
In a study designed to ascribe cause-effect relationships between an altered redox environment and reduced steroid formation, experimentally induced reduction in glutathione (GSH), an important antioxidant defense molecule of the Leydig cells, was shown to result in reduced steroidogenic function both in vitro and in vivo (Chen et al., 2010, 2008). As with many “aging” studies, these studies examined the acute effects of altering the redox environment in young cells, not effects occurring over time. Additionally, the redox environment of cells was experimentally altered by depleting an individual antioxidant (GSH, in this case), which may be misleading because of compensatory changes that might occur when one of many cellular antioxidants is experimentally altered.
Nrf2 (nuclear factor erythroid 2-related factor) is a master transcriptional factor that binds a common antioxidant response element in the promoters of various Nrf2 target genes (Cho et al., 2006; Giudice and Montella, 2006; Itoh et al., 2004a; Kobayashi and Yamamoto, 2006; Li and Kong, 2009; Osburn and Kensler, 2008; Zhang, 2006). Stimulation of Nrf2 activates an array of Nrf2-responsive genes and of their protein products, thus eliciting protection against a broad range of endogenous and exogenous factors that otherwise might disrupt cellular homeostasis through oxidative damage (Cho et al., 2005; Kwak et al., 2003; Lee et al., 2003). In the present study, we hypothesized that if alteration of the redox environment of aging Leydig cells indeed plays a significant role in age-related reductions in Leydig cell steroidogenesis, the experimental depletion (knockout) of Nrf2 should, over time, result in increased oxidative stress by inhibiting antioxidant protein synthesis, and this should result in decreases in testosterone production.
2. MATERIALS AND METHODS
2.1. Animals
Nrf2-deficient C57BL/6 mice (Nrf2−/−, Japan SLC, Inc.) were generated as described previously (Itoh et al., 1997). Mice were fed an AIN-76A diet and water ad libitum and housed under controlled conditions (23 ± 2°C; 12-hour light/dark periods). Experiments were conducted in accordance with the standards established by the United States Animal Welfare Act, set forth in NIH guidelines and the Policy and Procedures Manual of the Johns Hopkins University Animal Care and Use Committee.
2.2. Experimental procedures
Young (3 month-old, N=18), middle aged (8 month-old, N=12), and aged (24 month-old, N=12) wild-type (Nrf2+/+ ) and knockout (Nrf2−/−) mice were used in these studies. Blood was collected at about the same time each collection day to minimize variations in serum testosterone, and immediately placed on ice. Blood serum was prepared by centrifugation at 1500 g for 10 min (4 °C). The serum was frozen on dry ice and stored at −80 °C for subsequent analysis of testosterone and luteinizing hormone LH. One testis from each of 12 rats was used for the isolation of Leydig cells. The ability of the isolated cells to produce testosterone was assessed in vitro. One testis from each of 6 rats was frozen in liquid nitrogen for assay of total antioxidant activity (see below). The reminding contralateral testes were either fixed in 5% glutaraldehyde and embedded in Epon-Araldite for morphological analysis, or fixed in formaldehyde and embedded in paraffin for immnohistochemistry.
2.3. Leydig cell isolation and assessment of steroidogenic function
Leydig cells were isolated by a combination of Percoll and BSA density gradient centrifugation, as previously described (Salva et al 2001). In brief, the testes were decapsulated and digested in dissociation buffer (M-199 medium with 2.2 g/L HEPES, 1.0 g/L BSA, 2.2 g/L sodium bicarbonate) containing collagenase I (0.5 mg/mL) at 34°C, with slow shaking (90 cycles/min, 30 minutes). To separate the interstitial cells from the seminiferous tubules, digested testes were placed in a solution containing 1% BSA for one minute. The supernatants were collected and the interstitial cells were pelleted by centrifugation (1500g, 5 min). Leydig cells were purified by Percoll gradient separation (55% Percoll, 27,000g, 1hr) and then BSA gradient centrifugation (0–10% BSA, 450g, 10 min). The final purity of the Leydig cells, determined by staining the cells for 3β-hydroxysteroid dehydrogenase (3β-HSD) activity, was consistently about 90%.
Freshly isolated Leydig cells were suspended in M-199 culture media (0.1% BSA), plated in 96-well culture plates (about 104 cells/well), and incubated for 2h at 34°C with ovine LH (10 ng/ml), dibutryl cAMP (dbcAMP, 1mM), 22-hydroxycholesterol (22-HC, 25μM), or pregnenolone (P5, 25μM). After incubation, cells plus medium were frozen on dry ice and then stored at −80 °C for testosterone assay.
2.4. Serum testosterone and luteinizing hormone (LH) assay
Serum testosterone concentration and Leydig cell testosterone production was assayed by radioimmunoassay (RIA). The sensitivity of the assay was 10 pg/tube. The intra- and inter-assay coefficients of variation were 8.9% and 13.6%. Serum LH concentrations were determined by an enzyme linked immunosorbent assay (ELISA) kit (Biotang Inc., Lexington, MA). The minimum detectable dose of mouse LH was 0.08 ng/ml. The detection range was 0.08/ml −20 ng/ml. All samples were run in one assay with intra-assay coefficient of variation was below 15%.
2.5. Western blot analysis
Isolated Leydig cells were lysed with Tris-sodium dodecyl sulfate (SDS) buffer [62.5 mM Tris, 2% SDS, 50 mm dithiothreitol (pH 6.8)] and sonicated on ice. Lysates were mixed with 3× SDS loading buffer (New England BioLab, Ipswich, MA). Total protein from equal numbers of cells (1 × 105) was separated by 10% SDS-PAGE and then transferred onto a nitrocellulose membrane. After incubation with primary antibody (1:400) and horseradish peroxidase-conjugated secondary antibody (1:5000), signal was detected by use of the enhanced chemiluminescence Western blot kit from GE Healthcare Life Sciences (Piscataway, NJ). The bound antibodies on the membranes were stripped by Restore Western blot stripping buffer (Pierce, Rockford, IL), and the membranes were re-probed with new antibodies in the sequence STAR (1:1000, ABR-Affinity BioReagents, Golden, CO), CYP11A1 (1:1000, Chemicon International, Temecula, CA), and GAPDH (1:5000, Sigma-Aldrich, St. Louis, MO).
2.6. Testis morphology and immunostaining for nitrotyrosine
For morphological studies, testes were fixed with 5% glutaraldehyde in 0.05 M cacodylate buffer, postfixed in 1% buffered osmium tetroxide, and embedded in Epon-Araldite. Sections of 2 μm were stained with toluidine blue and examined using a Nikon Eclipse 800 microscope. For immunostaining of nitrotyrosine, testes were fixed in formalin and embedded in paraffin. Sections were incubated with nitrotyrosine antibody (rabbit polyclonal, Millipore) at a 1:200 dilution for 18 hours at −4 °C. Fluorescent secondary antibody (fluorescein anti-rabbit goat IgG; Life Technologies, Grand Island, NY) was used at a 1:1000 dilution for one hour. After washes, the nuclei were counter-stained with ethidium bromide (1 ug/ml). Images were obtained with a Nikon Eclipse 800 microscope equipped with a Princeton Instruments 5-Mhz cooled CCd camera, custom CRI color filter, and IP-Lab digital image analysis software (Scanalytics Inc., Fairfax, VA). As a negative control, primary antibody was replaced by normal rabbit serum.
2.7. Measurement of antioxidant levels in the testes
The total antioxidant capacity of testes was measured by using the Cayman Antioxidant Assay Kit (Cayman Chemical Co., Ann Arbor, MI). In brief, testes were homogenized in assay buffer (5 mM potassium phosphate, pH 7.4, containing 0.9% sodium chloride and 0.1% glucose). The homogenates were centrifuged at 10,000g for 15 min at 4 °C. Antioxidant capacity was measured in the supernatants. The Cayman Antioxidant Assay measures the capacity of antioxidants in the sample to prevent ABTS (2,2′-azino-di-[3-ethylbenzthiazoline sulphonate]) oxidation in comparison to Trolox, a water-soluble tocopherol analogue. Results are presented as molar Trolox equivalents (Huang et al., 2005). The final reaction was read on a DTX800 Multimode Detector (Beckman, Coulter, Inc., Fullterton, CA) at 405 nm wavelength.
2.8. Statistical analysis
Data are expressed as the mean ± SEM. Group means were evaluated by one-way ANOVA. If group differences were revealed by ANOVA (P < 0.05), differences between individual groups were determined with the Student-Neuman-Kuels test, using SigmaStat software (Systat Software Inc., Richmond, CA). Values were considered significant at P < 0.05.
3. RESULTS
3.1. Age-related changes in testis morphology in response to Nrf2 knockout
Knockout of Nrf2 (Nrf2−/−) did not affect the body weights of young (3 mo), middle-aged (8 mo) or old (24 mo) mice (Fig. 1A). However, testis weights in the middle-aged (8 mo) and old (24 mo) Nrf2−/− mice were significantly reduced compared to their wild-type (WT, Nrf2+/+) age-matched controls (Fig. 1B). Consistent with testis weights, there was no apparent difference in the morphology of the testes in the young knockout (3mo-KO) compared to the wild-type (3mo-WT) mice (Fig. 2). However, atrophic tubules were seen in the testes of middle-aged knockout mice (8mo-KO), but not in the wild-type controls (8mo-WT). By old age, atrophic tubules were widespread in the testes of knockout mice (24 mo-WT vs. 24 mo-KO). These results are consistent with a previous study reporting that Nrf2−/− mice of age 6 months had reduced testicular sperm counts compared to wild-type and heterozygous littermates (Nakamura et al., 2010).
Figure 1.
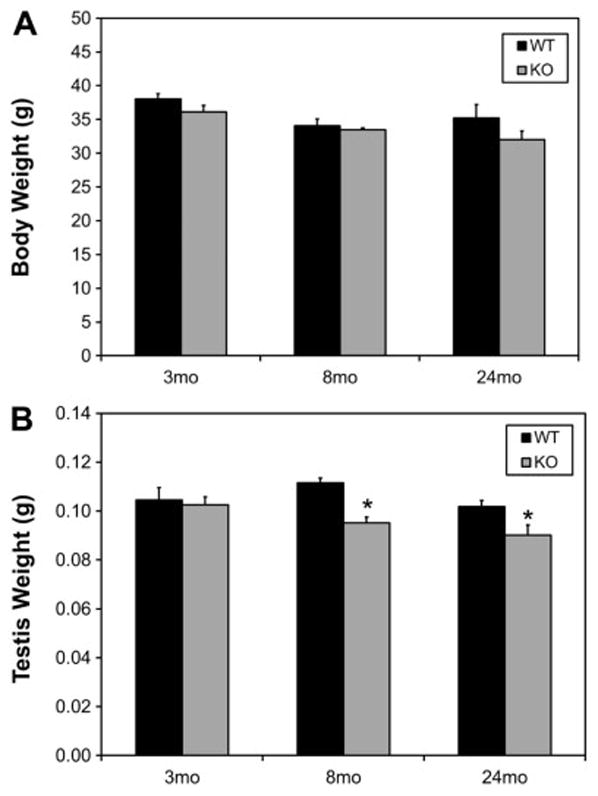
Effect of age and of Nrf2 knockout (KO) on the body (A) and testis (B) weights of young (3 month-old), middle-aged (8 month-old) and old (24 month-old) mice. WT signifies wild-type. Data are expressed as mean ± SEM of 12–18 animals per group. * Significant difference from the age-matched WT control, P<0.05.
Figure 2.
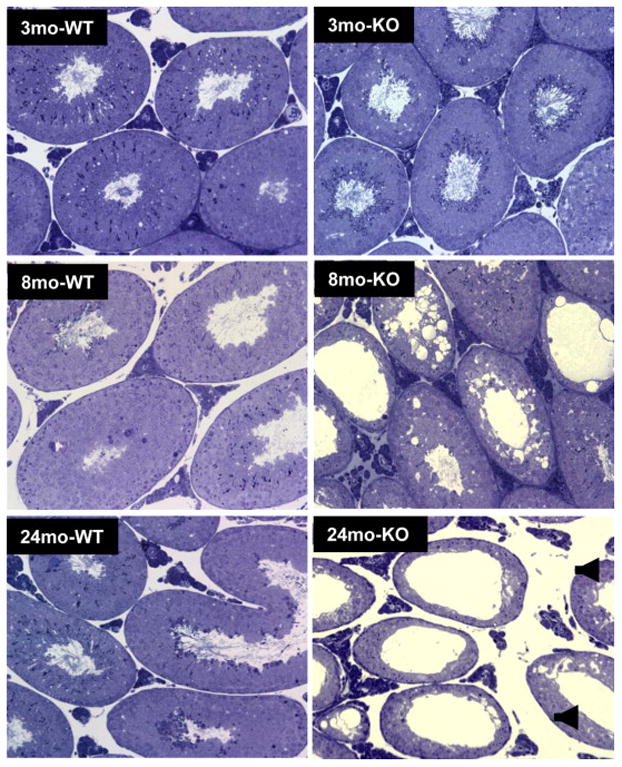
Effect of age and of Nrf2 knockout (KO) on the morphology of the testes of young (3 month-old), middle-aged (8 month-old) and old (24 month-old) wild-type (WT) and knockout (KO) mice.
3.2. Knockout of Nrf2 accelerates age-related reductions in serum testosterone levels and in Leydig cell steroid formation
In the wild-type mice, serum testosterone levels did not change through middle age (8 mo), but then declined significantly in the old (24 mo) mice (Fig 3A). In knockout mice, decrease in serum testosterone at an earlier age was seen. Thus, at 8 months there was no difference in the concentration of serum testosterone in wild-type but a significant decrease was seen in knockout mice; and by 24 months, an age at which serum testosterone levels had declined significantly in the wild-type mice, serum testosterone levels in the knockouts even decreased more extensively. To examine whether the changes in serum testosterone were caused by reduction in pituitary function, serum LH was measured (Fig. 3B). No significant differences were found across all groups; that is, neither age nor genotype affected serum LH concentrations significantly.
Figure 3.
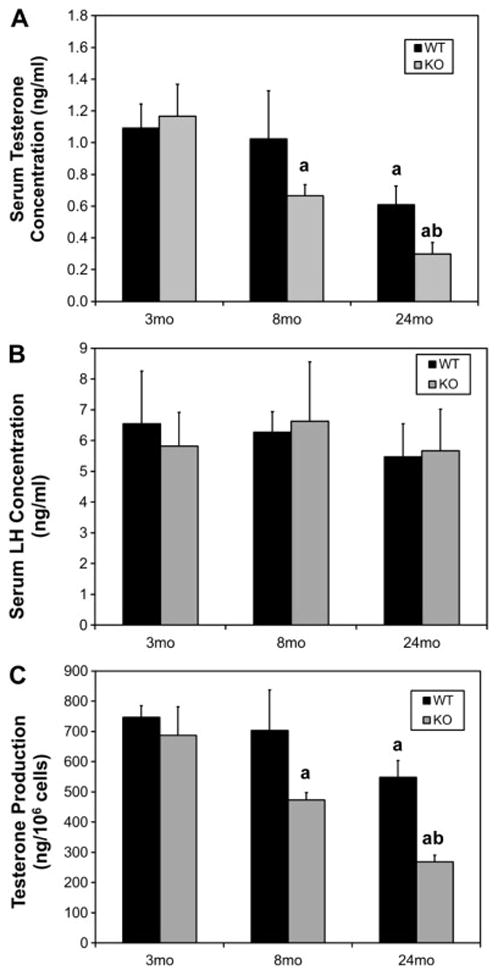
Effect of age and of Nrf2 knockout (KO) on serum testosterone (A) and LH (B) concentrations in young (3 month-old), middle-aged (8 month-old) and old (24 month-old) mice, and on testosterone production by Leydig cells isolated from the testes of these mice (C). For serum testosterone and LH measurements, data are expressed as mean ± SEM of 12–18 mice per group. For testosterone production by isolated cells, data are expressed as mean ± SEM of cells from 4–6 mice per group. a, Significant difference from young (3 mo) wild-type (WT) of the same genotype, P<0.05. b, Significant difference from age-matched WT, P<0.05.
To determine whether the decreases in serum testosterone levels in the aged wild-type and middle-aged and aged knockout mice resulted from changes in Leydig cell steroidogenic function, we assessed the ability of Leydig cells isolated from the testes of young, middle-aged and old wild-type and knockout mice to produce testosterone in response to LH. As seen in Figure 3C, testosterone production by the Leydig cells of wild-type mice decreased significantly by 24 months. In the knockout mice, significant, age-related reduction in testosterone production was seen in Leydig cells of 8 month-old mice, and there was a further decrease at 24 months. In each case, the ability of Leydig cells to produce testosterone was consistent with serum testosterone levels (compare Figs. 3A and 3C).
To begin to characterize the cellular changes in the steroidogenic pathway that might account for reduced steroid formation by Leydig cells of old wild-type and knockout mice, two key steroidogenic proteins, STAR and CYP11A1, were analyzed by Western blot. STAR is among the proteins involved in the rate-determining step in steroid formation, namely cholesterol translocation to the inner mitochondrial membrane where CYP11A1 converts it to pregnenolone (Miller 2013). Consistent with serum testosterone levels and cellular testosterone production, the levels of STAR and CYP11A1 were comparable in Leydig cells isolated from the testes of young wild-type and knockout mice (Fig 4A). By old age, the two proteins were reduced in Leydig cells from both wild-type and knockout old mouse testes, with the extent of reduction greater in the knockout mice. Differences also were seen in the activities of steroidogenic enzymes between the old wild-type and knockout mice (Fig 4B). For these studies, testosterone formation by Leydig cells isolated from the testes of old wild-type and old knockout mice was assessed after incubating the cells with LH, dbcAMP, 22-hydroxycholesterol (22HC) or pregnenolone (P5). In each case, cells from the knockout mice were found to produce significantly less testosterone than the wild-type cells.
Figure 4.
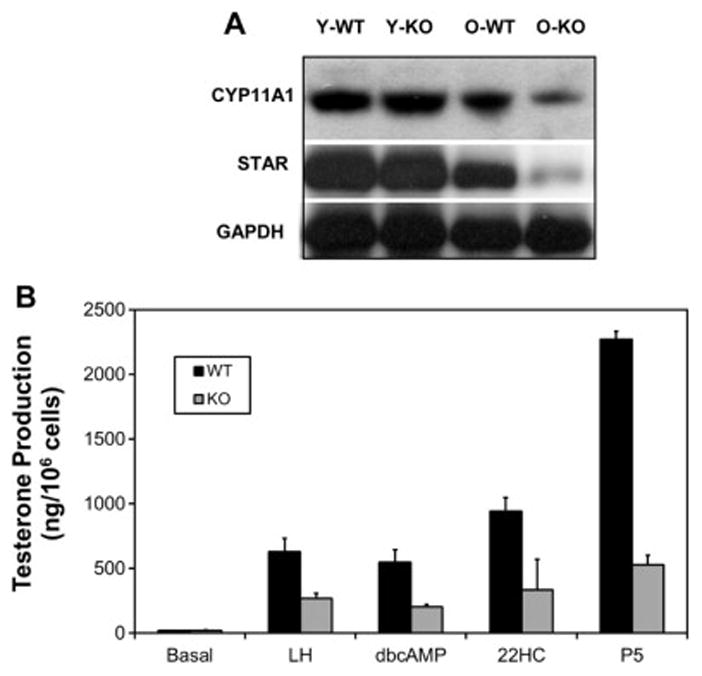
Effect of Nrf2 knockout (KO) on Leydig cell steroidogenic proteins and enzyme activities. A) Western blots of CYP11A1, STAR and GAPDH in Leydig cells from young (Y) and old (O) wild-type (WT) and knockout (KO). B) Testosterone production after incubating Leydig cells from old wild-type (WT) and knockout (KO) mice with LH, dbcAMP, 22-hydroxycholesterol (22HC) or pregnenolone.
3.3. Effect of knockout of Nrf2 on the testicular antioxidant/prooxidant environment
The antioxidant system of cells includes such enzymes as superoxide dismutase, glutathione peroxidase, and catalase, as well as vitamins C and E and glutathione (Finkel and Holbrook, 2000; Huang et al., 2005). These molecules are among those that function together to protect cells from damage by reactive oxygen or nitrogen radicals (Huang et al., 2005; Stadtman, 2001). We used the Cayman Antioxidant Assay, which measures the overall antioxidant activity of cells and tissues (Huang et al., 2005), to determine the effects of age and of Nrf2knockout on testicular antioxidant activity. As seen in Figure 5, antioxidant activity in wild-type mice did not change significantly through 8 months, but by 24 months there was a significant 30% drop from the 3 and 8 month-old values. In contrast to the wild-type mice, the knockout of Nrf2 resulted in a significant reduction in antioxidant activity as early as 3 months, and then a further reduction such that by age 24 months there was a 40% reduction in the Nrf2−/− testes compared to the wild-type controls. It was notable that the decrease in antioxidant capacity by age 3 months in the knockout mice preceded the decreases in serum testosterone and Leydig cell testosterone production, which occurred at 8 months (Fig. 3).
Figure 5.
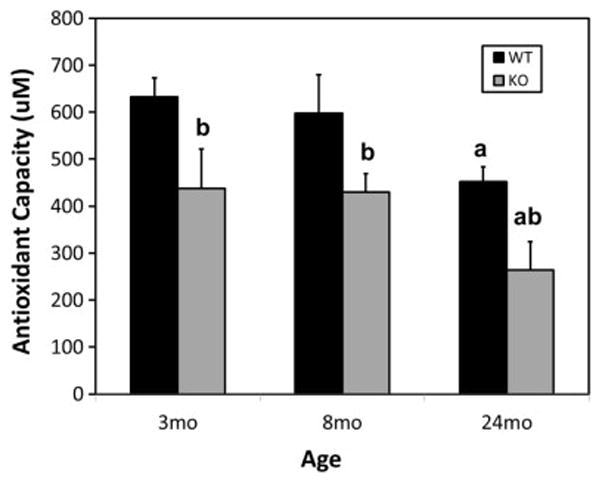
Effect of Nrf2 knockout on testicular antioxidant activities of young (3 month-old), middle-aged (8 month-old) and old (24 month-old) mice, as analyzed by the Cayman Antioxidant Assay. Data are expressed as mean ± SEM of preparations from 6–9 mice per group (n=6–9). a, Significant difference from young (3 mo) wild-type (WT) of the same genotype, P<0.05. b, Significant difference from age-matched WT, P<0.05.
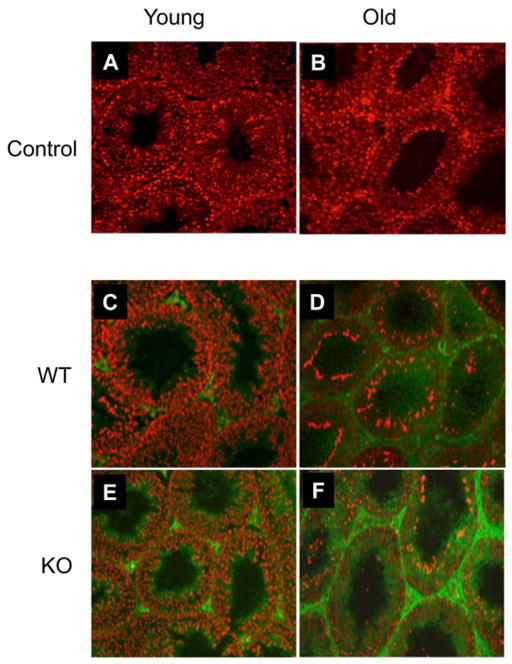
To determine whether the reduction in antioxidant activity in response to the Nrf2 knockout was reflected in an altered pro-oxidant testicular environment, the expression of nitrotyrosine residues, a marker of ROS, was assessed by immunofluorescence (Fig. 6). For these studies, sections were incubated with nitrotyrosine antibody, then with a fluorescent secondary antibody, and counterstained with ethidium bromide. As a negative control, the primary antibody was replaced by normal rabbit serum. No nitrotyrosine labeling was seen in the young (Fig. 6A) or old (Fig. 6B) controls. A modest, age-related increase in immunostaining of nitrotyrosine was seen in both the interstitial and seminiferous tubular compartments in the wild-type testes of old (Fig. 6D) as compared to young (Fig. 6C) mice. Increased nitrotyrosine concentration was seen in the testes of young Nrf2 knockout mice (Fig. 6E) in comparison to the young wild-type testis (Fig. 6C). A striking increase in nitrotyrosine immunostaining was seen in the old knockout (Fig. 6F) compared to old wild-type (Fig. 6D) or young KO (Fig. 6E) testes.
Figure 6.
Expression of protein nitrotyrosine residues in the testes of young and old mice, detected by immunofluorescence (green) with nuclear co-staining by ethidium bromide (red). A, B) Testes of young and old mice, respectively, in which the primary antibody was replaced by normal rabbit serum (negative controls). C, D) Testes of young and old wild-type (WT) mice, respectively. E, F) Testes of young and old knockout (KO) mice, respectively.
4. DISCUSSION
In rodents and men, serum levels of testosterone typically are reduced with aging, in both cases in response to a primary testicular defect (primary hypogonadism) and not in response to reduced LH (Chen et al., 1994; Liu et al., 2005; Takahashi et al 2007; Veldhuis et al., 2012; Zirkin and Chen, 2000). Leydig cells isolated from the testes of old Brown Norway rats were found to produce less testosterone in response to LH than cells from young rats (Chen et al., 1994). The cause of the relative insensitivity of the Leydig cells to LH, and thus reduced testosterone formation, remain uncertain. It is well known that aging cells, in general, can undergo deleterious changes in response to exposure to reactive oxygen-induced free radicals (Ames et al., 1993; Beckman and Ames, 1998; Raha and Robinson, 2000; Rebrin and Sohal 2008). Superoxide and other reactive oxygen species (ROS) are produced by the mitochondrial electron transport chain during oxidative phosphorylation (Murphy 2009). As in other cells, ROS are produced from this source in Leydig cells (Chen et al., 2001). In addition, the cytochrome P450 enzymes in Leydig and other steroid-forming cells catalyze the oxidation of metabolic intermediates in the steroidogenic pathway, thus generating additional free radicals (Hanukoglu 2006; Peltola et al., 1996). Mitochondrial superoxide production has been shown to increase in aging Leydig cells (Chen et al., 2001), and the antioxidant defense molecules superoxide dismutase-1 and -2, glutathione peroxidase, and GSH to decrease (Cao et al., 2004; Luo et al., 2006). Lipid peroxidation increases with age in Leydig (Cao et al., 2004) and adrenal cells (Azhar et al., 1995), two of the major steroidogenically active cells in the body. Such studies, taken together, suggest that imbalance in the oxidant/antioxidant environment of aging Leydig cells, presumably leading to oxidative damage to proteins, lipids, and/or DNA, may be the cause of reduced testosterone formation by these cells. However, cause-effect has yet to be proven.
There have been studies designed to address the effects of an altered redox environment on Leydig cell steroidogenic function experimentally. For example, a diet supplemented with the antioxidant vitamin E was shown to delay, though not prevent, age-related reductions in testosterone formation by Leydig cells of Brown Norway rats (Chen et al., 2005). Consistent with this, the acute experimental depletion of GSH in Leydig cells resulted in reduced steroidogenic function both in vitro and in vivo (Chen et al., 2010, 2008). In vitro studies showed that exposure to agents that increased intracellular ROS inhibited Leydig cell steroid production via effects on cholesterol transport proteins (Zhou et al., 2013) and steroidogenic enzymes (Georgiou et al., 1987; Quinn and Payne, 1986, 1984). Additionally, we and others demonstrated that LH stimulation of steroid formation increased oxidative stress in Leydig cells (Aggarwal et al., 2009; Beattie et al., 2013), while the long-term suppression of steroidogenesis via the suppression of LH was found to suppress age-related reductions in testosterone production (Chen and Zirkin, 1999). The results of these studies are consistent with the conclusion that persistent reactive oxygen exposure may cause reduced testosterone production by aging Leydig cells.
However, age-related reductions in steroidogenesis involves changes in a complex environment, over time, not the acute effects of changes in single antioxidant molecules. It was with this in mind that we turned to Nrf2 knockout mice. Under unstressed conditions, Nrf2 is bound to kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm of cells, and is degraded by ubiquitination (Itoh et al., 2004b; Zhang, 2006). Under conditions of oxidative stress, however, Nrf2 dissociates from Keap1 through modification of cysteine residues on the Keap1 protein, translocates to the nucleus, and there activates Nrf2- responsive genes involved in antioxidant expression and oxidant inactivation (Cho et al., 2006; Li and Kong, 2009). As Nrf2 is a master antioxidant regulator, knocking out Nrf2 reduces many antioxidant molecules, thus altering the redox environment in a manner that is reminiscent of physiological aging.
Knocking out Nrf2 had no significant effect on serum testosterone level or on testosterone formation by Leydig cells in young (3 month-old) mice, though by 3 months the antioxidant capacity of the testis had decreased significantly. By middle age (8 months), significantly reduced serum testosterone concentration and testosterone formation by Leydig cells were seen in the knockout but not in age-matched, wild-type mice. By old age (24 months), when the wild-type mice had reduced serum testosterone and Leydig cell testosterone formation, the reductions were more extensive in the knockout mice, with a 4-fold decrease in serum testosterone concentration, highly significant decrease in testosterone formation in vitro, decreased testosterone formation in response not only to LH but also to dbcAMP, 22-hydroxycholesterol or pregnenolone, and reduced CYP11A1 and STAR.
A plausible interpretation of these observations was that the knockdown of Nrf2 affected testicular cell antioxidant defense capacity, and that in time, doing so had detrimental effects on steroid formation that resulted in reduced testosterone formation. We wished to determine whether Nrf2 knockout in fact affected the antioxidant environment in the testis. Because Nrf2 has the potential to affect hundreds of antioxidant and phase II molecules, we measured total antioxidant capacity rather than specific antioxidant genes and proteins. In the wild-type mice, the testicular total antioxidant capacity changed in 24 month-old rats, but not earlier. Interestingly, there was a decrease in antioxidant capacity by age 3 months in the knockout mice, which preceded the decreases in serum testosterone and Leydig cell testosterone production seen at 8 months but not earlier. Indeed, antioxidant activities were significantly lower in the Nrf2 knockout testes in each of the young, middle-aged and aged groups; reductions of 30% were seen in the young and middle-aged knockout mice compared to age-matched wild-type mice, and the reduction at 24 months was over 50%. Immunocytochemical analyses of a common by-product of oxidative stress, nitrotyrosine, was used to determine the effect of decreased antioxidant capacity on peroxynitrite formation in vivo (Pacher et al., 2007; Szabo et al., 2007). Knocking out Nrf2 increased the expression of nitrotyrosine in both the interstitial and tubular compartments of the testis, with the most striking increases seen in old knockout animals.
These results support the contention that reductions in antioxidant activities and thus increased oxidative stress are related to, and may cause, the reduced testosterone formation that occurs with aging. It is particularly of interest in this regard that reduced antioxidant capacity and increased oxidative stress in the knockout mice preceded reduced testosterone formation. This is consistent with the idea that, with advancing time (aging), alterations in the redox environment in fact causes reduced testosterone formation.
5. CONCLUSIONS
The results presented herein provide experimental support for the hypothesis that increased oxidative stress, perhaps resulting from age-related decreases in antioxidant molecules and/or from increased ROS production, is closely associated with, and might cause, age-related reductions in Leydig cell testosterone formation. In the Nrf2 knockout mice, the reduced expression of numerous antioxidant molecules results in reduced antioxidant capacity and therefore an increasingly prooxidant environment, reminiscent of aging. Exposure of the Leydig cells to higher oxidative stress levels, over time, might be the cause of age-related reductions in steroidogenic function without effect on LH (primary hypogonadism). The mechanisms by which aging results in losses in antioxidant molecules, and how an altered redox environment affects the response of Leydig cells to LH and thus leads to reduced testosterone formation, remain uncertain.
HIGHLIGHTS.
Nrf2 knockout accelerated age-related reductions in Leydig cell steroidogenesis.
Nrf2 knockout significantly reduced the antioxidant capacity of the testis.
Nrf2 knockout resulted in significantly increased ROS.
Increased oxidative stress contributes to reduced testosterone production with age.
Acknowledgments
Grant support: This work was supported by NIH grant R37 AG21092 from the National Institute on Aging (B.R.Z), and by a National Natural Science Foundation of China grant NSFC81471411 (H.C).
ABBREVIATIONS
- ABTS
2,2′-azino-di-[3-ethylbenzthiazoline sulphonate]
- CYP11A1
Cytochrome P450, family 11A1; cholesterol side-chain cleavage enzyme
- dbcAMP
Dibutryl cAMP
- GSH
Glutathione
- 22-HC
22-Hydroxycholesterol
- ROS
Reactive oxygen species
- Nrf2
Nuclear factor erythroid 2-related factor
- P5
Pregnenolone
- STAR
Steroidogenic acute regulatory protein
- TSPO
Translocator protein
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- Aggarwal A, Misro MM, Maheshwari A, Sehgal N, Nandan D. Adverse effects associated with persistent stimulation of Leydig cells with hCG in vitro. Mol Reprod Dev. 2009;76:1076–1083. doi: 10.1002/mrd.21074. [DOI] [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo AB, O’Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, McKinlay JB. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2004;89:5920–5926. doi: 10.1210/jc.2003-031719. [DOI] [PubMed] [Google Scholar]
- Azhar S, Cao L, Reaven E. Alteration of the adrenal antioxidant defense system during aging in rats. J Clin Invest. 1995;96:1414–1424. doi: 10.1172/JCI118177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie MC, Chen H, Fan J, Papadopoulos V, Miller P, Zirkin BR. Aging and luteinizing hormone effects on reactive oxygen species production and DNA damage in rat Leydig cells. Biol Reprod. 2013;88:101–107. doi: 10.1095/biolreprod.112.107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol. 2004;88:61–67. doi: 10.1016/j.jsbmb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Chen H, Cangello D, Benson S, Folmer J, Zhu H, Trush MA, Zirkin BR. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function? Exp Gerontol. 2001;36:1361–1373. doi: 10.1016/s0531-5565(01)00118-8. [DOI] [PubMed] [Google Scholar]
- Chen H, Hardy MP, Huhtaniemi I, Zirkin BR. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J Androl. 1994;15:551–557. [PubMed] [Google Scholar]
- Chen H, Hardy MP, Zirkin BR. Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology. 2002;143:1637–1642. doi: 10.1210/endo.143.5.8802. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu J, Luo L, Baig MU, Kim JM, Zirkin BR. Vitamin E, aging and Leydig cell steroidogenesis. Exp Gerontol. 2005;40:728–736. doi: 10.1016/j.exger.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu J, Luo L, Zirkin BR. Dibutyryl cyclic adenosine monophosphate restores the ability of aged Leydig cells to produce testosterone at the high levels characteristic of young cells. Endocrinology. 2004;145:4441–4446. doi: 10.1210/en.2004-0639. [DOI] [PubMed] [Google Scholar]
- Chen H, Pechenino AS, Liu J, Beattie MC, Brown TR, Zirkin BR. Effect of glutathione depletion on Leydig cell steroidogenesis in young and old brown Norway rats. Endocrinology. 2008;149:2612–2619. doi: 10.1210/en.2007-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhou L, Lin CY, Beattie MC, Liu J, Zirkin BR. Effect of glutathione redox state on Leydig cell susceptibility to acute oxidative stress. Mol Cell Endocrinol. 2010;323:147–154. doi: 10.1016/j.mce.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zirkin BR. Long-term suppression of Leydig cell steroidogenesis prevents Leydig cell aging. Proc Natl Acad Sci U S A. 1999;96:14877–14881. doi: 10.1073/pnas.96.26.14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Debiase A, Yamamoto M, Kleeberger SR. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic Biol Med. 2005;38:325–343. doi: 10.1016/j.freeradbiomed.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8:76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- Culty M, Luo L, Yao ZX, Chen H, Papadopoulos V, Zirkin BR. Cholesterol transport, peripheral benzodiazepine receptor, and steroidogenesis in aging Leydig cells. J Androl. 2002;23:439–447. [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Georgiou M, Perkins LM, Payne AH. Steroid synthesis-dependent, oxygen-mediated damage of mitochondrial and microsomal cytochrome P-450 enzymes in rat Leydig cell cultures. Endocrinology. 1987;121:1390–1399. doi: 10.1210/endo-121-4-1390. [DOI] [PubMed] [Google Scholar]
- Giudice A, Montella M. Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention. Bioessays. 2006;28:169–181. doi: 10.1002/bies.20359. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004a;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004b;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- Lacombe A, Lelievre V, Roselli CE, Salameh W, Lue YH, Lawson G, Muller JM, Waschek JA, Vilain E. Delayed testicular aging in pituitary adenylate cyclase-activating peptide (PACAP) null mice. Proc Natl Acad Sci U S A. 2006;103:3793–3798. doi: 10.1073/pnas.0505827103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- Leers-Sucheta S, Stocco DM, Azhar S. Down-regulation of steroidogenic acute regulatory (StAR) protein in rat Leydig cells: implications for regulation of testosterone production during aging. Mech Ageing Dev. 1999;107:197–203. doi: 10.1016/s0047-6374(98)00149-3. [DOI] [PubMed] [Google Scholar]
- Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Reaven E, Azhar S. Age-related decline in the steroidogenic capacity of isolated rat Leydig cells: a defect in cholesterol mobilization and processing. J Steroid Biochem Mol Biol. 1993;46:39–47. doi: 10.1016/0960-0760(93)90207-d. [DOI] [PubMed] [Google Scholar]
- Liu PY, Takahashi PY, Roebuck PD, Iranmanesh A, Veldhuis JD. Age-specific changes in the regulation of LH-dependent testosterone secretion: assessing responsiveness to varying endogenous gonadotropin output in normal men. Am J Physiol Regul Integr Comp Physiol. 2005;289:R721–728. doi: 10.1152/ajpregu.00138.2005. [DOI] [PubMed] [Google Scholar]
- Luo L, Chen H, Trush MA, Show MD, Anway MD, Zirkin BR. Aging and the brown Norway rat leydig cell antioxidant defense system. J Androl. 2006;27:240–247. doi: 10.2164/jandrol.05075. [DOI] [PubMed] [Google Scholar]
- Luo L, Chen H, Zirkin BR. Temporal relationships among testosterone production, steroidogenic acute regulatory protein (StAR), and P450 side-chain cleavage enzyme (P450scc) during Leydig cell aging. J Androl. 2005;26:25–31. [PubMed] [Google Scholar]
- Luo L, Chen H, Zirkin BR. Leydig cell aging: steroidogenic acute regulatory protein (StAR) and cholesterol side-chain cleavage enzyme. J Androl. 2001;22:149–156. [PubMed] [Google Scholar]
- Luo L, Chen H, Zirkin BR. Are Leydig cell steroidogenic enzymes differentially regulated with aging? J Androl. 1996;17:509–515. [PubMed] [Google Scholar]
- Miller WL. Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol. 2013;379:62–73. doi: 10.1016/j.mce.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura BN, Lawson G, Chan JY, Banuelos J, Cortes MM, Hoang YD, Ortiz L, Rau BA, Luderer U. Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free Radic Biol Med. 2010;49:1368–1379. doi: 10.1016/j.freeradbiomed.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osburn WO, Kensler TW. Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mutat Res. 2008;659:31–39. doi: 10.1016/j.mrrev.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola V, Huhtaniemi I, Metsa-Ketela T, Ahotupa M. Induction of lipid peroxidation during steroidogenesis in the rat testis. Endocrinology. 1996;137:105–112. doi: 10.1210/endo.137.1.8536600. [DOI] [PubMed] [Google Scholar]
- Quinn PG, Payne AH. High affinity binding of substrate and effector ligands to testicular microsomal cytochrome P-450. J Steroid Biochem. 1986;25:943–949. doi: 10.1016/0022-4731(86)90327-4. [DOI] [PubMed] [Google Scholar]
- Quinn PG, Payne AH. Oxygen-mediated damage of microsomal cytochrome P-450 enzymes in cultured leydig cells. Role in steroidogenic desensitization. J Biol Chem. 1984;259:4130–4135. [PubMed] [Google Scholar]
- Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Sohal RS. Pro-oxidant shift in glutathione redox state during aging. Adv Drug Deliv Rev. 2008;60:1545–1552. doi: 10.1016/j.addr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salva A, Klinefelter GR, Hardy MP. Purification of rat leydig cells: increased yields after unit-gravity sedimentation of collagenase-dispersed interstitial cells. J Androl. 2001;22:665–671. [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- Takahashi PY, Votruba P, Abu-Rub M, Mielke K, Veldhuis JD. Age attenuates testosterone secretion driven by amplitude-varying pulses of recombinant human luteinizing hormone during acute gonadotrope inhibition in healthy men. J Clin Endocrinol Metab. 2007;92:3626–3632. doi: 10.1210/jc.2006-2704. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Liu PY, Keenan DM, Takahashi PY. Older men exhibit reduced efficacy of and heightened potency downregulation by intravenous pulses of recombinant human LH: a study in 92 healthy men. Am J Physiol Endocrinol Metab. 2012;302:E117–22. doi: 10.1152/ajpendo.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- Zhou L, Beattie MC, Lin CY, Liu J, Traore K, Papadopoulos V, Zirkin BR, Chen H. Oxidative stress and phthalate-induced down-regulation of steroidogenesis in MA-10 Leydig cells. Reprod Toxicol. 2013;42:95–101. doi: 10.1016/j.reprotox.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirkin BR, Chen H. Regulation of Leydig cell steroidogenic function during aging. Biol Reprod. 2000;63:977–981. doi: 10.1095/biolreprod63.4.977. [DOI] [PubMed] [Google Scholar]