Abstract
Per milligram of tissue, only the heart exceeds the kidney’s abundance of mitochondria. Not surprisingly, renal mitochondria are most densely concentrated in the epithelium of the nephron, at sites where the chemical work of moving solutes against electrochemical gradients places large and constant demands for ATP. Derangements of renal epithelial mitochondria appear to be a hallmark for diverse forms of acute kidney injury (AKI).
The pathogenesis of multiple-organ dysfunction syndrome (MODS) in sepsis is complex, but a substantial body of experimental and observational human data supports the twin concepts that mitochondrial dysfunction contributes to impaired filtration and that recovery of mitochondrial structure and function is essential for recovery from sepsis-associated AKI. These insights have suggested novel methods to diagnose, stratify, prevent, or even treat this common and deadly complication of critical illness.
This review will (1) describe the structure and functions of healthy mitochondria and how renal energy metabolism relates to solute transport; (2) provide an overview of the evidence linking mitochondrial pathology to renal disease; (3) summarize the mitochondrial lesions observed in septic AKI; (4) analyze the role of mitochondrial processes including fission/fusion, mitophagy, and biogenesis in the development of septic AKI and the recovery from this disease; and (5) explore the potential for therapeutically targeting mitochondria to prevent or treat septic AKI.
INTRODUCTION
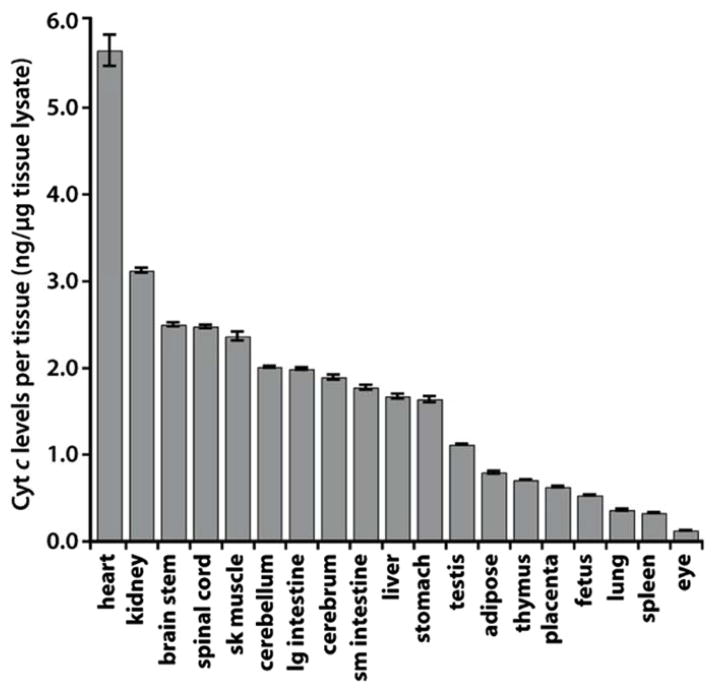
To purify blood, the kidney elegantly couples two remarkable functions: the bulk filtration of total body plasma every ~1hr and the selective, but massive, exchange of solutes and water between the filtered blood and the filtrate. Energy for the former derives from the hydraulic pressure of the beating heart. The latter is powered by ion pumps that generate powerful electrochemical gradients along the nephron. Since mitochondrial processes enable the most efficient conversion of substrates to ATP, it is perhaps not surprising that, per milligram of tissue, the heart and kidney consume the most oxygen and possess the greatest abundance of mitochondria (Figure 1)1.
Figure 1. Mitochondrial abundance across bodily organs.
Whole tissue lysates were prepared from mouse organs, and cytochrome C levels were measured by ELISA. The figure was adopted from Pagliarini et. al. Cell 2008.
In sepsis, the host response to infection clearly contributes to the severe manifestations of the disease, as evidenced by the fact that potent antimicrobials usually sterilize the blood, yet affected individuals often develop progressive multi-organ dysfunction syndrome (MODS). Among critically ill subjects, septic shock may be a contributing factor in nearly half of all acute kidney injury (AKI) cases 2.The exquisite vulnerability of the kidney to the septic milieu has long been felt to arise from the associated shock and hypoperfusion. However, while ischemia is undoubtedly a contributing factor, the correction of mean arterial pressure and macro-vascular blood flow is not restorative in septic AKI, compelling the search for intrarenal events that may be measurable and therapeutically targetable. Other articles in this Special Issue focus on glomeruli, microvasculature, and endothelium during sepsis. Below, we present background and evidence in support of the concept that mitochondrial dysfunction and injury may be a critical feature of septic AKI.
ENERGY METABOLISM, SOLUTE TRANSPORT, AND SEPSIS
Segments of the nephron that perform the most chemical work (i.e., highest sodium reabsorption) are happen to be the most densely loaded with mitochondria—namely, the proximal tubule and the thick ascending limb of Henle’s loop. The Na+-K+ ATPase found at the basolateral membrane of tubular cells generates this gradient by consuming 1 molecule of ATP to move 3 Na+ ions out of the cell and 2 K+ ions into the cell. Assuming NADH as the primary reducing equivalent in renal mitochondria, the following stoichiometry holds: 1 mol NADH generates 3 mol ATP and consumes ½ mol of O2. Therefore, consumption of 1 mol O2during oxidative phosphorylation in the mitochondrion should generate 6 mol ATP. We would therefore expect that a nephron consumes 1 mmol O2 for every 18 mEq Na+ reabsorbed. The tight correlation between O2 consumption and sodium reabsorption has enabled investigators to test this theoretical relationship. In intact kidneys, ~1 mmol oxygen appears to be required per 20–30 mEq Na+ reabsorbed 3, 4; the excess sodium reabsorption above the predicted threshold may be attributable to passive movement of Na+ out of the filtrate.
To generate ATP, the kidney oxidizes a variety of fuels, including glucose, fatty acids, and amino acids. Fuel utilization appears to vary by region of the nephron and based on the metabolic demands—for example, during chronic metabolic acidosis, increased glutamine extraction by the proximal tubule enables the accelerated generation of ammonium through an enzymatically catalyzed deamination reaction.
Biochemical studies suggest that the proximal tubule has the lowest capacity for glycolysis 5, whereas the thick ascending limb and more distal nephron segments produce substantial amounts of lactate 6. In the deep renal medulla, oxygen tension can reach 10 torr (compared to ~80 torr at the cortical surface), presumably favoring anaerobic metabolism. The proximal convoluted tubule also appears to be the exclusive site along the nephron for gluconeogenesis, as evidenced by the presence of phosphoenolpyruvatecarboxykinase (PEPCK), the rate-limiting enzyme in this pathway 5.
Mitochondrial injury clearly arises in human sepsis, as evidenced by biochemical and structural studies on tissue biopsies obtained in intensive care settings 7, 8. Since mitochondria are the chief consumers of oxygen in the kidney, and intact blood flow is critical to normal renal function, there is a tendency to conflate renal hypoxia and renal mitochondrial dysfunction. However, the question of whether the septic milieu induces renal hypoxia is complex for a number of reasons: (1) measurement in critically ill humans is difficult; (2) much like the human disease, experimental models can produce hyperperfusion or hypoperfusion depending on the noxious stimulus and timing of measurement; (3) the need for ATP is driven by sodium reabsorption, which, in turn arises when GFR delivers filtered sodium into the nephron lumen; (4) the energy metabolism of the septic nephron likely evolves over time and responds to changes inextrarenal host and pathogen factors9; and (5) regional variations of blood flow, oxygen shunting, and tubular epithelial metabolism within the kidney are difficult to assess in combination, let alone integrate into a single model10.
For example, using BOLD MRI in endotoxemic mice to show an absence of hypoxia 18 hrs after LPS, Tran, et al. have suggested that decreased oxygen delivery may be “matched” by decreased oxygen consumption, a scenario that could arise due to reduced GFR—thus reduced work of sodium reabsorption—or primary mitochondrial dysfunction due to the septic milieu 11. Using oxygen-sensitive phosphorescent dyes in the first 1–4 hrs after LPS, Johannes, et al., and Dyson et al, have found that oxygen consumption falls during endotoxemia, albeit not significantly so compared to control animals 12, 13.
Mitochondrial pathology in AKI
Ultrastructural changes in mitochondria are observed in kidney tubular cells during ischemic, nephrotoxic, as well as sepsis-associated AKI11, 14. The changes include decreased mitochondrial mass, disruption of cristae, and extensive mitochondrial swelling. Notably, these changes may represent different levels or stages of damage to mitochondria. As will be discussed in the next section, a decrease in mitochondrial mass can result from mitochondrial fragmentation that occurs as a result of the disruption of mitochondrial dynamics in AKI. While mitochondrial fragmentation is potentially reversible, mitochondrial swelling as a consequence of permanent permeability transition at the inner membrane is usually a late and irreversible event. In AKI, these structural changes are readily detectable. The affected tubules mainly include proximal tubules, but depending on AKI severity, distal tubules and thick ascending limb may show changes as well15.
Ultrastructural changes in mitochondria are often associated with functional decreases. For example, cisplatin nephrotoxicity induces a significant decrease of cytochrome c oxidase (COX) activity and a reduction in COX IV protein expression in the proximal tubules16. Furthermore, cisplatin treatment reduces mitochondrial MnSOD17, coupled with decreases in glutathione and succinate dehydrogenase (SDH) activity18, resulting in drastic decreases in antioxidant activity and ROS production from mitochondria. By using multiphoton microscopy, Hall and colleagues examined mitochondrial function in vivo during renal ischemia-reperfusion injury and demonstrated a marked increase in mitochondrial nicotinamide adenine dinucleotide (NADH) and the dissipation of mitochondrial membrane potential (ΔΨm) in proximal tubules, but not distal tubules19. Consistently, Funk and colleagues revealed the loss of mitochondrial respiratory proteins from proximal tubules in experimental models of glycerol-induced myoglobinuric and ischemic AKI20. The decreased proteins include NDUFB8, ATP synthase β, cytochrome c oxidase subunit I (COX I) and COX IV, and notably, they do not recover by at late time-points. Interestingly, these mitochondrial changes are associated with the increased expression of Drp1, a key fission protein in the regulation of mitochondrial dynamics.
Mitochondrial dysfunction is also known as an important pathogenic factor in sepsis-associated multiorgan failure, including septic AKI. Dysoxia, a phenomenon of increased tissue oxygen tensions but decreased utilization of oxygen, has been described in septic tissues21. The impaired oxygen utilization in septic tissues is mainly caused by mitochondrial dysfunction, resulting in reduced ATP production and bioenergetic failure. In line with this scenario, lipopolysaccharide (LPS)-induced endotoxemia is accompanied by declines of ATP and increased lactate levels in liver, kidney, and heart tissues22, 23. In LPS and CLP (cecal ligation and puncture) models of septic AKI, Tran et al. recently demonstrated mitochondrial swelling and disruption of cristae in renal tubular cells, which are associated with mitochondrial dysfunction11. It is noteworthy that the mitochondria-centered structural and metabolic or bioenergetic alterations occur prior to the onset of AKI, supporting a causative, pathogenic role of mitochondrial damage in this disease condition.
Mitochondrial dynamics in AKI
Despite the recognition of mitochondrial dysfunction in AKI, the underlying mechanism is largely unclear. Nonetheless, recent studies have unveiled the involvement of the disruption of mitochondrial dynamics. Mitochondrial dynamics describes the dynamic feature of mitochondria, which constantly undergo fission and fusion under physiological conditions. Mitochondrial dynamics may have multiple cellular and physiological functions. The fission or cleavage of defective or non-functional part of a mitochondrion may be important for the maintenance of the overall health of the organelle. The fission and fusion may facilitate the exchange of metabolites and substrates between mitochondria to ensure an optimal function of mitochondrial network. In addition, mitochondrial fission is necessary for the distribution of mitochondria to daughter cells during cell division 24, 25. Mitochondrial fusion involves multiple steps to tether the membranes of adjacent mitochondria to fuse, while fission divides a mitochondrion into two daughter mitochondria. Mitochondrial fusion depends on the dynamin-related GTPases, mitofusin1 (Mfn1) and mitofusin2 (Mfn2) at the mitochondrial outer membrane (MOM) and Opa1 on the inner membrane26. For fission, Drp1 is activated to move to mitochondria to initiate membrane cleavage although the molecular details remain elusive. It is now known that the dynamic homeostasis of mitochondrial fission and fusion is essential to mitochondrial function and cellular homeostasis and viability 24, 25.As a result, disruption of mitochondrial dynamics leads to the decline of mitochondrial function and loss of cell viability, i.e. cell death24, 25, 27.
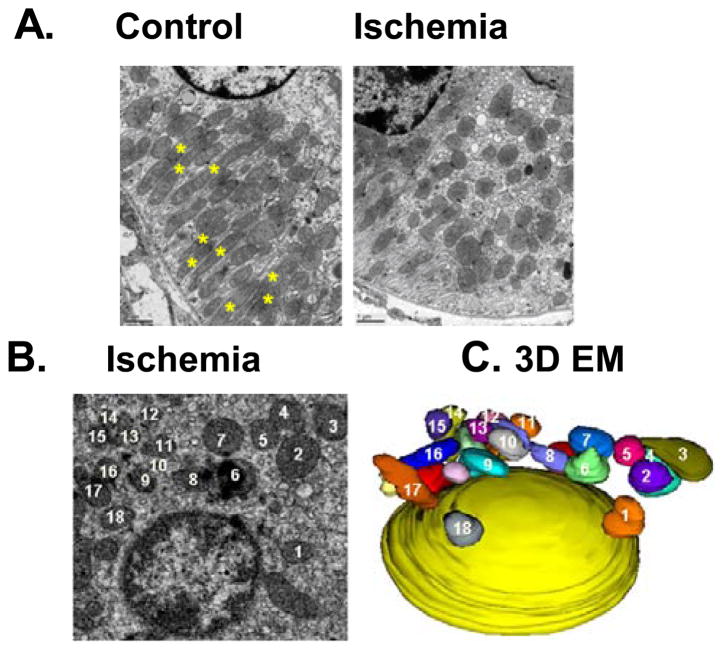
In 2009, Brooks and colleagues demonstrated the first evidence for the disruption of mitochondrial dynamics and its pathogenic role in a disease model. Using experimental models of AKI, mitochondrial fragmentation was shown to occur prior to renal tubular cell injury and death and tissue damage in kidneys during ischemic and cisplatin nephrotoxic AKI in mice14 (Figure 2). In cultured cells, inhibition of Drp-1 by dominant-negative mutants and siRNA blocked mitochondrial fragmentation and mitochondrial leakage, resulting in the protection against cell death by apoptosis. Furthermore, in mouse models of AKI, mdivi-1 as a pharmacological inhibitor of Drp-1, prevented mitochondrial fragmentation and protected kidneys against both ischemic and cisplatin nephrotoxic AKI. Mitochondrial fragmentation was also shown in mouse kidneys during AKI and importantly, suppression of mitochondrial fragmentation by mdivi-1 (Drp-1 inhibitor) led to the preservation of mitochondrial morphology and the amelioration of AKI14. Similar renoprotective effects of mdivi-1 were recently confirmed in glycerol-induced myoglobinuric AKI model 28. In further support, deficiency of Mfn2 was shown to trigger mitochondrial fragmentation in proximal tubule cells and increased Bax accumulation in or “attack” of mitochondria, resulting in exacerbated mitochondrial leakage of apoptotic factors during cell stress 29. In rat models of AKI, there were is up-regulation of Drp-1 and down-regulation of the fusion proteins such as mitofusins-2 20. The latest work by Xiao et al. further demonstrated the proteolysis and inactivation of Opa1, the key inner membrane fusion protein30. OPA1 proteolysis in ischemic AKI was diminished in OMA1-knockout mice, suggesting that this mitochondrial membrane-bound metallopeptidase is responsible for OPA1 inactivation in this experimental model. Changes of mitochondrial morphology, characterized by initial fragmentation of the organelles followed by ultrastructural alterations resulting in mitochondrial swelling and cristae deformation, were also observed in renal tubular cells during septic AKI 11. Together, these studies indicate that disruption of mitochondrial dynamics at both outer and inner membranes plays a crucial role in mitochondrial dysfunction and tubular cell injury and death in AKI.
Figure 2. Mitochondrial fragmentation in AKI.
C57BL/6 mice were subjected to 30 minutes of bilateral renal ischemia followed by 15 minutes of reperfusion (Ischemia), or control sham-operation (Control). Kidneys were fixed in situ via vascular perfusion and processed for electron microscopy (A, B). For 3D image (C), EM images of 100 serial sections were aligned for 3D reconstruction. Note: The numbered mitochondria in (B) and (C) correspond. The figure was modified from Brooks et al. JCI 2009 (Ref. 22).
As eluded above, mitochondrial fragmentation involves molecular regulation at both outer and inner membranes. At the outer membrane, fission is activated mainly by Drp1. Drp1 is a critical mitochondrial fission protein existing mainly in cytoplasm in physiological conditions or unstressed cells. In both in vitro and in vivo models of AKI, blockade of Drp-1 genetically and pharmacologically inhibited mitochondrial fragmentation and diminished mitochondrial damage, tubular apoptosis, and kidney injury, indicating a role of Drp-1 in the disruption of mitochondrial dynamics in AKI14. Mechanistically, Cho et al. demonstrated that Drp-1 was dephosphorylated in ATP-depleted renal tubular cells and ischemic kidney tissues and upon ATP-repletion or kidney reperfusion, Drp-1 became re-phosphorylated. Interestingly, Drp-1 dephosphorylation in stressed tubular cells was suppressed by cyclosporine A and FK506, suggesting the involvement of calcineurins in this dephosphorylation. Moreover, calcineurin inhibitors also partially protected against mitochondrial fragmentation and damage, accompanied by attenuation of apoptosis, suggesting the role of Drp-1 dephosphoration in Drp-1 activation during the disruption of mitochondrial dynamics in AKI 31. In addition to Drp-1-mediated fission, another important factor of mitochondrial fragmentation during cell stress is the arrest of mitochondrial fusion. In this aspect, Brooks et al. demonstrated that fusion arrest during apoptosis depends on Bak, a pro-apoptotic Bcl-2 family protein residing on mitochondrial outer membrane. In Bak-knockout cells, mitochondrial fragmentation was attenuated, and reconstitution of Bak into Bak-knockout cells could restore mitochondrial fragmentation. How does Bak regulate mitochondrial dynamics? Brooks et al. revealed the interaction of Bak with both mitofusins 1 and 2 in unstressed cells. Upon cell stress or apoptotic induction, Bak dissociates from mitofusins-2 and binds tightly with mitofusins-1. Importantly, this “binding partner switch” of Bak is a key to mitochondrial fragmentation because a Bak mutant that does not dissociated from mitofusins-2 cannot induce mitochondrial fragmentation32. Consistently, a role of Bak in mitochondrial fragmentation in AKI was recently demonstrated by Wei et al. by using Bak-deficient mice 33. At mitochondrial inner membrane, OPA1 is the fusion protein. As mentioned above, in ischemic AKI OPA1 is proteolytically inactivated by OMA1, resulting in the blockade of fusion, which contributes to mitochondrial fragmentation and consequent tubular apoptosis and kidney injury 30.
It is not fully understood how mitochondrial fragmentation can lead to mitochondrial damage. In this regard, a recent study has provided a clue 34.It was shown that mitofusin-deficient cells contained fragmented mitochondria under control culture conditions. Upon stress, these cells were much more sensitive to apoptosis. Furthermore, these cells showed enhanced or accelerated Bax insertion and oligomerization in mitochondria, leading to the release of apoptogenic factors such as cytochrome c 34. Thus, mitochondrial fragmentation may sensitize the organelles to Bax “attack”. Studies in the future need to determine whether this mechanism is applicable to other models of renal epithelial injury such as septic AKI.
Autophagy in AKI
Autophagy is a cellular process by which cytoplasmic cargos, such as protein aggregates and dysfunctional organelles, are sequestered within autophagosomes and then delivered to lysosomes for degradation. Under physiological conditions, autophagy is a main mechanism of nutrient turnover and retrieval. Autophagy deficiency may not only deprive the cell of nutrients but will also lead to the accumulation of protein aggregates and dysfunctional organelles that are detrimental to the cell. On the other hand, too much autophagy may digest healthy components of the cell resulting in cell injury and death. As a result, it plays a crucial role for in cellular homeostasis and viability and has been implicated in cell differentiation and animal development35. Recently, an emerging body of evidence indicates that autophagy is rapidly induced during various models of AKI and plays important roles in renoprotection36.
Ischemia/reperfusion (I/R) injury is a common cause of AKI which occurs in renal transplantation, trauma, sepsis, vascular obstruction including that during major surgery. In 2008, Suzuki et al. observed an increase in autophagosomes in renal tubular cells during mouse renal I/R and also in human kidney transplants37. In 2010, Jiang et al. verified that autophagy was induced during renal ischemia-reperfusion in mice and this induction was rapid and occurred prior to tissue damage or tubular apoptosis. In addition, inhibition of autophagy by 3-methyladinine exaggerated tubular apoptosis and AKI 38. In more recent studies, ischemic AKI was shown to be enhanced in renal tubule-specific autophagy gene-knockout mice, providing convincing evidence for a renoprotective role of autophagy 39, 40.
Autophagy has also been documented in AKI induced by nephrotoxins, such as cisplatin and cyclosporine41–43. In tubular epithelial cells, autophagy induction was shown to be a rapid response upon exposure to cisplatin, a widely used chemotherapy drug that induces AKI. Autophagy under this condition was protective as indicated by that observation that inhibition of autophagy increased tubular cell apoptosis41, 42. Notably, autophagy was also induced in kidney tubular cells in mice during cisplatin nephrotoxicity 41. Mechanistically, p53 and Bcl-2 proteins have been implicated in autophagic signaling 41. In Jiang, Takahashi and colleagues established renal tubule-specific autophagy gene-knockout mouse models and used these models to demonstrate that autophagy is renoprotective during AKI induced by cisplatin nephrotoxicity 39, 44 Consistently, Li et al. recently generated a mouse model with tubule-specific deletion of Rictor and showed that Rictor/mTORC2 induced autophagy through activating Akt signaling protected against cisplatin-induced AKI by promoting cell survival45.
In 2012, Hsiao et al. demonstrated a biphasic change of autophagy in the septic AKI model of cecal ligation and puncture (CLP) in rats46. Autophagy was shown to elevate at hours but then declined at 9 hours following CLP, where AKI was detected at 18 hours. Autophagy was shown in proximal tubules, and not in distal tubules. In the NRK-52E proximal tubule cell line, inhibition of autophagy increased tumor necrosis factor α-induced cell death, suggesting a renoprotective function of autophagy in septic AKI. In mouse models of septic AKI, Howell et al. further showed that temsirolimus (mTOR inhibitor) induced autophagy and protected against the development of AKI, even when added after established endotoxemia47. Collectively, these results suggest that autophagy is induced in various forms of AKI and plays a renoprotective role under the disease conditions. Although it remains largely unclear as to why autophagy is protective, autophagy may be important to the removal of damaged organelles under these conditions. One class of such organelles that are damaged and consequently may cause cell injury and death are mitochondria.
Mitophagy: regulation and involvement in AKI
Despite the recognition of the protective role of autophagy, it remains unclear how autophagy protects cells in stress or pathological conditions. Nonetheless, removal of damaged organelles, especially dysfunctional mitochondria, may contribute to the protective effect of autophagy. Damaged and dysfunctional mitochondria not only release pro-apoptotic factors—e.g., cytochrome c—but are a major source of the production of reactive oxygen species-ROS. Therefore, removal of these organelles via autophagy by a process called mitophage is postulated to be cytoprotective48.
Mitophagy selectively removes mitochondria under both physiological and pathological circumstances. It is required for the steady-state turnover of the mitochondria pool, for adjusting mitochondrial numbers following cellular metabolic needs, or during certain developmental stages in mammalian cells such as reticulocyte differentiation into red blood cells when undamaged mitochondria are removed49. On the other side, mitophagy is a critical component of mitochondrial quality control surveillance whereby it identifies and tags pathological stress-induced depolarized and dysfunctional mitochondria for prompt elimination. In this regard, mitophagy is a cellular defense or adaptation mechanism for purifying the mitochondrial pool to prevent excessive ROS accumulation, and mitochondrial apoptotic cascade under various pathological stress24. In addition, timely removal of damaged mitochondria is expected to prevent the release of mitochondrial DNA, which may stimulate a robust immune response or inflammation to contribute to pathogenesis50.
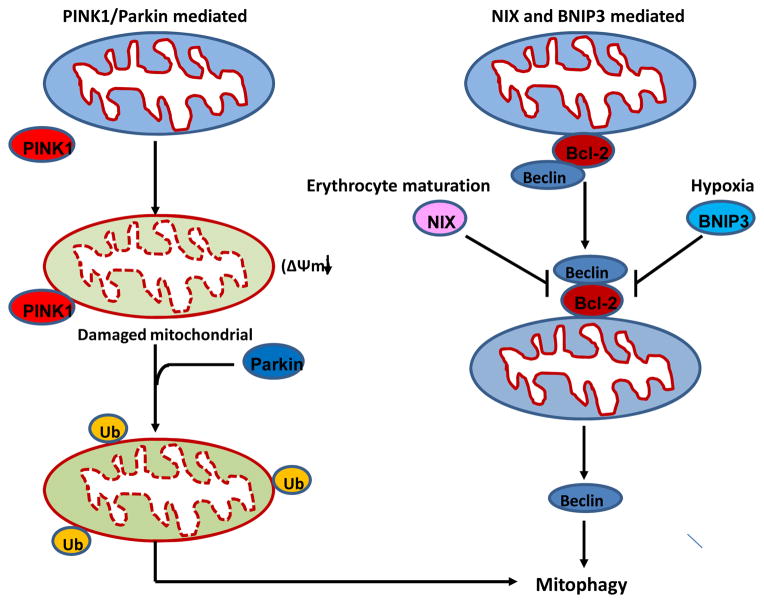
Currently, two main mechanisms of mitophagy have been described (Figure 3). In the first mechanism, mitophagy is mediated by PTEN-induced putative kinase protein1 (Pink1) and the E3 ubiquitin ligase Parkin-dependent pathway. Mutations in Pink1 and Parkin genes lead to early-onset autosomal recessive Parkinson’s disease, suggesting a possible link of defective mitophagy to the etiology of this neurodegenerative disease. In healthy mitochondria, Pink1 is constitutively imported into mitochondria and degraded by proteolysis to maintain a low level of expression. When a subset of mitochondria become impaired and depolarized, Pink1 proteolysis is suppressed resulting in Pink1 accumulation on mitochondrial outer membrane (MOM), which then recruits and activates Parkin from cytosol to the damaged mitochondria. One mechanism of Parkin recruitment by Pink1 has been shown in a recent study that Pink1 phosphorylates Mfn2, which then serves as a Parkin receptor to attract Parkin to damaged mitochondria51. On mitochondria, Parkin ubiquitinates various substrates in MOM, such as Mfn1, Mfn2 and voltage-dependent anion-selective channel protein 1 (VDAC1), to induce and promote autophagic removal of mitochondria49, 52. Recent studies also revealed that Parkin-mediated ubiquitination and mitophagy can be antagonized by certain mitochondria-localized deubiquitinase enzymes (DUBs), USP15 and 30, which block Parkin’s ability to drive mitophagy through removal of ubiquitin attached by Parkin onto damaged mitochondria 53, 54. The second mechanism of mitophagy is not mediated by Parkin but involves two Bcl-2 family proteins: BCL2/adenovirus E1B 19-kDa protein-interacting protein 3 (BNIP3) or Nip3-like protein X (NIX, also known as BNIP3L). During the maturation of red blood cells, all mitochondria contained in a reticulocyte are degraded by NIX-dependent mitophagy49. During hypoxia, BNIP3 induction via hypoxia-inducible factor-1 (HIF-1) promotes mitophagy in mouse embryo fibroblasts to prevent accumulation of ROS and cell death. Both NIX and BNIP3 may induce mitophagy by disrupting the interaction between Beclin1 and Bcl-255, 56. Of note, several tissue or cell types that has low or undetectable Parkin levels, such as Hela cells may also utilize a Parkin-independent mitophagy pathways, although the details remain unclear57.
Figure 3. Pathways of mitophagy.
Two main pathways of mitophagy are presented: (1) PINK/Parkin pathway. In healthy mitochondria, Pink1 is imported into mitochondria and degraded by the inner mitochondrial membrane protease PARL. In response to mitochondrial stress, mitochondrial membrane potential is dissipated, and full-length PINK1 accumulates on mitochondrial outer membrane. Pink1 then recruits Parkin to damaged mitochondria, and Parkin ubiquitinylates outer membrane proteins to induce and promote autophagic removal of mitochondria. (2) BNIP3/NIX pathway. In healthy cells, Bcl-2 interacts and inhibits Beclin 1. Under conditions such as erythrocyte maturation and hypoxia, Nix and BNIP3 are activated respectively. Both NIX and BNIP3 may interact with Bcl-2 to release Beclin 1, which induces autophagic removal of mitochondria.
Mitophagy is intimately linked to mitochondrial dynamics, which, as discussed above, is mainly characterized by fission and fusion. Specifically, mitophagy depends on mitochondrial fragmentation. As a result, mitochondrial dynamics and mitophagy form an integrated mitochondrial quality control axis, to monitor and purify the mitochondrial network. During fission, if a daughter mitochondrion loses membrane potential and integrity it may not fuse; instead, it is sensed and removed by mitophagy24, 58. Through this surveillance and clearance mechanism, mitochondrial damage control and cell homeostasis are maintained. However, in pathological conditions, once cellular stress is overwhelming and beyond the threshold of mitochondrial quality control, mitochondria undergo excessive fission (fragmentation) and mitophagy is impaired or suppressed, as a result, damaged mitochondria without prompt autophagic elimination are accumulated and induce the generation of excessive mitochondrial ROS, release of pro-apoptotic proteins and the subsequent initiation of mitochondrial apoptotic cascade, leading to cell injury and death. This process has been implicated in ageing and neurodegeneration in human59, 60.
In kidneys, mitophagy has been implicated in several disease models including both AKI and CKD. In a rabbit model of hyperglycemic critical illness, insufficient autophagy was observed in both liver and kidneys, which is correlated with persistent mitochondrial dysfunction and more severe organ damage, suggesting an important link between mitophagy and cell injury in kidneys during critical illness61. In an acid-load model of metabolic acidosis, a common complication of CKD, mitophagy was shown to be induced in proximal tubular cells, which protected mitochondrial respiratory function and alleviated mitochondrial swelling and depolarization, probably via a quality control mechanism of mitochondria62. During high-calorie diet induced renal injury, both autophagy and mitophagy were markedly inhibited along with abnormal mitochondrial morphology and notably calorie restriction could ameliorate mitochondrial oxidative damage by enhancing autophagy and mitophagy in kidneys63. During ischemia-reperfusion injury, mitophagy was demonstrated to have indispensible protective roles in vital organs such as brain, heart and kidney64–66, by the elimination of damaged mitochondria and consequent preservation of mitochondrial fidelity and functions. In renal ischemia-reperfusion, BNIP3 was up-regulated in a HIF-1 dependent way in proximal tubular cells. Interestingly, BNIP3 overexpression was shown to induce mitophagy, indicating that mitophagy maybe mediated via a HIF-1/BNIP3 pathway in tubular cells during AKI66. In kidney tissues of septic patients, increased autophagosomes juxtaposed with hydropic mitochondria with cristae damage in renal tubular cells was also observed, indicating the possibility of clearance of damaged mitochondria by mitophagy8. Collectively, these studies support the renoprotective effect of mitophagy in both acute and chronic kidney injury. Under these disease conditions, mitophagy may clear damaged mitochondria to prevent the activation of relevant mechanisms of cell injury and death in kidney cells and tissues. Thus, it is conceivable that during initial stage of kidney injury, mitophagy is elevated to ensure the quality control by removing damaged mitochondria. However, as the disease progresses, this selective autophagic process and quality control machinery may be overwhelmed or impaired, leading to the accumulation of damaged mitochondria, oxidative stress, and cell death. Therefore, a targeted approach by enhancing mitophagy during kidney injuries including septic AKI may be a promising therapeutic strategy for renoprotection.
MITOCHONDRIAL BIOGENESIS IN SEPTIC AKI
Mitochondrial biogenesis refers to the generation of new mitochondrial mass and replication of mitochondrial DNA. It is essential for the replacement of damaged mitochondrial proteins and for increasing energy-generating capacity when the need arises. Mitochondrial biogenesis appears to be an important process during the recovery from septic AKI.
PPARγ-co-activator-1α (PGC1α) is a nuclear-encoded protein that has been recognized as a positive regulator of mitochondrial biogenesis. Two related proteins called PGC1β and PGC-related co-activator (PRC) have been less well-studied in the context of sepsis. PGC1α activates an array of transcription factors including peroxisome proliferator activator receptors (PPARs), nuclear respiratory factors-1 and -2 (NRF1, NRF2), and estrogen-related receptor α (ERRα) (reviewed in 67). In addition to being a key mediator of mitochondrial biogenesis, PGC1α also drives other cellular programs, including the production of antioxidant enzymes and the elaboration of vascular growth factors to promote angiogenesis 68, 69.
Not surprisingly, PGC1α is most heavily expressed in the most metabolically active organs, including the brain, skeletal muscle, heart, and kidney 70. Within the kidney, the proximal tubular epithelium exhibits the highest PGC1α expression. During CLP or endotoxemia, PGC1α expression in the kidney falls in proportion to the severity of impaired filtration 11. Indeed, toxic and ischemic forms of AKI are also characterized by an initial fall in PGC1α20. If septic mice are resuscitated, the expression of PGC1α rebounds to normal levels, and the levels of downstream gene products, whether related to oxidative phosphorylation, fatty acid oxidation, or antioxidant defense, are tightly correlated to PGC1α expression11.
In theory, the fall in PGC1α expression could arise as a consequence of AKI—e.g., a fall in GFR leading to reduced sodium delivery, less ATP consumption, reduced activation of PGC1α through transcriptional and post-transcriptional mechanisms—or be a causal factor in the severity and/or recovery from septic AKI. PGC1α KO mice have normal renal function, mitochondrial ultrastructure, mitochondrial abundance, and absence of proteinuria 11. Although acute changes in a gene’s expression could produce different consequences than a conventional whole-body knockout (e.g., developmental compensation in the knockout mouse), these findings at least suggest that the fall in PGC1α during sepsis is insufficient to produce renal injury.
In non-renal experimental settings, the importance of PGC1α has become more apparent in stress situations, such as the study of heart failure 71, 72. Using a model of resuscitated sepsis (systemic LPS followed 18 hrs later by intraperitoneal isotonic fluid bolus and outcomes studied 24 hrs after resuscitation), Tran and colleagues observed that PGC1α knockout mouse had persistent AKI whereas their wild type littermates had recovered function 11. To rule out a renal impact of an extrarenal interaction between PGC1α deficiency and experimental sepsis, the authors created proximal tubular knockouts of PGC1α and again observed more protracted AKI when PGC1α expression was genetically ablated in part of the nephron. These findings suggest that the rebound in PGC1α expression as the animal recovers from systemic inflammation is critical to the functional recovery from septic AKI.
Why does PGC1α expression fall in the septic kidney and what facilitates its restoration as sepsis resolves? These questions have not been completely addressed, but the existing literature offers tantalizing clues. Independent studies have shown that various septic mediators induce a fall in PGC1α transcript and protein 11, 73, 74. MicroRNA-mediated transcript degradation has been proposed as an important regulator of PGC1α during sepsis 75. Expression of PGC1α is augmented in response to many stimuli—exercise and ATP depletion 76, nutritional status 77, cold exposure 70, hypoxia 68, hormones and pathways that trigger cAMP78, reactive oxygen species 69, and nitric oxide 79. The induction of PGC1α—and of mitochondrial biogenesis—during functional recovery from sepsis appears to be shared across organs affected by sepsis 80, 81. Several of these stimuli are greatly increased in sepsis, including ATP depletion and free radicals. Signaling events downstream of pattern recognition receptors that sense bacterial products may directly induce PGC1α transcription 82. Thus, the evolving balance of suppressive and inductive factors during the course of sepsis may dictate the temporal profile of PGC1α expression.
How does PGC1αeffect recovery in sepsis? As described earlier, although overt mitochondrial damage visible by EM is only present in the minority of tubular cells in models of sepsis, there appears to be a widespread reduction in the levels of Krebs’ cycle and oxidative phosphorylation enzymes 11. Therefore, it stands to reason that replacement of degraded or damaged mitochondrial proteins is likely one important effect of recovered PGC1α expression. Induction of anti-oxidant enzymes may be another 83. More exotic possibilities include PGC1α-induced cellular cross-talk, as has been observed, for example, in the angiogenic response to chronic ischemia 68. Because so many cellular programs are driven by this protein, the answer may be difficult to attribute to a single dominant downstream mechanism. The manifold disruptions induced by the septic milieu further complicate a reductionist approach.
Mitochondrial dysfunction: a therapeutic target in AKI
As presented above, there is ample evidence indicating that mitochondrial dysfunction contributes to the pathogenesis of AKI 19, 84. Mitochondrial damage leads to the opening of the mitochondrial permeability transition (MPT) pore, the depletion of mitochondrial membrane potential (ΔΨm), and a burst of excess reactive oxygen species (ROS) as a result of defective electron flux. When isolated renal proximal tubules were subjected to hypoxia/reperfusion, they developed severe deficits of mitochondrial function, decrease of ΔΨm, and persistent ATP depletion 85–87. In vivo, mitochondrial membrane potential decreased as early as 2 minutes after the onset of renal ischemia19. Therefore, following the occurrence of mitochondrial damage and dysfunction, oxidative stress and ATP depletion are among the typical signals that are associated closely with the process of AKI. Aberrant generation of reactive oxygen species (ROS) may contribute to the initiation and maintenance of tubular injury in AKI 88, and a profound reduction in intracellular ATP content invariably occurs early after ischemic AKI89.
The antioxidants that specifically target the mitochondria as either conjugation to a lipophilic cation (MitoQ) or incorporation into a mitochondrially targeted peptide (SS-31) showed the marked beneficial effects. They not only preserved mitochondrial structure and function, but also promoted the recovery of ATP supplies and inhibited the mitochondrial translocation of Drp1 and the associated mitochondrial fragmentation90, 91. Similarly, other antioxidants such as AH-SOD (a hexamethylenediamine-conjugated cationic superoxide dismutase derivative), SOD-mimetic, Selenium, Curcumin, alpha-Lipoic acid (LA) showed also protective effects on mitochondria during cisplatin-induced AKI92–96. Cyclosporine A is a pharmacologic inhibitor of Cyclophilin D, a key component of mitochondrial permeability transition (MPT), that has been shown to protect against renal injury in multiple experimental models of AKI 97. Interestingly, antimycin A or myxothiazol as the inhibitor of mitochondrial respiration complex can ameliorate cisplatin-induced p53 activation and exert the cytoprotective effects against apoptotic injury in cultured renal tubular cells 98. Moreover, mdivi-1 as the first selective inhibitor of the mitochondrial fission proteins Drp1 can attenuate mitochondrial fragmentation and block cytochrome c release, ROS production, and apoptosis 14, 99. Therefore, targeting mitochondrial dysfunction is a significant step forward in the long quest for renoprotective strategies in AKI.
CONCLUSIONS
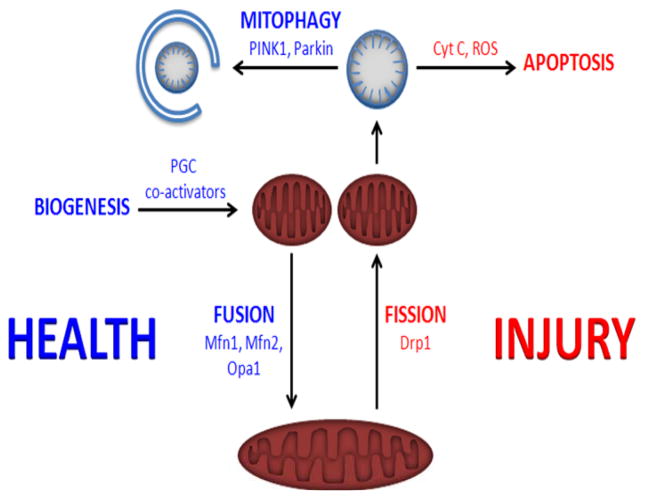
The healthy nephron relies on mitochondrially-generated ATP in order to facilitate the sodium-coupled reclamation of 99% filtered water. In both human and experimental acute kidney injury arising from toxic and ischemic etiologies, injury to the mitochondria appears to be an early event that precedes loss of GFR. In sepsis-associated AKI, tubular cell death is not a prominent finding, but molecular evidence of mitochondrial dysfunction is widespread. Studies in animal models suggest that mitochondrial injury in AKI not only contributes to the severity of AKI, but that intracellular processes to restore healthy mitochondrial mass may be critical to the recovery of renal function (Figure 4). Evolving strategies to target mitochondrial dysfunction therefore hold promise for the prevention and treatment of AKI.
FIGURE 4. The mitochondrial life cycle.
Activation of peroxisome proliferator gamma co-activators, such as PGC1α, is induced by diverse stimuli ranging from exercise to certain drugs. PGC1α promotes the biogenesis of mitochondria. Mitochondria can undergo fusion—directed by mitofusins (Mfn1,2) and Opa1—to form larger, more mature organelles. In healthy cells, fusion is balanced by fission—directed by dynamin-related protein 1 (Drp1)—in order to maintain a steady abundance of functional mitochondrial mass. In cells exposed to oxidant stress, abundant cytokines, hypoxia, or other sources of injury, the balance of mitochondrial dynamics tips toward fission and swelling of mitochondria. These mitochondria can release reactive oxygen species (ROS) and cytochrome C, leading to apoptosis. Alternatively, injured mitochondria can be safely cleared by mitophagy, a form of macro-autophagy regulated by proteins such as PINK1 and Parkin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex i disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Beginning Ending Supportive Therapy for the Kidney I. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 3.Deetjen P. Measurement of metabolism during renal work. The International journal of biochemistry. 1980;12:243–244. [PubMed] [Google Scholar]
- 4.Thurau K. Renal na-reabsorption and o2-uptake in dogs during hypoxia and hydrochlorothiazide infusion. Proc Soc Exp Biol Med. 1961;106:714–717. doi: 10.3181/00379727-106-26451. [DOI] [PubMed] [Google Scholar]
- 5.Vandewalle A, Wirthensohn G, Heidrich HG, Guder WG. Distribution of hexokinase and phosphoenolpyruvate carboxykinase along the rabbit nephron. Am J Physiol. 1981;240:F492–500. doi: 10.1152/ajprenal.1981.240.6.F492. [DOI] [PubMed] [Google Scholar]
- 6.Bagnasco S, Good D, Balaban R, Burg M. Lactate production in isolated segments of the rat nephron. Am J Physiol. 1985;248:F522–526. doi: 10.1152/ajprenal.1985.248.4.F522. [DOI] [PubMed] [Google Scholar]
- 7.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 8.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, Hotchkiss RS. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187:509–517. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Hassoun HT, Santora R, Rabb H. Organ crosstalk: The role of the kidney. Curr Opin Crit Care. 2009;15:481–487. doi: 10.1097/MCC.0b013e328332f69e. [DOI] [PubMed] [Google Scholar]
- 10.Evans RG, Ince C, Joles JA, Smith DW, May CN, O’Connor PM, Gardiner BS. Haemodynamic influences on kidney oxygenation: Clinical implications of integrative physiology. Clinical and experimental pharmacology & physiology. 2013;40:106–122. doi: 10.1111/1440-1681.12031. [DOI] [PubMed] [Google Scholar]
- 11.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM. Pgc-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121:4003–4014. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyson A, Bezemer R, Legrand M, Balestra G, Singer M, Ince C. Microvascular and interstitial oxygen tension in the renal cortex and medulla studied in a 4-h rat model of lps-induced endotoxemia. Shock. 2011 doi: 10.1097/SHK.0b013e3182169d5a. [DOI] [PubMed] [Google Scholar]
- 13.Johannes T, Mik EG, Nohe B, Raat NJ, Unertl KE, Ince C. Influence of fluid resuscitation on renal microvascular po2 in a normotensive rat model of endotoxemia. Crit Care. 2006;10:R88. doi: 10.1186/cc4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manny J, Livni N, Schiller M, Guttman A, Boss J, Rabinovici N. Structural changes in the perfused canine kidney exposed to the direct action of endotoxin. Isr J Med Sci. 1980;16:153–161. [PubMed] [Google Scholar]
- 16.Zsengeller ZK, Ellezian L, Brown D, Horvath B, Mukhopadhyay P, Kalyanaraman B, Parikh SM, Karumanchi SA, Stillman IE, Pacher P. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J Histochem Cytochem. 2012;60:521–529. doi: 10.1369/0022155412446227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanabe K, Tamura Y, Lanaspa MA, Miyazaki M, Suzuki N, Sato W, Maeshima Y, Schreiner GF, Villarreal FJ, Johnson RJ, Nakagawa T. Epicatechin limits renal injury by mitochondrial protection in cisplatin nephropathy. Am J Physiol Renal Physiol. 2012;303:F1264–1274. doi: 10.1152/ajprenal.00227.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharbangar A, Khynriam D, Prasad SB. Effect of cisplatin on mitochondrial protein, glutathione, and succinate dehydrogenase in dalton lymphoma-bearing mice. Cell Biol Toxicol. 2000;16:363–373. doi: 10.1023/a:1007648427024. [DOI] [PubMed] [Google Scholar]
- 19.Hall AM, Rhodes GJ, Sandoval RM, Corridon PR, Molitoris BA. In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury. Kidney Int. 2013;83:72–83. doi: 10.1038/ki.2012.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol. 2012;302:F853–864. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brealey D, Singer M. Mitochondrial dysfunction in sepsis. Curr Infect Dis Rep. 2003;5:365–371. doi: 10.1007/s11908-003-0015-9. [DOI] [PubMed] [Google Scholar]
- 22.Liaudet L, Fishman D, Markert M, Perret C, Feihl F. L-canavanine improves organ function and tissue adenosine triphosphate levels in rodent endotoxemia. Am J Respir Crit Care Med. 1997;155:1643–1648. doi: 10.1164/ajrccm.155.5.9154870. [DOI] [PubMed] [Google Scholar]
- 23.Levy B, Mansart A, Bollaert PE, Franck P, Mallie JP. Effects of epinephrine and norepinephrine on hemodynamics, oxidative metabolism, and organ energetics in endotoxemic rats. Intensive Care Med. 2003;29:292–300. doi: 10.1007/s00134-002-1611-0. [DOI] [PubMed] [Google Scholar]
- 24.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoppins S, Nunnari J. The molecular mechanism of mitochondrial fusion. Biochim Biophys Acta. 2009;1793:20–26. doi: 10.1016/j.bbamcr.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Zhan M, Brooks C, Liu F, Sun L, Dong Z. Mitochondrial dynamics: Regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. 2013;83:568–581. doi: 10.1038/ki.2012.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang WX, Wu WH, Qiu HY, Bo H, Huang SM. Amelioration of rhabdomyolysis-induced renal mitochondrial injury and apoptosis through suppression of drp-1 translocation. J Nephrol. 2013;26:1073–1082. doi: 10.5301/jn.5000268. [DOI] [PubMed] [Google Scholar]
- 29.Gall JM, Wang Z, Liesa M, Molina A, Havasi A, Schwartz JH, Shirihai O, Borkan SC, Bonegio RG. Role of mitofusin 2 in the renal stress response. PLoS One. 2012;7:e31074. doi: 10.1371/journal.pone.0031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao X, Hu Y, Quiros PM, Wei Q, Lopez-Otin C, Dong Z. Oma1 mediates opa1 proteolysis and mitochondrial fragmentation in experimental models of ischemic kidney injury. Am J Physiol Renal Physiol. 2014;306:F1318–1326. doi: 10.1152/ajprenal.00036.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho SG, Du Q, Huang S, Dong Z. Drp1 dephosphorylation in atp depletion-induced mitochondrial injury and tubular cell apoptosis. Am J Physiol Renal Physiol. 2010;299:F199–206. doi: 10.1152/ajprenal.00716.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci U S A. 2007;104:11649–11654. doi: 10.1073/pnas.0703976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei Q, Dong G, Chen JK, Ramesh G, Dong Z. Bax and bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int. 2013;84:138–148. doi: 10.1038/ki.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks C, Cho SG, Wang CY, Yang T, Dong Z. Fragmented mitochondria are sensitized to bax insertion and activation during apoptosis. Am J Physiol Cell Physiol. 2011;300:C447–455. doi: 10.1152/ajpcell.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livingston MJ, Dong Z. Autophagy in acute kidney injury. Semin Nephrol. 2014;34:17–26. doi: 10.1016/j.semnephrol.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki C, Isaka Y, Takabatake Y, Tanaka H, Koike M, Shibata M, Uchiyama Y, Takahara S, Imai E. Participation of autophagy in renal ischemia/reperfusion injury. Biochem Biophys Res Commun. 2008;368:100–106. doi: 10.1016/j.bbrc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 38.Jiang M, Liu K, Luo J, Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol. 2010;176:1181–1192. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S, Hartleben B, Kretz O, Wiech T, Igarashi P, Mizushima N, Walz G, Huber TB. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy. 2012;8:826–837. doi: 10.4161/auto.19419. [DOI] [PubMed] [Google Scholar]
- 41.Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 2008;74:631–640. doi: 10.1038/ki.2008.214. [DOI] [PubMed] [Google Scholar]
- 42.Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008;294:F777–787. doi: 10.1152/ajprenal.00590.2007. [DOI] [PubMed] [Google Scholar]
- 43.Pallet N, Bouvier N, Legendre C, Gilleron J, Codogno P, Beaune P, Thervet E, Anglicheau D. Autophagy protects renal tubular cells against cyclosporine toxicity. Autophagy. 2008;4:783–791. doi: 10.4161/auto.6477. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi A, Kimura T, Takabatake Y, Namba T, Kaimori J, Kitamura H, Matsui I, Niimura F, Matsusaka T, Fujita N, Yoshimori T, Isaka Y, Rakugi H. Autophagy guards against cisplatin-induced acute kidney injury. Am J Pathol. 2012;180:517–525. doi: 10.1016/j.ajpath.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Xu Z, Jiang L, Mao J, Zeng Z, Fang L, He W, Yuan W, Yang J, Dai C. Rictor/mtorc2 protects against cisplatin-induced tubular cell death and acute kidney injury. Kidney Int. 2014 doi: 10.1038/ki.2013.559. [DOI] [PubMed] [Google Scholar]
- 46.Hsiao HW, Tsai KL, Wang LF, Chen YH, Chiang PC, Chuang SM, Hsu C. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock. 2012;37:289–296. doi: 10.1097/SHK.0b013e318240b52a. [DOI] [PubMed] [Google Scholar]
- 47.Howell GM, Gomez H, Collage RD, Loughran P, Zhang X, Escobar DA, Billiar TR, Zuckerbraun BS, Rosengart MR. Augmenting autophagy to treat acute kidney injury during endotoxemia in mice. PLoS One. 2013;8:e69520. doi: 10.1371/journal.pone.0069520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacVicar T. Mitophagy. Essays Biochem. 2013;55:93–104. doi: 10.1042/bse0550093. [DOI] [PubMed] [Google Scholar]
- 49.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawlor KE, Vince JE. Ambiguities in nlrp3 inflammasome regulation: Is there a role for mitochondria? Biochim Biophys Acta. 2014;1840:1433–1440. doi: 10.1016/j.bbagen.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, Dorn GW., 2nd Pink1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Randow F, Youle RJ. Self and nonself: How autophagy targets mitochondria and bacteria. Cell Host Microbe. 2014;15:403–411. doi: 10.1016/j.chom.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bingol B, Tea JS, Phu L, Reichelt M, Bakalarski CE, Song Q, Foreman O, Kirkpatrick DS, Sheng M. The mitochondrial deubiquitinase usp30 opposes Parkin-mediated mitophagy. Nature. 2014;510:370–375. doi: 10.1038/nature13418. [DOI] [PubMed] [Google Scholar]
- 54.Cornelissen T, Haddad D, Wauters F, Van Humbeeck C, Mandemakers W, Koentjoro B, Sue C, Gevaert K, De Strooper B, Verstreken P, Vandenberghe W. The deubiquitinase usp15 antagonizes Parkin-mediated mitochondrial ubiquitination and mitophagy. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an hif-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Green DR, Levine B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell. 2014;157:65–75. doi: 10.1016/j.cell.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding WX, Guo F, Ni HM, Bockus A, Manley S, Stolz DB, Eskelinen EL, Jaeschke H, Yin XM. Parkin and mitofusins reciprocally regulate mitophagy and mitochondrial spheroid formation. J Biol Chem. 2012;287:42379–42388. doi: 10.1074/jbc.M112.413682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tatsuta T, Langer T. Quality control of mitochondria: Protection against neurodegeneration and ageing. EMBO J. 2008;27:306–314. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCoy MK, Cookson MR. Mitochondrial quality control and dynamics in Parkinson’s disease. Antioxid Redox Signal. 2012;16:869–882. doi: 10.1089/ars.2011.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gunst J, Derese I, Aertgeerts A, Ververs EJ, Wauters A, Van den Berghe G, Vanhorebeek I. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit Care Med. 2013;41:182–194. doi: 10.1097/CCM.0b013e3182676657. [DOI] [PubMed] [Google Scholar]
- 62.Namba T, Takabatake Y, Kimura T, Takahashi A, Yamamoto T, Matsuda J, Kitamura H, Niimura F, Matsusaka T, Iwatani H, Matsui I, Kaimori J, Kioka H, Isaka Y, Rakugi H. Autophagic clearance of mitochondria in the kidney copes with metabolic acidosis. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013090986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui J, Shi S, Sun X, Cai G, Cui S, Hong Q, Chen X, Bai XY. Mitochondrial autophagy involving renal injury and aging is modulated by caloric intake in aged rat kidneys. PLoS One. 2013;8:e69720. doi: 10.1371/journal.pone.0069720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X, Yan H, Yuan Y, Gao J, Shen Z, Cheng Y, Shen Y, Wang RR, Wang X, Hu WW, Wang G, Chen Z. Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy. 2013;9:1321–1333. doi: 10.4161/auto.25132. [DOI] [PubMed] [Google Scholar]
- 65.Andres AM, Hernandez G, Lee P, Huang C, Ratliff EP, Sin J, Thornton CA, Damasco MV, Gottlieb RA. Mitophagy is required for acute cardioprotection by simvastatin. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2013.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishihara M, Urushido M, Hamada K, Matsumoto T, Shimamura Y, Ogata K, Inoue K, Taniguchi Y, Horino T, Fujieda M, Fujimoto S, Terada Y. Sestrin-2 and bnip3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am J Physiol Renal Physiol. 2013;305:F495–509. doi: 10.1152/ajprenal.00642.2012. [DOI] [PubMed] [Google Scholar]
- 67.Weinberg JM. Mitochondrial biogenesis in kidney disease. J Am Soc Nephrol. 2011;22:431–436. doi: 10.1681/ASN.2010060643. [DOI] [PubMed] [Google Scholar]
- 68.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. Hif-independent regulation of vegf and angiogenesis by the transcriptional coactivator pgc-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 69.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the pgc-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 70.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 71.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: A question of balance. J Clin Invest. 2005;115:547–555. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking ppar-gamma coactivator 1alpha. Proc Natl Acad Sci U S A. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feingold KR, Wang Y, Moser A, Shigenaga JK, Grunfeld C. Lps decreases fatty acid oxidation and nuclear hormone receptors in the kidney. J Lipid Res. 2008;49:2179–2187. doi: 10.1194/jlr.M800233-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Downregulation of liver x receptor-alpha in mouse kidney and hk-2 proximal tubular cells by lps and cytokines. J Lipid Res. 2005;46:2377–2387. doi: 10.1194/jlr.M500134-JLR200. [DOI] [PubMed] [Google Scholar]
- 75.Sweeney TE, Suliman HB, Hollingsworth JW, Piantadosi CA. Differential regulation of the pgc family of genes in a mouse model of staphylococcus aureus sepsis. PLoS One. 2010;5:e11606. doi: 10.1371/journal.pone.0011606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jager S, Handschin C, St-Pierre J, Spiegelman BM. Amp-activated protein kinase (ampk) action in skeletal muscle via direct phosphorylation of pgc-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of pgc-1alpha and sirt1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 78.Wu Z, Huang X, Feng Y, Handschin C, Feng Y, Gullicksen PS, Bare O, Labow M, Spiegelman B, Stevenson SC. Transducer of regulated creb-binding proteins (torcs) induce pgc-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci U S A. 2006;103:14379–14384. doi: 10.1073/pnas.0606714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: The role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 80.Haden DW, Suliman HB, Carraway MS, Welty-Wolf KE, Ali AS, Shitara H, Yonekawa H, Piantadosi CA. Mitochondrial biogenesis restores oxidative metabolism during staphylococcus aureus sepsis. Am J Respir Crit Care Med. 2007;176:768–777. doi: 10.1164/rccm.200701-161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suliman HB, Carraway MS, Welty-Wolf KE, Whorton AR, Piantadosi CA. Lipopolysaccharide stimulates mitochondrial biogenesis via activation of nuclear respiratory factor-1. J Biol Chem. 2003;278:41510–41518. doi: 10.1074/jbc.M304719200. [DOI] [PubMed] [Google Scholar]
- 82.Sweeney TE, Suliman HB, Hollingsworth JW, Welty-Wolf KE, Piantadosi CA. A toll-like receptor 2 pathway regulates the ppargc1a/b metabolic co-activators in mice with staphylococcal aureus sepsis. PLoS One. 2011;6:e25249. doi: 10.1371/journal.pone.0025249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cherry AD, Suliman HB, Bartz RR, Piantadosi CA. Peroxisome proliferator-activated receptor gamma co-activator 1-alpha as a critical co-activator of the murine hepatic oxidative stress response and mitochondrial biogenesis in staphylococcus aureus sepsis. J Biol Chem. 2014;289:41–52. doi: 10.1074/jbc.M113.512483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brealey D, Karyampudi S, Jacques TS, Novelli M, Stidwill R, Taylor V, Smolenski RT, Singer M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol. 2004;286:R491–497. doi: 10.1152/ajpregu.00432.2003. [DOI] [PubMed] [Google Scholar]
- 85.Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci U S A. 2000;97:2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feldkamp T, Kribben A, Weinberg JM. Assessment of mitochondrial membrane potential in proximal tubules after hypoxia-reoxygenation. Am J Physiol Renal Physiol. 2005;288:F1092–1102. doi: 10.1152/ajprenal.00443.2004. [DOI] [PubMed] [Google Scholar]
- 87.Weinberg JM, Venkatachalam MA, Roeser NF, Saikumar P, Dong Z, Senter RA, Nissim I. Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am J Physiol Renal Physiol. 2000;279:F927–943. doi: 10.1152/ajprenal.2000.279.5.F927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109:665–678. doi: 10.1016/s0002-9343(00)00612-4. [DOI] [PubMed] [Google Scholar]
- 89.Devarajan P. Cellular and molecular derangements in acute tubular necrosis. Curr Opin Pediatr. 2005;17:193–199. doi: 10.1097/01.mop.0000152620.59425.eb. [DOI] [PubMed] [Google Scholar]
- 90.Solesio ME, Prime TA, Logan A, Murphy MP, Del Mar Arroyo-Jimenez M, Jordan J, Galindo MF. The mitochondria-targeted anti-oxidant mitoq reduces aspects of mitochondrial fission in the 6-ohda cell model of Parkinson’s disease. Biochim Biophys Acta. 2013;1832:174–182. doi: 10.1016/j.bbadis.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 91.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV. Mitochondria-targeted peptide accelerates atp recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22:1041–1052. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishikawa M, Nagatomi H, Nishijima M, Ohira G, Chang BJ, Sato E, Inoue M. Targeting superoxide dismutase to renal proximal tubule cells inhibits nephrotoxicity of cisplatin and increases the survival of cancer-bearing mice. Cancer Lett. 2001;171:133–138. doi: 10.1016/s0304-3835(01)00591-2. [DOI] [PubMed] [Google Scholar]
- 93.Wangila GW, Nagothu KK, Steward R, 3rd, Bhatt R, Iyere PA, Willingham WM, Sorenson JR, Shah SV, Portilla D. Prevention of cisplatin-induced kidney epithelial cell apoptosis with a cu superoxide dismutase-mimetic [copper2ii(3,5-ditertiarybutylsalicylate)4(ethanol)4] Toxicol In Vitro. 2006;20:1300–1312. doi: 10.1016/j.tiv.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 94.Sugiyama S, Hayakawa M, Kato T, Hanaki Y, Shimizu K, Ozawa T. Adverse effects of anti-tumor drug, cisplatin, on rat kidney mitochondria: Disturbances in glutathione peroxidase activity. Biochem Biophys Res Commun. 1989;159:1121–1127. doi: 10.1016/0006-291x(89)92225-0. [DOI] [PubMed] [Google Scholar]
- 95.Waseem M, Kaushik P, Parvez S. Mitochondria-mediated mitigatory role of curcumin in cisplatin-induced nephrotoxicity. Cell Biochem Funct. 2013;31:678–684. doi: 10.1002/cbf.2955. [DOI] [PubMed] [Google Scholar]
- 96.Lee YM, Bae SY, Won NH, Pyo HJ, Kwon YJ. Alpha-lipoic acid attenuates cisplatin-induced tubulointerstitial injuries through inhibition of mitochondrial bax translocation in rats. Nephron Exp Nephrol. 2009;113:e104–112. doi: 10.1159/000235754. [DOI] [PubMed] [Google Scholar]
- 97.Singh D, Chander V, Chopra K. Cyclosporine protects against ischemia/reperfusion injury in rat kidneys. Toxicology. 2005;207:339–347. doi: 10.1016/j.tox.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 98.Wang J, Biju MP, Wang MH, Haase VH, Dong Z. Cytoprotective effects of hypoxia against cisplatin-induced tubular cell apoptosis: Involvement of mitochondrial inhibition and p53 suppression. J Am Soc Nephrol. 2006;17:1875–1885. doi: 10.1681/ASN.2005121371. [DOI] [PubMed] [Google Scholar]
- 99.Ferrari LF, Chum A, Bogen O, Reichling DB, Levine JD. Role of drp1, a key mitochondrial fission protein, in neuropathic pain. J Neurosci. 2011;31:11404–11410. doi: 10.1523/JNEUROSCI.2223-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]