Abstract
The objective was to explore the relationship between the levels of serum and urinary free light chains (FLCs) during the progression of renal damage in multiple myeloma (MM) patients. We examined 91 cases of MM patients, detected levels of serum FLCs (sFLCs), urinary FLCs (uFLCs), and serum creatinine at the same time, and then compared sFLC and uFLC levels during normal and abnormal serum creatinine phases. Among the 91 MM patients, 22 patients had abnormal serum creatinine levels (no uremia), and 69 patients had normal serum creatinine levels. The levels of sFLCs and uFLCs in patients with abnormal serum creatinine were beyond normal, namely both serum and urine positive (serum+ and urine+), and the average concentrations of κFLCs and λFLCs were 516.76 and 604.67 mg/L, respectively. Of the 69 patients with normal creatinine levels, there were 39 and 30 cases of κ-type and λ-type MM, respectively. Of the κ-type patients, 11 cases were serum positive and urine negative (serum+ and urine−) with an average concentration of 55.47 mg/L, and 28 cases were serum positive and urine positive (serum+ and urine+) with an average concentration of 513.09 mg/L. Of the λ-type patients, 16 cases were serum positive and urine negative (serum+ and urine−) with an average concentration of 78.44 mg/L, and 14 cases were serum positive and urine positive (serum+ and urine+) with an average concentration of 518.08 mg/L. The levels of uFLCs did not parallel those of sFLCs. In addition to sFLC levels, renal function affected uFLC concentrations. As MM progressed, the concentration of sFLCs increased in a step-by-step manner, and the uFLCs changed from negative to positive to negative again. Therefore, the whole progression included three phases: sserum+ and urine−, serum+ and urine+, and then serum+ and urine−.
Keywords: Multiple myeloma, Serum free light chains, Urinary free light chains
Introduction
Multiple myeloma (MM) is a type of malignant plasma cell hyperplasia that originates from B lymphocytes. The incidence of MM is about one per 10,000 people per year in our country [1], thus accounting for about 1 % of all malignant neoplasms and 10 % percent of hematological malignances. The characteristics of the disease are over-hyperplasia and the accumulation of cloned plasma cells in the bone marrow, which secretes monoclonal immunoglobulin (Ig) or its fragments, which are accompanied by widespread osteolytic damage or osteoporosis. The special biological characteristics of MM are occult course, diverse symptoms, and invasion into multiple tissues and organs. These make early diagnosis, integrated management, and prevention very difficult. Until now, the disease is incurable.
An Ig molecule is composed of one heavy chain and two identical light chains. The heavy chain is divided into five types, and the light chains are divided into the kappa and lambda types. The speed at which light chains are synthesized is a little bit faster than that of heavy chains, because the molecular weight of the light chain is apparently smaller. When combined with heavy chains, about 40 % of light chains exist in blood, so there is normally a quantity of serum free light chains (sFLCs). In MM patients, malignant plasma cells produce monoclonal Ig or light/heavy chains, and significant increases in the serum concentrations of these components are detected. In particular, the concentrations of light chains are elevated. If the concentrations of sFLCs are beyond the renal resorption capacity, free light chains will be observed in the urine (uFLC). So, what is the relationship between sFLCs and uFLCs? Can we learn more about sFLCs by simply observing uFLCs? To answer these questions, we examined 91 cases of MM patients and studied the relationship between the concentrations of sFLCs and uFLCs during different phases of renal damage.
Materials and Methods
Patient Data
We examined 91 cases of MM at Tongji Hospital, which is affiliated with Tongji Medical College of Huazhong University of Science and Technology, from June 2011 to April 2012 (36–80 years, median: 52 years). Males and females comprised 45 and 46 cases, respectively. These included IgG-type (35), IgA-type (19), IgD-type (13), IgM-type (1), kappa light chain-type (10), and lambda light chain-type (13) cases. All patients met the diagnosis criteria for MM [2].
Research Methods
Detection of FLCs in the Serum and Urine
Serum FLCs were detected by using an immunoturbidimetric Freelite assay for kappa and lambda FLCs (The Binding Site, UK) and the Roche Modular Automatic Biochemical Analyzer. The normal ranges and reference intervals were as follows: sκFLC, 3.3–19.4 mg/L; sλFLC, 5.71–26.3 mg/L; and sFLC κ/λ, 0.26–1.65.
Urinary FLCs were detected as mentioned above. The normal ranges for uκFLC and uλFLC were 0.8–13.48 and 0.7–5.9 mg/L, respectively.
Detection of Serum Creatinine
Serum creatinine was detected by using the AEROSET-2300 automatic biochemical analyzer and supporting reagents. The reference interval was 45–84 µmol/L.
Results and Conclusions
Ninety-one MM cases were examined, and these included 46 kappa- and 45 lambda-type cases. Blood and urine samples were collected at the same time, and the detection results show that 22 patients (6 kappa- and 16 lambda-type cases) had abnormal serum creatinine levels with sFLC and uFLC concentrations that were both abnormally increased (namely serum+ and urine+). In the other 69 patients, serum creatinine levels were normal. Of these, 22 patients (11 kappa- and 11 lambda-type cases) had increased sFLC concentrations but normal uFLC concentrations (namely serum+ and urine−,).
Both sFLCs and uFLCs were increased in 42 patients (28 kappa- and 14 lambda-type cases), and the concentrations of FLCs in the serum and urine were normal in 4 lambda-type cases. Only one patient (kappa type) had normal levels of sFLCs but elevated uFLCs (namely serum and urine +).
Abnormal Serum Creatinine
Serum creatinine levels were abnormal in 22 patients. Six were kappa-type cases with sκFLC > 19.4 mg/L and uκFLC > 13.48 mg/L. Sixteen were lambda-type cases with sλFLC > 26.3 mg/L and uλFLC > 5.9 mg/L (serum+ and urine+). The average kappa-type sFLC concentration was 216.76 mg/L, and the average lambda-type sFLC concentration was 604.67 mg/L. None of these patients met the uremia criteria.
Normal Serum Creatinine
Kappa Type
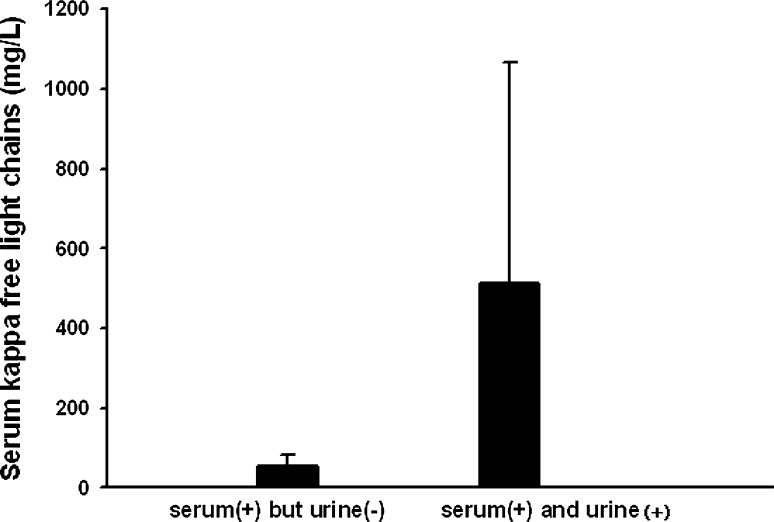
Eleven patients had levels of sκFLC > 19.4 mg/L and uκFLC < 13.48 mg/L (serum+ and urine−), and 28 patients had levels of sκFLC > 19.4 mg/L and uκFLC > 13.48 mg/L (serum+ and urine+). The mean sFLC value of the former and latter was 55.47 and 513.09 mg/L, respectively (Fig. 1).
Fig. 1.
Mean values of serum kappa FLCs in “serum (+) but urine (−)” and “serum (+) but urine (+)”
Lambda Type
Eleven patients had levels of sλFLC > 26.3 mg/L and uλFLC < 5.9 mg/L (serum+ and urine−), and 14 patients had levels of sλFLC > 26.3 mg/L and uλFLC > 5.9 mg/L (serum+ and urine+). The mean sFLC values of the former and latter were 78.44 and 518.08 mg/L, respectively (Fig. 2).
Fig. 2.
Mean values of serum lambda FLCs in “serum (+) but urine (−)” and “serum (+) but urine (+)”
Levels of sFLCs and uFLCs were both normal in four cases (lambda type), and only one patient (kappa type) had normal levels of sFLCs but elevated uFLCs.
Statistical Calculations
Normal Serum Creatinine
We applied the SPSS-19.0 software and the t test to the statistical calculation, and P < 0.05 represented statistical significance. The mean sFLC value of “serum positive but urine negative” was 55.47 mg/L, and the mean sFLC value of “both serum and urine positive” was 513.09 mg/L for kappa-type patients. There was a significant difference between the two groups (P = 0.01). As for lambda-type patients, the mean values were 78.44 and 518.08 mg/L, respectively. Similarly, they were significantly different (P = 0.02).
Therefore, in the normal creatinine group with consistent renal function damage and increases in sFLC concentrations, the levels of uFLCs did not parallel those of sFLCs.
Discussion
MM is a type of malignant plasma cell disease. The normal plasma cell makes up about 1 % of bone marrow cells, whereas the number of MM patients’ abnormal plasma cells can rise to above 90 % [2, 3]. Normally the synthesis of a heavy chain takes 18 min, and the synthesis of a light chain only takes 10 min. Therefore, when an Ig molecule is synthesized completely, there will be redundant light chains left. Ten percent of these will be catabolized by the renal tubules, 80 % are degraded by macrophages, and the other 10 % are discharged from body in the urine.
However, Ig synthesis in MM patients is significantly accelerated. One heavy chain can be synthesized in 2.5 min, and one light chain can be synthesized in 1 min. Therefore, there are almost two free light chains left, when one heavy chain is synthesized. When malignant plasma cells produce monoclonal Ig or light/heavy chains, their cell cycle enters into an infinitely proliferative state, and the speed of synthesis obviously accelerates. This leads to a significant increase in the levels of monoclonal Ig or light/heavy chains. In particular, concentrations of light chains in the serum or urine are elevated.
In normal individuals, about 500 mg sFLCs are produced every day and rapidly cleared by the kidneys, according to their molecular size. Monomeric κFLCs are cleared within 2–4 h when the glomerular filtration rate (GFR) is 40 %. Dimeric λFLCs are cleared within 3–6 h when the GFR is 20 % [4]. Almost all of the FLCs are filtered through the glomeruli and reabsorbed by the proximal convoluted tubule (PCT). About 1–10 mg FLCs are excreted into the urine every day, and these are thought to be sIgAs, which are produced by the distal convoluted tubule mucosal surfaces [5].
The daily renal re-absorption capacity for FLCs is 10–30 g. Even cells from MM patients produce plenty of FLCs, because the PCT has a powerful re-absorption capacity. When the sFLC concentration increases within some range, the uFLC concentration remains negative. However, when the sFLC concentration increases consistently and exceeds the re-absorption capacity of the PCT, the uFLC concentration becomes positive [6]. Therefore, the presence of uFLCs is dependent on renal function, and only sFLCs can really reflect the tumor burden. With MM progression, the concentration of sFLCs did not parallel that of uFLCs.
In our study, patients with normal serum creatinine and “serum+ and urine−” or “serum+ and urine+” patterns had average sκFLC concentrations of 55.47 or 513.09 mg/L, respectively. This difference was statistically significant. For lambda-type FLC patients, the mean concentrations were 78.44 or 518.08 mg/L, respectively, and this difference was also statistically significant. These reflect MM progression, as uFLCs could not be detected when FLCs were produced during the initial stage (first stage, serum positive but urine negative). As the disease progressed, uFLCs could be detected when the levels of sFLCs increased significantly (second stage, serum+ and urine+).
The levels of uFLCs increased in 22 patients with abnormal serum creatinine concentrations (glomerular damage). This was not only due to the high concentration of sFLCs (average sκFLC concentration: 516.76 mg/L and average sλFLC concentration: 604.67 mg/L), but it may also be a result of two events. First, renal function is still in the compensation period, and more light chains are filtered through residual functional nephrons. Second, glomerular ultrafiltration results in the leakage of albumin and other proteins, which may compete with FLCs to bind to brush border receptors. This binding reduces the absorption of light chains and leads to increases in the number of FLCs that enter into the distal convoluted tubules and are subsequently discharged in the urine. In other words, the concentration of FLCs depends upon the following aspects: glomerular filtrating rate, competition with other proteins for FLC receptors, and the absorptive capacity of renal tubules.
With the progression of the disease, a large number of sFLCs pass through the glomerular basement membrane and exceed the absorptive capacity of the proximal tubules. After entering the distal tubules, sFLCs are co-precipitated with Tamm-Horsfall protein to form waxy casts, which block the distal tubules and lead to interstitial inflammation. Finally, these casts destroy the basement membrane and renal interstitium. In addition, high concentrations of FLCs are directly toxic to tubular cells [7–10]. In theory, when MM patients are in the final stage of renal failure, the sFLCs are elevated significantly, but uFLCs are decreased because of the casts (third stage, serum+ and urine−). None of the patients in our study were in this category.
In our study, four patients were serum negative and urine negative. We analyzed the patients’ data one by one. The first patient had elevated sFLCs but normal uFLCs in the beginning, and his sFLC level became normal after chemotherapy. Therefore, we think that the normal sFLC and uFLC concentrations meant that the patient got better after therapy. The second patient had sFLC and uFLC levels that were normal in the beginning, and the sFLC concentration increased during his hospitalization. This patient’s normal levels of sFLC and uFLC were likely because he was in the early stage of the disease. The other two patients were diagnosed with non-secretory MM.
The last patient had normal sFLC levels but elevated uFLC levels. Because we did not have access to his other medical records, we speculated two possibilities: laboratory result error and MM clinical manifestation diversity. Because of diversity, the patient may suffer from plasma tumors in the bladder, which can cause uFLCs to be positive earlier than sFLCs.
In conclusion, levels of sFLCs and uFLCs did not parallel each other during the progression of MM. The process could be divided into three stages: “serum+ and urine−”, “serum+ and urine+”, and “serum+ and urine−”. The concentration of uFLCs was affected by not only the concentration of sFLCs but also renal function and other factors. Therefore, the level of uFLCs could not reflect the progression of MM, whereas the level of sFLCs could be used to indicate progression and monitor the improvement or recurrence of MM.
Footnotes
Zheng Li Xu and Chao Wu have contributed equally to this work.
References
- 1.Chen SL, Wu YJ, et al. (2004) Multiple myeloma. People’s Medical Publishing House, 63
- 2.Miettinen TA, Kekki M. Effect of impaired hepatic and renal function on Bence Jones protein catabolism in human subjects. Clin Chim Acta. 1967;18:395. doi: 10.1016/0009-8981(67)90036-8. [DOI] [Google Scholar]
- 3.Solomon A. Light chains of human immunoglobulins. Methods Enzymol. 1985;116:101–121. doi: 10.1016/s0076-6879(85)16008-8. [DOI] [PubMed] [Google Scholar]
- 4.Waldmann TA, Strober W, Mogielnicki RP. The renal handling of low molecular weight proteins. II. Disorders of serum protein catabolism in patients with tubular proteinuria, the nephrotic syndrome, or uremia. J Clin Invest. 1972;51:2162–2174. doi: 10.1172/JCI107023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo LM, Bakris GL, Comper WD. Renal handling of albumin: a critical review of basic concepts and perspective. Am J Kidney Dis. 2002;39:899–919. doi: 10.1053/ajkd.2002.32764. [DOI] [PubMed] [Google Scholar]
- 6.Bradwell AR, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet. 2003;361:489–491. doi: 10.1016/S0140-6736(03)12457-9. [DOI] [PubMed] [Google Scholar]
- 7.Herrera GA, Joseph L, Gu X, Hough A, Barlogie B. Renal pathologic spectrum in an autopsy series of patients with plasma cell dyscrasia. Arch Pathol Lab Med. 2004;128:875–879. doi: 10.5858/2004-128-875-RPSIAA. [DOI] [PubMed] [Google Scholar]
- 8.Sanders PW, Booker BB, Bishop JB, Cheung HC. Mechanisms of intranephronal proteinaceous cast formation by low molecular weight proteins. J Clin Invest. 1990;85:570–576. doi: 10.1172/JCI114474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders PW, Booker BB. Pathobiology of cast nephropathy from human Bence Jones proteins. J Clin Invest. 1992;89:630–639. doi: 10.1172/JCI115629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basnayake K, Ying WZ, Wang PX, Sanders PW. Immunoglobulin light chains activate tubular epithelial cells through redox signaling. J Am Soc Nephrol. 2010;21:1165–1173. doi: 10.1681/ASN.2009101089. [DOI] [PMC free article] [PubMed] [Google Scholar]