Abstract
Donor notification and counselling transforms the legal and ethical requirement of disclosure of transfusion transmissible infection (TTI) in a blood donor into practice. The present study was done to assess the response to the disclosure of TTI reactivity results in blood donors, assess the risk factors in blood donors and follow the compliance of the disclosure and clinical referral in a population of blood donors who are difficult to convince that they may be harbouring infections apparently in a healthy state today but with possible clinical disease consequences in the future. A retrospective study was conducted from April 2011 to November 2012. Screening was done using third generation ELISA kits used according to the manufacturer’s directions; these kits were approved for use in blood banks by the Drug Controller General of India. Those testing repeat reactive were referred for further confirmation and management. The total number of TTI reactive donors was 787 (0.93 %, N = 83,865). The observed response rate in the present study is 21.6 % (167, N = 787). The risk factors for acquiring infections in TTI reactive donors were statistically significant history of high risk behaviour (20.3 %) for human immunodeficiency virus infection and history of jaundice in themselves, family or close contacts (16.1 %) for hepatitis B virus infection. One hundred and ten (65.8 %) of the referred donors were on outpatient clinical care when post-referral follow up was conducted. The study emphasises on continuing sensitization of blood donation camp organisers to the need of privacy during blood donor selection. The study also stresses the need to strengthen the pre-donation counselling at outdoor blood donation at the same time raise awareness amongst blood donors about the importance of post-donation counselling and follow up.
Keywords: Blood donor, Notification, Counselling, Transfusion transmissible infection
Background
Donor notification and counselling achieves a number of major objectives; it protects the health of the donor, his family and community by preventing secondary transmission of infectious diseases to sexual partners and offspring’s, protects the safety of the blood supply by conveying the message that the individual should refrain from future blood donations, provides feedback about the effectiveness of donor selection procedures such as pre-donation education, medical history and confidential unit exclusion and also transforms the legal and ethical requirement of disclosure of transfusion transmissible infection (TTI) reactivity into practice [1].
Voluntary blood donors are considered as a safe source of blood, donate blood for altruism and are of the general opinion that they are free of diseases as they have been donating blood and thus may not be aware about the silent nature of some of these infectious agents that they may be harbouring [2]. Thus keeping this in mind, the need to notify blood donors about reactive test results as part of the legal and ethical obligation of blood transfusion services dates back since the first testing on donated blood was started for syphilis in the international scenario [1–3]. However, the system of notification was more streamlined with the recognition of the hepatitis and then the human immunodeficiency virus (HIV) and their consequent reactivity in blood donors.
In India, it is mandatory to test every unit of blood collected for hepatitis B surface antigen (HBsAg), hepatitis C antibodies (HCV), HIV 1 & 2 antibodies, syphilis and malaria as per the Drugs and Cosmetics Act of 1940 and the rules therein of 1945 amended from time to time [4]. National AIDS Control Organisation (NACO) and National Blood Transfusion Council (NBTC), Ministry of Health and Family Welfare, Government of India monitors the blood safety aspect in India. In accordance with the objective 4.18 of the action plan for blood safety the blood donor will be offered the option of knowing his TTI test status, by the blood bank when the blood donor questionnaire and consent from is filled. In the event that the donor (who wishes to know his TTI status) is found to be reactive to Hepatitis ‘B’ or Hepatitis ‘C’ or HIV, apart from discarding the blood unit in accordance with the existing regulations, the donor shall be requested to visit the blood bank personally by simply informing him/her that some of the immediate results are not conclusive, and need to be confirmed [5, 6]. Prior to this the disclosure of TTI reactivity results was not allowed in India [5–7].
The present study was done to assess the response to the disclosure of TTI reactivity results in blood donors, assess the risk factors for these infections in seroreactive blood donors and follow the compliance of the disclosure to clinical referral in a population of blood donors who are difficult to convince that they may be harbouring infections apparently in a healthy state today but with possible clinical disease consequences in the future.
Materials and Methods
A retrospective data analysis was performed at the Department of Transfusion Medicine of a tertiary care institute of northern India from April 2011 to November 2012. The ethics approval was taken from the institute ethics committee. The demographic data including age, gender, donor status (voluntary, replacement), and donation status (first time, repeat donor) were obtained from the blood donor registration card and records. This data is usually volunteered by the blood donor during the registration and may be actually written by them or by the personnel sitting for registration. Screening for TTI was done using third generation enzyme linked immunosorbent assay (ELISA) and Rapid kits used according to the manufacturer’s directions; these kits were approved for use in blood banks by the Drug Controller General of India. The tests were performed on semi-automated ELISA platform. Screening was done utilising kits from Bio Standard Diagnostics Pvt. Ltd., J. Mitra & Co. Pvt. Ltd., Transasia Bio-Medicals Ltd and Span Diagnostics Ltd. Screening for syphilis was done using kits from Span Diagnostics Ltd., Tulip Diagnostics (P) Ltd., Reckon Diagnostics P. Ltd. and Ranbaxy Laboratories Ltd.
The communications about initial reactive test results (ELISA and Rapid) were made telephonically. The donor was called and informed that the results of tests performed on donated blood were not conclusive; and a request for repeat blood sample testing was made. Consequent upon the presentation of blood donor a repeat blood sample was obtained after taking written informed consent and the ELISA and Rapid test was repeated with kits from a different manufacturer in order to label the blood donor as repeat reactive [5–8]. The donors found non-reactive on repeat testing were to be kept of follow up testing protocol. These donors were screened by ELISA every 3 months for 1 year in accordance with algorithm of the standard operating procedure (SOP) of the donor recall. The donors testing reactive on repeat testing were then counselled in a face to face interview and the interaction was based on clinical facts; they were explained about the screening nature and meaning of the test results and the need for further confirmation and possible present and future clinical implications. The blood donors were explained that they should not volunteer to donate again because of the transmissible nature of these infectious agents. The various modes of acquiring these infections were explained and a risk factor assessment of acquiring the infection was carried out using a structured proforma [1, 3].
The repeat reactive donors were referred to physicians for further management to the Department of Hepatology for Hepatitis B, C; Department of Venereology for syphilis and Integrated Counselling and Testing Centre (ICTC) of the Department of Immunopathology for HIV [5]. The post referral follow up was conducted telephonically from March 2013.
Statistical significance for comparison was determined using χ2 test. The percentage of reactivity observed was compared amongst voluntary versus replacement donors, male versus female donors and first time versus repeat donors. The statistical significance was also conducted amongst the reasons of acquiring the infections (risk factor assessment). The statistical tests were performed at a significance level of 0.05.
Results
Initial Reactive Donors
Donor demographics and pattern of reactivity in blood donor (initial reactive) and referred donors (repeat reactive) are depicted in Table 1. Total blood collected from April 2011 to November 2012 was 83,865 units, out of which 68,994 (82.3 %) was from voluntary donors (VD) and 14,871 (17.7 %) from replacement donors (RD), 75,773 (90.4 %) were males and 8,092 (9.6 %) were females and 51,828 (61.8 %) were first time donors whereas 32,037 (38.2 %) were repeat donors.
Table 1.
Demography and pattern of reactivity in blood donors and referred donors
| Category | Donors | Reactive donors (Initial reactive) (% of number of donors) | Referred donors (Repeat reactive) (% of reactive donors) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV | HCV | HBV | Syphilis | Total | HIV | HCV | HBV | Syphilis | Total | ||
| Total | 83,865 | 68 (0.08 %) | 268 (0.31 %) | 449 (0.54 %) | 2 (0.002 %) | 787 (0.93 %) | 9 (13.23 %) | 56 (20.89 %) | 101 (22.49 %) | 1 (50 %) | 167 (21.21 %) |
| Voluntary | 68,994 (82.26 %) | 47 (0.07 %) | 185 (0.27 %) | 328 (0.48 %) | 2 (0.003 %) | 562 (0.81 %) | 9 (19.14 %) | 52 (28.10 %) | 98 (29.87 %) | 1 (50 %) | 160 (28.46 %) |
| Replacement | 14,871 (17.73 %) | 21 (0.14 %) | 83 (0.55 %) | 121 (0.81 %) | 0 | 225 (1.51 %) | 0 | 4 (4.81 %) | 3 (2.47 %) | 0 | 7 (3.11 %) |
| P value | <0.0001 | NA | |||||||||
| Male | 75,773 (90.35 %) | 66 (0.08 %) | 263 (0.34 %) | 433 (0.57 %) | 2 (0.002 %) | 764 (1.01 %) | 9 (13.63 %) | 54 (20.53 %) | 97 (22.40 %) | 1 (50 %) | 161 (21.07 %) |
| Female | 8,092 (9.64 %) | 2 (0.02 %) | 5 (0.06 %) | 16 (0.19 %) | 0 | 23 (0.28 %) | 0 | 2 (40 %) | 4 (25 %) | 0 | 6 (26.08 %) |
| P value | <0.0001 | NA | |||||||||
| First time | 51,828 (61.79 %) | 22 (0.04 %) | 102 (0.20 %) | 203 (0.39 %) | 0 | 327 (0.63 %) | 1 (4.54 %) | 20 (19.60 %) | 39 (19.21 %) | 0 | 60 (18.34 %) |
| Repeat | 32,037 (38.20 %) | 46 (0.14 %) | 166 (0.52 %) | 246 (0.77 %) | 2 (0.006 %) | 460 (1.44 %) | 8 (17.39 %) | 36 (21.68 %) | 62 (25.20 %) | 1 (50 %) | 107 (23.26 %) |
| P value | <0.0001 | NA | |||||||||
P value < 0.05 was considered significant
NA not applicable
The total number of initial reactive donors was 787 (0.93 %, n = 83,865), out of which 562 (71.4 %) were VD with a reactivity rate of 0.81 % (n = 68,994) and 225 (28.6 %) were RD with a reactivity rate of 1.5 % (n = 14,871), the difference in reactivity was found to be statistically significant (P < 0.0001). Gender analysis of initial reactive donors revealed that 764 (97.1 %) were male donors with a reactivity rate of 1 % (n = 75,773), and 23 (2.9 %) were female donors with a reactivity rate of 0.28 % (n = 8,092), the difference in reactivity was found to be statistically significant (P < 0.0001). When reactivity and donation status was analysed it was observed that 460 (58.5 %) were repeat donors with a reactivity rate of 1.4 % (n = 32,037), and 327 (41.5 %) were first time donors with a reactivity rate of 0.6 % (n = 51,828), the difference in reactivity was found to be statistically significant (P < 0.0001).
Out of the 787 initial reactive; 449 (57 %) were reactive for HBsAg, 268 (34.1 %) were reactive for anti HCV antibodies, 68 (8.7 %) were reactive for anti-HIV1/2 antibodies and 2 (0.2 %) were reactive for RPR.
Donor Recall and Repeat Testing
Of the 787 initial reactive donors; 170 (21.6 %) could not be contacted telephonically because 120 (15.2 %) had either not provided contact telephone numbers, the number provided were not valid or the phone was not reachable and 50 (6.4 %) donors did not respond to our phone call despite three attempts to contact. Of the 617 (78.3 %) donors who were contacted, 450 (57.1 %) did not come for repeat sampling out of which 380 (48.2 %) had initially agreed to come and 70 (8.9 %) had refused to come as they were staying far off.
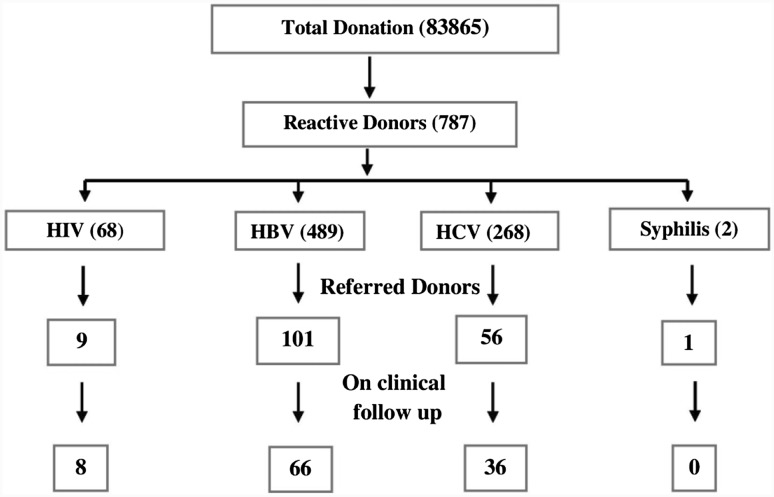
A total of 167 (21.2 %) blood donors reported and tested reactive on repeat testing and were referred for clinical follow up as in Fig. 1. Thus the response rate in the present study is 21.2 %. Amongst these, 161 (96.4 %) donors were male while six (3.6 %) donors were female, 160 (95.8 %) were VD and seven (4.2 %) were RD and 106 (63.5 %) were first time and 61 (36.5 %) were repeat donors.
Fig. 1.
Response rate and referral of reactive donors
Risk Factor Assessment
The risk factors assessment of acquiring the infection amongst the repeat reactive donors is depicted in the Table 2. Among the HBsAg repeat reactive donors; 24 (23.7 %) gave history of jaundice in themselves, in the family or close contacts, while 22 (21.7 %) gave history of injectable treatment without knowledge of sterility status (rural private treatment). Fifteen (14.8 %) donors gave history suggestive of high risk behaviour; and a similar number of donors gave history of either tattooing or ear piercing. Seven (6.9 %) were already aware of hepatitis B infection and three (2.9 %) donors gave history of receiving a phone call after previous blood donation but did not report to the concerned blood bank for follow up.
Table 2.
Risk factors of acquiring transfusion transmissible infection
| Risk factors | HIV (N = 9) (%) | HCV (N = 56) (%) | HBV (N = 101) (%) | Syphilis (N = 1) (%) | Total (N = 167) (%) |
|---|---|---|---|---|---|
| H/o injectable treatment without knowledge of sterilization status | 0 | 15 (26.78) | 22 (21.78) | 0 | 37 (22.15) |
| H/o high risk behavioura | 7*** (77.77) | 11 (19.64) | 15 (14.85) | 1 (100) | 34 (20.35) |
| H/o jaundice in themselves, family/close contacts | – | 3 (5.35) | 24*** (23.76) | 0 | 27 (16.16) |
| H/o tattooing or ear piercing | 0 | 7 (12.50) | 15 (14.85) | 0 | 22 (13.77) |
| H/o blood transfusion | 0 | 3 (5.35) | 1 (0.99) | 0 | 4 (2.39) |
| No significant history elicitedb | 2 (22.22) | 17 (30.36) | 24 (27.76) | 0 | 25 (14.97) |
HCV; three (5.35 %) reported h/o earlier reactivity status knowledge and four (7.14 %) reported h/o receiving call from blood bank after previous donation
HBV; seven (9.93 %) reported h/o earlier reactivity status knowledge and three (2.97 %) reported h/o receiving call from blood bank after previous donation
*** P value < 0.05
aIV drug abuse, unprotected sexual contact or multiple partners, long route truck driver
bHIV; 1(11.11 %) reported on being retroviral treatment during post test counselling
Among the anti-HCV repeat reactive donors; 15 (26.7 %) gave history of injectable treatment without knowledge of sterility status, whereas three (5.3 %) donors had history of jaundice in themselves, in the family or close contacts. Eleven (19.6 %) donors had history suggestive of high risk behaviour; out of which two (3.5 %) confirmed history of intravenous drug abuse. Seven donors (12.5 %) gave history of either tattooing or ear piercing. History of receiving phone call after last blood donation was elicited in four (7.1 %) donors, history of HCV reactivity was revealed by three (5.3 %) donors and three (5.3 %) gave history of blood transfusions.
Out of total anti-HIV repeat reactive donors, seven (77.8 %) had history suggestive of high risk behaviour, out of these five (55.6 %) donors gave history of sexual exposure without protection with multiple partners and two (22.2 %) donors were long route truck drivers by profession. One (11.1 %) donor disclosed that he was already on anti-retroviral treatment. The RPR reactive donor had a history of sexual exposure with multiple partners without protection.
The risk factors were revealed by the donors on post test counselling, and none of these were brought out during the blood donor selection. Had these histories been elicited/volunteered during the pre-donation screening it would have resulted in deferral of the blood donor.
Post Referral Follow Up
The post-referral follow up of referred donors is depicted in the Table 3. Of the 101 HBsAg repeat reactive donors referred, 27 (26.7 %) were instituted on medication, 39(38.6 %) were on follow up on outpatient basis but did not require therapy as of date. Of the 56 HCV repeat reactive donors referred, 20 (35.7 %) were instituted on medication, 16 (28.6 %) were on follow up on outpatient basis but did not require therapy as of date. Of the nine HIV repeat reactive donors referred, four (44.4 %) were on medication and an equal number of donors were follow up but did not require therapy as of date.
Table 3.
Follow up detail of referred donors
| Follow up data | On medication (%) | On follow up but not instituted on medicationa (%) | Did not report for clinical follow up (%) | Follow up lostb (%) |
|---|---|---|---|---|
| Anti HIV 1/2 (N = 9) | 4 (44.44 ) | 4 (44.44) | Nil | 1 (11.11) |
| HBsAg (N = 101) | 27 (26.73) | 39 (38.61) | 15 (14.85) | 20 (19.80) |
| Anti HCV (N = 56) | 20 (35.7) | 16 (28.56) | 7 (12.50) | 13 (23.21) |
| VDRL (N = 1) | Nil | Nil | Nil | 1 (100) |
| Total (N = 167) | 51 (30.53) | 59 (35.32) | 22 (13.17) | 35 (20.95) |
aBecause of confirmatory test pending, low viral load, CD 4 counts
bCould not be contacted on telephone to obtain follow up details
Discussion
The observed response rate in the present study of 21.6 % (study on 83,865 donors) is low in comparison to other studies from India and abroad. Kaur et al. and Agarwal et al. reported a response rate of 34.9 % (study on 15,844 donors) and 59.8 % (study on 48,386 donors) from north India, Patel et al. reported a response rate of 60.4 % (study on 20,865 donors) from western India, and Suman et al. reported a response rate of 70.3 % (study on 22,573 donors) from south India [9–12]. Kleinman et al. reported 42 % (study on 4,141 donors) response to their email questionnaire in the United States [13]. Kerzman et al. reported 40 % (study on 201 donors) response during telephone interviews conducted with blood donors identified as HCV positive following blood donation from Israel [14]. Roshan et al. observed a response of 71.7 % (HCV), 58.9 % (HBV), 64 % (HIV) and 67.1 % (VDRL) respectively (65.4 % overall) from Malaysia [15]. However it was observed in the study by Roshan et al. that because blood donors knew about the policy of blood banks of informing the donor in case of reactivity on testing, blood donations could be used as a medium to avail free testing facility [15]. The possible explanation for the low response in the present study can be explained on the large volume of blood collection, firstly that about 21.6 % of the donors could not be contacted due to wrong phone number or non-answering of the call. Secondly about 48.2 % (initially agreed) and 8.9 % (refused) to come for repeat sampling/testing probably because of distance/time/money concern. Since our department is designated both as the regional blood centre and a model blood bank in this region with the annual blood collection of more than 50,000 units, we travel up to 3–4 h distance in the effort to ensure adequate blood and blood components, therefore the donors who reside at distance from the transfusion centre are reluctant to come all the way spending money and time over an issue that they presume is not of enough importance. Moreover the response rate depends upon the donors understanding and perception about the testing and notification of tests carried out on donated blood; Choudhury et al. observed that only 53 % of the donors were aware that the blood transfusion services are supposed to inform them of reactive test result in southern India; moreover 57 % of donors expressed the desire to know their TTI status every time they donated blood, irrespective of the result [3]. One in two (50 %) of the blood donors thought that they did not have blood-borne infections in the study by Choudhury et al.
The present study supports the universal concept of voluntary blood source being safer by the observation of a statistically significant higher percentage of initial reactivity in RD when compared with VD. Moreover a statistically significant higher percentage of initial reactivity was observed in male donors when compared with female donors. A notable observation of serious concern, was that higher TTI reactivity was observed in repeat donors (1.4 %, n = 32,037) as compared to first time donors (0.63 %, n = 51,828) as depicted in Table 1. This is in sharp contrast to what is usually reported in literature [16–18]. However the Retrovirus Epidemiology Donor Study (REDS) reports that incidence rates of TTI are not necessarily lower in whole blood donors who donate regularly and more frequently. Observation of more TTI reactivity in donors of repeat category as compared to the first time donors is thus supported [19]. This could be because these donors were either nonreactive on their previous screening (seroconverted during the last donation) or were not informed of the reactivity status of their previous donations or were nonreactive by the rapid screening system for TTI that is being followed in peripheral blood bank settings. Moreover blood donors are a floating population in the urban world as the frequently move their residence due to better job prospects, and thus keep changing their location of blood donation and telephone contact numbers (which accounted for not being able to contact about 21.6 % of donors in the present study). Even the rural blood donor population may donate at different peripheral blood banks where the TTI testing in our country may still be done by rapid methods and even the notification protocol may not be in place in such small volume collection peripheral blood bank settings. Therefore this blood donor will mention being a repeat donor in his registration card, even though he may be donating for the first time at a different location. Majority of the blood collected at our centre is voluntary (82.3 %), the voluntary blood donation in our country is largely dependent on outdoor blood donation camps and the camp atmosphere is mostly a social get-together wherein the focus is to bring maximum volunteers for recruitment as blood donors, however in this situation because of the time constraint of the donors they tend to crowd around or pressurise the blood transfusion services staff to hasten their blood collection process. In such a situation ensuring enough privacy for blood donor screening becomes difficult. Doll et al. reported that 31 % of HIV positive donors mentioned lack of privacy during blood donor screening; while 20 % were of the opinion that they would have given a different answer if they had been provided more privacy [20]. This provides us insight that we still have a long way to go in informing, educating blood donors about blood safety and that donor screening needs privacy in order to extract correct information from the donor. In these circumstances the theoretical advantage of being a voluntary blood donor in contrast to the pressure on the replacement blood donor of hiding information so as to ascertain blood for relative or friend is nullified. Pre-donation screening of blood donors with the blood donors questionnaire and consent form format of NBTC is an integral part of blood safety and there is a need for well trained and competent medical officers so that their communication is clear and comprehensible and most importantly, privacy and confidentiality should be tried to be maintained. Gradual sensitization of outdoor blood donation camp organisers to the need of privacy during blood donor selection in order strengthen the pre-donation counselling in the countries that are still building a volunteer donor base.
Data on post-donation risk factor assessment in reactive donors is limited and not available in any other study from India. During post donation counselling the risk factors for acquiring infections in TTI reactive donors was history of jaundice in themselves, family or close contacts (16.1 %) was found to be statistically significant in HBsAg reactive donors, this is supported by the fact that a majority of HCV infection does not manifest with icterus (Table 2). History of high risk behaviour (77.8 %) was statistically significant in anti-HIV reactive donors reiterating it as among one of the most common cause of transmission of HIV infection. The risk factors were revealed by the donors on counselling, and none of these were brought out during the blood donor selection. Had this been revealed earlier at the time of donor screening or had they self-excluded themselves before donation could have led to decrease in the infectious pool and saved time, money and manpower. Therefore there is a need to gradual sensitise the blood donors to the need of diligently reading the questionnaire or answering the questions truthfully. An abbreviated donor screening may not be feasible at least in developing countries at present even in the repeat donor.
One hundred and six first time donors were counselled, thus stopping them from future donations and preventing further spread of infection to their family and to the community. They were given appropriate medical advice and timely medical care. Sixty one repeat donors who were donating blood regularly without any information of harbouring infections were also advised to refrain from donations thus preventing future donations from these donors and the consequent benefit of not discarding these blood units after repeated testing and wastage of finance and manpower. The possible chance of these units escaping detection from testing due to any unforeseen reason and the transfusion of the blood components to patients in that scenario and the possible exposure to any of the transfusion services personnel could thus be prevented by proper counselling.
Follow up was possible in only 110 (65.8 %) of the referred donors and 35 (20.9 %) could not be contacted to obtain this information on telephone and 22 (13.1 %) did not report for the post referral clinical follow up. These 110 (65.2 %) of the referred donors were on clinical management, including 51 (30.5 %) who were on medication and 59 (35.3 %) who did not require therapy till date. Therapy was not instituted due to reasons like confirmatory test pending, low viral load or the confirmatory test negative and normal CD 4 counts in case of reactivity for HIV. Agarwal et al. reported 182 donors (43.7 %) on clinical follow up after 6 months [12]. Thus the percentage of referred donors (110 donors, 65.85 %) on regular follow up is more in our study. All referred donors expressed gratitude towards the concern shown by the department staff and treating physicians with one exception; this donor reacted in a denial mode and refused any further testing and clinical management.
The present role of the blood transfusion services in TTI testing, donor notification, counselling and referral can actually be compared to a miniature public health model. The screening of asymptomatic blood donors serves as a mode of secondary prevention in terms of finding undiagnosed infections. The referral for further confirmation and management serves as a mode of tertiary prevention in form of intervention to reduce the disease burden. Extensive information, education about modes of acquiring these TTI’s as part of blood donor motivation, recruitment and retention activity on mass public scale serves as mode of primary prevention by raising awareness and reducing the risks.
Conclusion
Blood donor notification, counselling and referral clearly emerges as one of the single largest platform from where asymptomatic people could be informed of abnormal test results related to infectious agents that they could be harbouring silently today at least in the developing countries [13]. The early diagnosis of these infections facilitates institution of therapy in time which could result in prevention of significant morbidity and mortality in future, not to mention the cost of healthcare that could be prevented and quality of life that can be modified if they respond, and comply for follow up and management.
The study emphasises on continuing sensitization of blood donation camp organisers to the need of privacy during blood donor selection. The study also stresses the need to strengthen the pre-donation counselling at outdoor blood donation at the same time raise awareness amongst blood donors about the importance of post-donation counselling and follow up.
Acknowledgments
We acknowledge that the study could not delineate the various reasons for the significantly high number of repeat donors noted in the initial reactive category. This study was based upon retrospective data review, where the discriminating details of previous donations were not readily available.
Conflict of Interest
There is no conflict of interest amongst any of the authors and no affiliation with any funding source.
References
- 1.Bianco C, Kessler D. Donor notification and counseling. Management of blood donors with positive test results. Vox Sang. 1994;67(Suppl 3):255–259. doi: 10.1111/j.1423-0410.1994.tb04588.x. [DOI] [PubMed] [Google Scholar]
- 2.Choudhury LP, Tetali S. Notification of transfusion transmitted infection. Indian J Med Ethics. 2008;5:58–60. doi: 10.20529/IJME.2008.022. [DOI] [PubMed] [Google Scholar]
- 3.Miller R, Hewitt PE, Warwick R, Moore MC, Vincent B. Review of counselling in a transfusion service: the London (UK) experience. Vox Sang. 1998;74:133–139. doi: 10.1046/j.1423-0410.1998.7430133.x. [DOI] [PubMed] [Google Scholar]
- 4.The Drugs and Cosmetics Act, 1940 and the Drugs and Cosmetics Rules, 1945, as amended up to 30th June, 2005. Schedule F. Part XIIB. Government of India. Ministry of Health and Family Welfare. Department of Health. pp 268–288. http://www.cdsco.nic.in/Drugs&CosmeticAct.pdf. Accessed 4 April 2013
- 5.National AIDS Control organisation (2007) An action plan for blood safety. National AIDS Control organisation. Ministry of Health and Family Welfare. Government of India, p 31–32. http://www.nacoonline.org/upload/Final%20Publications/Blood%20Safety/An%20Action%20Plan%20for%20blood%20safety.pdf. Accessed 8 May 2013
- 6.Mudur G. India announces plan to inform HIV infected blood donors. BMJ. 2002;325:1380. doi: 10.1136/bmj.325.7377.1380/d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangla B. India: HIV-positive blood donors. Lancet. 1993;341:1527–1528. doi: 10.1016/0140-6736(93)90653-X. [DOI] [PubMed] [Google Scholar]
- 8.National AIDS Control Organization (2007) Manual on quality standards for HIV testing laboratories. National AIDS Control Organization, Ministry of Health and Family Welfare, Government of India, pp 9–11. http://www.nacoonline.org/NACO/Quick_Links/Publication/Blood_Safety__Lab_Services. Accessed 8 May 2013
- 9.Kaur G, Kaur P, Basu S, Kaur R, Sharma S. Donor notification and counseling: experience and challenges. Transfus Apheres Sci. 2013;49:291–294. doi: 10.1016/j.transci.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal N. Response rate of blood donors in the Uttarakhand region of India after notification of reactive test results on their blood samples. Blood Transfus. 2012;5:1–3. doi: 10.2450/2012.0100-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel P, Patel S, Bhatt J, Bhatnagar N, Gajjar M. Evaluation of response to donor notification of reactive transfusion transmitted infections (TTIs) result. NJIRM. 2012;3:20–25. [Google Scholar]
- 12.Suman FR, Krishnamoorthy R, Panicker VK, Alexander S, Ida S. Is it essential to inform the positive donor? A 2-year study in a tertiary care hospital. J Nat Sci Biol Med. 2011;2:185–187. doi: 10.4103/0976-9668.92330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleinman S, Wang B, Wu Y, Glynn SA, Williams A, Nass C, et al. Retrovirus epidemiology donor study. The donor notification process from the donor’s perspective. Transfusion. 2004;44:658–666. doi: 10.1111/j.1537-2995.2004.03347.x. [DOI] [PubMed] [Google Scholar]
- 14.Kerzman H, Green MS, Shinar E. Predictors for non-compliance of notified hepatitis C virus-positive blood donors with recommendation to seek medical counselling. Vox Sang. 2009;96:20–28. doi: 10.1111/j.1423-0410.2008.01111.x. [DOI] [PubMed] [Google Scholar]
- 15.Roshan TM, Rosline H, Ahmed SA, Rappiah M, Khattak MN. Response rate of Malaysian blood donors with reactive screening test to transfusion medicine unit calls. Southeast Asian J Trop Med Public Health. 2009;40:1315–1321. [PubMed] [Google Scholar]
- 16.Li C, Xiao X, Yin H, He M, Li J, Dai Y, et al. Prevalence and prevalence trends of transfusion transmissible infections among blood donors at four Chinese regional blood centers between 2000 and 2010. J Transl Med. 2010;10:176. doi: 10.1186/1479-5876-10-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagalo MB, Sanou M, Bisseye C, Kaboré MI, Nebie YK, Kienou K, et al. Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses and syphilis among blood donors in Koudougou (Burkina Faso) in 2009. Blood Transfus. 2011;9:419–424. doi: 10.2450/2011.0112-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tessema B, Yismaw G, Kassu A, Amsalu A, Mulu A, Emmrich F, et al. Seroprevalence of HIV, HBV, HCV and syphilis infections among blood donors at Gondar University Teaching Hospital, Northwest Ethiopia: declining trends over a period of five years. BMC Infect Dis. 2010;10:111. doi: 10.1186/1471-2334-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schreiber GB, Glynn SA, Busch MP, Sharma UK, Wright DJ, Kleinman SH. Retrovirus epidemiology donor study. Incidence rates of viral infections among repeat donors: are frequent donors safer? Transfusion. 2001;41:730–735. doi: 10.1046/j.1537-2995.2001.41060730.x. [DOI] [PubMed] [Google Scholar]
- 20.Doll LS, Petersen LR, White CR, Ward JW. Human immunodeficiency virus type 1-infected blood donors: behavioral characteristics and reasons for donation. The HIV Blood Donor Study Group. Transfusion. 1991;31:704–709. doi: 10.1046/j.1537-2995.1991.31892023494.x. [DOI] [PubMed] [Google Scholar]