Abstract
Background
Atrial fibrillation (AF) has been linked with an increased risk of cognitive impairment and dementia.
Purpose
To complete a meta-analysis of studies examining the association between AF and cognitive impairment.
Data Sources
Electronic search of 5 large databases and hand search of article references.
Study Selection
Prospective and non-prospective studies reporting adjusted risk estimates for the relationship between AF and cognitive impairment.
Data Extraction
Two abstracters independently extracted data on study characteristics, risk estimates, methods of AF and outcome ascertainment, and methodological quality.
Data Synthesis
Twenty one studies were included in the meta-analysis. AF was significantly associated with a higher risk of cognitive impairment independent of stroke history (relative risk (RR) [95% confidence interval (CI)] =1.34 [1.13, 1.58]), in patients with first-ever or recurrent stroke (RR [95%] =2.7 [1.82, 4.00]) and in a broader population including patients with or without a history of stroke (RR [95% CI] =1.4 [1.19, 1.64]). However, there was significant heterogeneity among studies of the broader population (I2 =69.4 %). Limiting the analysis to prospective studies yielded similar results (RR [95% CI] =1.36 [1.12, 1.65]). Restricting the analysis to studies of dementia eliminated the significant heterogeneity (P value =0.137) but did not alter the pooled estimate substantially (RR [95% CI] = 1.38 [1.22, 1.56]).
Limitations
There is an inherent bias due to confounding variables in observational studies. There was significant heterogeneity among included studies.
Conclusions
Evidence suggests that AF is associated with a higher risk of cognitive impairment and dementia, with or without a history of clinical stroke. Further studies are required to elucidate the relationship between AF and subtypes of dementia as well as the etiology of cognitive impairment.
Keywords: Atrial Fibrillation, Dementia, Cognitive Impairment, Meta-analysis
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in the United States (US), affecting more than 2.7 million Americans in 2010. Of all US men and women ≥ 40 years of age, 25% will develop AF during their lifetime. In addition, the prevalence of AF is rising dramatically as the population ages (1).
Moreover, the prevalence of cognitive impairment and dementia is also rising in association with the increased longevity of the population and the accumulation of risk factors for cognitive impairment (2). Three putative risk factors for cognitive impairment are heart failure (3), diabetes (4, 5) and hypertension (6, 7), which are also known risk factors for AF (1). Several longitudinal studies and meta-analyses reported positive associations between these factors and cognitive decline (8). Notably, heart failure(3) and diabetes (5) were associated with a greater than 1.5 times increased risk of cognitive dysfunction and dementia, respectively. Mild cognitive impairment is characterized by an objective long-term memory impairment that does not adversely affect activities of daily living, while dementia is defined by a memory impairment and at least one other impairment in cognitive function that is severe enough to interfere with daily life. Of the many different types of dementia, Alzheimer’s disease is the most prevalent, affecting 1 in 8 people over the age of 65 years; vascular dementia and Lewy body dementia are the next most common causes (9, 10). Given the significant burden that cognitive impairment and dementia have on patients, families, and the health care system (10), it is crucial to identify its major risk factors to facilitate implementation of appropriate preventive measures.
Recently, a growing body of evidence has linked AF with an increased risk of cognitive impairment and dementia (11-13). However, the association has not been consistent across studies (14-16). While AF increases the risk of stroke by a factor of 4 to 5 (17), it is not clear whether cognitive impairment in the context of AF is solely mediated through an increased risk of stroke or whether other factors are responsible. A recent review reported a significant association between AF and post-stroke dementia in patients with first-ever or recurrent stroke (18). However, the researchers did not attempt to estimate the association of dementia independent of a stroke history. Elucidating this association could be particularly helpful in understanding the underlying mechanisms that link AF with cognitive impairment. Therefore, we performed a comprehensive systematic review of the literature to explore and elucidate the association between AF and cognitive impairment (independent of stroke and in patients with first-ever or recurrent stroke).
Methods
Data Sources and Searches
We followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (19) to perform a meta-analysis of observational studies that reported an association between AF and cognitive impairment. Five large databases MEDLINE (Ovid interface), PsycINFO, Cochrane library (Ovid SP), CINAHL, and EMBASE were electronically searched from their inception to Sep 18, 2012. An electronic search was performed by one of the investigators (S.K.) with help from a qualified librarian, using text and explosion of Medical Subject Headings (Online Appendix 1). No language restriction was applied. To ensure a comprehensive search of the literature, we also manually searched the reference lists of the included studies and previously published systematic reviews and meta-analyses. We contacted the authors when required data were ambiguous or missing.
Study Selection
Prospective and non-prospective studies reporting the relationship between AF and cognitive impairment or total dementia were included in this systematic review. We excluded: 1) reviews, editorials, letters, case series, case reports, and conference proceedings; 2) studies evaluating cognitive decline after open heart surgery in patients with AF; 3) studies lacking a control group; 4) studies in which the presence or absence of dementia was only assessed by an informant review; 5) post-mortem studies; 6) studies in which the control group was selected from patients with other types of arrhythmia; 7) studies with inappropriate outcome measures (any outcomes other than cognitive impairment);and 8) studies that provided only unadjusted or crude analyses. The last exclusion criterion is particularly important because crude estimates of the association between atrial fibrillation and cognitive impairment are likely to be highly biased by confounding variables (such as age), and therefore misleading.
Outcome measures
The primary outcome of interest was cognitive impairment (from mild to severe dementia). The secondary outcomes were cognitive impairment and dementia, separately.
Data Extraction and Quality Assessment
The following data from eligible studies were extracted in duplicate by two independent abstracters: first author, year, design (case-control, cross-sectional, prospective cohort), comparison groups, inclusion and exclusion criteria, total sample size, number of subjects in the AF group and in the no AF group, and number of events within each group, population characteristics (e.g., age, number of females, history of stroke), outcome, methods of AF, outcome and stroke ascertainment, type of relative risk (RR) estimate (odds ratio, risk ratio, and hazard ratio), the RR estimate and its 95% confidence interval (CI), adjusted analysis (classified as minimal if adjusted for age and as multivariate if adjusted for at least two potential confounding variables in addition to age), along with a list of variables used in the adjusted analysis. Any disagreements or discrepancies were resolved by consensus.
The quality of included studies was assessed using an adaptation of two published checklists (20, 21) with the seven criteria most relevant to included studies, with only six criteria applicable to non-prospective studies: 1) was AF the main exposure of interest? (yes/no); 2) were the inclusion and exclusion criteria clearly stated? (yes/no); 3) potential for misclassification of AF based on AF ascertainment method: was an electrocardiogram used for AF diagnosis? (Yes/No/Unclear); 4) potential for misclassification of outcome based on outcome ascertainment method: for instance, using multiple neuropsychological tests for assessment of cognitive impairment was considered superior to using single MMSE test; and, using criteria from the Diagnostic and Statistical Manual of Mental Disorders third or fourth edition (22, 23) for assessment of dementia was judged superior to using codes from the International Classification of Diseases ninth or tenth revision (24, 25) from patient discharge files or data registries; 5) was temporality clear (i.e. was AF diagnosis made before the outcome?) (“yes” for prospective studies and “no” for non-prospective studies); 6) potential for attrition bias in prospective studies (≥10% versus <10% lost-to-follow-up); 7) Potential for confounding bias based on level of adjustment in multivariate models: minimal adjustment for age versus multivariate adjustment for age and at least two other potential confounding variables such as heart failure, hypertension and diabetes mellitus. Quality criteria were extracted in duplicates by two abstracters and discrepancies were resolved by a third reviewer (J.R. or T.S.). Superiority or acceptability of diagnostic methods for dementia and cognitive impairment was confirmed by one of the senior authors (T.S.).
When duplicates were identified, the most recent study was included unless the earlier version of the study reported the multivariable adjusted-risk estimate, in which case the earlier version was included. When both cross-sectional and prospective association of AF and cognitive impairment were reported, we only included the prospective assessment. When associations with cognitive impairment and dementia were both reported, for the main analysis, we used the broader definition of outcome that included the other outcome (e.g., dementia is a subset of cognitive impairment).
Data Synthesis and Analysis
Random effects models using the DerSimonian and Laird method (26) were incorporated to estimate the pooled RR of the association between AF and cognitive impairment or dementia. The random effects model was used to account for both within- and between-studies variances. To evaluate the association independent of stroke history, we performed a meta-analysis of studies that either excluded patients with a history of stroke or adjusted for this co-morbidity in the multivariate adjusted model. To investigate the association between AF and cognitive impairment ascertained by the Mini Mental State Examination (MMSE), (the most widely used screening tool in practice), we performed a sensitivity analysis restricted to the studies that used the MMSE to define cognitive impairment (MMSE score of ≤24) or cognitive decline (MMSE decline≥3 points during follow-up). Due to the methodological differences between prospective and non-prospective studies, we performed all the analyses within study designs and reported the pooled estimates only when separate analyses justified the combination. The main analysis combined dementia outcomes with cognitive impairment. To justify the combination and to report a separate pooled estimate for dementia, a subgroup analysis was performed separating studies of dementia from cognitive impairment. Heterogeneity was assessed using the P value from Q-statistics and was quantified by Higgins I-squared statistics where an I-squared value of 30% to 60% was considered to represent a moderate level of heterogeneity (27). Publication bias was evaluated by using Egger’s regression test and illustrated using a funnel plot. A forest plot was used to graphically display the effect size in each study as well as in the pooled estimate. A P value<0.05 was considered significant. All the analyses were performed in Stata/IC 12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP). The funding sources played no role in the design, conduct, and analysis of the study or in the decision to submit the manuscript for publication.
Results
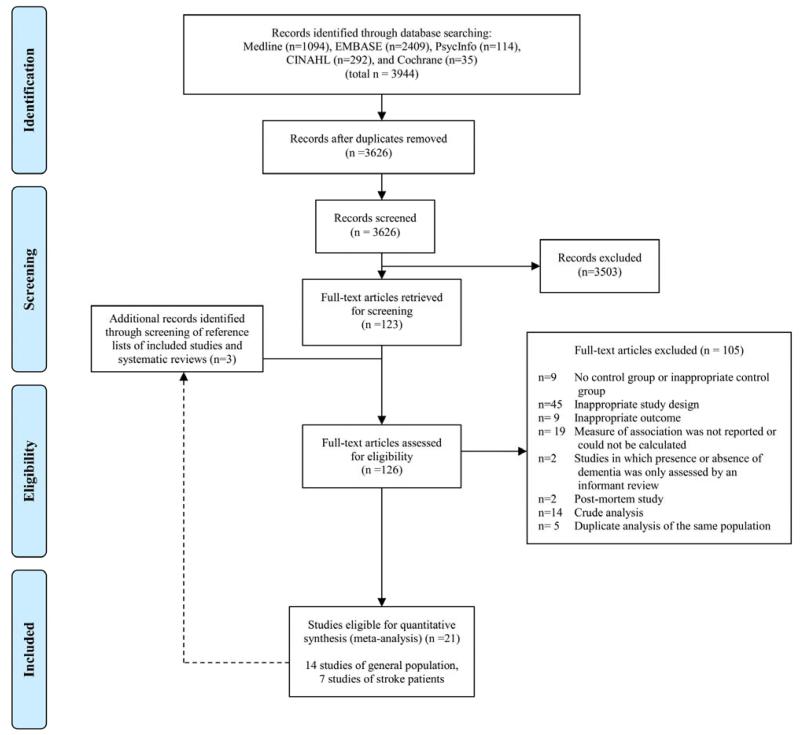
Of 3944 retrieved articles, 123 abstracts were chosen for full-text screening, including one Chinese and one Italian study that were translated to English. Among the 123 studies reviewed, 21 met the inclusion criteria. Three additional reports were eligible for full text screening when the reference lists of the included studies and previously published review papers were scanned, however, none met our inclusion criteria (Appendix Figure 1). Of the 21 included studies, 7 studies specifically examined the association of AF with post-stroke cognitive impairment or dementia and 14 reported the association between AF and cognitive impairment or dementia in a broader population (including patients with or without a history of stroke).
AF and Cognitive Impairment in Patients with or without History of Stroke
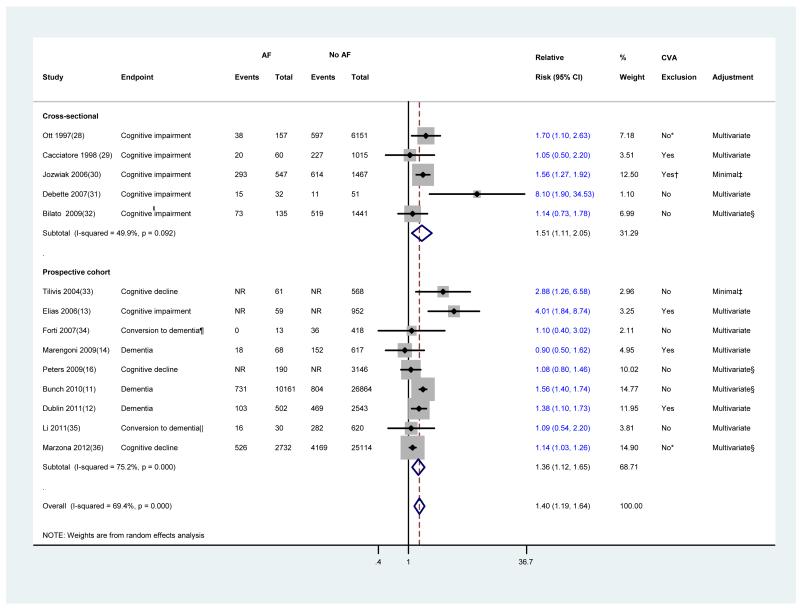
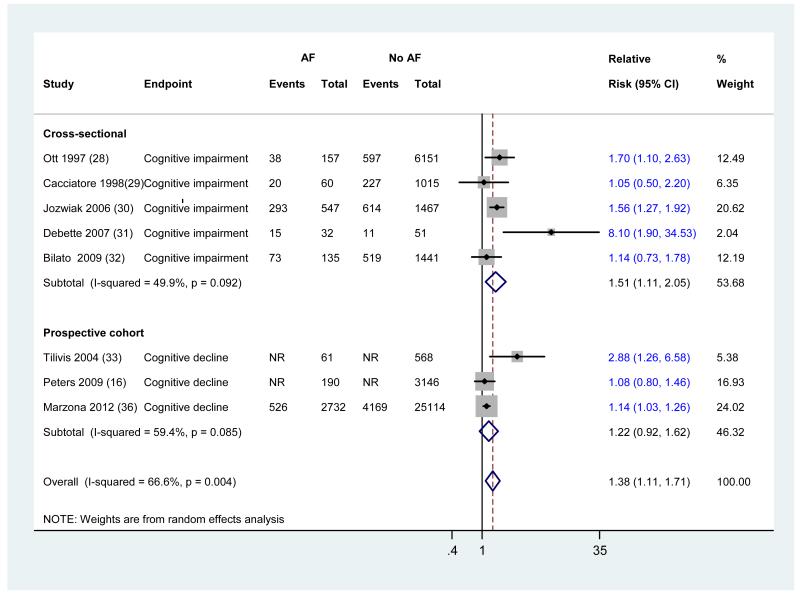
Fourteen studies (5 cross-sectional, and 9 prospective studies) investigated the association between AF and dementia or cognitive impairment. The characteristics of these studies are tabulated in Appendix Table 1. Results, description of the multivariate models, methods of AF, stroke and outcome ascertainments are described in Appendix Table 2. In a combined analysis of all 14 studies (Figure 1), AF was significantly associated with the risk of developing cognitive impairment (RR [95% CI] =1.40 [1.19, 1.64]). The adjusted prospective estimate was virtually the same as the adjusted cross-sectional estimate, justifying their combination. However, as anticipated, there was significant heterogeneity among studies. The overall heterogeneity resulted mainly from variability among prospective studies. Such heterogeneity might have originated from variances in characteristics of the participants (e.g., age and co-morbidities), methods of AF ascertainment, and outcome measures (Appendix Table 2). Among the 14 included studies, the most common method of AF ascertainment was the electrocardiogram followed by the International Classification of Diseases codes. The remaining studies either did not report the AF ascertainment method or used physical examination and medical history. Cognitive impairment was most commonly assessed by the use of the MMSE, and dementia diagnosis was most commonly confirmed by the Diagnostic and Statistical Manual of Mental Disorders criteria. The International Classification of Diseases codes and neuropsychological batteries were used in the remainder of the studies for the diagnosis of dementia. Stroke diagnosis was mainly self-reported or determined by medical records and rarely confirmed by imaging evaluations.
Figure 1.
Meta-analysis of 14 studies evaluating the association between atrial fibrillation and cognitive impairment in patients with or without history of stroke
Studies are sorted by publication year. Diamond represents the pooled risk estimate. NR: not reported.
* Patients with history of stroke were excluded in a subgroup analysis.
† Patients with no focal neurologic deficits (i.e. previous strokes, head injuries, head neurosurgery, tumors of the central nervous system, and so forth) were only considered for this meta-analysis.
‡ Minimal adjustment should include at least adjustment for age.
§ History of stroke was included as a covariate in the multivariate adjusted model.
¶ Conversion from normal cognition to dementia.
║Conversion from mild cognitive impairment to dementia.
Sensitivity and Subgroup Analyses and Assessment of Heterogeneity
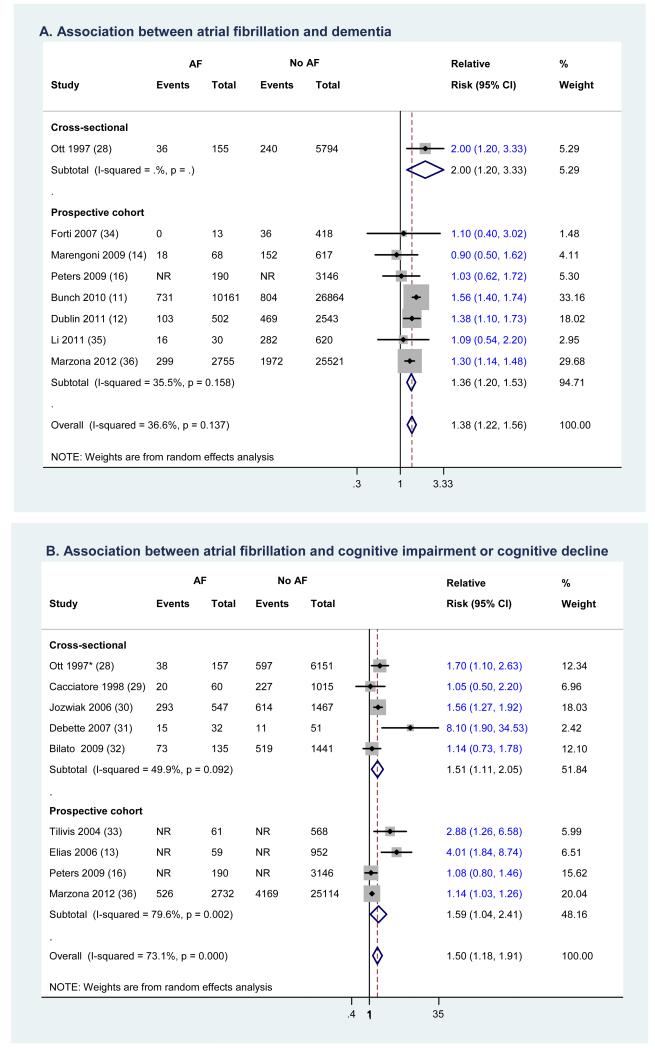
In view of the significant heterogeneity observed in this meta-analysis, we incorporated a random effects model and performed several sensitivity analyses to assess the robustness of the results. The pooled estimates were virtually the same for prospective and cross-sectional studies (Figure 1). However, significant heterogeneity was observed among prospective studies. This heterogeneity may be due in part to variances in outcome measures. Restricting the analysis to studies of dementia (Figure 2 A), which is more reliably diagnosed than cognitive impairment, eliminated the significant heterogeneity without changing the pooled estimate substantially (RR [95% CI] =1.38 [1.22, 1.56]). Limiting the analysis to the 8 studies that ascertained cognitive impairment or decline by MMSE score ≤24 or MMSE decline≥3 points, did not appreciably change the results (RR [95% CI] = 1.38 [1.11, 1.71]) (Appendix Figure 2). We assessed the effect of any single study on the pooled estimate by removing one study at a time. Removing no single study changed the significance of the pooled estimate or heterogeneity. Investigating subtypes of dementia failed to demonstrate a significant association between AF and Alzheimer’s disease (RR [95% CI] =1.22 [0.96, 1.56]); however, the association was significant for vascular dementia (RR [95% CI] =1.72 [1.27, 2.32]).
Figure 2.
Separating dementia outcomes from cognitive impairment
Studies are sorted by publication year. Diamond represents the pooled risk estimate. NR: not reported.
* Patients with dementia were excluded.
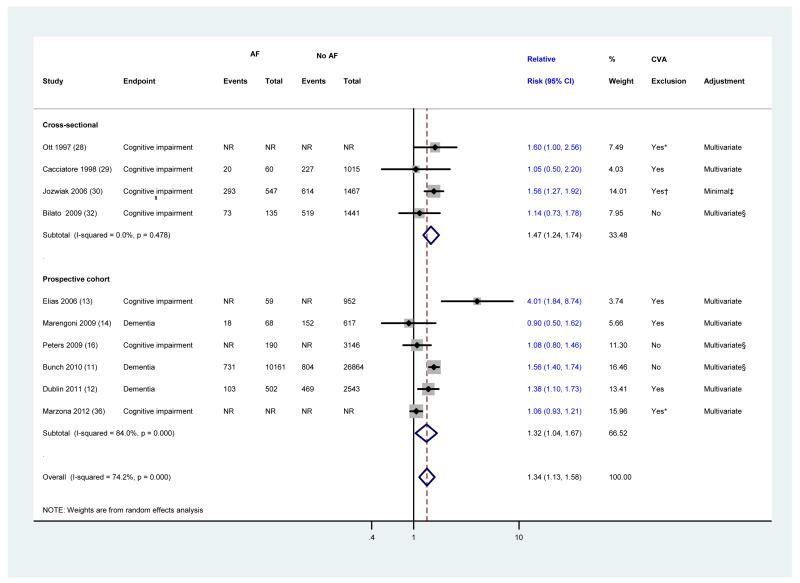
AF and Cognitive Impairment Independent of Stroke History
Limiting the analysis to participants without a history of a stroke and to studies that adjusted for this co-morbidity in multivariate analyses did not appreciably affect the primary results (RR [95% CI] =1.34 [1.13, 1.58]) (Figure 3). Furthermore, restricting the analysis to studies that specifically excluded patients with a history of stroke did not alter the results (RR [95% CI] =1.37 [1.08, 1.73]).
Figure 3.
The association between atrial fibrillation and cognitive impairment independent of stroke history
Studies are sorted by publication year. Diamond represents the pooled risk estimate. NR: not reported.
* Patients with history of stroke were excluded in a subgroup analysis.
† Patients with no focal neurologic deficits (i.e. previous strokes, head injuries, head neurosurgery, tumors of the central nervous system, and so forth) were only considered for this meta-analysis.
‡ Minimal adjustment should include at least adjustment for age.
§ History of stroke was included as a covariate in the multivariate adjusted model.
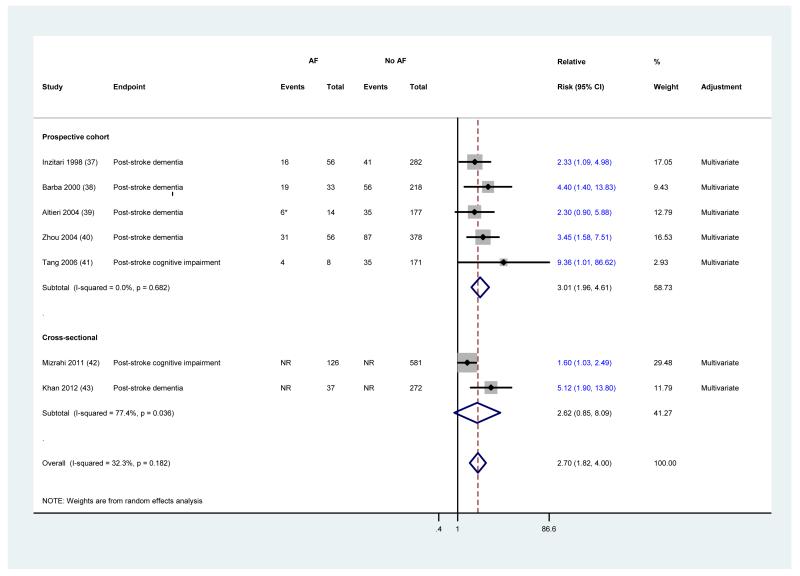
AF and Post-Stroke Cognitive Impairment
The association between AF and post-stroke cognitive impairment or dementia was reported in 7 studies. The characteristics of these studies are tabulated in Appendix Table 3. Overall, AF was associated with a more than two-fold increase in the risk of developing post-stroke cognitive impairment or dementia (RR [95%] =2.7 [1.82, 4.00]) (Figure 4). Although prospective and cross-sectional studies showed overlapping risk estimates, the association was stronger within prospective studies (RR [95%] =3.01 [1.96, 4.61]). Additionally, prospective studies were more homogeneous than non-prospective ones. Appendix table 4 describes the results of individual studies, multivariate models, and methods of AF, outcome and stroke ascertainment. Almost all studies of post-stroke cognitive impairment or dementia confirmed the diagnosis of stroke by detailed imaging studies.
Figure 4.
Meta-analysis of 7 studies evaluating the association between atrial fibrillation and post-stroke cognitive impairment in patients with recurrent or first-ever stroke
Studies are sorted by publication year. Diamond represents the pooled risk estimate. NR: not reported.
* During the follow-up 5 additional patients developed atrial fibrillation (2 were diagnosed with post-stroke dementia)
Quality of Included Studies
The quality of the prospective and cross-sectional studies was assessed by 7 and 6 objective criteria, respectively. Studies which meet a higher number of quality criteria have a more favorable methodological quality and less risk of bias. Appendix Table 5 describes the quality criteria of studies that evaluated patients with or without history of stroke. Seven prospective studies (11-14, 16, 34, 36) had favorable methodological quality with adequate adjustment for confounding factors but variable methods of AF and outcome ascertainment. There was no single criterion that would stand out as the main problem in these prospective studies. The quality of one prospective study (33) was poor due to the potential for misclassification of AF and outcome, and risk of attrition and confounding bias. Among the non-prospective studies, 3 (29-31) had an overall higher risk of bias mostly due to the potential for misclassification of AF and outcome. Two (28, 32) had better overall methodological quality with adequate adjustment for confounding variables and accurate diagnosis of AF. Eliminating studies which met 3 or fewer quality criteria had little effect on the pooled estimate (RR [95% CI] = 1.32 [1.12, 1.57]).
Appendix Table 6 describes the quality criteria of post-stroke studies. Among the prospective studies, none evaluated AF as their main exposure of interest and all except one (39) were at risk of attrition bias. Overall, non-prospective studies (42, 43) were of poor quality mainly due to potential for misclassification of AF and outcome. Restricting the analysis to studies which met 3 or fewer quality criteria did not substantially change the results (RR [95% CI] = 3.01 [1.96, 4.61]).
Publication bias
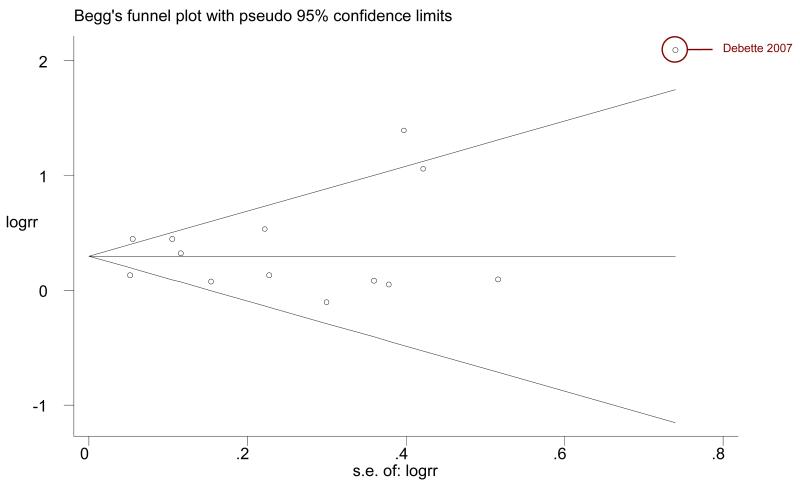
The funnel plot resembles a symmetrical funnel for the 14 studies of patients with or without a history of stroke, which rules out publication bias (Appendix Figure 3). We used Egger’s regression test to objectively assess the symmetry of the plot. The estimated bias coefficient was 0.64 (P value =0.40) which excludes publication bias. The Egger’s regression test was also performed for the 7 studies of post-stroke cognitive impairment. The estimated bias coefficient was 2.46 (P value=0.009) which suggests the presence of publication bias. Excluding the smallest study with the most unbalanced result (41) did not significantly change the association between AF and post-stroke cognitive impairment or dementia (RR [95% CI] = 2.57 [1.75, 3.79]).
Discussion
Our findings suggest a significant association between AF and cognitive impairment or dementia independent of stroke, in patients with first-ever or recurrent stroke, and in a broader population including patients with or without a history of stroke. Restricting the analysis to dementia outcomes, which are more accurately diagnosed than cognitive impairment, eliminated the heterogeneity but did not change the significance of the association.
Several mechanisms have been proposed for the association between AF and cognitive impairment. One explanation is the presence of shared risk factors (e.g., hypertension, congestive heart failure, diabetes) between AF and cognitive impairment (8). Further, these risk factors tend to accumulate as the population ages. However, this observation fails to explain the association between AF and cognitive impairment in longitudinal studies that controlled for such co-morbidities (13, 36). Another potential mechanism is a hypercoagulable state (44) in patients with AF, as well as stasis of blood in the left atrium that may lead to formation of thrombi in the left atrial appendage and ultimately to clinical and sub-clinical strokes (45, 46). The results of this meta-analysis cannot rule out the possibility of silent stroke as a potential mechanism of the association. However, one study that excluded patients with a history of stroke by detailed imaging also showed an association between AF and cognitive impairment (47). This observation is particularly important as it highlights the need for further studies to elucidate new mechanisms for this association. Other potential but unproven mechanisms include: brain hypoperfusion due to beat-to-beat variability in the length of the cardiac cycle and reduced cardiac output (48); the pro-inflammatory state in AF (49, 50); and periventricular white matter lesions (51).
Our study has several strengths. We performed a comprehensive search of literature without language restriction, contacted authors for clarifications in case of ambiguity, and requested additional data when necessary. Second, data extraction was performed by two independent investigators. Third, we performed several sensitivity analyses to assess the robustness of our results. There was a consistent significant association between AF and cognitive impairment. Fourth, to our knowledge, this is the first study that collected and presented separate data for dementia and cognitive impairment outcomes. Fifth, the studies included in this report were from geographically diverse regions (Asia, North and South America, Europe, and Australia), thus increasing generalizability. Sixth, this meta-analysis has substantial statistical power to detect a clinically meaningful association because of the large number of events observed. Finally, we used multiple objective criteria to assess the quality of individual studies. This allowed us to identify studies with a higher risk of bias from those with lower risk of bias.
This review has several limitations. A significant heterogeneity was observed in the prospective studies of patients with or without history of stroke. However, we attempted to account for both within- and between-studies variability by using a random effects model. Also, to investigate different endpoints as a source of heterogeneity, we separated dementia outcomes from cognitive impairment in a sensitivity analysis and found no significant heterogeneity in studies of dementia. Some degree of subjectivity is inevitable in assessing the quality of studies of cognitive impairment and dementia due to the wide range of diagnostic tools available. Six of the 21 studies included in this report met 3 or fewer quality criteria, mainly because of a higher potential for misclassification of AF or outcome, inadequate adjustment for potential confounders, and the presence of attrition bias. However, exclusion of these studies in sensitivity analyses had little effect on the reported results. We have reported a significant association between atrial fibrillation and cognitive impairment independent of stroke history. However, it is important to note that a history of stroke was mainly self-reported or derived from medical records and rarely confirmed by imaging evaluations. Therefore, the reported association is only independent of clinically overt stroke and the possibility of silent stroke cannot be ruled out. The results of the Egger’s tests suggested an absence of publication bias in the 14 studies of patients with or without a history of stroke, and a presence of publication bias in the 7 studies of patients with stroke. The Egger’s test has limited power in detecting publication bias especially when the number of studies included in the meta-analysis is small. Conversely, in some instances P values from the Egger’s test are erroneously very small (suggesting publication bias) due to a correlation between the standard error of the log RR and the size of the RR. This is more likely to happen when the effect size is large or when there is significant between-study heterogeneity or when the number of events per study is small(52). Therefore, over-interpretation of Egger’s test should be avoided. Finally, the results of the subgroup analysis separating Alzheimer’s disease from vascular dementia should be interpreted with caution because accurate distinction between Alzheimer’s disease and vascular dementia can only be made through autopsy data and epidemiologic studies have limited ability to reliably separate dementia subtypes. In addition, patients often present with features of both types of dementia and, finally, subgroup analyses are usually underpowered to detect significant associations owing to the limited number of studies included.
Although this meta-analysis must be interpreted in the context of the limitations of the studies included, the current study provides the most comprehensive evidence to date on the potential effects of AF on cognitive impairment. This analysis also highlights critical gaps in our knowledge about the mechanisms underlying the association between AF and cognitive impairment. The finding of this association warrants further well-designed longitudinal studies with better adjustment for potential confounders and with detailed information on subtypes of dementia, as well as clinical trials designed to evaluate interventions that may postpone or reduce the risk of cognitive impairment in patients with AF. On the basis of this systematic review and meta-analysis of all available data, future research should make a careful distinction between different types of dementia and investigators should consider cognitive function as a new outcome to be assessed in interventional studies for the treatment of AF.
Acknowledgments
We would like to thank Jose Sarmiento, M.D., M.P.H. from Harvard School of Public Health, Kasra Moazzami, M.D., M.P.H. from Massachusetts General Hospital who performed duplicate data extraction; Hang Lee, Ph.D., Brian Healy, Ph.D. from Harvard Catalyst who provided biostatistical consultation; Susan Landry who edited a draft of this manuscript; Julie Goodman Ph.D., and Donald Halstead, B.A. from Harvard School of Public Health for their help and support. None of these individuals discloses any conflict of interest or takes responsibility for the content of this manuscript. We would also like to thank the following individuals who provided us with additional data from their published studies: Jared Bunch, M.D., Alessandra Marengoni, M.D., Yan-Jiang Wang, M.D., Ph.D., Sascha Dublin, M.D., Ph.D., and Ruth Peters, M.D.
Financial Support: Supported in part by the Deane Institute for Integrative Research in Atrial Fibrillation and Stroke at the Massachusetts General Hospital.
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
Online Appendix 1
Search Strategy
Ovid MEDLINE(R) 1946 to Present with Daily Update
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations September 18, 2012
#1 mental processes/ or anticipation, psychological/ or exp cognition/ or executive function/ or intention/ or exp learning/ or exp perception/ or exp thinking/ or exp delirium, dementia, amnestic, cognitive disorders/ or exp amnesia/ or exp cognition disorders/ or exp consciousness disorders/ or exp delirium/ or exp dementia/ or exp dementia/ or alzheimer disease/ or exp dementia, vascular/ or exp frontotemporal lobar degeneration/ or lewy body disease/ or exp neurodegenerative diseases/ or Frontotemporal Dementia/ or exp Dementia, Multi-Infarct/ or exp memory disorders/ or exp amnesia/ or psychomotor agitation/ or (cognitive disorders or cognitive impairment or memory loss or amnesia or amnestic or delirium or dementia or Alzheimer or vascular dementia or frontotemporal lobar degeneration or lewy body disease or neurodegenerative diseases or frontotemporal Dementia or MultiInfarct Dementia).ti,ab. 1006376
#2 exp atrial fibrillation/ or exp atrial flutter/ or (atrial fibrillation or atrial flutter or auricular fibrillation or auricular flutter or AFIB).ti,ab. 43836
#1 AND #2 →1094
PsycINFO 1967 to September Week 2 2012
#1 exp cognitive ability/ or exp cognitive appraisal/ or exp cognitive assessment/ or exp cognition/ or exp cognitive impairment/ or exp cognitive processes/ or exp dementia/ or exp alzheimer’s disease/ or exp neurodegenerative diseases/ or exp vascular dementia/ or exp delirium/ or exp dementia with lewy bodies/ or exp memory disorders/ or exp amnesia/ or (cognitive disorders or cognitive impairment or memory loss or amnesia or amnestic or delirium or dementia or Alzheimer or vascular dementia or frontotemporal lobar degeneration or lewy body disease or neurodegenerative diseases or frontotemporal Dementia or MultiInfarct Dementia).ti,ab. 390596
#2 exp atrial fibrillation/ or exp auricular fibrillation/ or (atrial fibrillation or atrial flutter or auricular fibrillation or auricular flutter or AFIB).ti,ab. 562
#1 AND #2 →114
EBM Reviews - Cochrane Database of Systematic Reviews 2005 to August 2012
EBM Reviews - Database of Abstracts of Reviews of Effects 3rd Quarter 2012
EBM Reviews - Cochrane Central Register of Controlled Trials September 2012
EBM Reviews - Cochrane Methodology Register 3rd Quarter 2012
#1 mental processes/ or anticipation, psychological/ or exp cognition/ or executive function/ or intention/ or exp learning/ or exp perception/ or exp thinking/ or exp delirium, dementia, amnestic, cognitive disorders/ or exp amnesia/ or exp cognition disorders/ or exp consciousness disorders/ or exp delirium/ or exp dementia/ or exp dementia/ or alzheimer disease/ or exp dementia, vascular/ or exp frontotemporal lobar degeneration/ or lewy body disease/ or exp neurodegenerative diseases/ or Frontotemporal Dementia/ or exp Dementia, Multi-Infarct/ or exp memory disorders/ or exp amnesia/ or psychomotor agitation/ or (cognitive disorders or cognitive impairment or memory loss or amnesia or amnestic or delirium or dementia or Alzheimer or vascular dementia or frontotemporal lobar degeneration or lewy body disease or neurodegenerative diseases or frontotemporal Dementia or MultiInfarct Dementia).ti,ab. 34076
#2 exp Atrial Flutter/ or exp Atrial Fibrillation/ or (atrial fibrillation or atrial flutter or auricular fibrillation or auricular flutter or AFIB).ti,ab. 3289
#1 AND #2 →35
CINAHL
#1“cognitive disorders” OR “cognitive impairment” OR “memory loss” OR “amnesia” OR “amnestic” OR “delirium” OR “dementia” OR “Alzheimer” OR “vascular dementia” OR “frontotemporal lobar degeneration” OR “lewy body disease” OR “neurodegenerative diseases” OR “frontotemporal Dementia” OR “MultiInfarct Dementia” 31461
OR
#2 (MH “Delirium, Dementia, Amnestic, Cognitive Disorders+”) OR (MH “Cognition Disorders+”) OR (MH “Mental Processes+”) 162413
AND
#3 (MH “Atrial Fibrillation”) OR (MH “Atrial Flutter”) OR “atrial fibrillation” OR “atrial flutter” OR “AFIB” OR “auricular fibrillation” OR “auricular flutter” 9153
(#1 OR #2) AND #3 →292
EMBASE
#1’cognitive disorders’:ab,ti OR ’cognitive impairment’:ab,ti OR ’memory loss’:ab,ti OR ‘amnesia’:ab,ti OR ‘amnestic’:ab,ti OR ‘delirium’:ab,ti OR dementia:ab,ti OR ‘alzheimer’:ab,ti OR ‘vascular dementia’:ab,ti OR ‘frontotemporal lobar degeneration’:ab,ti OR ‘lewy body disease’:ab,ti OR ‘neurodegenerative diseases’:ab,ti OR ‘frontotemporal dementia’:ab,ti OR ‘multiinfarct dementia’:ab,ti OR ‘memory’/exp OR ‘cognition’/exp OR ‘executive function’/exp OR ‘learning’/exp OR ‘perception’/exp OR ‘delirium’/exp OR ‘dementia’/exp OR ‘alzheimer disease’/exp OR ‘degenerative disease’/exp OR ‘lewy body’/exp OR ‘multiinfarct dementia’/exp OR ‘frontotemporal dementia’/exp OR ‘amnesia’/exp OR ‘cognitive defect’/exp AND [humans]/lim AND [embase]/lim 772,728
#2 ‘heart atrium fibrillation’/exp OR ‘heart atrium fibrillation’ OR ‘heart atrium flutter’/exp OR ‘heart atrium flutter’ OR ‘atrial fibrillation’:ab,ti OR ‘atrial flutter’:ab,ti OR ‘auricular fibrillation’:ab,ti OR ‘auricular flutter’:ab,ti OR afib:ab,ti AND [humans]/lim AND [embase]/lim 50,150
#1 AND #2 →2,409
Total=3944
Appendix Figure 1.
Flow diagram of selection process.
Appendix Figure 2.
Meta-analysis of the association between atrial fibrillation and Mini-Mental State Examination (MMSE) score ≤24 or MMSE decline≥ 3 points
Studies are sorted by publication year. Diamond represents the pooled risk estimate. NR: not reported.
Appendix Figure 3.
Funnel plot for assessment of publication bias among the 14 studies evaluating patients with or without history of stroke
Appendix Table 1. Characteristics of the 14 included studies evaluating the association between atrial fibrillation and cognitive impairment in patients with or without history of stroke.
| Author Year | Design & Settings (Comparison Groups) |
N | Female,% | Age, Mean (SD) | CVA Exclusion |
Country |
|---|---|---|---|---|---|---|
|
Ott 1997 (28) |
Cross-sectional community cohort (cognitive impairment vs. no cognitive impairment, and dementia vs. no cognitive impairment ) |
6584 | 59.2 | 69.2 (9.1) | No* | The Netherlands |
|
Cacciatore 1998 (29) |
Cross-sectional community survey (MMSE <24 vs. MMSE ≥24) |
1075 | 55.3 | 73.9 (6.2) | Yes | Italy |
|
Tilivis 2004 (33) |
Prospective cohort with up to 10 yrs of follow-up (change in cognitive function over time, in patients with AF vs. no AF) |
650† | 73.61 | Age at entry 75 (37%) 80 (32.7%) 85 (30.2% ) |
No | Finland |
|
Elias 2006 (13) |
Prospective cohort of the Framingham Offspring Heart Study with assessment of cognitive function an average of 8 mos after the AF surveillance period (chronic or paroxysmal AF vs. no AF followed for development of dementia) |
1011 | 0 | No AF:60.5 (9.4) AF: 68.1 (7.0) |
Yes | United States |
|
Jozwiak 2006 (30) |
Cross-sectional study (4 comparison groups : AF alone; AF with FNDs; FNDs alone; neither AF nor FNDs) |
2314‡ | 65 | 76§ (71- 81) ║ | Yes¶ | Poland |
|
Debette 2007 (31) |
Cross-sectional study of HF patients with LVEF ≤45% (MMSE <24 vs. MMSE ≥24) |
83 | 30.1 | 62§ (17-98) ** | No | France |
|
Forti 2007 (34) |
Prospective cohort with 3 and 4 yrs of follow-up for patients with MCI and normal cognitive function, respectively (evaluating conversion to dementia, comparing converters vs. nonconverters) |
Normal:4 31 MCI: 180 |
Normal: 63 MCI: 51 |
Normal: 75.2 (9.0) MCI: 75.7 (8.3) |
No | Italy |
|
Bilato 2009 (32) |
Cross-sectional assessment of participants in the Progetto Veneto Anziani (Pro.V.A.) study (comparing cognitive impairment in patients with AF vs. no AF) |
1,576 | 61.5 | Men: 77 (8) Women: 76 (7) |
No | Italy |
|
Marengoni 2009 (14) |
Prospective cohort with 6 yrs of follow- up (AF vs. no AF ) |
685 | 75.6 †† | 83.6 (4.1) †† | Yes‡‡ | Sweden |
|
Peters 2009 (16) |
Prospective cohort of hypertensive elderly with mean follow-up of 2 yrs (development of dementia in patients with AF vs. no AF ) |
3336 | 60.4 | >=80 | No | United Kingdom |
|
Bunch 2010 (11) |
Prospective cohort with mean follow-up of 5 yrs (development of dementia in patients with AF vs. no AF ) |
37,025 | 39.9 | 60.6 (17.9) | No | United States |
|
Dublin 2011 (12) |
Prospective cohort of community- dwelling adults with mean follow-up of 6.8 yrs (development of dementia in AF vs. No AF) |
3,045 | 60 | 75.3 (6.18) †† | Yes | United States |
|
Li 2011 (35) |
Prospective cohort of patients with MCI with mean follow-up of 5 yrs (evaluating conversion to AD ) |
837§§ | Convertors: 62.8 Non- convertors: 54.5 |
66.5 (7.12) †† | No | China |
|
Marzona 2012 (36) |
Prospective cohort (post-hoc analysis of two randomized controlled trials) with median follow-up of 56 mos (AF vs. no AF ) |
31,506 | 29.7 | 66.5 (7.2) | No* | 40 countries |
Studies are ordered based on the publication year.
Excluded patients with stroke history in a secondary analysis.
Only 629 were included in the analysis.
Including patients in all the four comparison groups.
Median age.
Inter-quartile range.
Two comparison groups for this meta-analysis were: AF alone vs. neither AF nor focal neurologic deficits.
Age range.
Per contact with the author.
Only excluded patients with history of stroke at baseline and did not exclude incident stroke cases during the follow up.
638 completed the follow-up.
SD: Standard Deviation; CVA: Cerebrovascular Accidents; MMSE: Mini-mental State Examination; AF: Atrial fibrillation; FND: Focal Neurologic Deficit; HF: Heart Failure; LVEF: Left Ventricular Ejection Fraction; MCI: Mild Cognitive Impairment; AD: Alzheimer’s disease
Appendix Table 2. Results, multivariate models, methods of AF, stroke and outcome ascertainments in 14 studies of patients with or without a history of stroke.
| Author Year | Outcomes | Outcome ascertainment | AF ascertainment | Stroke ascertainment | Results | Variables in multivariate model |
|---|---|---|---|---|---|---|
|
Ott 1997 (28) |
Cognitive impairment without dementia, total Dementia, AD, VD |
Cognitive impairment by MMSE score<26 Dementia by MMSE and Cambridge Examination for mental Disorders of elderly combined with an informant interview and brain MRI, diagnosis confirmed by neurologists or neuropsychologist based on DSM-III criteria. AD by NINCDS-ADRDA criteria VD by NINDS-AIREN criteria |
Standard 12-lead ECG analyzed with the Modular ECG Analysis System (MEANS) software |
Interviewing participants or the informants and inquiring about a history of clinically overt stroke, verified by medical records |
Significant association between AF and cognitive impairment: OR [95%CI] =1.7 [1.1-2.6] Significant association between AF and total dementia: OR [95%CI] =2.0 [1.2-3.4] No significant association between AF and either VD or AD in multivariate adjusted analysis |
Age, sex, myocardial infarction, blood pressure, peripheral atherosclerosis, diabetes mellitus, education, antihypertensives, beta-blocker, digoxin, verapamil, anticoagulants, thyroid drugs |
|
Cacciatore 1998 (29) |
Cognitive impairment |
Italian MMSE score <24 | Physical examination |
Medical history and clinical assessment by trained physician |
No significant association between AF and cognitive impairment (multivariate adjusted OR [95%CI] =1.05 [0.5, 2.19]) |
Age, sex, congestive heart failure, diabetes, hypertension, education, GDS score, alcohol consumption, smoking, heart rate, blood pressure |
|
Tilivis 2004 (33) |
Cognitive impairment and cognitive decline |
Cognitive impairment was defined as MMSE score <24. Cognitive decline was determined by a minimum of 4-point decrease in MMSE score or an increase in CDR class |
NR* | NR* | Significant association between AF and 5 yr cognitive decline: Multivariate RR[95% CI]=2.88 [1.26–6.06] |
Age and baseline MMSE score |
|
Elias 2006 (13) |
Global cognitive ability and several sub- domains |
A battery of multiple neurologic tests evaluated by Framingham Study neuropsychological review panel |
ECG or Holter reading confirmed by a Framingham cardiologist |
Repeated screening of all participants for stroke by physical examination, repeated CT or MRI in suspected patients |
Significant association between AF and performance at or below the 25th percentile (OR [95%CI] = 4.01 [1.84, 8.74]) |
Age, education, blood pressure, cigarettes/day, alcohol, BMI, total cholesterol, depressed mood, electrocardiographic left ventricular hypertrophy, diabetes, cardiovascular disease, and antihypertensive treatment |
|
Jozwiak 2006 (30) |
General cognitive function |
MMSE score ≤23 | Physical examination and resting ECG |
Medical history and detailed neurologic examination in all patients, CT in a subset of patients |
Significant association between AF and cognitive impairment (multivariate OR [95% CI] = 1.56 [1.27–1.92]) |
Age, sex |
|
Debette 2007 (31) |
Overt cognitive impairment |
MMSE score <24 | NR (a history of AF was considered in all patients) |
Medical history (defined by WHO criteria) |
Significant association between AF and risk of overt cognitive impairment (multivariate OR [95% CI] = 8.1 [1.9–34.6]) |
Age, sex, schooling>8 y, NYHA class IV, Plasma hemoglobin<12.3, cause of heart failure (ischemic, nonischemic, undetermined) |
|
Forti 2007 (34) |
Conversion from MCI to dementia, conversion from normal cognition to dementia |
MMSE and an extensive neuropsychological battery by two examiners with third examiner for discrepancies for diagnosis of MCI. Incident dementia diagnosed by follow up clinical and neuropsychological evaluations. NINCDS-ADRDA criteria for AD |
Medical history confirmed by clinical evaluation, and previous medical records (when available) |
NR (all participants were interviewed and underwent physical examination) |
In patients with MCI, there was a significant association between AF and dementia (multivariate HR [95% CI] = 4.63 [1.72-12.46]) but such an association was not present in the cognitively normal group (multivariate HR [95% CI] = 1.10 [0.40-3.03]) |
Age, sex, education, baseline MMSE score, blood pressure, BMI, serum folate |
|
Bilato 2009 (32) |
Cognitive impairment |
MMSE<24 | 10 Sec ECG evaluated by 2 cardiologists and validated by a third cardiologist |
NR (Medical record review and general physical examination) |
Cognitive impairment was significantly more prevalent in patients with AF but the association was not significant in multivariate model (multivariate adjusted OR [95%] CI = 1.14 [0.73–1.80]) |
Age, sex, heart failure, myocardial infarction, angina pectoris, diabetes mellitus, peripheral artery disease, disability in basic activity daily living, stroke, chronic obstructive pulmonary disease |
|
Marengoni 2009 (14) |
Total dementia, AD |
MMSE for global cognitive function, DSM-III criteria for dementia |
Physician diagnosis (by auscultation) medical records, medical drug use, and ICD-9 code |
ICD-9 and ICD-10 codes for incident stroke from Stockholm Inpatient Register |
No significant association between AF and dementia (multivariate HR [95%CI]=0.9 [0.5, 1.7]) No significant association between AF and AD ( multivariate HR [95%CI]= 0.8 [0.4, 1.5]) |
Age, sex, education, baseline MMSE score, hypertension, anti-thrombotic medications, and APO-E genotype |
|
Peters 2009 (16) |
Dementia, cognitive decline |
DSM-IV criteria for dementia diagnosis Cognitive decline was defined as decrease in MMSE score to <24 or by >3 points annually |
Reported by the investigators at the baseline visit after taking an ECG from the patient † |
Previous stroke was ascertained by local investigators via patient interview and medical records. † |
Multivariate HR [95% CI] for the association between AF and dementia: 1.031 [0.619, 1.718] Multivariate HR [95% CI] for the association between AF and cognitive decline : 1.08 [0.798, 1.463] |
Sex, geographic recruitment area, BMI, randomized trial treatment group, previous stroke, heart failure, diabetes mellitus, total cholesterol, HDL cholesterol, creatinine, glucose, hemoglobin |
|
Bunch 2010 (11) |
VD, AD, SD, ND, total dementia |
ICD-9 codes to identify dementia and its subtypes |
Diagnostic ICD-9 codes, ECG database of all Intermountain Healthcare hospitals |
Patients records from inpatients and outpatients clinical visits |
Significant association between AF and total dementia (Multivariate OR[95% CI] 1.56[ 1.40-1.74]), and between AF and VD (multivariate OR [95% CI]=1.73 [1.27-2.36])† Significant association between AF and AD only in patients younger than 70 yo (multivariate OR=2.30 [1.40-3.79]) |
Confounding variables included in the models were chosen based on 10% change in hazard ratios and all models included history of cerebrovascular accidents † |
|
Dublin 2011 (12) |
All-cause dementia and possible or probable AD |
DSM-IV criteria for dementia (by a multidisciplinary committee) and NINDS-AIREN criteria for AD |
At least 2 documented ICD-9 codes within 12 mos |
Self-reported history of stroke; ICD-9 codes and self-report for incident stroke |
Significant association between AF and all-cause dementia (adjusted HR [95% CI]=1.38 [1.10-1.73] as well as AF and AD (adjusted HR [95% CI]=1.50 [1.16–1.94]) |
Age, incident stroke, sex, education, diabetes mellitus, hypertension, blood pressure, coronary heart disease, and congestive heart failure |
|
Li 2011 (35) |
Conversion from MCI to AD |
Modified DSM-IV for dementia. NINCDS-ADRDA criteria for AD NINDS-AIREN criteria for VD Demented patients were further evaluated with CT or MRI |
ICD-9 codes | CVD defined by history, presence of focal neurologic signs or brain imaging including strategic or multiple lesions, or diffuse white matter lesions, or TIA |
No significant association between AF and conversion to AD (Multivariate adjusted HR [95% CI]=1.088 [0.538–2.201]) |
Age, sex, education, occupation, depressive symptoms, APO E4, baseline MMSE, ADL score |
|
Marzona 2012 (36) |
Cognitive decline, dementia |
Cognitive decline: a decrease in MMSE score ≥3 Dementia: defined as new dementia diagnosis, reported severe cognitive impairment or MMSE≤23 |
12-lead ECG‡ | History of stroke was determined by using patient reports. Incident stroke was determined by an adjudication committee |
Significant association between atrial fibrillation and cognitive decline ( multivariate HR [95%CI]=1.14 [1.03, 1.26) Significant association between atrial fibrillation and dementia ( multivariate HR [95%CI]=1.30 [1.14,1.49]) |
Age; education; sex; baseline MMSE score; blood pressure; history of stroke or transient ischemic attack, hypertension, diabetes and myocardial infarction; levels of microalbuminuria, macroalbuminuria and creatinine; statins, beta-blockers, angiotensin-converting enzyme inhibitors, antiplatelets or oral anticoagulants; changes in systolic blood pressure during follow-up; smoking; BMI; physical activity; sleep apnea; and alcohol consumption |
Studies are ordered based on the publication year.
At entry all participants underwent physical examination by a neurologist and a cardiologist and the patient records were collected.
Per contact with the authors.
Data obtained from rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials (53).
AF: Atrial Fibrillation; AD: Alzheimer’s Disease; VD: Vascular Dementia; MMSE: Mini-mental State Examination; DSM: The Diagnostic and Statistical Manual of Mental Disorders; NINCDS-ADRDA: The National Institute of Neurological and Communicable Disease and Stroke- Alzheimer’s Disease and Related Disorders Association; NINDS-AIREN: National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l’Enseignement en Neurosciences; ECG: Electrocardiogram; OR: Odds Ratio; CI: Confidence Interval; GDS: Geriatric Depression Scale; CDR: The Clinical Dementia Rating; NR: Not Reported; RR: Relative Risk; CT: Computed Tomography ; MRI: Magnetic Resonance Imaging; WHO: World Health Organization; NYHA: The New York Heart Association Functional Classification; MCI: Mild Cognitive Impairment; HR: Hazard Ratio; BMI: Body Mass Index; ICD: The International Classification of Diseases; HDL: High Density Lipoprotein; SD: Senile Dementia; ND: Non-specific Dementia; CVD: Cerebrovascular Diseases; TIA: Transient Ischemic Attack; ADL: Activities of Daily Living.
Appendix Table 3. Characteristics of the 7 included studies evaluating the association between atrial fibrillation and post-stroke cognitive impairment or dementia.
| Author Year | Design & Settings | N | Female,% | Age, Mean (SD) | Country |
|---|---|---|---|---|---|
|
Inzitari 1998 (37) |
Prospective cohort of stroke patients with dementia assessment 1 year after stroke |
339* | 47.9 | Dementia: 76.2 (9.4), No-dementia: 70.0 (11.5) |
Italy |
|
Barba 2000 (38) |
Prospective cohort of patients with either ischemic or hemorrhagic stroke with 3 mos of follow-up |
251† | 47 | 69 (13) | Spain |
|
Altieri 2004 (39) |
Prospective cohort of patients with hemorrhagic or ischemic stroke with mean (SD) follow-up of 45.3 (9.2) mos |
191 | 30.9 | 71.3 (8.9) | Italy |
|
Zhou 2004 (40) |
Prospective cohort of patients with ischemic stroke with 3 mos of follow- up |
434 | 47.2 | Dementia: 73.8 (7.5) No dementia: 65.3 (6.8) |
China |
|
Tang 2006 (41) |
Prospective cohort of patients with first-ever or recurrent stroke with 3 mos of follow-up |
179‡ | 44.1 | 73 (7.5) | China |
|
Mizrahi 2012 (42) |
Cross-sectional study of patients with ischemic stroke admitted to stroke rehabilitation with assessment of cognitive function within 1 week of admission |
707 | 42.9 | 74.11 (9.29) | Israel |
|
Khan 2012 (43) |
Cross-sectional study of patients with ischemic or hemorrhagic stroke admitted to Aga Khan University Hospital with assessment of cognitive function within 1 to 12 mos of admission |
309 | 62.1 | 61.75 (21-90) § | Pakistan |
Studies are ordered based on the publication year.
Number alive for interview at 1 year follow-up (>10% lost to follow-up).
Available for interview at three month follow-up (>10% lost to follow-up).
Number included in the final analysis (>10% lost to follow-up).
Inter-quartile range.
Appendix Table 4. Results, multivariate models, methods of AF, stroke and outcome ascertainments in 7 post-stroke studies.
| Author Year |
Outcomes | Outcome ascertainment |
AF ascertainment | Stroke ascertainment | Results | Variables included in the multivariate model |
|---|---|---|---|---|---|---|
|
Inzitari 1998 (37) |
Post-stroke dementia |
ICD-10 codes and interview with a proxy informant (method was validated in two different studies) Minimum required duration for memory and intellectual deficit was 6 mos |
Chronic AF by at least one ECG and/or clinical verification |
Stroke was defined by WHO criteria and every stroke diagnosis was confirmed by a neurologist |
Significant association between AF and post-stroke dementia multivariate OR [95% CI ] = 2.33 (1.09–4.98) |
Age> 72 yrs, ,Prestroke Rankin>2, Previous stroke, Aphasia, Urinary incontinence |
|
Barba 2000(38) |
Post-stroke dementia |
DSM-IV criteria for post stroke dementia, DSM- III-R criteria for previous dementia and dementia stage, and NINDS-AIREN criteria for VD, cognitive status assessment with SS- IQCODE |
Clinical diagnosis and ECG after the acute phase |
Clinical diagnosis based on presence of acute focal signs and cerebral dysfunction lasting ≥24 hrs, with CT scan available for 93% of cases |
Significant association between AF and post-stroke dementia: multivariate OR [95% CI ]= 4.4 [1.4 -14.3] |
Age, AF, Nephropathy, Psychiatric disease, Canadian neurological scale, SS-IQCODE |
|
Altieri 2004 (39) |
Post-stroke dementia |
ICD-10 codes for dementia NINCDS-ADRDA for AD NINDS-AIREN for VD |
NR | Clinical diagnosis based on presence of acute focal signs and cerebral dysfunction lasting ≥24 hrs, confirmed by CT or MRI |
No significant association between AF and post-stroke dementia : Multivariate HR[95% CI] = 2.3 [0.9–5.7] |
Age, cortical atrophy, multiple lesions, education, subcortical atrophy, leukoariosis |
|
Zhou 2004 (40) |
Post-stroke dementia |
Modified DSM-IV criteria plus several neuropsychological tests |
NR (based on previous AF diagnosis or treatment) |
Clinical diagnosis based on presence of acute focal signs and cerebral dysfunction lasting ≥24 hrs and CT or MRI |
Significant association between AF and stroke-related dementia : OR[95% CI] = 3.45 [1.584–7.512 ] |
Age, educational level, drinking, prior stroke, dysphasia, and left carotid territory infarction |
|
Tang 2006 (41) |
Post-stroke cognitive impairment |
Not meeting DSM-IV criteria for dementia but scoring ≤ the boundary score on the MMSE |
ECG | Clinical presentation or brain CT |
Significant association between AF and post-stroke cognitive impairment after adjustment for potential confounders: OR [95% CI]= 9.363 [1.012-86.622] |
Sex, NIHSS dysarthria score , Urinary incontinence , Education Cerebral atrophy index , Prestroke IQCODE score |
|
Mizrahi 2012 (42) |
Post-stroke cognitive impairment |
MMSE score <24 | ICD-9 | Clinical diagnosis based on presence of acute focal signs and cerebral dysfunction lasting ≥24 hrs confirmed by CT or MRI |
AF was significantly associated with post-stroke cognitive impairment (multivariate adjusted OR [95%CI] =1.6 [1.03, 2.47]) |
Age, sex, ischemic heart disease, hypertension, diabetes mellitus, hyperlipidemia, dementia, Parkinson’s disease, previous stroke |
|
Khan 2012(43) |
Post-stroke dementia |
Blessed Dementia Scale (BDS) |
NR | Stroke was defined by the WHO definition and diagnosis was supported by CT or MRI |
AF was significantly associated with post-stroke dementia (multivariate adjusted OR [95%CI] =5.12 [1.9, 13.3]) |
Not explicitly mentioned (variables with biological significance and P value< 0.25 in the univariate analysis were included in multivariate logistic regression model) |
Studies are ordered based on the publication year.
AF: Atrial Fibrillation; ICD: The International Classification of Diseases; ECG: Electrocardiogram; WHO: World Health Organization; OR: Odds Ratio; CI: Confidence Interval; DSM: The Diagnostic and Statistical Manual of Mental Disorders; NINDS-AIREN: National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l’Enseignement en Neurosciences; VD: Vascular Dementia; SS-IQCODE: Shortened Spanish version of the Informant Questionnaire on Cognitive Decline in the Elderly; CT: Computed Tomography; NINCDS-ADRDA: The National Institute of Neurological and Communicable Disease and Stroke-Alzheimer’s Disease and Related Disorders Association; AD: Alzheimer’s Disease; NR: Not Reported; MRI: Magnetic Resonance Imaging; HR: Hazard Ratio; MMSE: Mini-mental State Examination; NIHSS, National Institutes of Health Stroke Scale; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly.
Appendix Table 5. Assessment of selected quality criteria in 14 studies of patients with or without a history of stroke.
| Author Year | Was AF the primary exposure of interest? |
Inclusion & exclusion criteria clearly stated? |
Was ECG used as one of the methods for AF ascertainment? |
Method of Outcome ascertainment |
Tempo rality clear? |
Lost to follow up, % |
Adjustment | Number of quality criteria met* |
|---|---|---|---|---|---|---|---|---|
| Ott 1997 (28) | Yes | Yes | Yes | Superior† | No | N/A | Multivariate | 5 |
| Cacciatore 1998 (29) | No | Yes | No | Acceptable | No | N/A | Multivariate | 2 |
| Tilivis 2004 (33) | No | No | Unclear | Acceptable | Yes | > 10% | Minimal | 1 |
| Elias 2006 (13) | Yes | Yes | Yes | Superior | Yes | Unclear | Multivariate | 6 |
| Jozwiak 2006 (30) | Yes | Yes | Yes | Acceptable | No | N/A | Minimal | 3 |
| Debette 2007 (31) | No | Yes | Unclear | Acceptable | No | N/A | Multivariate | 2 |
| Forti 2007 (34) | Yes | Yes | No | Superior | Yes | Unclear | Multivariate | 5 |
| Bilato 2009 (32) | Yes | Yes | Yes | Acceptable | No | N/A | Multivariate | 4 |
| Marengoni 2009 (14) | Yes | Yes | No | Superior | Yes | >10% | Multivariate | 5 |
| Peters 2009 (16) | No | Yes | Yes‡ | Superior† | Yes | Unclear | Multivariate | 5 |
| Bunch 2010 (11) | Yes | Yes | Yes§ | Acceptable | Yes | Unclear | Multivariate | 5 |
| Dublin 2011 (12) | Yes | Yes | No | Superior | Yes | <10%‡ | Multivariate | 6 |
| Li 2011 (35) | No | Yes | No | Superior | Yes | >10% | Multivariate | 4 |
| Marzona 2012 (36) | Yes | Yes | Yes | Acceptable | Yes | <10% | Multivariate | 6 |
Studies are ordered based on the publication year.
The purpose of the numbers reported in this column is to provide an overview of how studies compare to each other in terms of methodological quality. We made every effort to include the most comprehensive and relevant quality criteria; however, there is no standard basis for quality assessment of observational studies. Although we find it unlikely that the classification of studies would dramatically change by using different quality criteria. It should be kept in mind that these numbers could vary depending on the items chosen for quality assessment (54, 55). Therefore, readers are encouraged to focus on each individual quality criterion rather than the overall quality scores in the assessment of bias.
Superior for dementia acceptable for cognitive impairment.
Per contact with the author.
ECG database of all Intermountain Healthcare hospitals and ICD-9 codes were used for AF diagnosis.
Appendix Table 6. Assessment of selected quality criteria in 7 studies of post-stroke cognitive impairment or dementia.
| Author Year | Was AF the primary exposure of interest? |
Inclusion & exclusion criteria clearly stated? |
Was ECG used as one of the methods for AF ascertainment? |
Method of Outcome ascertainment |
Temporality clear? |
Lost to follow up, % |
Adjustment | Number of quality criteria met* |
|---|---|---|---|---|---|---|---|---|
| Inzitari 1998 (37) | No | No | Yes | Superior | Yes | >10% | Multivariate | 4 |
| Barba 2000 (38) | No | Yes | Yes | Superior | Yes | >10% | Multivariate | 5 |
| Altieri 2004 (39) | No | Yes | Unclear | Superior | Yes | <10% | Multivariate | 5 |
| Zhou 2004 (40) | No | Yes | Unclear | Superior | Yes | >10% | Multivariate | 4 |
| Tang 2006 (41) | No | Yes | Yes | Acceptable | Yes | >10% | Multivariate | 4 |
| Mizrahi 2012 (42) | Yes | Yes | No | Acceptable | No | N/A | Multivariate | 3 |
| Khan 2012 (43) | No | Yes | Unclear | Acceptable | No | N/A | Multivariate | 2 |
Studies are ordered based on the publication year.
The purpose of the numbers reported in this column is to provide an overview of how studies compare to each other in terms of methodological quality. We made every effort to include the most comprehensive and relevant quality criteria; however, there is no standard basis for quality assessment of observational studies. Although we find it unlikely that the classification of studies would dramatically change by using different quality criteria. It should be kept in mind that these numbers could vary depending on the items chosen for quality assessment (54, 55). Therefore, readers are encouraged to focus on each individual quality criterion rather than the overall quality scores in the assessment of bias.
Footnotes
Potential Conflicts of Interest:
SK: None
TAS: Royalties (modest) from Mosby/Elsevier and McGraw-Hill for editing textbooks; Honoraria (modest) from Reed-Elsevier for speaking on topics related to general hospital psychiatry; Salary as employee of the Academy of Psychosomatic Medicine (significant) for editing (Psychosomatics).
MM: Biosense Webster Consultant; Research grants from Biosense Webster, Boston Scientific, MC10, Voyage Medical
JNR: Advanced Medical Education-Consultant (significant); Astellas/Cardiome-Consultant (significant); Atricure-Consultant (significant); Biosense Webster-Consultant (modest) & Fellowship Support (significant); Boston Scientific- Fellowship Support (significant); Bristol-Myers Squibb-Consultant(significant); CardioFocus-Clinical Oversight Committee (no compensation); CardioInsight-Scientific Advisory Board (modest); InfoBionic-Scientific Advisory Board and equity (modest); Medtronic-Consultant (modest) & Fellowship Support (significant); Pfizer-Consultant and Scientific Steering Committee (modest); Portola-Consultant & equity (modest); Sanofi-Aventis-Consultant (modest); St. Jude Medical -Fellowship Support (significant); Third Rock Ventures- Consultant (significant).
Disclaimer:
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the defnitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to Annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the offcial version of record.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1-2):125–32. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail. 2007;9(5):440–9. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28(3):726–35. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- 5.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42(5):484–91. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 6.Elias MF, Wolf PA, D’Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol. 1993;138(6):353–64. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- 7.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56(1):42–8. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 8.Duron E, Hanon O. Vascular risk factors, cognitve decline, and dementia. Vasc Health Risk Manag. 2008;4(2):363–81. doi: 10.2147/vhrm.s1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebert LE, Beckett LA, Scherr PA, Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15(4):169–73. doi: 10.1097/00002093-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Alzheimer’s Association Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8(2):131–68. doi: 10.1016/j.jalz.2012.02.001. 2012. [DOI] [PubMed] [Google Scholar]
- 11.Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer’s dementia. Heart Rhythm. 2010;7(4):433–7. doi: 10.1016/j.hrthm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Dublin S, Anderson ML, Haneuse SJ, Heckbert SR, Crane PK, Breitner JCS, et al. Atrial fibrillation and risk of dementia: A prospective cohort study. J Am Geriatr Soc. 2011;59(8):1369–75. doi: 10.1111/j.1532-5415.2011.03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias MF, Sullivan LM, Elias PK, Vasan RS, D’Agostino RB, Sr, Seshadri S, et al. Atrial Fibrillation Is Associated With Lower Cognitive Performance in the Framingham Offspring Men. J Stroke Cerebrovasc Dis. 2006;15(5):214–22. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Marengoni A, Qiu C, Winblad B, Fratiglioni L. Atrial fibrillation, stroke and dementia in the very old: A population-based study. Neurobiol Aging. 2011;32(7):1336–7. doi: 10.1016/j.neurobiolaging.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Park H, Hildreth A, Thomson R, O’Connell J. Non-valvular atrial fibrillation and cognitive decline: a longitudinal cohort study. Age Ageing. 2007;36(2):157–63. doi: 10.1093/ageing/afl164. [DOI] [PubMed] [Google Scholar]
- 16.Peters R, Poulter R, Beckett N, Forette F, Fagard R, Potter J, et al. Cardiovascular and biochemical risk factors for incident dementia in the hypertension in the very elderly trial. J Hypertens. 2009;27(10):2055–62. doi: 10.1097/HJH.0b013e32832f4f02. [DOI] [PubMed] [Google Scholar]
- 17.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 18.Kwok CS, Loke YK, Hale R, Potter JF, Myint PK. Atrial fibrillation and incidence of dementia: A systematic review and meta-analysis. Neurology. 2011;76(10):914–22. doi: 10.1212/WNL.0b013e31820f2e38. [DOI] [PubMed] [Google Scholar]
- 19.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.Thompson S, Ekelund U, Jebb S, Lindroos AK, Mander A, Sharp S, et al. A proposed method of bias adjustment for meta-analyses of published observational studies. Int J Epidemiol. 2011;40(3):765–77. doi: 10.1093/ije/dyq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tooth L, Ware R, Bain C, Purdie DM, Dobson A. Quality of reporting of observational longitudinal research. Am J Epidemiol. 2005;161(3):280–8. doi: 10.1093/aje/kwi042. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Third Edition, revised. American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- 23.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 24.Classifications of Diseases, Functioning, and Disability: International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) National Center for Health Statistics; Hyattsville (MD): Sep 21, 2010. updated. Available from: http://www.cdc.gov/nchs/icd/icd9cm.htm. [Google Scholar]
- 25.Classifications of Diseases, Functioning, and Disability: International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) National Center for Health Statistics; Hyattsville (MD): Jan 04, 2010. updated. Available from: http://www.cdc.gov/nchs/icd/icd10cm.htm. [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Deeks J, Higgins J, Altman D. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Available from: http://www.cochrane-handbook.org/ [Google Scholar]
- 28.Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. Stroke. 1997;28(2):316–21. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- 29.Cacciatore F, Abete P, Ferrara N, Calabrese C, Napoli C, Maggi S, et al. Osservatorio Geriatrico Campano Study Group Congestive heart failure and cognitive impairment in an older population. J Am Geriatr Soc. 1998;46(11):1343–8. doi: 10.1111/j.1532-5415.1998.tb05999.x. [DOI] [PubMed] [Google Scholar]
- 30.Jozwiak A, Guzik P, Mathew A, Wykretowicz A, Wysocki H. Association of atrial fibrillation and focal neurologic deficits with impaired cognitive function in hospitalized patients >or=65 years of age. Am J Cardiol. 2006;98(9):1238–41. doi: 10.1016/j.amjcard.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 31.Debette S, Bauters C, Leys D, Lamblin N, Pasquier F, de Groote P. Prevalence and determinants of cognitive impairment in chronic heart failure patients. Congest Heart Fail. 2007;13(4):205–8. doi: 10.1111/j.1527-5299.2007.06612.x. [DOI] [PubMed] [Google Scholar]
- 32.Bilato C, Corti MC, Baggio G, Rampazzo D, Cutolo A, Iliceto S, et al. Prevalence, functional impact, and mortality of atrial fibrillation in an older Italian population (from the Pro.V.A. study) Am J Cardiol. 2009;104(8):1092–7. doi: 10.1016/j.amjcard.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 33.Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of Cognitive Decline and Mortality of Aged People over a 10-Year Period. J Gerontol A Biol Sci Med Sci. 2004;59(3):268–74. doi: 10.1093/gerona/59.3.m268. [DOI] [PubMed] [Google Scholar]
- 34.Forti P, Maioli F, Pisacane N, Rietti E, Montesi F, Ravaglia G. Atrial fibrillation and risk of dementia in non-demented elderly subjects with and without mild cognitive impairment (MCI) Arch Gerontol Geriatr. 2007;44(Suppl 1):155–65.35. doi: 10.1016/j.archger.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Wang YJ, Zhang M, Xu ZQ, Gao CY, Fang CQ, et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76(17):1485–91. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]
- 36.Marzona I, O’Donnell M, Teo K, Gao P, Anderson C, Bosch J, et al. Increased risk of cognitive and functional decline in patients with atrial fibrillation: results of the ONTARGET and TRANSCEND studies. CMAJ. 2012;184(6):E329–36. doi: 10.1503/cmaj.111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inzitari D, Di Carlo A, Pracucci G, Lamassa M, Vanni P, Romanelli M, et al. Incidence and determinants of poststroke dementia as defined by an informant interview method in a hospital-based stroke registry. Stroke. 1998;29(10):2087–93. doi: 10.1161/01.str.29.10.2087. [DOI] [PubMed] [Google Scholar]
- 38.Barba R, Martinez-Espinosa S, Rodriguez-Garcia E, Pondal M, Vivancos J, Del Ser T. Poststroke dementia: Clinical features and risk factors. Stroke. 2000;31(7):1494–501. doi: 10.1161/01.str.31.7.1494. [DOI] [PubMed] [Google Scholar]
- 39.Altieri M, Di Piero V, Pasquini M, Gasparini M, Vanacore N, Vicenzini E, et al. Delayed poststroke dementia: A 4-year follow-up study. Neurology. 2004;62(12):2193–7. doi: 10.1212/01.wnl.0000130501.79012.1a. [DOI] [PubMed] [Google Scholar]
- 40.Zhou DH, Wang JY, Li J, Deng J, Gao C, Chen M. Study on frequency and predictors of dementia after ischemic stroke: the Chongqing stroke study. J Neurol. 2004;251(4):421–7. doi: 10.1007/s00415-004-0337-z. [DOI] [PubMed] [Google Scholar]
- 41.Tang WK, Chan SSM, Chiu HFK, Ungvari GS, Wong KS, Kwok TCY, et al. Frequency and clinical determinants of poststroke cognitive impairment in nondemented stroke patients. J Geriatr Psychiatry Neurol. 2006;19(2):65–71. doi: 10.1177/0891988706286230. [DOI] [PubMed] [Google Scholar]
- 42.Mizrahi EH, Waitzman A, Arad M, Adunsky A. Atrial fibrillation predicts cognitive impairment in patients with ischemic stroke. Am J Alzheimers Dis Other Demen. 2011;26(8):623–6. doi: 10.1177/1533317511432733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan M, Ahmed B, Ahmed M, Najeeb M, Raza E, Khan F, et al. Functional, cognitive and psychological outcomes, and recurrent vascular events in Pakistani stroke survivors: a cross sectional study. BMC Res Notes. 2012;5:89. doi: 10.1186/1756-0500-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barber M, Tait RC, Scott J, Rumley A, Lowe GD, Stott DJ. Dementia in subjects with atrial fibrillation: hemostatic function and the role of anticoagulation. J Thromb Haemost. 2004;2(11):1873–8. doi: 10.1111/j.1538-7836.2004.00993.x. [DOI] [PubMed] [Google Scholar]
- 45.Ezekowitz MD, James KE, Nazarian SM, Davenport J, Broderick JP, Gupta SR, et al. The Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators Silent cerebral infarction in patients with nonrheumatic atrial fibrillation. Circulation. 1995;92(8):2178–82. doi: 10.1161/01.cir.92.8.2178. [DOI] [PubMed] [Google Scholar]
- 46.Feinberg WM, Seeger JF, Carmody RF, Anderson DC, Hart RG, Pearce LA. Epidemiologic features of asymptomatic cerebral infarction in patients with nonvalvular atrial fibrillation. Arch Intern Med. 1990;150(11):2340–4. [PubMed] [Google Scholar]
- 47.Farina E, Magni E, Ambrosini F, Manfredini R, Binda A, Sina C, et al. Neuropsychological deficits in asymptomatic atrial fibrillation. Acta Neurol Scand. 1997;96(5):310–6. doi: 10.1111/j.1600-0404.1997.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 48.Lavy S, Stern S, Melamed E, Cooper G, Keren A, Levy P. Effect of chronic atrial fibrillation on regional cerebral blood flow. Stroke. 1980;11(1):35–8. doi: 10.1161/01.str.11.1.35. [DOI] [PubMed] [Google Scholar]
- 49.Anderson JL, Allen Maycock CA, Lappe DL, Crandall BG, Horne BD, Bair TL, et al. Frequency of elevation of C-reactive protein in atrial fibrillation. Am J Cardiol. 2004;94(10):1255–9. doi: 10.1016/j.amjcard.2004.07.108. [DOI] [PubMed] [Google Scholar]
- 50.Crandall MA, Horne BD, Day JD, Anderson JL, Muhlestein JB, Crandall BG, et al. Atrial fibrillation and CHADS2 risk factors are associated with highly sensitive C-reactive protein incrementally and independently. Pacing Clin Electrophysiol. 2009;32(5):648–52. doi: 10.1111/j.1540-8159.2009.02339.x. [DOI] [PubMed] [Google Scholar]
- 51.de Leeuw FE, de Groot JC, Oudkerk M, Kors JA, Hofman A, van Gijn J, et al. Atrial fibrillation and the risk of cerebral white matter lesions. Neurology. 2000;54(9):1795–801. doi: 10.1212/wnl.54.9.1795. [DOI] [PubMed] [Google Scholar]
- 52.Sterne J, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Available from: http://www.cochrane-handbook.org/ [Google Scholar]
- 53.Teo K, Yusuf S, Sleight P, Anderson C, Mookadam F, Ramos B, et al. Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. Am Heart J. 2004;148(1):52–61. doi: 10.1016/j.ahj.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 54.Greenland S. Invited commentary: a critical look at some popular meta-analytic methods. Am J Epidemiol. 1994;140(3):290–6. doi: 10.1093/oxfordjournals.aje.a117248. [DOI] [PubMed] [Google Scholar]
- 55.Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282(11):1054–60. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]