Introduction
Enteral tube feeding is the preferred method of nutrition for patients unable to take nutrition by mouth, but who maintain adequate gastrointestinal function, especially in the hospital setting.1,2 Following total laryngectomy, enteral feeding through a nasogastric tube (NGT) is maintained for a period of days to weeks to allow for adequate nutritional support to allow post-operative healing. The anastomosis and proper healing of the neopharynx is critical for post-operative swallow function, speech, and prevention of wound complications, such as fistulae formation. A NGT may be used post-operatively and is typically removed after the patient passes a barium swallow leak test. In the perioperative setting, the NGT allows for non-invasive and non-permanent enteral access that is readily reversible and well tolerated.3–5
Traditionally, an NGT is placed in the operating room (OR) at the time of laryngectomy. Despite best efforts at securing the NGT, reinsertion may be necessary due to clogging, misplacement, or accidental removal. Several techniques are available for replacement, including blind insertion, placement with the assistance of a fiberoptic nasolaryngoscope or pediatric gastroscope, or direct visualization in the OR. Any technique used in the acute post-operative setting should minimize trauma to the neopharyngeal suture line in an effort to prevent dehiscence and leak. We have found fluoroscopic guidance for NGT replacement in the laryngectomy patient to be safe, effective, and reliable.
The otolaryngology and radiology literature have ample studies comparing techniques of NGT placement.6–11 However, few studies specifically address the specific challenge of gaining enteral access in the post-operative laryngectomy patient. Furthermore, there is a lack of discussion of fluoroscopic guidance of NGT placement that specifically addresses the passage of the NGT over the neopharynx. At the most basic level, fluoroscopic guided NGT replacement provides an additional minimally invasive technique for gaining enteral access when bedside endoscopy or direct visualization in the OR is not feasible. The technique may also be appropriate in any patient with complicated anatomy following head and neck reconstructive surgery.
In this paper, we describe relevant anatomy seen in lateral neck films of the post-laryngectomy patient, case examples, and the specific set-up for and technique of otolaryngologist-assisted fluoroscopic guided NGT placement. Our technique employs a team approach, including an otolaryngologist, radiologist, radiology technologist, and the patient. An otolaryngologist familiar with patient-specific procedure and anatomy is ideally equipped to perform the NTG placement.
Methods and Materials
The oropharynx and both nares are anesthetized with aerosolized 1% lidocaine with neo-synephrine solution. The fluoroscopic tower is used in the upright position. The patient is positioned between the x-ray source and the image intensifier in the lateral position. (Figure 1) The patient may either sit or stand. Proper positioning of the patient in the lateral position, as opposed to the anterior-posterior position, allows the otolaryngologist to keep his/her hands out of the x-ray beam and the most advantageous view of the neopharynx.
Figure 1. Fluoroscopy Set-Up.
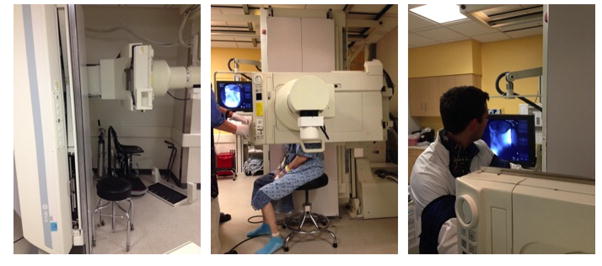
Patient is positioned between the x-ray beam and recording cassette, which may also double as the table in the fluoroscopy suite. Set-up may vary depending on the hospital and it may be best to request the same set-up as a lateral neck film.
At our institution, we use a Coviden Kangaroo™ nasogastic feeding tube, which has a weighted tip. The NGT is then lubricated and inserted by the otolaryngologist into the nasal cavity. At this point, initial fluoroscopic images are obtained. In order to reduce radiation exposure to the person placing the NGT, the image should be at lowest magnification, cones should be used to restrict the image to the region of interest, and the placer of the NGT should keep hands out of the beam. After reaching the nasopharynx, a film is taken by the radiologist and reviewed by the team. This initial film will provide key information as to the patient’s post-operative anatomy. (Figure 2.)
Figure 2. Relevant anatomy seen on lateral film in laryngectomy patient without and without NGT.
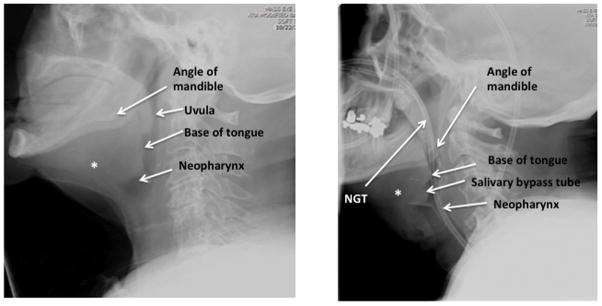
Left panel demonstrates proximity of neopharynx to spinal cord in a patient without NGT. Right panel depicts a different patient with NGT coursing though neopharynx. * Depicts likely former location of hyoid bone prior to laryngectomy which is notably absent.
Once the initial image is captured (last image hold is sufficient for this), the NGT is further advanced, and the course of the NGT is monitored with intermittent fluoroscopic guidance. As the NGT traverses the neopharynx, it will typically course in one of two directions – posteriorly towards the esophagus or anteriorly towards the tracheostoma. If the NGT courses anteriorly, it should be withdrawn slightly to the nasopharynx and another attempt made to approach the esophagus. (Figure 3.) Minimal resistance should be felt as the NGT is advanced. If necessary, the NGT may be twisted 180 degrees while simultaneously advancing it to allow access into the proximal esophagus. Additional, a small amount of contrast may be given orally to better delineate post-operative anatomy and visualize proper route of NGT. (Water-soluble contrast should be used, as the risk of extravasation is high.)
Figure 3. Anterior and Posterior Placement of NGT.
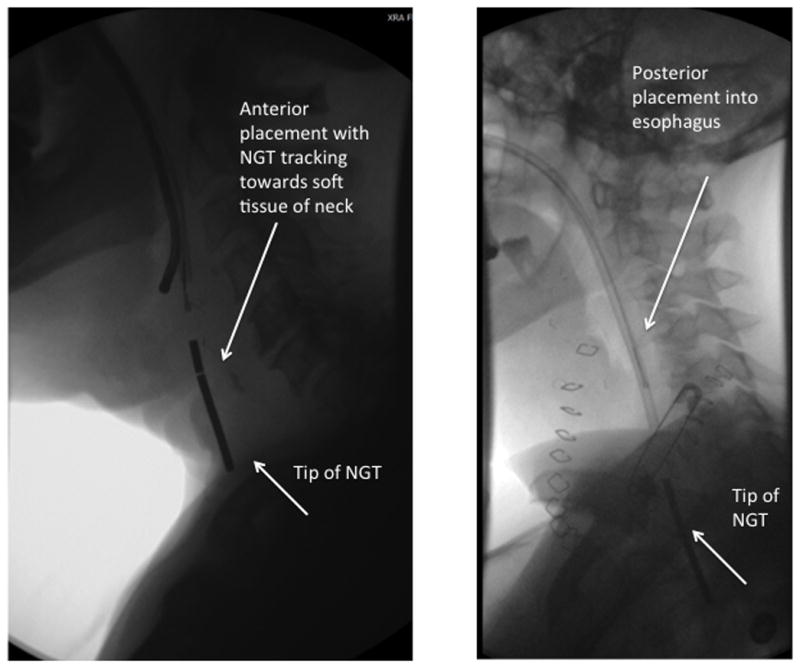
Left panel: Anterior placement tracking towards soft tissue of the neck. Note increased distance between vertebral bodies and NGT as compared with the correct positioning in the esophagus. This NGT should be retracted slightly to the level of the nasopharynx and attempt made at a more posterior placement. Right panel: Posterior placement. NGT placement demonstrates course within the esophagus.
Following successful passage of the NGT into the esophagus, it is advanced to the pre-measured length, and a fluoroscopic image is taken to confirm placement in the stomach. No contrast is needed as the tip is radiopaque. The NGT is then secured in place with a 2-0 silk suture through the membranous septum of the nose and supported with tape along the nasal dorsum.
Results
Over the past three years, we have completed 15 otolaryngologist-assisted fluoroscopic guided NGT placements. Of this patient cohort, 13/15 underwent total laryngectomy. Out of the patients that underwent total laryngectomy, 7 also had radial forearm free flap reconstruction, 1 had a pectoralis major myocutaneous flap reconstruction, and 1 had a supraclavicular flap reconstruction. Of our total patient cohort, 14/15 underwent successful NGT placement in the fluoroscopy suite. One patient had to return to the OR for placement after unsuccessful attempts at fluoroscopic NGT placement. The patient had a salivary bypass tube in place, contributing to challenging NGT placement. There were no complications with NGT placement. (Table 1)
Table 1.
Cohort of Patients that Underwent Fluoroscopic NGT Guidance over the Past Three Years at Massachusetts Eye and Ear Infirmary
| Patient Number | Age | Gender | Original Location of Malignancy | Operation | Reconstruction | Successful NGT Placement |
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 54 | M | Larynx | TL | RFFF | Yes |
| 2 | 67 | F | Larynx | TL | Primary closure | Yes |
| 3 | 43 | M | BOT | No TL | RFFF | Yes |
| 4 | 65 | M | Larynx | TL | Pec | Yes |
| 5 | 85 | M | Larynx | TL | Primary closure | Yes |
| 6 | 51 | F | Larynx | TL | RFFF | Yes |
| 7 | 66 | F | Larynx | TL | RFFF | Yes |
| 8 | 82 | M | Larynx | TL | Primary closure | Yes |
| 9 | 71 | M | Hypopharynx | TL | RFFF | No* |
| 10 | 87 | F | Oral Tongue | No TL | RFFF | Yes |
| 11 | 89 | F | Larynx | TL | RFFF | Yes |
| 12 | 78 | M | Larynx | TL | RFFF | Yes |
| 13 | 69 | F | Larynx | TL | RFFF | Yes |
| 14 | 58 | M | BOT | TL | SF | Yes |
| 15 | 73 | F | Larynx | TL | Primary closure | Yes |
Bot=Base of tongue; RFFF=radial forearm free flap; Pec=pectoralis major myocutaneous flap; SF=supraclavicular flap.
Salivary bypass tube made NGT placement challenging, and patient subsequently brought to operating room for placement under direct visualization.
Discussion
The technique of otolaryngologist-assisted fluoroscopic guided NGT placement is a straightforward and minimally invasive approach. One of the key advantages of this technique is comfort for both the patient and the otolaryngologist. The patient is able to sit or stand comfortably and avoids the need for concurrent NGT and fiberoptic laryngoscope in the nasal cavity. This technique also avoids the need for instrumenting the oropharynx with Magill forceps for NGT guidance. The otolaryngologist is able to bimanually direct the NGT, with increased control. In addition, the otolaryngologist may track the tip of the NGT throughout the aerodigestive tract ensuring minimal risk to the neopharynx anastomosis. Confirmatory abdominal films are performed at the same time, minimizing the time before patients can begin using their NGT for medications and nutrition.
Our patient cohort did well with successful placement of the NGT via fluoroscopic guidance. Placement was quick and confirmation of the NGT in the stomach was immediate. No additional pain medication or sedation was typically needed.
Literature exists on the topic of fluoroscopic guided NGT placement, but few studies discuss this technique in the post-laryngectomy patient population. Indeed, a variety of techniques exist for feeding tube placement. While many of these techniques may work well, several may be cumbersome for the patient and otolaryngologist. Further, there is a significant time and cost to bring a patient to the OR, and there are risks related to general anesthesia, especially in patients with free tissue transfers. At our institution, NGT placement is initially attempted at bedside under direct visualization with the use of a flexible nasolaryngoscope. However, if post-operative anatomy or patient discomfort makes endoscopy challenging, fluoroscopic guidance is readily performed.
As with every procedure, it is critical to assemble and check all equipment and perform a standard timeout. The otolaryngologist is often a guest in the fluoroscopy suit or may be consulting in an unfamiliar hospital. The radiologist or technician may be familiar with “routine” NGT placement in non-head and neck surgery patients and may desire to attempt a different technique. In these circumstances, it is important for the otolaryngologist to explain the steps of the procedure and rationale to all parties involved. Given the otolaryngologist’s familiarity with the post-operative head and neck anatomy, he or she is ideally situated to place the NGT efficiently.
Fluoroscopic- guided NGT placement has a few limitations. Similar to other techniques, fluoroscopic guidance requires a certain degree of patient cooperation, as the positioning of patient is key. We would not recommend it for patients that are unable to sit or stand unassisted. Further, as seen in our patient cohort, patients with salivary bypass tubes may limit the passage of the NGT and should be addressed prior to the procedure.
Conclusion
The cervical anatomy in post-operative laryngectomy patients can be challenging and is best understood by the otolaryngologist. Obtaining enteral access through a neopharyngeal reconstruction presents a unique dilemma that can be addressed by bringing the patient to the fluoroscopy suite for otolaryngologist-assisted fluoroscopic guided NGT placement. This study demonstrates a minimally invasive technique for NGT placement that has several advantages over standard approaches and may be employed in complicated head and neck patients. In patients where endoscopic-based NGT placement is unsuccessful and a return to the OR for direct visualization is untenable, fluoroscopic guidance provides a safe intermediate step with high rate of success.
Footnotes
FINANCIAL DISCLOSURE: None
CONFLICT OF INTEREST: None
References
- 1.Wood AJJ, Souba W. Nutritional Support New England Journal of Medicine. 1997;336:42–48. doi: 10.1056/NEJM199701023360107. [DOI] [PubMed] [Google Scholar]
- 2.Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta-analysis1. Am J Clin Nutr. 2001;74:534–542. doi: 10.1093/ajcn/74.4.534. [DOI] [PubMed] [Google Scholar]
- 3.Magné N, Marcy P, Foa C, et al. Comparison between nasogastric tube feeding and percutaneous fluoroscopic gastrostomy in advanced head and neck cancer patients. Eur Arch Otorhinolaryngol. 2001;258:89–92. doi: 10.1007/s004050000311. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill JP, Shaha AR. Nutrition management of patients with malignancies of the head and neck. The Surgical clinics of North America. 2011;91:631–639. doi: 10.1016/j.suc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Varkey P, Tang WR, Tan NC. Nutrition in head and neck cancer patients. Seminars in plastic surgery. 2010;24:325–330. doi: 10.1055/s-0030-1263074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang JC, Hilden K, Holubkov R, DiSario JA. Transnasal endoscopy vs. fluoroscopy for the placement of nasoenteric feeding tubes in critically ill patients. Gastrointestinal endoscopy. 2005;62:661–666. doi: 10.1016/j.gie.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Foote J, Kemmeter P, Prichard P, et al. A randomized trial of endoscopic and fluoroscopic placement of postpyloric feeding tubes in critically ill patients. Journal of Parenteral and Enteral Nutrition. 2004;28:154–157. doi: 10.1177/0148607104028003154. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez ED, Balfe DM. Fluoroscopically Guided Nasoenteric Feeding Tube Placement: Results of a 1-year Study. Radiology. 1991;178:759–762. doi: 10.1148/radiology.178.3.1899727. [DOI] [PubMed] [Google Scholar]
- 9.Hwang JY, Shin JH, Lee YJ, et al. Fluoroscopically guided nasojejunal enteral tube placement in infants and young children. AJR American journal of roentgenology. 2009;193:545–548. doi: 10.2214/AJR.08.1341. [DOI] [PubMed] [Google Scholar]
- 10.Joffe A, Grant M, Wong B, Gresiuk C. Validation of a blind transpyloric feeding tube placement technique in pediatric intensive care: Rapid, simple, and highly successful. Pediatr Crit Care Med. 2000;1:151–155. doi: 10.1097/00130478-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Deschler D, Lustig L. Gelcap-Assisted Fiberoptic Nasogastric Feeding Tube Placement. Ear, Nose & Throat Journal. 1996;75:102. [PubMed] [Google Scholar]


