Summary
Cells acclimate to fluctuating environments by utilizing sensory circuits. One common sensory pathway used by bacteria is two-component signaling (TCS), composed of an environmental sensor (the sensor kinase, SK) and a cognate, intracellular effector (the response regulator, RR). The squid symbiont Vibrio fischeri uses an elaborate TCS phosphorelay containing a hybrid SK, RscS, and two RRs, SypE and SypG, to control biofilm formation and host colonization. Here, we found that another hybrid SK, SypF, was essential for biofilms by functioning downstream of RscS to directly control SypE and SypG. Surprisingly, although wild-type SypF functioned as a SK in vitro, this activity was dispensable for colonization. In fact, only a single non-enzymatic domain within SypF, the HPt domain, was critical in vivo. Remarkably, this domain within SypF interacted with RscS to permit a bypass of RscS’s own HPt domain and SypF’s enzymatic function. This represents the first in vivo example of a functional SK that exploits the enzymatic activity of another SK, an adaptation that demonstrates the elegant plasticity in the arrangement of TCS regulators.
Keywords: biofilm/Euprymna, scolopes/hybrid, sensor, kinase/two-component, signaling/Vibrio fischeri
Introduction
For organisms to survive, they must appropriately respond to the assorted environments they experience. To do this, they use signaling pathways that link environmental signals with relevant intracellular outputs. One type of cellular circuitry found in most bacteria, some archaea, and a few eukaryotic species, is the two-component signaling (TCS) pathway (reviewed in (Stock et al., 2000, Wuichet et al., 2010)). The basic TCS architecture consists of two types of proteins: a sensor kinase (SK) and a response regulator (RR). Typically, a specific environmental ligand binds a cell membrane-bound SK, which autophosphorylates on a conserved histidine within a HisKA domain using ATP as the phosphoryl donor. It then donates this phosphoryl group to a conserved aspartate in the REC (receiver) domain within a cognate RR, an event that is catalyzed by the enzymatic activity of the REC domain. Often the RR has an effector domain, such as a DNA binding or enzymatic domain, whose activity is activated or deactivated once the REC domains becomes phosphorylated (Galperin, 2010). This two-protein arrangement connected by a single His-Asp phosphotransfer event remains the most common TCS architecture found in bacteria; however, some TCS pathways consist of a complicated phosphorelay involving more than one phosphotransfer event (His-Asp-His-Asp) between two or more cognate TCS proteins. Often these phosphorelays include a “hybrid” SK, which contains a second site of phosphorylation within a covalently attached REC domain. Some hybrid SKs also possess a third site of phosphorylation, a histidine within a C-terminal histidine-containing phosphotransfer (HPt) domain. To date, most hybrid SKs with autokinase activity require these additional sites of phosphotransfer to effectively donate the phosphoryl group to their cognate RR (Hsu et al., 2008, Jourlin et al., 1997, Takeda et al., 2001, Tsuzuki et al., 1995, Uhl & Miller, 1996). It is believed that these extra phosphotransfer events represent checkpoints that control whether a cell initiates physiological changes under particular conditions (Jung et al., 2012, West & Stock, 2001).
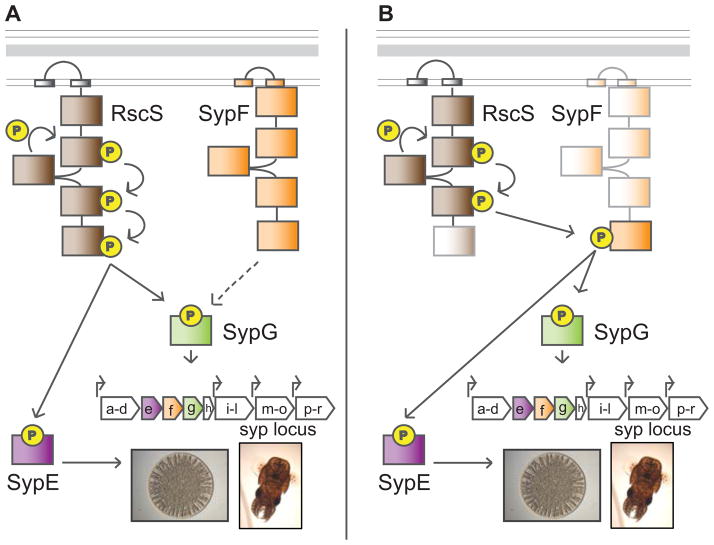
One developmental process in bacteria that is often governed by TCS circuits is the formation of biofilms, or matrix-encased communities of cells (Ferrieres & Clarke, 2003, Gooderham & Hancock, 2009, Hamon & Lazazzera, 2001, Huang et al., 2013, Irie et al., 2004, Li et al., 2002, Petrova & Sauer, 2009, Stipp et al., 2013, Su & Ganzle, 2014, Zhang et al., 2004). It is believed that environmental signals activate or deactivate specific TCS pathways to disfavor the independent, planktonic state and favor the assembly of a community (McLoon et al., 2011, Mulcahy & Lewenza, 2011, Ventre et al., 2006). Environments that induce biofilm development can include host tissues, where these communities are implicated in initiation and persistence of colonization by both pathogenic and commensal bacteria (Reviewed in (Heindl et al., 2014, Joo & Otto, 2012, Percival & Suleman, 2014, Ramey et al., 2004, Yildiz & Visick, 2009)). One unique model system used to study biofilms in the context of a natural host is the symbiosis between the bacterium, Vibrio fischeri, and the Hawaiian bobtail squid, Euprymna scolopes (Reviewed in (McFall-Ngai, 2014, Stabb & Visick, 2013)). Successful colonization requires that V. fischeri cells form and disperse from a biofilm to enter the symbiotic organ, known as the light organ (Nyholm et al., 2000, Yip et al., 2006). This biofilm depends on the production of the symbiosis polysaccharide (Syp PS) generated by proteins encoded by the 18-gene syp locus (Shibata et al., 2012, Yip et al., 2006). Control over Syp production occurs via a complex TCS cascade. Previous work indicated that the hybrid SK, RscS, senses an unknown signal that leads to the phosphorylation of two downstream RRs, SypE and SypG (reviewed in (Visick, 2009)) (Fig 1A). SypG functions as a transcription factor to directly promote transcription of the syp locus, while SypE functions downstream of syp transcription to control production of Syp PS (Morris et al., 2011, Morris & Visick, 2013, Ray et al., 2013, Yip et al., 2005). Both sypE and sypG are located within the syp locus, whereas rscS is located elsewhere in the chromosome and was proposed to be horizontally acquired (Mandel et al., 2009, Visick & Skoufos, 2001, Yip et al., 2005). The current model is that, after its acquisition, RscS evolved the ability to activate SypG and inactivate SypE, thus allowing V. fischeri to utilize Syp for colonization of E. scolopes.
Figure 1. Syp biofilm regulation.
Biofilm formation and host colonization by V. fischeri is controlled by a complex two-component signaling (TCS) pathway. (A) Previous model: the hybrid sensor kinase (SK), RscS, functions upstream of two response regulators (RR), SypE and SypG, to promote biofilm formation on agar plates (depicted as a wrinkled colony) and biofilm formation during colonization (represented by an image of a squid, the host for V. fischeri). Phospho-SypG functions as a transcription factor to activate the transcription of the syp locus at four promoters, and SypE inhibits biofilms at a level below syp transcription. When phosphorylated, SypE is no longer inhibitory. The sypE and sypG genes reside within the syp locus. Between sypE and sypG lies an additional hybrid SK gene, sypF, with an unclear role in biofilms. (B) Revised model: the C-terminal HPt domain of SypF functions between RscS and the two RRs, SypE and SypG, thus bypassing the requirement for the C-terminal domain of RscS. The faded colors indicate domains found to be non-essential for wrinkled colony formation and colonization.
RscS may not be the only SK that regulates the Syp pathway. Located between the RR genes sypE and sypG is an additional hybrid SK gene, sypF (Fig 1), a genetic configuration that is typical for TCS proteins that function together. Indeed, our previous work suggested that SypF could control biofilm formation: although overproduction of wild-type SypF had no effect on biofilms, overproduction of an active variant of SypF, termed SypF*, induced biofilm formation (Darnell et al., 2008). This variant contained two mutations, one of which was located three amino acids upstream from the conserved site of phosphorylation (Fig 2A) (Darnell et al., 2008). However, it remained unknown whether the phenotype of SypF* was physiologically relevant, whether SypF had any role in host colonization, or how input from two SKs (an unusual arrangement for TCS pathways), SypF and RscS, might dictate the control of biofilms.
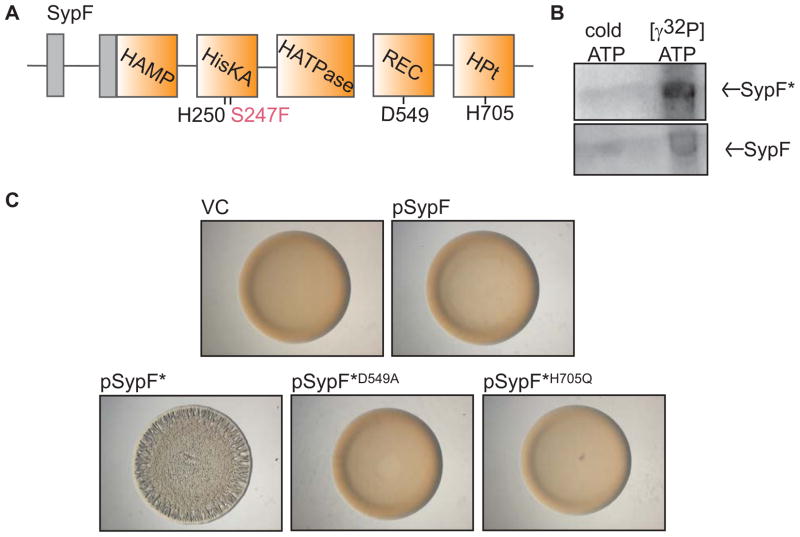
Figure 2. Function of SypF* as a SK.
(A) Cartoon of the predicted functional domains within SypF, including HAMP, HisKA, HATPase_c, REC, and HPt domains (orange boxes) as well as transmembrane regions (gray boxes) flanking a putative periplasmic loop. Conserved putative sites of phosphorylation are indicated below in black type. SypF* contains two mutations. The critical mutation, S247F, is indicated in pink type. (B) Autoradiograph of purified MBP-SypF* (above) and wild-type MBP-SypF (below) after incubation with unlabeled ATP or [γ-32P]-ATP. (C) Colony morphology of wild-type (WT) V. fischeri strain ES114 containing vector control (VC, pKV69) or various SypF and SypF* overproduction plasmids as follows: pSypF (pCLD54), pSypF* (pCLD29), pSypF*D549A (pANN61), and pSypF*H705Q (pANN62). Cultures of the indicated strains were spotted on agar plates and colony morphology was assessed after 24 h.
In this study, we found that SypF is critical for biofilm formation and host colonization by functioning as the direct donor of phosphoryl groups to the downstream RRs SypE and SypG. Surprisingly, although SypF could autophosphorylate in vitro, only one non-enzymatic domain of SypF was required for biofilms and colonization. Instead of its own enzymatic domains, SypF relied on the catalytic activity of the upstream SK, RscS to control biofilms and colonization. SypF thus represents the first example of a hybrid SK that has the ability to function as a histidine kinase, yet, in vivo, it forfeits this activity to an upstream SK. This interaction between the recently acquired RscS protein and the more conserved SypF protein demonstrates the flexibility of TCS architectures, and provides insight into how these regulatory circuits might evolve to allow a bacterium to take advantage of a new niche, such as host tissues.
Results
SypF* functions as a canonical hybrid sensor kinase
In culture, biofilm formation by V. fischeri is induced upon overproduction of any of three TCS proteins: the SK RscS, the RR SypG (in the absence of inhibitory RR protein SypE), and a mutant version of the SK SypF (SypF*) (Darnell et al., 2008, Hussa et al., 2008, Yip et al., 2006). Our long-standing model proposes that RscS directly controls SypG and SypE (Fig 1A). As a result, the role of SypF in controlling biofilm formation has been unclear, especially since overproduction of only SypF*, but not wild-type SypF, could induce biofilm formation (Darnell et al., 2008). Within SypF*, two mutations exist (S247F and V439I). The former is located 3 residues away from the predicted site of autophosphorylation (H250) (Fig 2A). In this position, the substitution of the small serine side chain with the bulky phenylalanine side chain could affect the ability of H250 to be phosphorylated. Thus, it was inferred that SypF* exists in a kinase “active” conformation (Darnell et al., 2008). This result, along with the strong conservation of sequences known to catalyze kinase and phosphotransfer reactions (Supplemental Fig S1A, B), suggested that SypF* functions as a SK. To test this hypothesis, we purified the cytoplasmic portion of SypF*, assessed whether it could autophosphorylate in vitro, and found that it could under the tested conditions (Fig 2B).
To determine if SypF* could function as a hybrid SK, we tested whether biofilm formation required predicted sites of phosphorylation in the REC (D549) and HPt domains (H705) (Fig 2A). We generated individual mutations of D549 and H705, overexpressed these mutant alleles in otherwise wild-type cells, and assessed the ability of the resulting strains to form wrinkled colonies, an in vitro indicator of biofilm formation. Whereas cells that overproduced SypF* (pSypF*) formed wrinkled colonies, those containing either pSypF*D549A or pSypF*H705Q formed smooth colonies (Fig 2C). Thus, similar to canonical hybrid SKs, SypF* required these sites of phosphorylation to function.
To confirm that the SypF* variants were produced, we generated constructs that produced FLAG epitope-tagged versions of SypF*, SypF*D549A or SypF*H705Q, as well as two additional mutants, SypF*H250Q (in the HisKA domain) and SypFS247F (containing only one of the two mutations present in SypF*). We then used western blot analysis to assess the levels of these proteins and colony morphology to assess their ability to induce biofilm formation. Importantly, we found that the steady-state levels of all these SypF variants were similar (Supplementary Fig S2B). However, the FLAG tag somewhat diminished the biofilm-inducing activity of SypF* (Supplementary Fig S2A, compare pSypF* to pSypF*-FLAG). Regardless, the H250Q, D549A, and H705Q mutants failed to induce the formation of wrinkled colonies. In contrast, the SypFS247F mutant promoted wrinkled colony development to approximately the same extent as SypF*, demonstrating that this substitution was sufficient for the activity of SypF*. Together, our data support the hypothesis that SypF* functions as a canonical hybrid SK.
sypF is required for biofilm formation and syp transcription
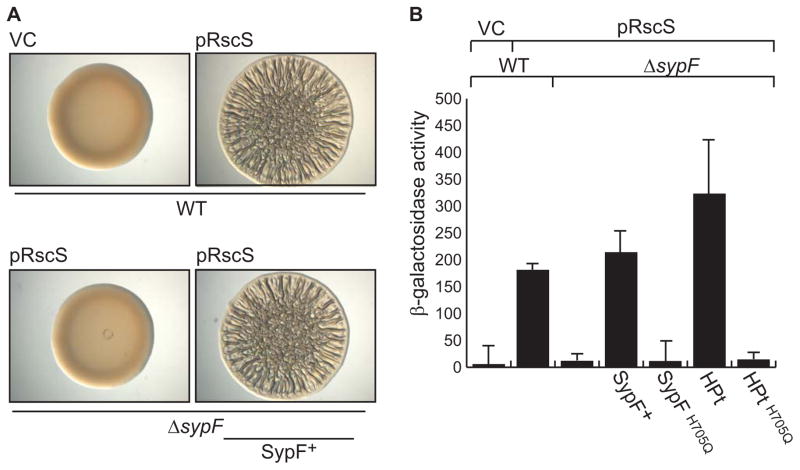
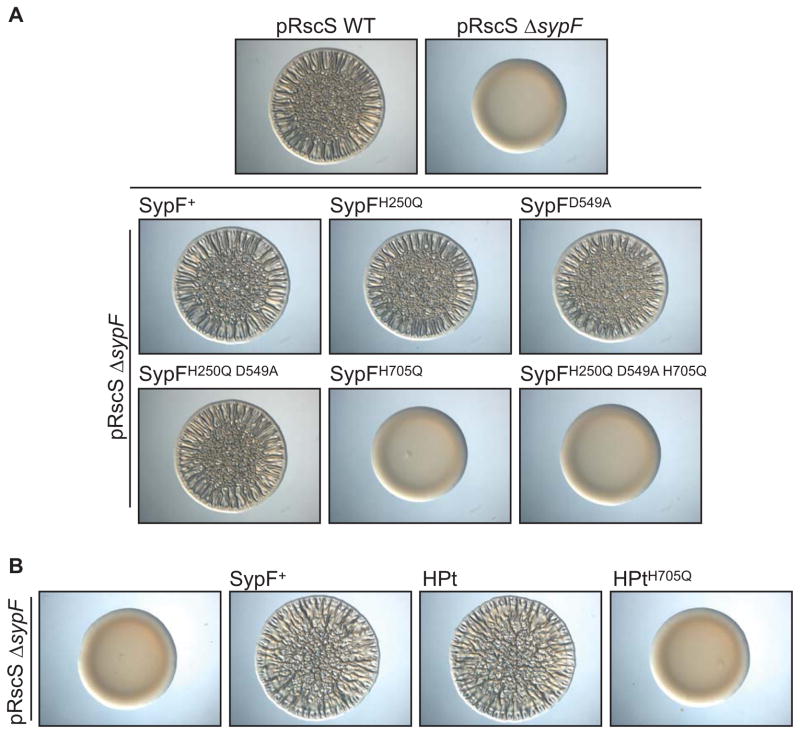
We next asked where SypF might function in the Syp pathway to control biofilm formation. We first determined where it functioned relative to RscS, the other hybrid SK. To do this, we deleted sypF from the chromosome and assessed whether this affected the ability of RscS to induce wrinkled colonies. Whereas RscS overproduction induced the formation of wrinkled colonies by the wild-type strain, it failed to do so in the sypF mutant, which formed smooth colonies indistinguishable from the vector control (Fig 3A). Complementation of the sypF deletion with a wild-type copy of sypF in single copy restored wrinkled colony formation. These data suggest that SypF works below RscS in the regulatory hierarchy.
Figure 3. Role of SypF in RscS-induced biofilm formation and syp transcription.
RscS-induced (A) biofilm formation and (B) syp transcription was assessed by overproducing RscS from a plasmid (pARM7). (A) Colony morphology of wild-type cells (WT, KV4389), a ΔsypF strain (KV6921), or the complemented ΔsypF strain (KV6659). These cells contained either vector control (VC, pKV282) or pRscS, as is indicated in the figure. Cultures of the indicated strains were spotted on an agar plate and colony morphology was assessed after 39 hours. (B) PsypA-lacZ reporter activity in WT cells, the ΔsypF strain, and in ΔsypF strains producing the SypF proteins. The strains used for this experiment contained either VC or pRscS as indicated in the figure. The PsypA-lacZ reporter base strains used are as follows: WT (KV7410); ΔsypF (KV7412), ΔsypF sypF+ (KV7386), ΔsypF sypFH705Q (KV7387), ΔsypF sypF-HPt (KV7377), and ΔsypF sypF-HPtH705Q (KV7413). Error bars represent the standard deviation.
Because RscS-induced biofilm formation required sypF, we asked whether RscS-induced syp transcription would similarly require sypF. Thus, we evaluated the impact of the sypF deletion on the activity of a PsypA-lacZ reporter. In the wild-type background, RscS induced expression of the PsypA-lacZ reporter relative to the vector control. In the sypF deletion background, however, RscS failed to induce the reporter (Fig 3B). Finally, provision of the wild-type sypF allele in trans complemented the defect. We conclude that RscS requires SypF to induce syp transcription, and propose a model wherein SypF functions downstream of RscS in the Syp TCS pathway (Fig 1B).
SypF directly controls SypG and SypE
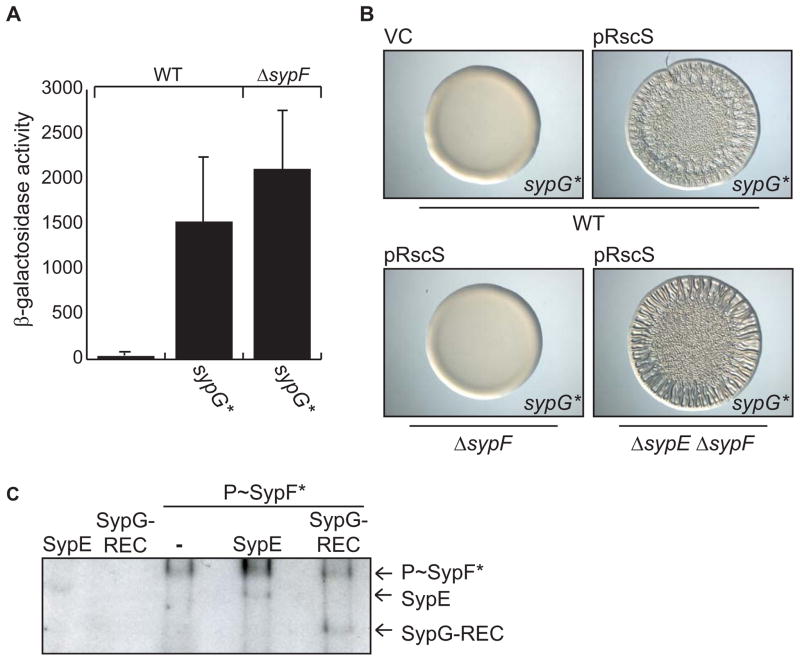
RscS is proposed to act upstream of two RRs, SypG and SypE (Hussa et al., 2008, Morris et al., 2011, Yip et al., 2006). We thus asked whether SypF functioned between RscS and one or both of these downstream RRs (Fig 1B). Because RscS required SypF to promote syp transcription, we first asked if SypF functions above SypG, the direct transcriptional activator of the syp locus (Ray et al., 2013). If so, then it should be possible to bypass the requirement for sypF using an active SypG variant that no longer requires activation by an SK. We generated strains that produced SypG*, a SypG protein in which the conserved site of phosphorylation (aspartate 53) was converted to a glutamate. This mutation mimics the phosphorylated state of other RRs (Freeman & Bassler, 1999, Sanders et al., 1992, Sanders et al., 1989) and has been shown to increase the activity of SypG (Hussa et al., 2008). Indeed, single-copy expression of sypG* was sufficient to induce syp transcription in the wild-type background (Fig 4A) and in the absence of sypF (Fig 4A). These data support a model in which SypF functions between RscS and SypG to control syp transcription (Fig 1B).
Figure 4. Determining where SypF functions in the Syp pathway.
(A) SypG*-induced PsypA-lacZ reporter activity in WT (KV7230) or ΔsypF (KV7231) strains. Error bars represent standard deviation. (B) Wrinkled colonies of WT V. fischeri strains producing SypG* (KV6527) with vector control (VC, pKV282) or pRscS (pARM7) (top two panels) and of pRscS, SypG*-producing ΔsypF (KV6526) and ΔsypE ΔsypF (KV6586) strains (bottom two panels). Cultures were spotted and colony morphology was assessed after 19 hours. (C) in vitro phosphotransfer assay. Left two lanes: GST-SypE or MBP-SypG-REC incubated with radiolabelled ATP. Right three lanes: phospho-SypF* incubated with or without GST-SypE or MBP-SypG-REC.
RscS also functions upstream of SypE, the RR that controls biofilms below syp transcription; phosphorylation of SypE switches off its inhibitory activity, thus allowing biofilms to develop (Morris et al., 2011) (Fig 1A). To determine if SypF also functions upstream of SypE, we evaluated RscS-induced wrinkled colony formation in a SypG*-producing sypF deletion strain like the one used above. Because expression of sypG* overcomes the requirement for SypF in syp transcription, we anticipated that this strain would produce wrinkled colonies only if SypF is not also required to inactivate SypE. As controls, we evaluated the production of wrinkled colonies by sypF+ cells. As predicted from previous work (Hussa et al., 2008), single copy expression of sypG* in an otherwise wild-type background failed to induce wrinkled colony formation due to inhibition by SypE; however, expression of both rscS and sypG* in wild-type cells induced wrinkled colony formation (Fig 4B). This demonstrates that, in this strain background, rscS expression is sufficient to turn off the inhibitory activity of SypE. In contrast, expression of both rscS and sypG* in the sypF mutant failed to induce this phenotype. This observation suggests that sypF has an additional role in promoting biofilms, potentially by inactivating SypE. Indeed, a sypE sypF double mutant formed wrinkled colonies with rscS and sypG* expression (Fig 4B). We infer from these data that RscS works through SypF to control the activities of both SypG and SypE (See model in Fig 1B).
To more directly assess the ability of SypF to interact with and control SypG and SypE, we evaluated whether SypF could donate phosphoryl groups to these RRs in vitro. We purified the REC domain of SypG and the full-length form of SypE, and added these purified proteins to reactions containing phosphorylated SypF*. In support of the genetic data, we detected phosphorylated forms of SypE and SypG-REC after incubation with phospho-SypF* (Fig 4C). These data indicate that SypF can directly interact with and phosphorylate these two RR proteins.
RscS-induced biofilm formation does not require conserved SypF residues
The above evidence indicate that RscS functions through SypF to control the activity of SypG and SypE. This is an unusual regulatory set-up for TCS systems with multiple SKs; thus, the mechanism by which SypF functions after RscS to control biofilms remained unclear. Specifically, we wondered if wild-type SypF could function as a SK like SypF* and, if so, if that SK activity was necessary for RscS-dependent activation of the pathway. To ask the first question, we purified the cytoplasmic portion of wild-type SypF and assessed whether it could autophosphorylate in vitro. Indeed, in the presence of radiolabeled ATP, SypF exhibited autophosphorylation activity (Fig 2B).
To determine whether RscS-induced biofilm formation requires SypF to function as a SK, we generated mutations in each predicted site of phosphorylation of wild-type SypF. We then assessed whether the mutant proteins could complement the sypF mutant for wrinkled colony formation. As shown previously (Fig 3A), overproduction of RscS in the sypF mutant failed to induce biofilm formation (Fig 5A), but this defect could be restored with a wild-type copy of sypF expressed in single copy from the chromosome. Surprisingly, mutating the first conserved histidine (H250Q), the conserved aspartate (D549A), or both together (H250Q D549A), did not negatively impact complementation: strains with these proteins retained their ability to form wrinkled colonies (Fig 5A). However, a SypF mutant disrupted for all three putative sites of phosphotransfer (H250, D549, and H705) failed to promote wrinkled colony formation, indicating that the last site of phosphotransfer may be required under these conditions. Indeed, SypFH705Q, which contained a single substitution in the conserved site of phosphorylation within the HPt domain, did not complement the sypF deletion (Fig 5A). Analogous results were seen when assessing whether this mutant protein could complement a sypF deletion for syp transcription (Fig 3B). Finally, we observed similar steady-state levels for epitope-tagged versions of the wild-type and mutant SypF proteins via western blotting (Supplementary Fig S2C). Thus, the negative results for SypFH705Q and the triple mutant were not due to gross protein instability. Together, these data indicate that SypF does not function as a canonical hybrid SK under conditions that promote wrinkled colony development. Instead, SypF appears to require only H705 within its HPt domain to function.
Figure 5. Function of the SK activity of wild-type SypF.
(A) RscS-induced (pARM7) wrinkled colony formation in WT cells or sypF deletion strains with or without sypF alleles expressed in single copy. Strains used are as follows: WT (KV4389); ΔsypF (KV6921); ΔsypF sypF+ (KV6659); ΔsypF sypFH250Q (KV6896); ΔsypF sypFD549A (KV6692); ΔsypF sypFH705Q (KV7085); ΔsypF sypFH250Q D549A (KV7154); ΔsypF sypFH250Q D549A H705Q (KV7155). Strains were spotted on agar plates and colony morphology was assessed after 39 hours. (B) RscS-induced (pARM7) wrinkled colony phenotype of a ΔsypF strain (KV6291), or the ΔsypF strain containing full-length sypF (KV6659), sypF-HPt (KV7226) or sypF-HPtH705Q (KV7485) after 39 hours.
RscS requires only the HPt domain of SypF
Because RscS-induced biofilm formation and syp transcription only required H705 in SypF, but not H250 or D549, we wondered whether the domain that contains H705, the HPt domain, was sufficient to promote these phenotypes. Indeed, sypF in other Vibrio species encodes a single HPt domain rather than a full-length SK (Supplementary Fig S3). We thus cloned this domain and assessed complementation. We found that the HPt protein alone permitted RscS-induced biofilm formation (Fig 5B) and syp transcription in a sypF deletion mutant (Fig 3B). In contrast, when the HPt domain contained a mutation in the site of phosphorylation, it did not complement the sypF deletion. These data suggest that the HPt domain in SypF is the sole domain to engage in phosphotransfer reactions that control biofilm formation induced by RscS.
RscS directly utilizes the HPt domain of SypF
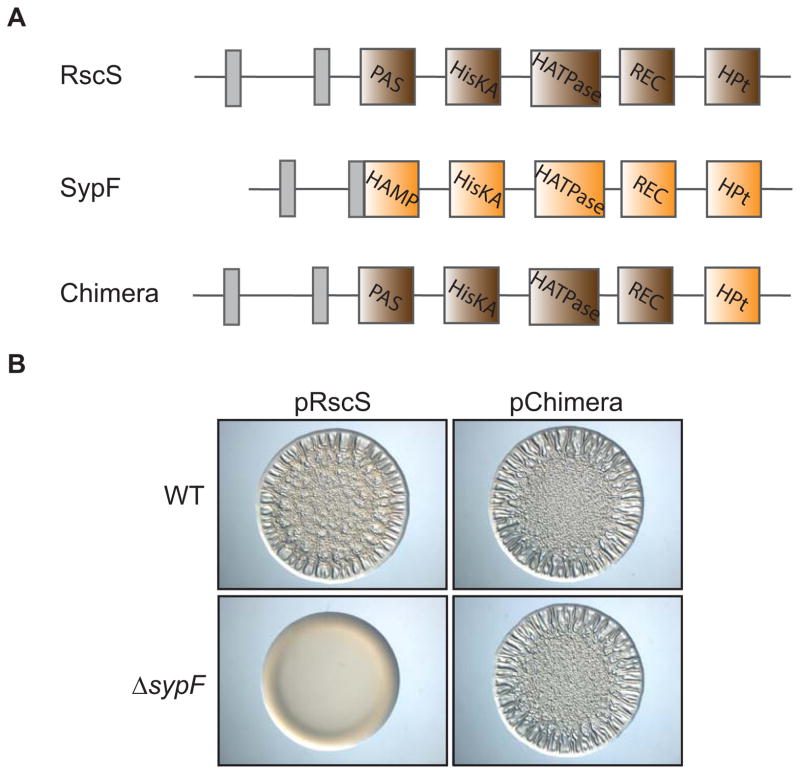
The requirement for only the HPt domain of wild-type SypF was surprising because single domain HPt proteins do not exhibit enzymatic activity. Therefore, they must receive a phosphoryl group from an upstream protein to donate phosphoryl groups to downstream RRs. Interestingly, previous data suggested that RscS, a hybrid SK with three predicted sites of phosphorylation, did not require the last site of phosphorylation in its HPt domain to promote biofilms (Geszvain & Visick, 2008). Thus, we hypothesized that RscS donates phosphoryl groups to the HPt domain of SypF, which then passes phosphoryl groups to the two downstream RRs, SypG and SypE (Fig 1B). To test this hypothesis, we generated a chimeric protein that contained the N-terminal portion of RscS (lacking its HPt domain) and the C-terminal HPt domain of SypF (Fig 6A.). We introduced the plasmid that produces this chimera into wild-type and sypF deletion backgrounds, and then assessed whether the chimeric protein was sufficient to induce biofilms even in the absence of sypF. In accordance with our hypothesis, the chimera induced wrinkled colonies in both backgrounds (Fig 6B). Together, these data suggest that neither RscS nor SypF require the full complement of their own phosphotransfer domains, but instead rely on each other for the signal transduction that leads to biofilm formation.
Figure 6. Interaction between RscS and SypF in the Syp biofilm pathway.
(A) Cartoon image comparing the predicted functional domains of RscS (brown: PAS, HisKA, HATPase_c, REC, Hpt), SypF (orange: HAMP, HisKA, HATPase_c, REC, HPt), and an RscS-SypF chimera that contains the N-terminal regions of RscS and the HPt domain of SypF. Grey boxes indicate transmembrane regions that flank a putative periplasmic domain. (B) Wrinkled colony formation of WT (ES114) or sypF deletion (KV5367) cells overproducing RscS (pARM7) or the RscS-SypF chimera (pANN69). Indicated strains were spotted and grown for 22 hours.
Requirement for SypF during host colonization
Our ability to assess the function of SypF in culture depends on the plasmid-based production of regulators such as RscS and SypF*. Use of those two different regulators, however, yielded conflicting results about how SypF regulates biofilms. More specifically, SypF* required all three sites of phosphorylation to induce wrinkled colony formation, whereas RscS-induced phenotypes only required a single conserved site of phosphorylation within the HPt domain of SypF. We thus wanted to define a clear role for SypF and its putative enzymatic domains during biofilm formation using a more physiologically relevant approach. To do this, we assayed the importance of sypF and its conserved sites of phosphorylation for V. fischeri to colonize its squid host. Importantly, colonization is an in vivo phenotype that requires biofilm formation, but does not rely on the overproduction of regulatory proteins.
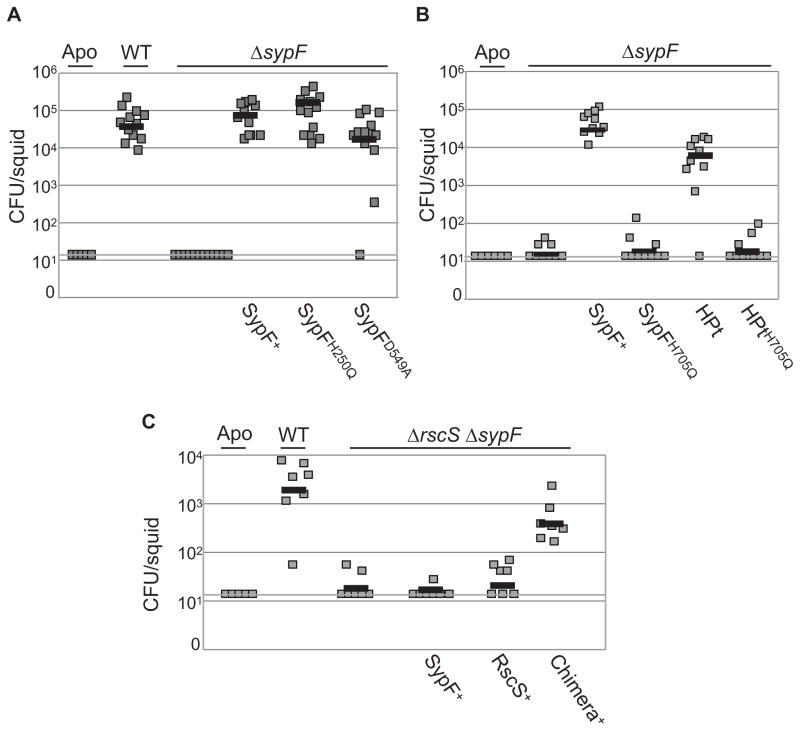
We first assessed the requirement of sypF for this phenotype by incubating the sypF deletion mutant with aposymbiotic squid for 18 h and then determining the number of V. fischeri cells in each squid. As expected, wild-type V. fischeri could colonize; however, the sypF mutant exhibited a severe colonization defect that could be complemented by providing wild-type sypF in single copy in trans (Fig 7A, B). This evidence indicates that sypF is required for host colonization.
Figure 7. Mechanism by which SypF functions in vivo.
(A, B, C) Indicated strains of V. fischeri were incubated with aposymbiotic (Apo) juvenile squid for 18 hours, and colonization of E. scolopes was determined by calculating colony-forming units (CFU) of V. fischeri in each squid. (Limit of detection, CFU = 14). (A, B) Colonization comparison between WT cells and sypF deletion strains expressing sypF alleles in single copy. Strains used are as follows: WT (KV4389), ΔsypF (KV6921); ΔsypF sypF+ (KV6659); ΔsypF sypFH250Q (KV6896); ΔsypF sypFD549A (KV6692); ΔsypF sypFH705Q (KV7085); ΔsypF sypF-HPt (KV7226), and ΔsypF sypF-HPtH705Q (KV7485). Panels A and B represent independent experiments. (C) Colonization phenotype of WT cells (KV4389), a ΔrscS ΔsypF strain (KV7657), and the ΔrscS ΔsypF strain that produces SypF (KV7656), RscS (KV7654), or the chimera (KV7651).
We next identified the domains/amino acids within SypF that are important for host colonization. We found that, similar to the RscS-induced wrinkled colony experiments, cells that produced SypFH250Q or SypFD549A successfully colonized the squid whereas cells producing SypFH705Q did not (Fig 7A, B). Additionally, production of the HPt domain of SypF alone allowed the sypF deletion mutant to colonize E. scolopes unless the HPt domain contained a mutation within the site of phosphorylation (Fig 7B). These results indicate that SypF does not function as an SK to promote colonization, and that the RscS-induced wrinkled colony phenotype is more physiologically relevant than the SypF*-induced phenotype.
Finally, to confirm our findings that RscS and SypF function in an unusual phosphorelay to promote biofilm formation, we asked whether the rscS-sypF chimera, expressed from the chromosome of a double rscS sypF mutant, was proficient to promote colonization. Because rscS and sypF are individually required for colonization ((Visick & Skoufos, 2001) (Fig 7A, B)), it was not surprising that the rscS sypF double mutant failed to colonize the squid, and introducing either rscS or sypF alone into this strain did not restore host colonization (Fig 7C). However, in support of our model for biofilm regulation, the chimeric allele mostly complemented the rscS sypF mutant for colonization. Together, these data confirm that the HPt domain of SypF functions between RscS and SypG/SypE to control biofilms, and that the enzymatic activity of SypF is largely dispensable for this signaling cascade during host colonization.
Discussion
TCS is a universal mechanism that bacteria use to link environmental signals with an intracellular response. At the apex of these pathways is the SK, a receptor that senses an environmental ligand to initiate physiological changes within the cell. Bioinformatic analyses readily identify SK proteins based on highly conserved enzymatic residues involved in histidine autokinase activity (Kim & Forst, 2001, Kofoid & Parkinson, 1988, Nixon et al., 1986, Stock et al., 1988). Canonical SKs containing a single phosphorylatable residue, the site of histidine-autophosphorylation, are predicted in most bacterial genomes. In contrast, hybrid SKs are predicted in about 1/3 of bacterial genomes (Galperin, 2005, Zhang & Shi, 2005). Hybrid SKs enforce an extra level of regulatory complexity in TCS, as their additional sites of phosphorylation are thought to function as checkpoints that fine-tune whether a physiological output is instigated under particular environmental conditions. The vast majority of hybrid SKs that autophosphorylate require each additional phosphorylation site to promote effective regulation of downstream phenotypes (Goodman et al., 2009, Hsu et al., 2008, Jourlin et al., 1997, Takeda et al., 2001, Tsuzuki et al., 1995, Uhl & Miller, 1996). SypF is an exception to this rule.
Our genetic and biochemical studies demonstrated that SypF controls biofilm formation by functioning directly above both SypG and SypE, confirming its importance in the Syp regulatory cascade. Complicating these results, however, was the irrefutable evidence that another hybrid SK, RscS, also controlled biofilms, an uncommon arrangement for TCS cascades. In other TCS pathways with multiple SKs, such as the Vibrio harveyi luminescence (Lux) and Bacillus subtilis sporulation cascades, these SKs function as separate inputs into downstream regulators (Henke & Bassler, 2004, Jiang et al., 2000). Thus, we initially proposed that SypF and RscS, together, control the activity of the downstream RRs. This hypothesis was supported by the observations that in culture, overproducing either RscS or the SypF variant, SypF*, induced wrinkled colony formation (Darnell et al., 2008, Yip et al., 2006), and that both RscS (Geszvain & Visick, 2008) and SypF* (Fig 2) required sites of autophosphorylation to induce this phenotype. However, although SypF* could function as a hybrid SK in the cell, this activity seemed not to be physiologically relevant. In particular, only the single, non-enzymatic HPt domain of SypF was required to promote host colonization, an in vivo phenotype that does not require the artificial overexpression of regulatory genes. Similarly, we observed that RscS-induced wrinkled colonies required the HPt domain of SypF, but no N-terminal, enzymatic regions of SypF. Combined with our data that an RscS-SypF chimera is sufficient to promote colonization, we conclude that (1) SypF does not function as a SK under biofilm-promoting conditions, (2) SypF* activity is not physiologically relevant, and (3) SypF functions downstream of RscS and thus RscS and SypF do not provide separate inputs into the Syp pathway. We propose a mechanism in which RscS bypasses its own HPt domain and preferentially hijacks the HPt domain of SypF to affect the activity of the downstream RRs, SypE and SypG, to control biofilms (Fig 1B).
Why might SypF*, but not SypF, function as an SK in vivo? We maintain our previous conclusion that, in the cell, SypF* is in a kinase “on” state (Darnell et al., 2008). SKs generally function as homodimers, and histidine kinase activity requires that the ATP hydrolyzing domain (HATPase_c) interact with the HisKA domain, which contains the conserved, phosphorylatable histidine, in cis (Casino et al., 2009, Pena-Sandoval & Georgellis, 2010) or in trans (Ashenberg et al., 2013, Dago et al., 2012, Marina et al., 2005, Pan et al., 1993). This histidine side chain is generally solvent exposed, allowing it to interact with and receive phosphoryl groups from the HATPase_c domain. Our observation that the S247F mutation within wild-type SypF generates the SypF* phenotype confirmed that this mutation is sufficient to alter the enzymatic activity of SypF within the cell. Serine 247 is located three amino acids away from the site of phosphorylation. Perhaps this mutation changes the position of the downstream histidine, placing it in a location to be more readily phosphorylated by the HATPase_c domain. Although our genetic data support this conclusion, it remains to be determined whether SypF* has higher catalytic activity than SypF in the cell.
What is unprecedented about the Syp pathway is that wild-type SypF apparently relies on the enzymatic activity of a different SK as a source of its phosphoryl group in vivo. This result is especially surprising considering the evidence that SypF exhibits autokinase activity in vitro. Similarly, the Eps pathway in Myxococcus xanthus contains a hybrid SK, EpsC, that exhibits SK activity in vitro, but does not require residues involved in autophosphorylation in vivo (Schramm et al., 2012). In vitro evidence suggested that another hybrid SK, EpsA, could phosphorylate the REC domain of EpsC, but whether this mechanism occurs in vivo remains to be determined (Schramm et al., 2012). Together, SypF and EpsC contradict the assumption that an enzymatically-competent SK must function as so in vivo. Furthermore, the fact that SypF instead uses the enzymatic activity of RscS is a unique result. We propose that this may be a mechanism more common than is currently appreciated; there are examples of SKs that do not require all sites of phosphorylation to promote a phenotype [e.g., (Chand et al., 2011, Laskowski & Kazmierczak, 2006)], but it remains to be tested whether they have histidine kinase activity or whether an interacting partner exists to supply phosphates.
If V. fischeri does not require SypF to function as an SK to promote biofilms, then why is full-length sypF maintained in the genome? This question is especially perplexing given the observation that the syp locus in some other species of Vibrio encodes SypF as a single HPt domain (Supplementary Fig S3). One explanation is that, in V. fischeri, sypF is fated toward degeneracy, but the 5′ sequences have not had sufficient time to be negatively selected for and lost. If this is sypF’s fate, then the Syp TCS would end up similar to the Rcs pathway in Escherichia coli, where the hybrid SK, RcsC, donates phosphoryl groups to the HPt domain in a degenerate SK, RcsD (Takeda et al., 2001). Alternatively, conditions found in later stages of colonization or outside of squid colonization could require that SypF utilize its enzymatic domains. V. fischeri is a marine organism found on ocean sediment and in association with a number of aquatic animals besides E. scolopes (Haygood, 1993, Lee & Ruby, 1992, Mandel et al., 2009, Ortigosa et al., 1994, Ramesh et al., 1989, Ruby & Lee, 1998, Ruby & Nealson, 1976, Yetinson & Shilo, 1979). Perhaps in these other contexts SypF functions as a bona fide SK to induce formation of the Syp or a Syp-like biofilm. With this hypothesis in mind, the RscS-SypF interaction brings to light the intriguing possibility that domains within the same signaling network could have discrete roles depending on environmental conditions surrounding the cell. It should be noted that, although the HPt domain of SypF alone and the RscS-SypF chimera allowed for V. fischeri to colonize E. scolopes, these proteins did not promote the same efficiency of colonization as seen with wild-type V. fischeri. This suggests that there may be other, more subtle roles for the N-terminal domains of SypF or the HPt domain of RscS during colonization. For example, many SKs exhibit both kinase and phosphatase activity (Aiba et al., 1989, Casino et al., 2009, Freeman et al., 2000, Huynh et al., 2010, Ninfa & Magasanik, 1986, Yang & Inouye, 1993), so SypF could utilize both of these activities to permit fine-tuning of the Syp phosphorelay. Similarly, perhaps the transmembrane regions within SypF allow for membrane localization, which may be important for efficient signaling in the Syp pathway. The relative importance of these additional domains during colonization awaits exploration.
Continued research into TCS has unveiled an increasing number of TCS architectures with two or more interacting SKs [e.g., (Goodman et al., 2009, He et al., 2013, Kong et al., 2013, Schramm et al., 2012)]; however, the environmental pressures that selected for these interactions remain unknown. Conversely, V. fischeri has given researchers some clues as to how the complex Syp pathway may have evolved. In V. fischeri, there are at least two genetic loci required for in vivo biofilms: the syp locus and rscS. Whereas the syp locus is conserved in V. fischeri, only a subset of V. fischeri strains contains rscS (Mandel et al., 2009). This suggests that the acquisition of rscS eventually granted V. fischeri access to the light organ of E. scolopes. sypF is conserved in V. fischeri, but perhaps for colonization purposes, RscS functionally replaced the enzymatic activity of SypF, and the HPt domain of SypF was positively selected for to provide an additional regulatory checkpoint. If only a small number of environments require the Syp biofilm, then it seems reasonable that this intricate TCS arrangement evolved to prevent inappropriate activation of a complex developmental process.
Flexibility in the arrangement of TCS allows all domains of life to precisely regulate their physiology to manage a vast repertoire of environments. The unique architecture of Syp, for example, has allowed V. fischeri to expand its niche to include the light organ of E. scolopes, thus outcompeting all other bacterial strains found in the local environment. Therefore, Syp demonstrates not only the plasticity of TCS pathways, but also provides a potential model for how a bacterium may adapt to conquer new environments and guarantee proliferation of its progeny.
Experimental Procedures
Bacterial strains and media
The bacterial strains used in this study are listed in Table 1 and were derived from ES114, a wild-type V. fischeri strain isolated from E. scolopes (Boettcher & Ruby, 1990). V. fischeri derivatives were generated using previously described conjugation (Visick & Skoufos, 2001) mutagenesis (Le Roux et al., 2007, Shibata et al., 2012), and transposon (Tn7) chromosomal-insertion (McCann et al., 2003) methods. V. fischeri cells were grown in Luria-Bertani Salt (LBS) media (Graf et al., 1994), Seawater Tryptone (SWT) media (Boettcher & Ruby, 1990), or HEPES Minimal Media (HMM) (Ruby & Nealson, 1977). E. coli strains used for molecular genetics in this study include: ER2508 (NEB), TAM1 λ pir (Active Motif), π3813 (Le Roux et al., 2007), CC118 λ pir (Herrero et al., 1990), and GT115 (Invivogen). E. coli strains were grown in LB (Davis et al., 1980). Solid media contained 1.5% agar. For V. fischeri, antibiotics were added to the following concentrations when necessary: erythromycin (Erm) at 5 μg ml−1, tetracycline (Tet) at 5 μg ml−1 in LBS or 30 μg ml−1 in SWT and HMM, or chloramphenicol (Cm) at 2.5 μg ml−1. The following antibiotics were added to E. coli media where appropriate: Cm at 25 μg ml−1, Tet at 15 μg ml−1, kanamycin (Kan) at 50 μg ml−1, or ampicillin (Ap) at 100 μg ml−1.
Table 1.
Strains and key plasmids used in this study
| Strains | ||
|---|---|---|
| Strain | Relevant genotype | Source or Reference |
| ES114 | Wild-type V. fischeri | (Boettcher & Ruby, 1990) |
| KV3246 | attTn7::PsypA-lacZ | (Morris & Visick, 2013) |
| KV4389 | attTn7::ermR | (Morris et al., 2011) |
| KV5367 | ΔsypF | This study |
| KV6351 | ΔrscS ΔsypF | This study |
| KV6526 | ΔsypF attTn7::sypG*-FLAG | This study |
| KV6527 | attTn7::sypG*-FLAG | This study |
| KV6586 | ΔsypE ΔsypF attTn7::sypG*-FLAG | This study |
| KV6659 | ΔsypF attTn7::sypF-FLAG | This study |
| KV6692 | ΔsypF attTn7::sypFD549A-FLAG | This study |
| KV6896 | ΔsypF attTn7::sypFH250Q-FLAG | This study |
| KV6921 | ΔsypF attTn7::ermR | This study |
| KV7085 | ΔsypF attTn7::sypFH705Q-FLAG | This study |
| KV7230 | attTn7::sypG*-FLAG PsypA-lacZ | This study |
| KV7231 | ΔsypF attTn7::sypG*-FLAG PsypA-lacZ | This study |
| KV7154 | ΔsypF attTn7::sypFH705Q D549A-FLAG | This study |
| KV7155 | ΔsypF attTn7::sypFH705Q D549A H705Q-FLAG | This study |
| KV7226 | ΔsypF attTn7::sypF-HPt-FLAG | This study |
| KV7371 | IG (yeiR-glmS)::PsypA-lacZ 1 | This study |
| KV7372 | ΔsypF IG (yeiR-glmS)::PsypA-lacZ 1 | This study |
| KV7377 | ΔsypF IG (yeiR-glmS)::PsypA-lacZ attTn7::sypF- HPt-FLAG1 | This study |
| KV7386 | ΔsypF IG (yeiR-glmS)::PsypA-lacZ attTn7::sypF- FLAG1 | This study |
| KV7387 | ΔsypF IG (yeiR-glmS)::PsypA-lacZ attTn7::sypFH705Q-FLAG1 | This study |
| KV7410 | IG (yeiR-glmS)::PsypA-lacZ attTn7::ermR 1 | This study |
| KV7412 | ΔsypF IG (yeiR-glmS)::PsypA-lacZ attTn7::ermR 1 | This study |
| KV7413 | ΔsypF IG (yeiR-glmS)::PsypA-lacZ attTn7::sypF- HPtH705Q-FLAG 1 | This study |
| KV7485 | ΔsypF attTn7::sypF-HPtH705Q-FLAG | This study |
| KV7651 | ΔsypF ΔrscS attTn7::rscS-sypF chimera | This study |
| KV7654 | ΔsypF ΔrscS attTn7::rscS | This study |
| KV7656 | ΔsypF ΔrscS attTn7::sypF | This study |
| KV7657 | ΔsypF ΔrscS attTn7::ermR | This study |
| Key Plasmids | ||
|---|---|---|
| Plasmid | Description | Source or Reference |
| pANN61 | pKV69 + sypF*D549A generated using primer 1295 | This study |
| pANN62 | pKV69 + sypF*H705Q generated using primer 1569 | This study |
| pANN69 | pCLD292 + rscS-sypF chimera-FLAG generated using primers 1899, 1900, 1901, 1882 | This study |
| pARM7 | pKV282 + rscS | (Morris et al., 2011) |
| pCLD29 | pKV69 + sypF* | (Darnell et al., 2008) |
| pCLD54 | pKV69 + sypF | (Darnell et al., 2008) |
| pKV69 | Vector; CmR, TetR | (Visick & Skoufos, 2001) |
| pKV282 | Vector, TetR | (Morris et al., 2011) |
IG (yeiR-glmS): intergenic (IG) region between the yeiR and glmS genes directly upstream of the Tn7 site in the chromosome
the original sypF* sequence was removed from pCLD29 using restriction enzymes before the insertion of indicated DNA sequences
Plasmid construction
Plasmids used in this study are indicated in Table 1 and Table S1. Plasmids were generated using either restriction digest-based cloning or Gibson assembly cloning [New England Biolabs (NEB)]. In some cases, DNA sequences of interest were amplified via PCR using the indicated primers and inserted into the pJET1.2 cloning vector. DNA sequences were subcloned into the pKV363 suicide vector used for gene deletions, the pKV69 overexpression plasmid, or the pEVS107 mini-Tn7 delivery vector using standard molecular techniques. Alternatively, sequences were amplified using the indicated primers and then inserted into a mobilization vector using the Gibson Assembly approach (NEB). For site-directed mutagenesis of sypF, sypG, or sypF*, either Gibson Assembly or the Quick-Change Site-Directed Mutagenesis Kit (Stratagene) with the primer(s) indicated in Table S2 was used.
Wrinkled colony assay
V. fischeri cells were grown overnight shaking at 28°C in LBS Tet and then subcultured and grown to an optical density of 600 nm (OD600) of 0.2. 10 μl of the culture were spotted on LBS plates containing Tet to maintain plasmid selection. All spots were grown at room temperature (24°C) and images were captured at the indicated time points using a Zeiss stemi 2000-C dissecting microscope.
β-galactosidase measurements
V. fischeri strains were grown overnight in triplicate at 24°C with shaking in HMM with Tet. Cultures were back-diluted into fresh medium to an OD600 of 0.2 and then grown for 24 hours. 1 ml was removed and β-galactosidase activity was measured as described (Miller, 1972). Protein levels were assessed using previously described methods (Lowry et al., 1951) and the data are reported as β-galactosidase activity mg−1 of protein.
Western blot procedure
Overnight samples of V. fischeri cells were standardized by OD600 and lysed with 2X sample buffer (100mM Tris pH 6.8, 4% SDS, 20% glycerol, 12% β-mercaptoethanol, 0.01% bromophenol-blue). When higher concentrations of cells were needed to assess SypF-FLAG levels expressed in single copy, samples were lysed with B-PER (Thermo Scientific) with 10 mg mL−1 DNase. Lysates were resolved on an SDS-polyacrylamide gel (10%, 29:1 acrylamide: N, N′-methylene-bis-acrylamide, 375 mM Tris pH 8.6, 0.1% SDS), transferred to a PVDF membrane, and subjected to standard western blot procedures using an anti-flag primary antibody (Sigma-Aldrich) and a HRP conjugated secondary antibody (Sigma-Aldrich). Proteins were visualized using SuperSignal West Pico Chemiluminescent Substrate with subsequent exposure to film.
Protein production
Sequences encoding the REC domain of SypG and the cytoplasmic form of SypF were amplified by PCR and cloned into pMAL-c5x using Gibson Assembly to generate N-terminal maltose binding protein (MBP) fusion proteins. Plasmids were transformed into the ER2508 strain (NEB), a BL21 derivative that does not express native MBP. To purify cytoplasmic MBP-SypF (pANN48) and MBP-SypF* (pANN74), 1 liter of Amp-containing LB was inoculated with the appropriate E. coli strain and grown to an OD600 of 0.7 at 37°C. Protein production was induced with 0.1 mM IPTG at 18°C overnight. Cells were harvested by centrifugation (10,000 × g) for 10 min and lysed using B-PER detergent (Thermo Scientific) with 100 μl 20 mg ml−1 lysozyme (Thermo Scientific), 20 μl 10 mg ml−1 DNase (Sigma) and 50 μl 100 μM PMSF (Sigma). Lysates were cleared by centrifugation at 16,000 × g for 20 min. Supernatant was applied to an amylose-resin column (NEB), washed three times with 1X Phosphate Buffered Saline (PBS) (137 mM NaCl, 2.7 mM KCl, 10 mM NaH2CO4, 1.8 mM KH2PO4, pH 7.4), and eluted with 10 mM maltose. An Amicon 30k filter device (Millipore) equilibrated with storage buffer (50 mM Tris pH 8, 50 mM KCl, 50% glycerol) was used to exchange the elution buffer with storage buffer and to concentrate the purified protein. To purify MBP-SypG-REC (pANN49), a similar approach as above was taken, except 500 ml of cells at an OD600 of 0.5 were induced with 0.5 mM IPTG at 24°C overnight. To purify GST-SypE (pARM141) we modified the methods from Morris and Visick (Morris & Visick, 2013) as follows: briefly, pARM141 expressed from the ER2508 strain was used because this improved solubility of GST-SypE. This E. coli strain was grown to an OD600 of 0.5 and then induced with 0.4 mM IPTG overnight. Cells were harvested and lysed with Bugbuster (Novagen), and the supernatants were applied to Glutathione Sepharose 4B columns. Bound proteins were eluted with 10 mM glutathione. GST-SypE was concentrated and the elution buffer was exchanged with storage buffer using an Amicon 30k filter device (Millipore). Purified proteins were assessed by resolving samples on a 10% SDS-polyacrylamide gel with subsequent Coomassie Brilliant Blue R-250 protein staining (Thermo-Scientific) or western immunoblotting procedures as described above using anti-GST or anti-MBP primary antibodies (Sigma).
in vitro assays
Autokinase reaction: 2 μg/μL of purified MBP-SypF or MBP-SypF* were incubated in kinase buffer [50 mM Tris-HCl pH 8, 50 mM KCl, 5 mM MgCl2, and 5 μCi [γ32P]-ATP (3000 Ci mmol−1)] for 30 minutes at 28°C. In reactions without radiolabelled ATP, the same volume of 2 mM cold ATP was added. Samples were stopped with 5X sample buffer (250 mM Tris-HCl pH 6.8, 10% SDS, 20% glycerol, 3% β-mercaptoethanol, 0.01% bromophenol-blue), electrophoresed through a 10% SDS-polyacrylamide gel which was dried for 2 h and then exposed to film for 24–48 h. Phosphotransfer reactions: phospho-MBP-SypF or phospho-MBP-SypF* was obtained as described above. Equimolar concentrations of GST-SypE or MBP-SypG-REC were added and the reactions were incubated for 30 min. As a negative control, GST-SypE or MBP-SypG-REC was incubated in the same buffer conditions for 30 min but in the absence of a kinase. To assess levels of phosphorylated proteins, autoradiographs were generated as described above.
Colonization assay
V. fischeri strains were grown on agar plates overnight and then inoculated and grown to early log phase in liquid SWT media without shaking at 28°C. Aposymbiotic juvenile squid were collected shortly after hatching and placed in artificial sea water (ASW) (Instant Ocean, United Pet Group) that contained V. fischeri strains at a concentration of 1000 cells ml−1. Colonization was allowed to proceed for 18 hours at which point individual E. scolopes were homogenized in 70% ASW. Serial dilutions of the homogenates were plated on SWT to determine the CFU of V. fischeri per squid. Limit of detection is 14 CFUs of V. fischeri per squid. Experiments involving E. scolopes animals were carried out using approaches described in an Animal Component of Research Protocol (ACORP) approved by Loyola University’s Institutional Animal Care and Use Committee (IACUC) (LU #107314, 201297).
Supplementary Material
Acknowledgments
We thank Jonathan Willett and members of the T. Bae lab for their invaluable insights into biochemical approaches used to study TCS regulators, and Stephen Johnston, Addeline Boettcher, Kevin Quirke, and Shikhar Tomur for their assistance in genetic studies. We would also like to acknowledge Alan Wolfe and the Visick lab for their critique of this manuscript. This work was supported by the NIH Grant GM59690 awarded to K.L.V. and a grant from Loyola’s Research Funding Committee LU#205978 awarded to K.L.V.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Aiba H, Mizuno T, Mizushima S. Transfer of phosphoryl group between two regulatory proteins involved in osmoregulatory expression of the ompF and ompC genes in Escherichia coli. J Biol Chem. 1989;264:8563–8567. [PubMed] [Google Scholar]
- Ashenberg O, Keating AE, Laub MT. Helix bundle loops determine whether histidine kinases autophosphorylate in cis or in trans. J Mol Biol. 2013;425:1198–1209. doi: 10.1016/j.jmb.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher KJ, Ruby EG. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J Bacteriol. 1990;172:3701–3706. doi: 10.1128/jb.172.7.3701-3706.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casino P, Rubio V, Marina A. Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell. 2009;139:325–336. doi: 10.1016/j.cell.2009.08.032. [DOI] [PubMed] [Google Scholar]
- Chand NS, Lee JS, Clatworthy AE, Golas AJ, Smith RS, Hung DT. The sensor kinase KinB regulates virulence in acute Pseudomonas aeruginosa infection. J Bacteriol. 2011;193:2989–2999. doi: 10.1128/JB.01546-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dago AE, Schug A, Procaccini A, Hoch JA, Weigt M, Szurmant H. Structural basis of histidine kinase autophosphorylation deduced by integrating genomics, molecular dynamics, and mutagenesis. Proc Natl Acad Sci U S A. 2012;109:E1733–1742. doi: 10.1073/pnas.1201301109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell CL, Hussa EA, Visick KL. The putative hybrid sensor kinase SypF coordinates biofilm formation in Vibrio fischeri by acting upstream of two response regulators, SypG and VpsR. J Bacteriol. 2008;190:4941–4950. doi: 10.1128/JB.00197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RW, Botstein D, Roth JR Cold Spring Harbor Laboratory of Quantitative Biology. Advanced bacterial genetics: a manual for genetic engineering. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1980. p. x.p. 251. [Google Scholar]
- Ferrieres L, Clarke DJ. The RcsC sensor kinase is required for normal biofilm formation in Escherichia coli K-12 and controls the expression of a regulon in response to growth on a solid surface. Mol Microbiol. 2003;50:1665–1682. doi: 10.1046/j.1365-2958.2003.03815.x. [DOI] [PubMed] [Google Scholar]
- Freeman JA, Bassler BL. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol Microbiol. 1999;31:665–677. doi: 10.1046/j.1365-2958.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- Freeman JA, Lilley BN, Bassler BL. A genetic analysis of the functions of LuxN: a two-component hybrid sensor kinase that regulates quorum sensing in Vibrio harveyi. Mol Microbiol. 2000;35:139–149. doi: 10.1046/j.1365-2958.2000.01684.x. [DOI] [PubMed] [Google Scholar]
- Galperin MY. A census of membrane-bound and intracellular signal transduction proteins in bacteria: bacterial IQ, extroverts and introverts. BMC Microbiol. 2005;5:35. doi: 10.1186/1471-2180-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperin MY. Diversity of structure and function of response regulator output domains. Curr Opin Microbiol. 2010;13:150–159. doi: 10.1016/j.mib.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geszvain K, Visick KL. The hybrid sensor kinase RscS integrates positive and negative signals to modulate biofilm formation in Vibrio fischeri. J Bacteriol. 2008;190:4437–4446. doi: 10.1128/JB.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooderham WJ, Hancock RE. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa. FEMS Microbiol Rev. 2009;33:279–294. doi: 10.1111/j.1574-6976.2008.00135.x. [DOI] [PubMed] [Google Scholar]
- Goodman AL, Merighi M, Hyodo M, Ventre I, Filloux A, Lory S. Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes Dev. 2009;23:249–259. doi: 10.1101/gad.1739009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J, Dunlap PV, Ruby EG. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J Bacteriol. 1994;176:6986–6991. doi: 10.1128/jb.176.22.6986-6991.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon MA, Lazazzera BA. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol Microbiol. 2001;42:1199–1209. doi: 10.1046/j.1365-2958.2001.02709.x. [DOI] [PubMed] [Google Scholar]
- Haygood MG. Light organ symbioses in fishes. Crit Rev Microbiol. 1993;19:191–216. doi: 10.3109/10408419309113529. [DOI] [PubMed] [Google Scholar]
- He K, Marden JN, Quardokus EM, Bauer CE. Phosphate flow between hybrid histidine kinases CheA(3) and CheS(3) controls Rhodospirillum centenum cyst formation. PLoS Genet. 2013;9:e1004002. doi: 10.1371/journal.pgen.1004002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindl JE, Wang Y, Heckel BC, Mohari B, Feirer N, Fuqua C. Mechanisms and regulation of surface interactions and biofilm formation in Agrobacterium. Front Plant Sci. 2014;5:176. doi: 10.3389/fpls.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke JM, Bassler BL. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J Bacteriol. 2004;186:6902–6914. doi: 10.1128/JB.186.20.6902-6914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero M, de Lorenzo V, Timmis KN. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JL, Chen HC, Peng HL, Chang HY. Characterization of the histidine-containing phosphotransfer protein B-mediated multistep phosphorelay system in Pseudomonas aeruginosa PAO1. J Biol Chem. 2008;283:9933–9944. doi: 10.1074/jbc.M708836200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TP, Lu KM, Chen YH. A novel two-component response regulator links rpf with biofilm formation and virulence of Xanthomonas axonopodis pv. citri. PLoS One. 2013;8:e62824. doi: 10.1371/journal.pone.0062824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussa EA, Darnell CL, Visick KL. RscS functions upstream of SypG to control the syp locus and biofilm formation in Vibrio fischeri. J Bacteriol. 2008;190:4576–4583. doi: 10.1128/JB.00130-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh TN, Noriega CE, Stewart V. Conserved mechanism for sensor phosphatase control of two-component signaling revealed in the nitrate sensor NarX. Proc Natl Acad Sci U S A. 2010;107:21140–21145. doi: 10.1073/pnas.1013081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie Y, Mattoo S, Yuk MH. The Bvg virulence control system regulates biofilm formation in Bordetella bronchiseptica. J Bacteriol. 2004;186:5692–5698. doi: 10.1128/JB.186.17.5692-5698.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Shao W, Perego M, Hoch JA. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol Microbiol. 2000;38:535–542. doi: 10.1046/j.1365-2958.2000.02148.x. [DOI] [PubMed] [Google Scholar]
- Joo HS, Otto M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem Biol. 2012;19:1503–1513. doi: 10.1016/j.chembiol.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourlin C, Ansaldi M, Mejean V. Transphosphorylation of the TorR response regulator requires the three phosphorylation sites of the TorS unorthodox sensor in Escherichia coli. J Mol Biol. 1997;267:770–777. doi: 10.1006/jmbi.1997.0919. [DOI] [PubMed] [Google Scholar]
- Jung K, Fried L, Behr S, Heermann R. Histidine kinases and response regulators in networks. Curr Opin Microbiol. 2012;15:118–124. doi: 10.1016/j.mib.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Kim D, Forst S. Genomic analysis of the histidine kinase family in bacteria and archaea. Microbiology. 2001;147:1197–1212. doi: 10.1099/00221287-147-5-1197. [DOI] [PubMed] [Google Scholar]
- Kofoid EC, Parkinson JS. Transmitter and receiver modules in bacterial signaling proteins. Proc Natl Acad Sci U S A. 1988;85:4981–4985. doi: 10.1073/pnas.85.14.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Chen L, Zhao J, Shen T, Surette MG, Shen L, Duan K. Hybrid sensor kinase PA1611 in Pseudomonas aeruginosa regulates transitions between acute and chronic infection through direct interaction with RetS. Mol Microbiol. 2013;88:784–797. doi: 10.1111/mmi.12223. [DOI] [PubMed] [Google Scholar]
- Laskowski MA, Kazmierczak BI. Mutational analysis of RetS, an unusual sensor kinase-response regulator hybrid required for Pseudomonas aeruginosa virulence. Infect Immun. 2006;74:4462–4473. doi: 10.1128/IAI.00575-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux F, Binesse J, Saulnier D, Mazel D. Construction of a Vibrio splendidus mutant lacking the metalloprotease gene vsm by use of a novel counterselectable suicide vector. Appl Environ Microbiol. 2007;73:777–784. doi: 10.1128/AEM.02147-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Ruby EG. Detection of the Light Organ Symbiont, Vibrio fischeri, in Hawaiian Seawater by Using lux Gene Probes. Appl Environ Microbiol. 1992;58:942–947. doi: 10.1128/aem.58.3.942-947.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Lau PC, Tang N, Svensater G, Ellen RP, Cvitkovitch DG. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J Bacteriol. 2002;184:6333–6342. doi: 10.1128/JB.184.22.6333-6342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mandel MJ, Wollenberg MS, Stabb EV, Visick KL, Ruby EG. A single regulatory gene is sufficient to alter bacterial host range. Nature. 2009;458:215–218. doi: 10.1038/nature07660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina A, Waldburger CD, Hendrickson WA. Structure of the entire cytoplasmic portion of a sensor histidine-kinase protein. EMBO J. 2005;24:4247–4259. doi: 10.1038/sj.emboj.7600886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J, Stabb EV, Millikan DS, Ruby EG. Population dynamics of Vibrio fischeri during infection of Euprymna scolopes. Appl Environ Microbiol. 2003;69:5928–5934. doi: 10.1128/AEM.69.10.5928-5934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall-Ngai M. Divining the essence of symbiosis: insights from the squid-vibrio model. PLoS Biol. 2014;12:e1001783. doi: 10.1371/journal.pbio.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoon AL, Kolodkin-Gal I, Rubinstein SM, Kolter R, Losick R. Spatial regulation of histidine kinases governing biofilm formation in Bacillus subtilis. J Bacteriol. 2011;193:679–685. doi: 10.1128/JB.01186-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in molecular genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1972. p. xvi.p. 466. [Google Scholar]
- Morris AR, Darnell CL, Visick KL. Inactivation of a novel response regulator is necessary for biofilm formation and host colonization by Vibrio fischeri. Mol Microbiol. 2011;82:114–130. doi: 10.1111/j.1365-2958.2011.07800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AR, Visick KL. The response regulator SypE controls biofilm formation and colonization through phosphorylation of the syp-encoded regulator SypA in Vibrio fischeri. Mol Microbiol. 2013;87:509–525. doi: 10.1111/mmi.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy H, Lewenza S. Magnesium limitation is an environmental trigger of the Pseudomonas aeruginosa biofilm lifestyle. PLoS One. 2011;6:e23307. doi: 10.1371/journal.pone.0023307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninfa AJ, Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci U S A. 1986;83:5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon BT, Ronson CW, Ausubel FM. Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc Natl Acad Sci U S A. 1986;83:7850–7854. doi: 10.1073/pnas.83.20.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm SV, Stabb EV, Ruby EG, McFall-Ngai MJ. Establishment of an animal-bacterial association: recruiting symbiotic vibrios from the environment. Proc Natl Acad Sci U S A. 2000;97:10231–10235. doi: 10.1073/pnas.97.18.10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortigosa M, Garay E, Pujalte MJ. Numerical taxonomy of vibrionaceae isolated from oysters and seawater along an annual cycle. Systematic and Applied Microbiology. 1994;17:216–225. [Google Scholar]
- Pan SQ, Charles T, Jin S, Wu ZL, Nester EW. Preformed dimeric state of the sensor protein VirA is involved in plant--Agrobacterium signal transduction. Proc Natl Acad Sci U S A. 1993;90:9939–9943. doi: 10.1073/pnas.90.21.9939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Sandoval GR, Georgellis D. The ArcB sensor kinase of Escherichia coli autophosphorylates by an intramolecular reaction. J Bacteriol. 2010;192:1735–1739. doi: 10.1128/JB.01401-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival SL, Suleman L. Biofilms and Helicobacter pylori: Dissemination and persistence within the environment and host. World J Gastrointest Pathophysiol. 2014;5:122–132. doi: 10.4291/wjgp.v5.i3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova OE, Sauer K. A novel signaling network essential for regulating Pseudomonas aeruginosa biofilm development. PLoS Pathog. 2009;5:e1000668. doi: 10.1371/journal.ppat.1000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh A, Loganathan BG, Venugopalan VK. Season distribution of luminous bacteria in the sediments of a tropical estuary. J Gen Appl Microbiol. 1989;35:363–368. [Google Scholar]
- Ramey BE, Koutsoudis M, von Bodman SB, Fuqua C. Biofilm formation in plant-microbe associations. Curr Opin Microbiol. 2004;7:602–609. doi: 10.1016/j.mib.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Ray VA, Eddy JL, Hussa EA, Misale M, Visick KL. The syp enhancer sequence plays a key role in transcriptional activation by the σ54-dependent response regulator SypG and in biofilm formation and host colonization by Vibrio fischeri. J Bacteriol. 2013;195:5402–5412. doi: 10.1128/JB.00689-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby EG, Lee KH. The Vibrio fischeri-Euprymna scolopes Light Organ Association: Current Ecological Paradigms. Appl Environ Microbiol. 1998;64:805–812. doi: 10.1128/aem.64.3.805-812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby EG, Nealson KH. Symbiotic association of Photobacterium fischeri with the marine luminous fish Monocentris japonica; a model of symbiosis based on bacterial studies. Biol Bull. 1976;151:574–586. doi: 10.2307/1540507. [DOI] [PubMed] [Google Scholar]
- Ruby EG, Nealson KH. A luminous bacterium that emits yellow light. Science. 1977;196:432–434. doi: 10.1126/science.850787. [DOI] [PubMed] [Google Scholar]
- Sanders DA, Gillece-Castro BL, Burlingame AL, Koshland DE., Jr Phosphorylation site of NtrC, a protein phosphatase whose covalent intermediate activates transcription. J Bacteriol. 1992;174:5117–5122. doi: 10.1128/jb.174.15.5117-5122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DA, Gillece-Castro BL, Stock AM, Burlingame AL, Koshland DE., Jr Identification of the site of phosphorylation of the chemotaxis response regulator protein, CheY. J Biol Chem. 1989;264:21770–21778. [PubMed] [Google Scholar]
- Schramm A, Lee B, Higgs PI. Intra- and interprotein phosphorylation between two-hybrid histidine kinases controls Myxococcus xanthus developmental progression. J Biol Chem. 2012;287:25060–25072. doi: 10.1074/jbc.M112.387241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Yip ES, Quirke KP, Ondrey JM, Visick KL. Roles of the structural symbiosis polysaccharide (syp) genes in host colonization, biofilm formation, and polysaccharide biosynthesis in Vibrio fischeri. J Bacteriol. 2012;194:6736–6747. doi: 10.1128/JB.00707-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabb EV, Visick KL. Vibrio fischeri: a bioluminescent light-organ symbiont of the bobtail squid Euprymna scolopes. In: Rosenberg E, editor. The prokaryotes. New York: Springer; 2013. pp. 497–532. [Google Scholar]
- Stipp RN, Boisvert H, Smith DJ, Hofling JF, Duncan MJ, Mattos-Graner RO. CovR and VicRK regulate cell surface biogenesis genes required for biofilm formation in Streptococcus mutans. PLoS One. 2013;8:e58271. doi: 10.1371/journal.pone.0058271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock A, Chen T, Welsh D, Stock J. CheA protein, a central regulator of bacterial chemotaxis, belongs to a family of proteins that control gene expression in response to changing environmental conditions. Proc Natl Acad Sci U S A. 1988;85:1403–1407. doi: 10.1073/pnas.85.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- Su MS, Ganzle MG. Novel two-component regulatory systems play a role in biofilm formation of Lactobacillus reuteri rodent isolate 100–23. Microbiology. 2014;160:795–806. doi: 10.1099/mic.0.071399-0. [DOI] [PubMed] [Google Scholar]
- Takeda S, Fujisawa Y, Matsubara M, Aiba H, Mizuno T. A novel feature of the multistep phosphorelay in Escherichia coli: a revised model of the RcsC --> YojN --> RcsB signalling pathway implicated in capsular synthesis and swarming behaviour. Mol Microbiol. 2001;40:440–450. doi: 10.1046/j.1365-2958.2001.02393.x. [DOI] [PubMed] [Google Scholar]
- Tsuzuki M, Ishige K, Mizuno T. Phosphotransfer circuitry of the putative multi-signal transducer, ArcB, of Escherichia coli: in vitro studies with mutants. Mol Microbiol. 1995;18:953–962. doi: 10.1111/j.1365-2958.1995.18050953.x. [DOI] [PubMed] [Google Scholar]
- Uhl MA, Miller JF. Integration of multiple domains in a two-component sensor protein: the Bordetella pertussis BvgAS phosphorelay. EMBO J. 1996;15:1028–1036. [PMC free article] [PubMed] [Google Scholar]
- Ventre I, Goodman AL, Vallet-Gely I, Vasseur P, Soscia C, Molin S, Bleves S, Lazdunski A, Lory S, Filloux A. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc Natl Acad Sci U S A. 2006;103:171–176. doi: 10.1073/pnas.0507407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visick KL. An intricate network of regulators controls biofilm formation and colonization by Vibrio fischeri. Mol Microbiol. 2009;74:782–789. doi: 10.1111/j.1365-2958.2009.06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visick KL, Skoufos LM. Two-component sensor required for normal symbiotic colonization of Euprymna scolopes by Vibrio fischeri. J Bacteriol. 2001;183:835–842. doi: 10.1128/JB.183.3.835-842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AH, Stock AM. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem Sci. 2001;26:369–376. doi: 10.1016/s0968-0004(01)01852-7. [DOI] [PubMed] [Google Scholar]
- Wuichet K, Cantwell BJ, Zhulin IB. Evolution and phyletic distribution of two-component signal transduction systems. Curr Opin Microbiol. 2010;13:219–225. doi: 10.1016/j.mib.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Inouye M. Requirement of both kinase and phosphatase activities of an Escherichia coli receptor (Taz1) for ligand-dependent signal transduction. J Mol Biol. 1993;231:335–342. doi: 10.1006/jmbi.1993.1286. [DOI] [PubMed] [Google Scholar]
- Yetinson T, Shilo M. Seasonal and geographic distribution of luminous bacteria in the eastern Mediterranean Sea and the Gulf of Elat. Appl Environ Microbiol. 1979;37:1230–1238. doi: 10.1128/aem.37.6.1230-1238.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Visick KL. Vibrio biofilms: so much the same yet so different. Trends Microbiol. 2009;17:109–118. doi: 10.1016/j.tim.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip ES, Geszvain K, DeLoney-Marino CR, Visick KL. The symbiosis regulator rscS controls the syp gene locus, biofilm formation and symbiotic aggregation by Vibrio fischeri. Mol Microbiol. 2006;62:1586–1600. doi: 10.1111/j.1365-2958.2006.05475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip ES, Grublesky BT, Hussa EA, Visick KL. A novel, conserved cluster of genes promotes symbiotic colonization and sigma-dependent biofilm formation by Vibrio fischeri. Mol Microbiol. 2005;57:1485–1498. doi: 10.1111/j.1365-2958.2005.04784.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shi L. Distribution and evolution of multiple-step phosphorelay in prokaryotes: lateral domain recruitment involved in the formation of hybrid-type histidine kinases. Microbiology. 2005;151:2159–2173. doi: 10.1099/mic.0.27987-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lei Y, Khammanivong A, Herzberg MC. Identification of a novel two-component system in Streptococcus gordonii V288 involved in biofilm formation. Infect Immun. 2004;72:3489–3494. doi: 10.1128/IAI.72.6.3489-3494.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.