Abstract
Background
Bile acid sequestrants have been used for many years to treat hypercholesterolemia by increasing hepatic conversion of cholesterol to bile acids, thereby inducing hepatic LDL receptor expression and clearance of apoB-containing particles. In order to further understand the underlying molecular mechanisms linking gut-liver signaling and cholesterol homeostasis, mouse models defective in ileal apical membrane bile acid transport (Asbt null) and ileal basolateral membrane bile acid transport (Ostα null) were studied under basal and hypercholesterolemic conditions.
Key Messages
Hepatic conversion of cholesterol to bile acids is the major pathway for cholesterol catabolism and a major mechanism for cholesterol elimination. Blocking ileal apical membrane bile acid transport (Asbt null mice) increases fecal bile acid excretion, hepatic Cyp7a1 expression and the relative proportion of taurocholate in the bile acid pool, but decreases ileal FGF15 expression, bile acid pool size, and hepatic cholesterol content. In contrast, blocking ileal basolateral membrane bile acid transport (Ostα null mice) increases ileal FGF15 expression, reduces hepatic Cyp7a1 expression, and increases the proportion of tauro-β-muricholic acid in the bile acid pool. In the hypercholesterolemic apoE null background, plasma cholesterol levels and measurements of atherosclerosis were reduced in Asbt/apoE null mice but not in Ostα/apoE null mice.
Conclusions
Blocking intestinal absorption of bile acids at the apical versus basolateral membrane differentially affects bile acid and cholesterol metabolism, including the development of hypercholesterolemia-associated atherosclerosis. The molecular mechanism likely involves altered regulation of ileal FGF15 expression.
Keywords: Enterohepatic circulation, Intestine, liver, cholesterol, atherosclerosis
Introduction
Bile acids are synthesized from cholesterol via 2 major pathways, the “classical” neutral pathway (Cholesterol 7α-hydroxylase, Cyp7a1, pathway) that favors cholic acid biosynthesis, and an “alternative” acidic pathway (Sterol 27-hydroxylase pathway) that favors biosynthesis of chenodeoxycholic acid in humans and biosynthesis of muricholic acid in mice. After their synthesis, bile acids are secreted and stored in the gall bladder [1]. Following a lipid-rich meal, the gall bladder empties its contents into the small intestine, where bile acids facilitate the digestion and absorption of dietary fat and cholesterol [2]. In the distal small intestine, bile acids are almost quantitatively reabsorbed and carried in the portal venous circulation back to the liver, where they are taken up, transported across the hepatocyte, and resecreted into bile [1]. The major transporters that function to maintain the EHC of bile acids have been identified [1]. In ileum, the apical sodium-dependent bile acid transporter (Asbt or ibat; Slc10a2) mediates uptake of bile acids from the gut lumen across the apical brush border membrane of the enterocyte [3], whereas the heteromeric transporter Ostα-Ostβ (Ost = Organic solute transporter) mediates intestinal basolateral bile acid export [4]. Ostα-Ostβ is comprised of two subunits, a 352 amino acid polytopic membrane protein (Ostα, Slc51a), and a 154 amino acid type I membrane protein (Ostβ, Slc51b) [5].
Intestinal Bile Acid Transport and Cholesterol Metabolism
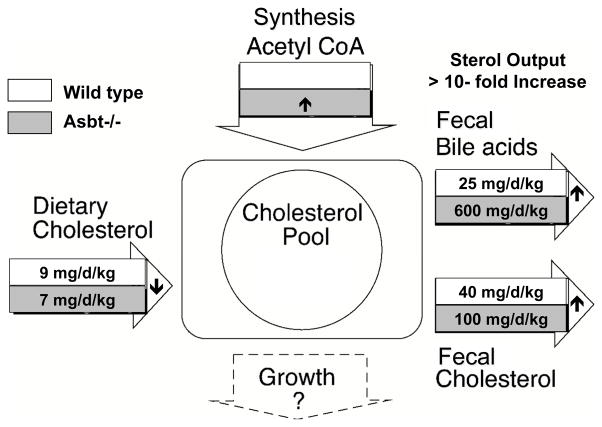
In order to maintain the bile acid pool size under normal physiological conditions [6], hepatic conversion of cholesterol to bile acids balances the fecal excretion. This process is the major route for cholesterol catabolism and accounts for almost half of the cholesterol eliminated from the body each day [7]. Disruption of the enterohepatic circulation (EHC) of bile acids stimulates their de novo synthesis from cholesterol. The hepatic demand for cholesterol is met by increasing hepatic cholesterol synthesis and plasma clearance of lipoproteins such as LDL. Indeed, this mechanism is thought to be responsible for the lower plasma cholesterol levels and reduced cardiovascular disease associated with partial ileal bypass surgery in the Program on the Surgical Control of Hyperlipidemias (POSCH) study [8] or with the use of bile acid sequestrants in the Lipid Research Clinics Coronary Primary Prevention Trial (LRC-CPPT) [9]. The POSCH study is notable for being one of the first lipid-lowering clinical trials to show a statistically significant decrease in cardiovascular disease mortality, underscoring the beneficial effect of aggressively lowering plasma cholesterol levels [10]. Similar to treatment with bile acid sequestrants or ileal bypass, patients with ASBT mutations malabsorb bile acids and maintain decreased plasma LDL cholesterol levels [11]. In Asbt null mice, intestinal return of bile acids to the liver is significantly impaired, and hepatic Cyp7a1 mRNA expression and cholesterol conversion to bile acids is significantly increased [12,13]. Careful analysis of the cholesterol balance in wild type versus Asbt null mice revealed that cholesterol excretion as neutral and acidic sterols is increased almost 10-fold [12]. Cholesterol turnover in Asbt null mice is shown schematically in Fig. 1. The increase in cholesterol excretion is due to several mechanisms including increased fecal neutral sterol excretion secondary to decreased intestinal cholesterol absorption or possibly increased direct intestinal cholesterol excretion, and increased fecal bile acid excretion.
Fig. 1. Cholesterol Balance in Wild Type and Asbt null Mice.
Schematic depicting sterol turnover in three-month old male wild type and Asbt null mice (129S6/SvEv background). The dietary cholesterol input was calculated by measuring daily food consumption and adjusting for the percent cholesterol absorption, as measured using the fecal dual isotope ratio method. Aliquots of quantitative fecal collections were used to measure bile acids and neutral sterols by colorimetric assay and gas-liquid chromatography, respectively. Cholesterol synthesis from acetyl CoA was not measured directly, but hepatic expression of HMG CoA reductase mRNA was increased in the Asbt null mice versus wild type mice. The whole body cholesterol pool (cholesterol content) was not measured, but is predicted to be unchanged and approximately 2200 mg per kg body weight [40]. Growth rates for the three-month old wild type and Asbt null mice appeared to be comparable. The small arrows indicate the direction of change in Asbt null versus wild type mice. The balance is calculated using the results from reference [12] and reproduced with permission.
Gut-Liver Signaling, and Metabolism of Bile Acids and Cholesterol
Although generally assumed that bile acid sequestrants act by reducing delivery of bile acids to the liver [14], recent data suggests that bile acid sequestrant effects on gut-liver signaling may also be important. The regulation of hepatic Cyp7a1 expression and bile acid synthesis is complex [6]. Whereas a role for hepatic FXR in regulating Cyp7a1 expression has been appreciated for more than a decade [15,16], intestinal FXR’s contribution was recognized only more recently [17,18]. In that FXR-FGF15 gut-liver signaling pathway, bile acids are taken up by ileal enterocytes where they activate FXR to induce expression of fibroblast growth factor (FGF) 15 (the mouse ortholog of human FGF19) [17]. FGF15 is then released into the portal circulation, and carried to the liver where it binds its receptor, the FGFR4/β-klotho complex on the surface of hepatocytes. This activates a signaling cascade that down-regulates expression of Cyp7a1 and other genes critical for hepatic bile acid biosynthesis [19,20].
In Asbt null mice, the contributions of altered hepatic versus altered ileal bile acid signaling could not be discerned. However, the Ostα null mouse, generated previously to test the hypothesis that Ostα-Ostβ is the major intestinal basolateral membrane bile acid transporter, provided such an opportunity. Both Asbt and Ostα null mice exhibit impaired ileal bile acid absorption, decreased return of bile acids to the liver and a decreased bile acid pool size [21,22]. However hepatic Cyp7a1 expression and bile acid synthesis is paradoxically suppressed in Ostα null mice, secondary to elevated ileal FGF15 production [23]. These results are significant with regard to the mechanism of action of bile acid sequestrants and also the potential side effect of dietary constituents, supplements, or drugs. Blocking intestinal absorption of bile acids at the enterocyte apical brush border membrane (e.g., bile acid sequestrants, dietary fiber, Asbt inhibitors) suppresses ileal FGF15 expression, induces hepatic bile acid synthesis, and reduces plasma cholesterol levels. Conversely, blocking basolateral membrane export may induce ileal FGF15 expression, inhibit hepatic bile acid synthesis, and potentially raise plasma cholesterol levels. Ostα-Ostβ’s broad substrate specificity raises the concern that dietary constituents, dietary supplements, or drugs could act as Ostα-Ostβ inhibitors to slow bile acid export, activate FXR to induce intestinal FGF15/19 expression, and raise plasma cholesterol levels. Precedence already exists for part of this pathway. Cafestol, a component in unfiltered coffee that has been described as one of the most potent cholesterol-elevating compounds known in the human diet, is believed to operate by directly activating intestinal FXR to induce FGF19 expression and reduce hepatic Cyp7a1 expression [24]. Increased plasma LDL cholesterol levels have also been observed in a clinical trial using the synthetic FXR agonist, obeticholic acid [25].
Intestinal Bile Acid Transport and Atherosclerosis
The differences in sterol metabolism observed with blocking ileal apical membrane versus basolateral membrane bile acid transport may translate to altered susceptibility to development of hypercholesterolemia and atherosclerosis. Although wild type mice are relatively resistant to atherosclerosis, the genetically modified LDLr null and apoE null mice reproducibly develop hypercholesterolemia and atherosclerotic lesions, and are commonly used as experimental models to study the underlying mechanisms [26,27]. Since bile acid sequestrants have been used for many years to treat hypercholesterolemia [28], it is surprising that there are relatively few published studies using LDLr or apoE null mice to examine the molecular mechanisms underlying their effects on the development of hypercholesterolemia and atherosclerosis.
Effect of Blocking Ileal Apical Brush Border Membrane Bile Acid Transport on the Development of Hypercholesterolemia and Atherosclerosis
The effect of inactivation of the Asbt on development of hypercholesterolemia and atherosclerosis in female LDLr and apoE null mice maintained for 16 weeks on an atherogenic diet has been reported recently [29]. In that study, introducing the Asbt null allele into the LDLr null or apoE null backgrounds resulted in significant reductions in total plasma cholesterol, apoB-containing lipoprotein (VLDL plus LDL) cholesterol, and the plasma cholesterol exposure (area under the curve) over the 16-week dietary challenge. In agreement with reductions in the apoB-containing lipoprotein fraction (VLDL and LDL), it was not surprising to find that aortic total cholesterol and cholesteryl ester content, markers of atherosclerosis [30], were also significantly reduced in LDLr and apoE null mice lacking the Asbt. The reduction in plasma cholesterol levels in the Asbt/LDLr and Asbt/apoE null mice appears to be secondary to the increased hepatic cholesterol demand for bile acid synthesis. Numerous other mouse studies have demonstrated a central role for hepatic Cyp7a1 and bile acid synthesis in cholesterol homeostasis, including the findings that the apoB-containing lipoprotein cholesterol and atherosclerosis are reduced in Cyp7a1 transgenic mice [31] and in models that induce hepatic Cyp7a1 expression, such as the Retnla transgenic mice [32]. The findings for the Asbt/LDLr and Asbt/apoE null mice also agree with previous reports of LDLr null mice or apoE null mice treated with a bile acid sequestrant [33,34], and apoE null, LDLr/apoE null, and SR-BI/apoE null mice treated with small molecule inhibitors of the Asbt [35–37]. Notably, the finding that blocking intestinal apical membrane bile acid absorption reduces plasma cholesterol levels even in the absence of LDL receptors and apoE [35] suggests that the atheroprotective effects are related to induction of a negative cholesterol balance and operates via mechanisms in addition to classical hepatic lipoprotein clearance [33]. The question remains to be answered whether this involves non-biliary mechanisms for cholesterol elimination such as trans-intestinal cholesterol excretion [38,39]. The recent study of the Asbt/apoE null mice also provided insight to the role of gut-liver signaling in this process. In agreement with the proposed role of the FXR-FGF15 pathway as a negative regulator of hepatic bile acid synthesis, there was a strong inverse correlation between the levels of mRNA expression for ileal FGF15 and hepatic Cyp7a1 in these models. However even more intriguing, there was a strong direct correlation between ileal FGF15 mRNA expression and total plasma cholesterol levels, and between ileal FGF15 mRNA expression and aortic cholesterol content, a measure of atherosclerosis [29]. The question of whether a similar relationship exists between plasma levels of FGF19 (human ortholog of mouse FGF15) and markers of atherosclerosis exists in humans has not yet been examined.
Effect of Blocking Ileal Basolateral Membrane Bile Acid Transport on the Development of Hypercholesterolemia and Atherosclerosis
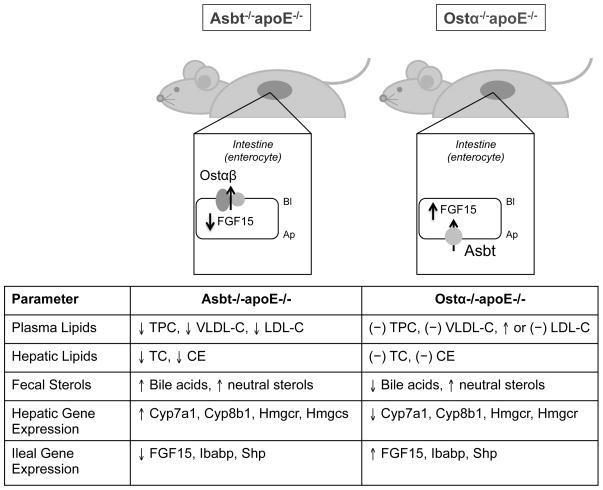
It is generally assumed that different interventions that block intestinal return of bile acids to the liver should have similar effects on cholesterol homeostasis [7]. However the interventions examined to date, such as ileal bypass, bile acid sequestrants, and Asbt inhibitors, only blocked enterohepatic cycling of bile acids at the intestinal apical brush border membrane. As such, it was not possible to isolate individual contributions of ileal enterocyte and hepatocyte signaling to the phenotype. To further understand these underlying mechanisms, the effects of Ostα deficiency were studied in the apoE null mice [29]. In contrast to the findings in the Asbt/apoE null mice, blocking intestinal bile acid absorption by inactivating Ostα failed had no effect on hepatic cholesterol content, and the expression of genes important for cholesterol and bile acid synthesis, such as HMG-CoA reductase, HMG-CoA synthase, Cyp7a1 and Cyp8b1, were unaffected or reduced in Ostα/apoE null mice. Finally in Ostα/apoE null mice, ileal expression of FGF15 also tended to be higher or similar to the levels in apoE null mice, and significantly higher than Asbt/apoE null mice. In aggregate, this evidence supports a model (Figure 2) where repression of ileal FGF15 expression and induction of hepatic bile acid synthesis is required for the atheroprotective effects associated with the interruption of the enterohepatic circulation of bile acids.
Fig. 2. Phenotypic Differences Between Asbt/apoE and Ostα/apoE null mice.
The arrows indicated the direction of change in Asbt/apoE null mice or Ostα/apoE null mice versus the matched apoE null mice. Asbt/apoE null mice exhibit decreased plasma total cholesterol (TPC), VLDL cholesterol (VLDL-C), and LDL cholesterol (LDL-C), and a decreased hepatic total cholesterol (TC) and cholesteryl ester (CE). The increased hepatic demand for cholesterol was secondary to a significant increase in hepatic Cyp7a1 expression, which was inversely correlated with significantly reduced levels of ileal FGF15 expression. Plasma and hepatic cholesterol levels were not significantly changed in Ostα/apoE null mice versus apoE null mice. In contrast to a block in intestinal bile acid absorption at the apical brush border membrane, disrupting the enterohepatic circulation of bile acids at the basolateral membrane failed to increase hepatic demand for cholesterol by inducing hepatic Cyp7a1 expression and bile acid synthesis.
Acknowledgments
I thank Drs. Astrid Kosters and Anuradha Rao for critical reading of the manuscript. This project was supported by NIH DK047987 and an American Heart Association Mid-Atlantic Affiliate Grant-in-aid.
Footnotes
Conflicts of Interest
Dr. Dawson has served as a consultant for Lumena Pharmaceuticals in the past.
References
- 1.Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westergaard H, Dietschy JM. The mechanism whereby bile acid micelles increase the rate of fatty acid and cholesterol uptake into the intestinal mucosal cell. J Clin Invest. 1976;58:97–108. doi: 10.1172/JCI108465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong MH, Oelkers P, Craddock AL, Dawson PA. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J Biol Chem. 1994;269:1340–1347. [PubMed] [Google Scholar]
- 4.Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter alpha-beta, ostalpha-ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, Seward DJ, Li L, Boyer JL, Ballatori N. Expression cloning of two genes that together mediate organic solute and steroid transport in the liver of a marine vertebrate. Proc Natl Acad Sci U S A. 2001;98:9431–9436. doi: 10.1073/pnas.161099898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang JY. Bile acids: Regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 8.Buchwald H, Varco RL, Matts JP, Long JM, Fitch LL, Campbell GS, Pearce MB, Yellin AE, Edmiston WA, Smink RD, Jr, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. Report of the program on the surgical control of the hyperlipidemias (posch) N Engl J Med. 1990;323:946–955. doi: 10.1056/NEJM199010043231404. [DOI] [PubMed] [Google Scholar]
- 9.The lipid research clinics coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 10.Buchwald H, Rudser KD, Williams SE, Michalek VN, Vagasky J, Connett JE. Overall mortality, incremental life expectancy, and cause of death at 25 years in the program on the surgical control of the hyperlipidemias. Ann Surg. 2010;251:1034–1040. doi: 10.1097/SLA.0b013e3181deb4d0. [DOI] [PubMed] [Google Scholar]
- 11.Oelkers P, Kirby LC, Heubi JE, Dawson PA. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (slc10a2) J Clin Invest. 1997;99:1880–1887. doi: 10.1172/JCI119355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson PA, Haywood J, Craddock AL, Wilson M, Tietjen M, Kluckman K, Maeda N, Parks JS. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278:33920–33927. doi: 10.1074/jbc.M306370200. [DOI] [PubMed] [Google Scholar]
- 13.Jung D, Inagaki T, Dawson PA, Kliewer SA, Mangelsdorf DJ, Moschetta A. Fxr agonists and fgf15 reduce fecal bile acid excretion in a mouse model of bile acid malabsorption. J Lipid Res. 2007 doi: 10.1194/jlr.M700351-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 15.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors fxr, shp-1, and lrh-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 17.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Lundasen T, Galman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260:530–536. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. Differential regulation of bile acid homeostasis by the farnesoid x receptor in liver and intestine. J Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid x receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012;56:1034–1043. doi: 10.1002/hep.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anuradha Rao JH, Craddock Ann L, Belinsky Martin G, Kruh Gary D, Dawson Paul A. The organic solute transporter α-β, ostα-ostβ is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0712328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballatori N, Fang F, Christian WV, Li N, Hammond CL. Ostalpha-ostbeta is required for bile acid and conjugated steroid disposition in the intestine, kidney, and liver. Am J Physiol Gastrointest Liver Physiol. 2008;295:G179–G186. doi: 10.1152/ajpgi.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan T, Rao A, Haywood J, Kock ND, Dawson PA. Mouse organic solute transporter alpha deficiency alters fgf15 expression and bile acid metabolism. J Hepatol. 2012;57:359–365. doi: 10.1016/j.jhep.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricketts ML, Boekschoten MV, Kreeft AJ, Hooiveld GJ, Moen CJ, Muller M, Frants RR, Kasanmoentalib S, Post SM, Princen HM, Porter JG, Katan MB, Hofker MH, Moore DD. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane x receptors. Mol Endocrinol. 2007;21:1603–1616. doi: 10.1210/me.2007-0133. [DOI] [PubMed] [Google Scholar]
- 25.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid x receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582. e571. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 26.Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-e-deficient mouse: A decade of progress. Arterioscler Thromb Vasc Biol. 2004;24:1006–1014. doi: 10.1161/01.ATV.0000128849.12617.f4. [DOI] [PubMed] [Google Scholar]
- 27.Veniant MM, Withycombe S, Young SG. Lipoprotein size and atherosclerosis susceptibility in apoe(−/−) and ldlr(−/−) mice. Arterioscler Thromb Vasc Biol. 2001;21:1567–1570. doi: 10.1161/hq1001.097780. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd J, Packard CJ, Bicker S, Lawrie TD, Morgan HG. Cholestyramine promotes receptor-mediated low-density-lipoprotein catabolism. N Engl J Med. 1980;302:1219–1222. doi: 10.1056/NEJM198005293022202. [DOI] [PubMed] [Google Scholar]
- 29.Lan T, Haywood J, Dawson PA. Inhibition of ileal apical but not basolateral bile acid transport reduces atherosclerosis in apoe(−)/(−) mice. Atherosclerosis. 2013;229:374–380. doi: 10.1016/j.atherosclerosis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veniant MM, Sullivan MA, Kim SK, Ambroziak P, Chu A, Wilson MD, Hellerstein MK, Rudel LL, Walzem RL, Young SG. Defining the atherogenicity of large and small lipoproteins containing apolipoprotein b100. J Clin Invest. 2000;106:1501–1510. doi: 10.1172/JCI10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyake JH, Duong-Polk XT, Taylor JM, Du EZ, Castellani LW, Lusis AJ, Davis RA. Transgenic expression of cholesterol-7-alpha-hydroxylase prevents atherosclerosis in c57bl/6j mice. Arterioscler Thromb Vasc Biol. 2002;22:121–126. doi: 10.1161/hq0102.102588. [DOI] [PubMed] [Google Scholar]
- 32.Lee MR, Lim CJ, Lee YH, Park JG, Sonn SK, Lee MN, Jung IH, Jeong SJ, Jeon S, Lee M, Oh KS, Yang Y, Kim JB, Choi HS, Jeong W, Jeong TS, Yoon WK, Kim HC, Choi JH, Oh GT. The adipokine retnla modulates cholesterol homeostasis in hyperlipidemic mice. Nature communications. 2014;5:4410. doi: 10.1038/ncomms5410. [DOI] [PubMed] [Google Scholar]
- 33.Meissner M, Wolters H, de Boer RA, Havinga R, Boverhof R, Bloks VW, Kuipers F, Groen AK. Bile acid sequestration normalizes plasma cholesterol and reduces atherosclerosis in hypercholesterolemic mice. No additional effect of physical activity. Atherosclerosis. 2013;228:117–123. doi: 10.1016/j.atherosclerosis.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 34.Terasaka N, Miyazaki A, Kasanuki N, Ito K, Ubukata N, Koieyama T, Kitayama K, Tanimoto T, Maeda N, Inaba T. Acat inhibitor pactimibe sulfate (cs-505) reduces and stabilizes atherosclerotic lesions by cholesterol-lowering and direct effects in apolipoprotein e-deficient mice. Atherosclerosis. 2007;190:239–247. doi: 10.1016/j.atherosclerosis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Galman C, Ostlund-Lindqvist AM, Bjorquist A, Schreyer S, Svensson L, Angelin B, Rudling M. Pharmacological interference with intestinal bile acid transport reduces plasma cholesterol in ldl receptor/apoe deficiency. FASEB J. 2003;17:265–267. doi: 10.1096/fj.02-0341fje. [DOI] [PubMed] [Google Scholar]
- 36.Bhat BG, Rapp SR, Beaudry JA, Napawan N, Butteiger DN, Hall KA, Null CL, Luo Y, Keller BT. Inhibition of ileal bile acid transport and reduced atherosclerosis in apoe−/− mice by sc-435. J Lipid Res. 2003;44:1614–1621. doi: 10.1194/jlr.M200469-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Braun A, Yesilaltay A, Acton S, Broschat KO, Krul ES, Napawan N, Stagliano N, Krieger M. Inhibition of intestinal absorption of cholesterol by ezetimibe or bile acids by sc-435 alters lipoprotein metabolism and extends the lifespan of sr-bi/apoe double knockout mice. Atherosclerosis. 2008;198:77–84. doi: 10.1016/j.atherosclerosis.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tietge UJ, Groen AK. Role the tice?: Advancing the concept of transintestinal cholesterol excretion. Arterioscler Thromb Vasc Biol. 2013;33:1452–1453. doi: 10.1161/ATVBAHA.113.301562. [DOI] [PubMed] [Google Scholar]
- 39.Temel RE, Brown JM. Biliary and nonbiliary contributions to reverse cholesterol transport. Curr Opin Lipidol. 2012;23:85–90. doi: 10.1097/MOL.0b013e3283508c21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dietschy JM, Turley SD. Control of cholesterol turnover in the mouse. J Biol Chem. 2002;277:3801–3804. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]