Abstract
Objective
The purpose of this study is to evaluate the long-term survival following gastric bypass using propensity-matched controls.
Methods
We identified all patients who either received a GBP or met criteria to receive a GBP between Jan 1, 2002 and Dec 31, 2003. Propensity matching was performed. Long-term, all-cause mortality data was collected and evaluated using Kaplan-Meier curves.
Results
430 GBP cases and 5323 controls were identified from the enrollment period. Ultimately, 802 cases and controls (1:1 matching, 93.2% match rate) were identified using propensity matching. Median follow-up was similar between groups. Overall mortality was lower for the GBP group (OR: 0.48, 95% CI: 0.29, 0.78). GBP demonstrated significantly increased survival when compared to controls (p-value = 0.002). Similar patterns were noted among diabetics.
Conclusions
We have demonstrated that gastric bypass provides a clear long-term survival advantage compared to non-surgical propensity-matched controls.
Keywords: Obesity, Survival, Gastric Bypass, Propensity
Introduction
Recent evaluation of the National Health and Nutrition Examination Survey (NHANES) observed that the prevalence of adult obesity in the United States in 2012 was 34.9%, and has remained relatively constant over the past decade (1). Other estimates suggest a more concerning forecast, with the rate of adult obesity approaching 51% by 2030 (2). These concerns have prompted the United States Department of Health and Human Services to classify obesity as a marker of overall health, with the goal of reducing obesity by the year 2020 (3).
Bariatric surgery offers an opportunity for improved survival in this population via drastic risk modification. However, long-term (> 10 year) evaluations of outcomes following bariatric surgery in the literature are limited. Fewer still have incorporated propensity matching as a primary portion of their analysis. The purpose of this study was to create a historical cohort of gastric bypass cases and propensity-matched controls in order to evaluate long-term mortality in both diabetic and non-diabetic patients.
Material and Methods
Our Institutional Review Board for Health Sciences Research (IRB-HSR) approved this study. We identified obese and morbidly obese patients at our tertiary care center that met criteria for a gastric bypass between January 1, 2002 and December 31, 2003. Appropriate demographics, comorbidities, and insurance status were identified and recorded. Patients were classified as either “cases” or “controls” based upon receipt of a roux-en-y gastric bypass. Only data known during the two-year period was recorded. Due to limitations in our medical record system during the 2002–2003 time period, data required to accurately calculate body mass index (BMI) was not reliably recorded in the control group. We therefore used ICD-9 codes to identify morbidly obese patients (278.0, 278.00, & 278.01). These ICD-9 codes have been previously used as a substitute for BMI in studies of this type (4).
We utilized a propensity score-matching algorithm based on the likelihood of receiving a gastric bypass. All variables known during the two-year period were included in the regression analysis. Automated stepwise selection was used to limit the number of predictor variables. Matching was conducted on a 1:1 basis using the “greedy” method. Cases and controls were each used only once. Once the matched cohort was identified, survival data through February 2014 (most recent available) was collected from the Social Security Death Master File (SSDMF).
Standard univariate analysis was conducted using Wilcoxon Rank Sum, Chi-Square, and Fisher’s exact tests where appropriate. Thirty-day, 1-year, 5-year, and 10-year mortality rates are listed in addition to overall mortality. Survival analysis was conducted using Kaplan-Meier curves. Statistical significance was set at p-values < 0.05. Statistical analysis was conducted using SAS software, version 9.3 (SAS Institute, Cary, NC).
Results
We identified 5,753 patients eligible for gastric bypass during the study period. Four hundred and thirty (7.5%) received a gastric bypass. Our propensity model identified 401 matched pairs for a 93.2% match rate (c-statistic = 0.85; HLT = 0.71). Patients who underwent a gastric bypass demonstrated considerable heterogeneity compared to unmatched controls. However, after matching, there were no significant differences between cases and controls in any category, effectively eliminated treatment allocation bias based on known variables (Supplemental Table A). Details of the propensity model are listed in Supplemental Table B.
Median follow up for the gastric bypass group was 11.9 years compared to 11.8 years for the controls (p-value = 0.06). Among survivors, only one patient had follow-up of less than 10 years (9.5 years). This was due to missing data making it impossible to match with the SSDMF. Instead, the last date that this patient was known to be alive through interaction with our health system was used instead.
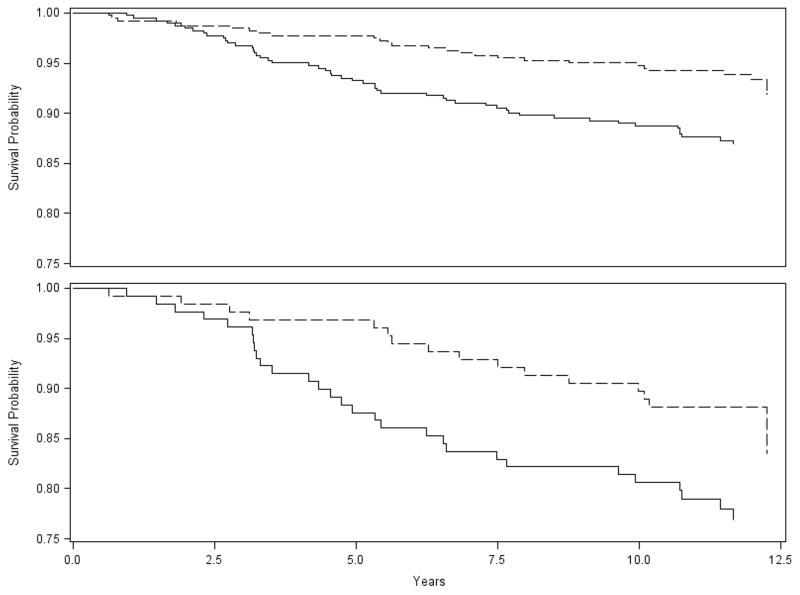
Mortality within 30 days of surgery for cases or within 30 days of enrollment for the controls was zero for both groups. One-year mortality was 0.7% for cases compared to 0.2% for controls (n = 3 for cases, n = 1 for controls; Fisher’s exact test p-value 0.62). Patients who received a gastric bypass had significantly lower rates of overall mortality (6.5% vs. 12.7%) than matched comparators. Similar findings were observed for diabetic patients (Table 1). Survival was significantly improved in the gastric bypass group for both the overall cohort (Figure 1; p-value = 0.002) and our diabetic subset (Figure 1; p-value = 0.03). There appears to be no survival benefit in the first 2 years in the overall cohort and in the first 3 years in the diabetic cohort.
Table 1.
Propensity-Matched Outcomes
| Overall Outcomes | Controls | Cases | Odds Ratio (95% CI) | p-value |
|---|---|---|---|---|
| Median Length of Follow-up (Years) | 11.8 (10.9- 12.2) | 11.9 (11.2- 12.3) | -- | 0.06 |
| 30-day Mortality | 0 | 0 | -- | N/A |
| 1-year Mortality | 1 (0.2%) | 3 (0.7%) | 3.02 (0.31–29.10) | 0.62 |
| 5-year Mortality | 27 (6.7%) | 9 (2.2%) | 0.32 (0.15–0.69) | 0.003 |
| 10-year Mortality | 46 (11.5%) | 21 (5.4%) | 0.43 (0.25–0.73) | 0.002 |
| Overall Mortality | 51 (12.7%) | 26 (6.5%) | 0.48 (0.29–0.78) | 0.003 |
| Diabetic Outcomes | ||||
| 30-day Mortality | 0 | 0 | -- | N/A |
| 1-year Mortality | 1 (0.8) | 1 (0.8) | 0.98 (0.06–15.91) | 0.99 |
| 5-year Mortality | 16 (12.4%) | 4 (3.1%) | 0.23 (0.08–0.71) | 0.01 |
| 10-year Mortality | 25 (19.4%) | 13 (10.2%) | 0.47 (0.23–0.98) | 0.04 |
| Overall Mortality | 29 (22.5%) | 16 (12.6%) | 0.49 (0.26–0.97) | 0.04 |
Figure 1. Survival for Full Cohort and Diabetic Patients.
Top Graph = Survival for full cohort (p-value = 0.002)
Bottom Graph = Survival for diabetic patients only (p-value = 0.03)
Gastric bypass cases = dashed line. Non-surgical controls = solid line
Discussion
There are several studies supporting the notion that bariatric surgery reduces long-term mortality (4–9). Adams and colleagues published an analysis of long-term mortality in gastric bypass patients as compared to matched non-surgical controls. Their study retrospectively followed 7,925 matched patient pairs over an average of 7.1 years demonstrating a clear survival advantage for those patients that received a gastric bypass (6). However, as acknowledged by the authors, their study was not able to control for treatment allocation bias. Christou et al matched patients based upon age, sex, and a diagnosis of morbid obesity, but suffered from similar limitations (7). Another study by Flum et al utilized a propensity-matching mechanism on a subset of their study patients to evaluate 5-year survival, but the characteristics of their propensity model were not published (4). Our study adds to the literature by implementing a fully described propensity-matched analysis, thereby minimizing the effect of treatment allocation bias. Additionally, our study is the only one in the literature with true 10-year follow-up on the entire cohort.
Although variable follow-up periods make direct comparison difficult, our observed mortality rates appear to be similar to those reported elsewhere in the literature (4, 6, 9). Buchwald et al reported that retrospective and observational studies had higher mortality rates than other study designs, however, our 30-day mortality rates were still among the lowest in the literature (4, 10). In the particular case of the Swedish Obesity Study, any differences may be due to case mix, since only 13% of their patients underwent a gastric bypass (6, 9). Our mortality rate was higher than that reported by both Peeters and Christou (7, 8). However, the follow up period for the surgical cohort in Peeters’ study was fairly short (median 3.6 years), and Christou only analyzed five-year survival (7, 8). The differences in 5-year post-surgical survival between our study and Christou’s remain unclear, but may be due to underlying differences in our patient populations. Survival for the control group in Peeters’ study over a median of 12.8 years, however, was similar to ours (8). Our exclusive use of a medical cohort as a source of control patients may result in higher mortality rates for that group than observed in other studies (6, 7). However, our odds ratios for mortality are similar to those reported in the meta-analysis by Pontiroli (5).
Zhang et al demonstrated that the presence of diabetes significantly reduced survival following surgery (11). Other authors have previously demonstrated reliable diabetic remission rates following bariatric surgery (12). Our study demonstrates that these patients also obtain a survival benefit although it may not be evident until 3 years after surgery.
The Swedish Obesity Study found that bariatric surgery did not become an independent risk factor for survival until 13 years after the procedure (9). Our analysis demonstrates that surgery imparts an appreciable difference in mortality beginning at about 2 years after surgery. Again, this difference may be attributable to the small percentage of gastric bypass patients included in the SOS. Our findings are similar to those of Christou and colleagues, who also noted a divergence beginning at approximately 2 years post-surgery (7). Adams and Christou both demonstrated that bariatric surgery reduced the risk of cardiovascular related mortality (6, 7). We believe that the benefits of risk modification via bariatric surgery take some period of time to accumulate and exert a noticeable difference in mortality.
Adams demonstrated increased rates of suicide among gastric bypass patients (6). We find this observation concerning. While we are limited in our ability to determine cause of death in our study, the logistic regression used in our propensity-matching algorithm identified the presence of either a psychiatric disorder or substance abuse issue to be independently predictive of the receipt of a gastric bypass (Odds Ratio: 2.02; 95% CI: 1.61 – 2.55). In contrast, Christou and colleagues noted decreased hospitalizations for psychiatric and mental health reasons in their surgical cohort (7). We believe that the potential associations between mental illness and bariatric surgery warrant further study.
Many of the studies evaluating survival in this population have used a Cox proportional hazards model (4, 6, 8, 9). However, comparing a substantial surgical intervention with a non-surgical cohort may violate the proportional hazards assumption. As demonstrated in our data, the proportional risk or “hazard” of mortality is not constant at all time points. The risk of death is increased within the first year following surgery. Any survival advantage is achieved later. Other studies may suffer the same limitation, as this is an observation inherent with this type of surgery and study design. Indeed, Flum notes a similar pattern in his discussion (4). It is unclear to what degree, if any, the hazard ratios reported by other studies result in biased assessments. When attempting to verify the proportional hazards assumptions for our own data, we noted that the hazard ratios differed only slightly from our reported odds ratios. Ultimately, our data did not fit the assumptions required to perform a Cox proportional hazards model.
Our study has several limitations. First, as a single institution, retrospective study, our findings may not be broadly generalizable. Second, while we were pleased with the functioning of our propensity model, the prospect remains that our model may not account for the full degree of variability within our data. Third, our status as a tertiary care center and geographic location result in a large referral area spanning several states. Rural and out-of-state patients may not necessarily receive all of their care at our institution. These factors impact our ability to reliably determine cause of death in a large percentage of cases. Additionally, cause of death is not included in our data from the SSDMF. Finally, due to institution-specific limitations in our medical record system over the almost 12 year study period; we are unable to include exact BMI data. All analyses were conducted using ICD-9 codes for obesity and morbid obesity.
Conclusion
We describe the only analysis of true long-term mortality in gastric-bypass patients using a propensity-matched analysis. Echoing the findings of other studies, we demonstrate that the gastric bypass significantly improves survival compared to propensity-matched controls. These findings add to the growing consensus that bariatric surgery is the current standard of care for reducing long-term mortality in obese and morbidly obese patients.
Supplementary Material
Table A: Demographics, Comorbidities, and Insurance Status Before and After Propensity Matching
Table B: Details of Multivariate Logistic Regression Analysis for the Likelihood of Receiving a Gastric Bypass
Summary.
Patients who received a gastric bypass were compared with propensity-matched non-surgical controls. Our analysis indicates a significant survival advantage to receiving a gastric bypass.
Acknowledgments
Funding Source
This work was funded by a National Institutes of Health training grant (T32 AI078875). The funding source had no involvement in any part of this study.
This study would not have been possible without the efforts and assistance of Mr. Kenneth Scully in the Department of Public Health.
Footnotes
Conception and design: CAG & PTH. Data acquisition: CAG. Analysis and interpretation: CAG, SWD, & PTH. Manuscript drafting and editing for important intellectual content: All Authors
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and severe obesity forecasts through 2030. American journal of preventive medicine. 2012;42:563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 3.United states department of health and human services. 2020 leading health indicators. 2014 May 23; Available from: http://www.healthypeople.gov/2020/LHI/2020indicators.aspx.
- 4.Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: A population-based analysis. Journal of the American College of Surgeons. 2004;199:543–551. doi: 10.1016/j.jamcollsurg.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery. A systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Annals of surgery. 2011;253:484–487. doi: 10.1097/SLA.0b013e31820d98cb. [DOI] [PubMed] [Google Scholar]
- 6.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 7.Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, MacLean LD. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Annals of surgery. 2004;240:416–423. doi: 10.1097/01.sla.0000137343.63376.19. discussion 423–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peeters A, O’Brien PE, Laurie C, Anderson M, Wolfe R, Flum D, MacInnis RJ, English DR, Dixon J. Substantial intentional weight loss and mortality in the severely obese. Annals of surgery. 2007;246:1028–1033. doi: 10.1097/SLA.0b013e31814a6929. [DOI] [PubMed] [Google Scholar]
- 9.Sjostrom L. Review of the key results from the swedish obese subjects (sos) trial - a prospective controlled intervention study of bariatric surgery. Journal of internal medicine. 2013;273:219–234. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 10.Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: A systematic review and meta-analysis. Surgery. 2007;142:621–632. doi: 10.1016/j.surg.2007.07.018. discussion 632–625. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Mason EE, Renquist KE, Zimmerman MB. Factors influencing survival following surgical treatment of obesity. Obesity surgery. 2005;15:43–50. doi: 10.1381/0960892052993422. [DOI] [PubMed] [Google Scholar]
- 12.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. The American journal of medicine. 2009;122:248–256. e245. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A: Demographics, Comorbidities, and Insurance Status Before and After Propensity Matching
Table B: Details of Multivariate Logistic Regression Analysis for the Likelihood of Receiving a Gastric Bypass