Abstract
Innate immune responses in the cornea mainly play an important role to mobilize multiple interrelated pathways of corneal lipid, which involve in inflammatory corneal diseases. Signaling lipid mediators derived from arachidonic acid (AA) control cell proliferation, apoptosis, metabolism, and migration, are known as eicosanoids, phosphoinositides, sphingolipids, and fatty acids. Emerging evidences have highlighted the implication of lipid mediators in both injury and repair mechanisms in the cornea. Recently, the role of AA and its metabolites to induce proinflammatory mediators and inflammatory cell infiltration in the pathogen-infected cornea and to cause severe keratitis have been revealed. In this review, we focus on the novel roles of AA downstream signaling in the corneal inflammatory diseases and also the biological relevance of AA signaling in the therapeutic strategies for targeting sight-threatening diseases.
Keywords: Innate immune, Arachidonic acid, Lipid, Cornea, Inflammation, cPLA2α, Acanthamoeba, Keratitis, MIP-133, TLRs
Introduction
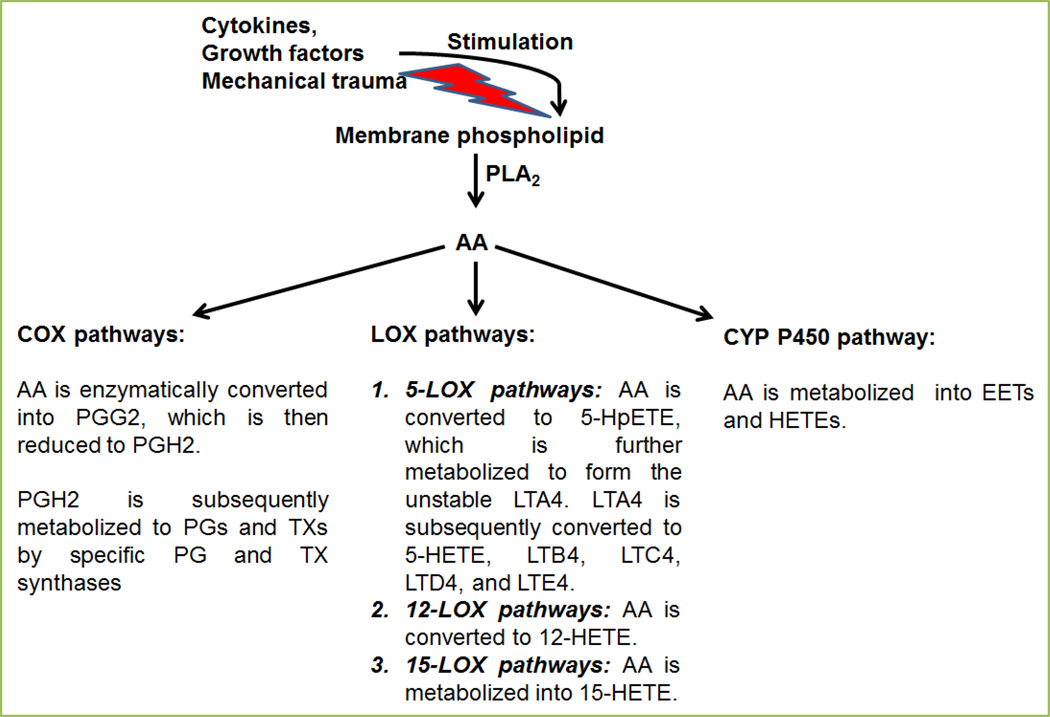
Arachidonic acid (AA, C20H32O2) is an omega-6 (n-6) polyunsaturated fatty acid (PUFA), esterified on the sn-2 acyl position of phospholipids (especially, phosphatidylethanolamine, phosphatidylcholine, and phosphatidylinositides) in the outer and intracellular membranes of mammalian cells [1]. Arachidonic acid serves as the precursor for the bioactive lipid mediators, the eicosanoids. The eicosanoid biosynthesis in mammalian cells is induced by the activation of phospholipase A2 (PLA2) and the release of AA from membrane phospholipids upon stimulation by growth factors, cytokines or mechanical trauma [1, 2]. Arachidonic acid is subsequently transformed to eicosanoids by cyclooxygenase (COX, which produces prostaglandins [PGs] and thromboxane [TX]), lipoxygenase (LOX, that leads to the formation of leukotriens [LTs]), and the cytochrome P450 monooxygenases (CYP P450, which generates hydroxyeicosatetraenoic acid [HETEs] and epoxyeicosatetraenoic acid [EETs]) pathways [1, 2] (Fig. 1).
Figure 1. Enzymatic pathways for the synthesis of eicosanoids derived from arachidonic acid (AA).
Arachidonic acid is synthesized by the enzymatic activity of phospholipase A2 (PLA2) from cellular membrane upon stimulation by cytokines, growth factors or mechanical trauma. Arachidonic acid is then converted to eicosanoids via the three major pathways: the COX, the LOX, and the cytochrome P450 monooxygenase pathways.
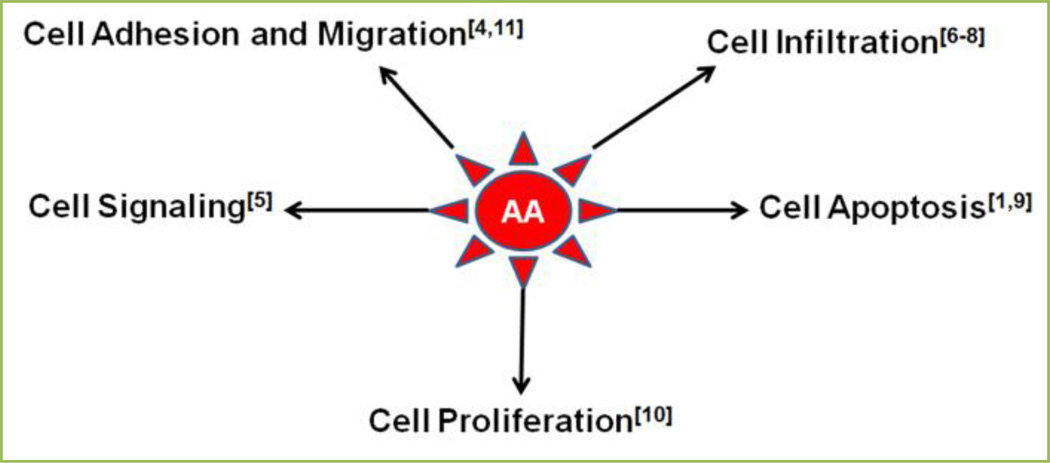
Eicosanoids (PGs, LTs, and TX) production is considerably increased during inflammation. They interact to the specific G protein-coupled receptors (GPCRs) and trigger their biological effects [1]. Eicosanoids are naturally acknowledged for their capacity to provoke biological responses, vascular homeostasis, protection of the gastric mucosa and platelet aggregation, regulation of immunopathological processes ranging from inflammatory responses to chronic tissue remodeling, asthma, rheumatoid arthritis, cancer, and autoimmune disorders [3]. Metabolites of AA are well known in several biological processes which are important for the physiological responses and the pathological processes of the cell [4–11] (Fig. 2).
Figure 2.
Important roles of arachidonic acid (AA) in a variety of cellular functions.
Eicosanoids regulate many important aspects of immunity, including pathogen recognition through the extracellular and the intracellular receptors (Fc, mannose or Toll-like receptors [TLRs]), antibody formation, cytokine production, cell proliferation, differentiation, migration, and antigen presentation [3]. Cells of the innate immune system, such as dendritic cells (DCs), macrophages, and polymorphonuclear neutrophils (PMNs) produce eicosanoids which act at nanomolar concentrations on target cells [3]. Immune cells trigger their effects in an autocrine and paracrine fashion, and affect the function of neighboring cells. It has been documented that eicosanoids and their specific receptors cooperate with other important signaling molecules, mainly cytokines and chemokines, and have an important role in regulating physiological processes in both homeostatic and inflammatory conditions [3]. Biosynthetic pathways of eicosanoid production are clinically relevance because their derivatives are associated in the pathogenesis of several immune related disorders. Thus, numerous therapeutic approaches based on eicosanoids and their receptors are being used, and several are on the horizon; however, pharmacological inhibition of eicosanoid biosynthesis might simultaneously be beneficial and harmful [3].
We will discuss the emphasized properties of AA and its derived eicosanoids, and their increasing role in disease pathogenesis, focusing their connection in inflammatory corneal diseases.
Role of innate immune system in the recruitment of arachidonic acid signaling
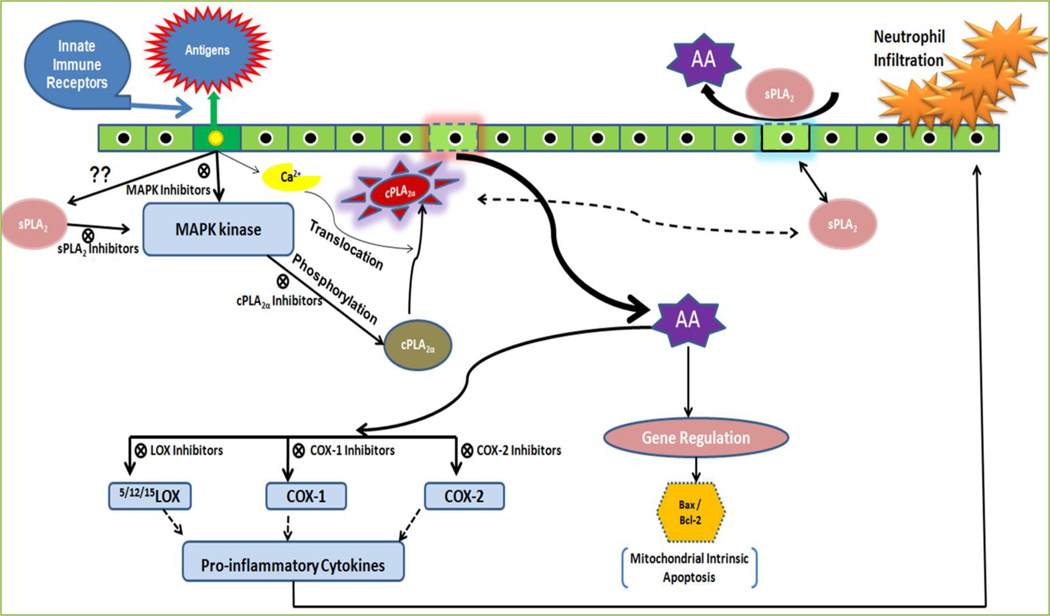
Involvement of innate immunity in the activation of signaling pathways of eicosanoids’ production in cells is not completely understood; however, it is clear that the calcium-dependent cytosolic group IVA PLA2α (cPLA2α) is an important enzyme in AA mobilization, and that, depending on cell type and activation conditions, regulatory cross-talk mechanisms exist between cPLA2α, and Ca2+-dependent secreted PLA2 (sPLA2), and Ca2+-independent cytosolic PLA2 (iPLA2) enzymes present in the cells [12]. Numerous agents that exert effects on cells through innate immune receptors provoke a series of signals which lead to increased PLA2 activity [12]. The PLA2 signaling mechanism developed over the years for major immunoinflammation (Fig. 3). This schematic presentation contemplates a scenario where the concerted role of two distinct PLA2s lead to a complete AA signaling. cPLA2α seems to be dominant over sPLA2 action in AA release; however, sPLA2 may primarily act to amplify the inflammatory response by providing additional signals for full activation of cPLA2α, the reader is kindly referred to reference [12] for the depth review on the subject. Recently, we investigated the cPLA2α induced AA signaling in the pathogenesis of Acanthamoeba keratitis. This signaling is effectively attenuated by cPLA2α specific inhibitors which therapeutically mitigate corneal inflammatory responses in vivo Chinese hamster in Acanthamoeba infection [13, 14] (Fig. 4).
Figure 3. Arachidonic acid (AA) signal transduction is activated by the phosphorylation of cPLA2α during the recognition of inflammation or antigen via innate immune receptors.
Inhibitors of various cascades therapeutically may play an important role in disease management.
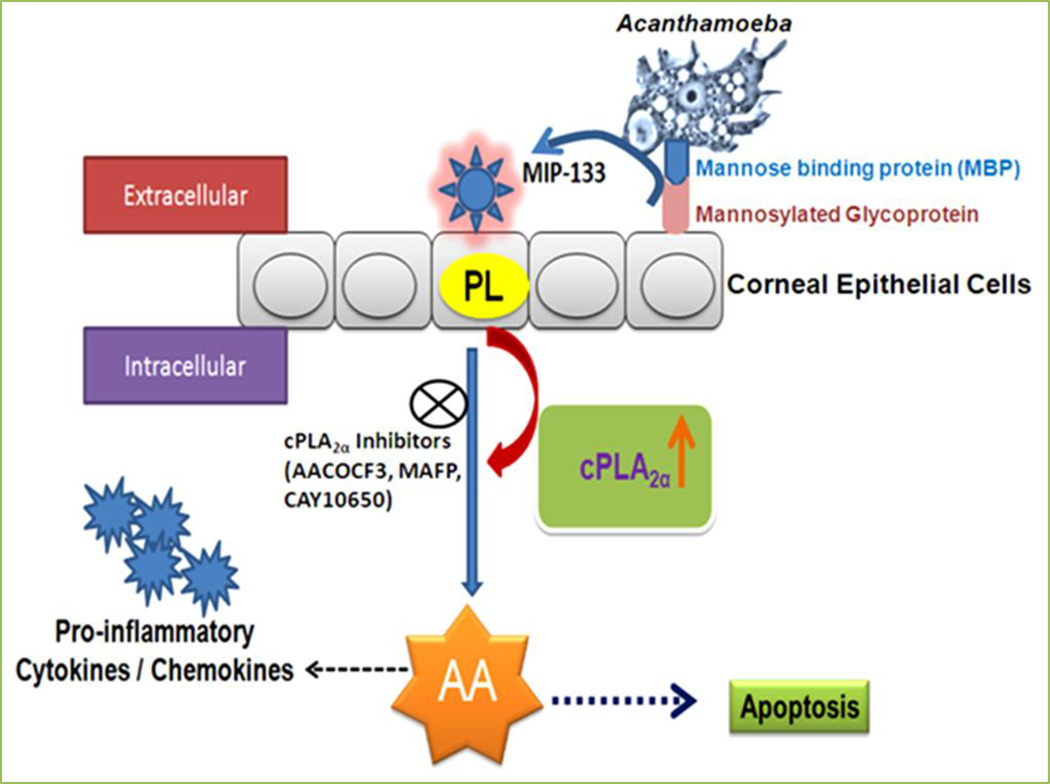
Figure 4. Role of Acanthamoeba cytopathic protein, MIP-133, in the pathogenesis of Acanthamoeba keratitis.
Acanthamoeba binds to the corneal surface by mannose binding protein (MBP). This binding of Acanthamoeba to corneal epithelial cells induces release of the mannose-induced 133 kDa protease (MIP-133). MIP-133 interacts with phospholipids on plasma membrane of human corneal epithelial (HCE) cells and Chinese hamster corneal epithelial (HCORN) cells, and activates cytosolic phospholipase A2α (cPLA2α). cPLA2α is involved in apoptosis, arachidonic acid (AA) release, and activation of proinflammatory cytokines/chemokines from HCE and HCORN cells. cPLA2α inhibitors (AACOCF3, MAFP, and CAY10650) may be a therapeutic target in Acanthamoeba keratitis [13,14].
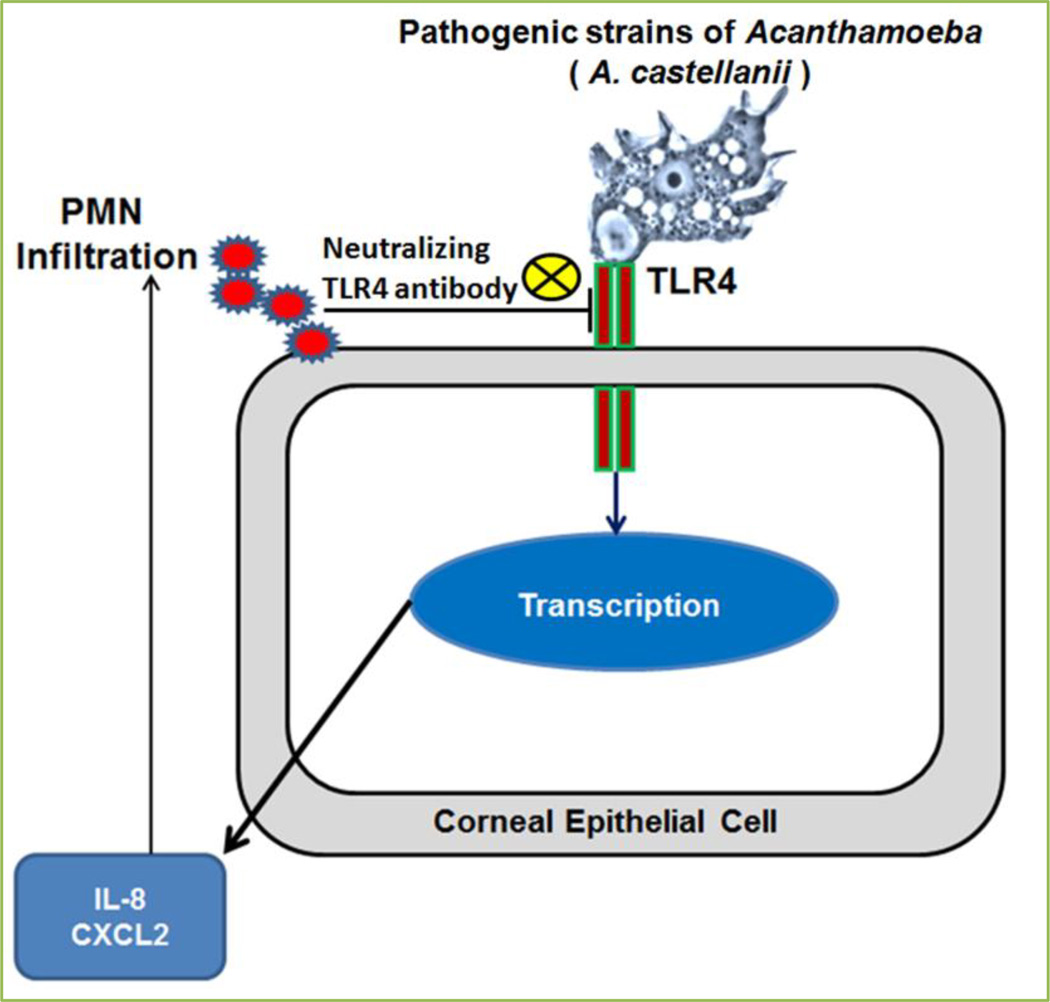
Toll-like receptors (TLRs) are important tool of innate immune system of cells and constituents of the first line of defense against invading pathogens. TLRs recognize a broad range of pathogens via their specific family members based on amino acid sequences, including TLR1-TLR10 (all related to human); TLR11-TLR13 (all related to mice) [15, 16]. Out of all, only TLR3 and TLR9 families express exclusively in endosomal compartments and are recognized as intracellular receptors [17]. Recognition of invading pathogens by TLR2, TLR3, TLR4, TLR7, TLR8, and TLR9 in various cell types and AA mobilization via the stimulation of cPLA2α and sPLA2 signaling, have been extensively studied[18–37]. We investigated the recognition of TLR4 by pathogenic but not non-pathogenic species of Acanthamoeba in human corneal epithelial (HCE) cells and Chinese hamster corneal epithelial (HCORN) cells in vitro and in vivo Chinese hamster corneas [38]. This recognition induces inflammatory responses in cornea and demonstrates TLR4 signaling in the pathogenesis of Acanthamoeba keratitis (Fig. 5). This study was not focused to investigate the involvement of AA downstream signaling activated through TLR4 recognition by Acanthamoeba trophozoites; however, it provoked further study to explore TLR4 induced AA signaling in corneal inflammation by pathogenic species of Acanthamoeba which supported by the previous investigations [30–32, 35] that TLR4 regulates cPLA2α-AA downstream signaling in inflammation.
Figure 5. Pathogenesis of Acanthamoeba keratitis is induced by the activation of TLR4 pathway.
Recognition of TLR4 by pathogenic Acanthamoeba Spp., A. castellanii, induces secretion of proinflammatory cytokines from corneal epithelial cells and increases infiltration of inflammatory cells in cornea [38].
Arachidonic acid in cornea
Most of the available evidence about AA metabolites in the eye arises from studies on the conjunctiva, uvea, and intraocular pressure (IOP) [39–42]. However, Bito et al.[41] and Taylor et al.[43] explored the AA metabolites formation in isolated corneal epithelial and corneal fibroblast cells. Bazan et al. [44] demonstrated that in healthy cornea, prostaglandin E2 (PGE2) is the chief cyclooxygenase product produced in the stroma, while thromboxane B2 (TXB2) predominated in the endothelium and epithelium; moreover, the main lipoxygenase product, mono-hydroxyeicosatetraenoic acid (mono-HETE) and 12-HETE, were detected in the epithelium and in the stroma, respectively. While lipoxygenase products could not be detected in control endothelium. In contrast of healthy cornea, membrane lipid in epithelium and endothelium decreased after injury, these decreases were observed in phosphatidylinositol, phosphatidylcholine, and phosphatidylethanolamine. However, at the same time, cyclooxygenase reaction products, particularly prostaglandin F2α (PGF2α) was increased in the epithelium. Moreover, they observed that prostaglandins (6-keto-PGFlα, TxB2, PGF2α, and PGE2) levels in the stroma and in the epithelium; while PGF2α, PGE2, and PGD2 were increased in endothelium after injury. Increased lipoxygenase products were observed in the endothelium, stroma, and epithelium after wounding. Thus, Bazan et al. [44] first time demonstrated that the metabolism of AA is altered by cryogenic injury and resulting changes differ in the corneal epithelium, stroma, and endothelium.
Hurst et al. [45] further demonstrated that (12S)-HETE, but not (12R)-HETE, is accumulated in the corneal all layers (epithelial, stromal, and endothelial) in response to alkali injury and cryogenic injury. These findings support Bazan et al. [44] studies and reflecting the possibility of activation of 12-lipoxygenase. Gupta et al. [46] showed that lipoxygenase activity is a significant factor in regulating corneal epithelial wound healing in the rat, by influencing epithelial cell migration. Also, Nakamura et al. [11] demonstrated 5-lipoxygenase involvement in corneal epithelial migration. Stoltz et al. [47] showed that corneal epithelial hypoxia in response to contact lens wear outcomes in the time-dependent formation of NADPH-CYP P450-dependent arachidonate metabolites, (12R)-HETE and (12R)-HETrE. Biologically (12R)-HETE blocks Na+/K+-ATPase, augments corneal thickness, and diminishes IOP, while (12R)-HETrE causes vasodilation, neutrophil chemoattraction, and angiogenesis. However, origin of 12-HETE and 12-HETrE had a debate as they arise from the fact that both lipoxygenase and CYP P450 have the ability to synthesize these two compounds from AA. Whereas lipoxygenase produces only the (S) enantiomer, CYP P450 has the capacity to synthesize a racemic mixture with the (R) enantiomer predominating in the corneal epithelium of certain species (e.g., porcine) as well as in hepatic tissues, ciliary epithelium, and inflamed epidermis [47]. Moreover, cyclooxygenase or lipooxygenase inhibitors (indomethacin, diclofenac, and BW755C) were unaffected on the formation of 12-HETE and 12-HETrE while their formation is blocked by CYP P450 enzyme blockers such as SKF-525A, carbon monoxide (CO), and clotrimazole [47]. For the detailed study of CYP P450 in AA metabolism, reader requested for the review reference [48].
Recently, cyclooxygenase-2 (COX-2) expression was revealed in all the corneal layers, epithelium, stromal cells, and endothelium in keratitis dog eyes [49]. Takahira et al. [50] showed that the application of AA blocked the inactivating voltage-gated K+ current and increased the noisy, sustained K+ current in the rabbit corneal epithelium and revealed an inactivating voltage-gated K+ channel in the corneal epithelium. Arachidonic acid metabolism is essential for the functional activity of cornea. Thus, further investigations are needed to explore the biochemical roles of AA metabolites in the biology of cornea.
Cytosolic Ca2+-dependent PLA2α-induced arachidonic acid signaling in corneal epithelial cell-derived proinflammatory mediators
Cytosolic Ca2+-dependent PLA2α (cPLA2α) out of 5 sub-groups, α-ζ[51–53], has been investigated comprehensively because it is the only PLA2 that shows specificity for hydrolysis of sn-2 arachidonic acid (AA) from membrane phospholipids for eicosanoids biosynthesis in response to diverse extracellular stimuli,[54, 55] and is controlled by phosphorylation and an intracellular calcium ([Ca2+]i) increase[51]. Phosphorylation of cPLA2α by MAPKs is required for cPLA2α-induced AA production in stimulated cells [54, 55]. Several studies revealed the twofold role of PLA2s in ocular diseases, which may be associated to their enzymatic activities or to regulatory roles comprising signaling and protein-protein interactions [56]. We observed the functional activity of cPLA2α in vitro HCE cells and HCORN cells, and in vivo Chinese hamster corneas which induces pathogenesis of Acanthamoeba keratitis [13, 14]. We investigated that cPLA2α enzyme activity at both gene expression and protein production level is significantly upregulated by Acanthamoeba-cytopathic protein (MIP-133) in HCE and HCORN cells [13, 14].
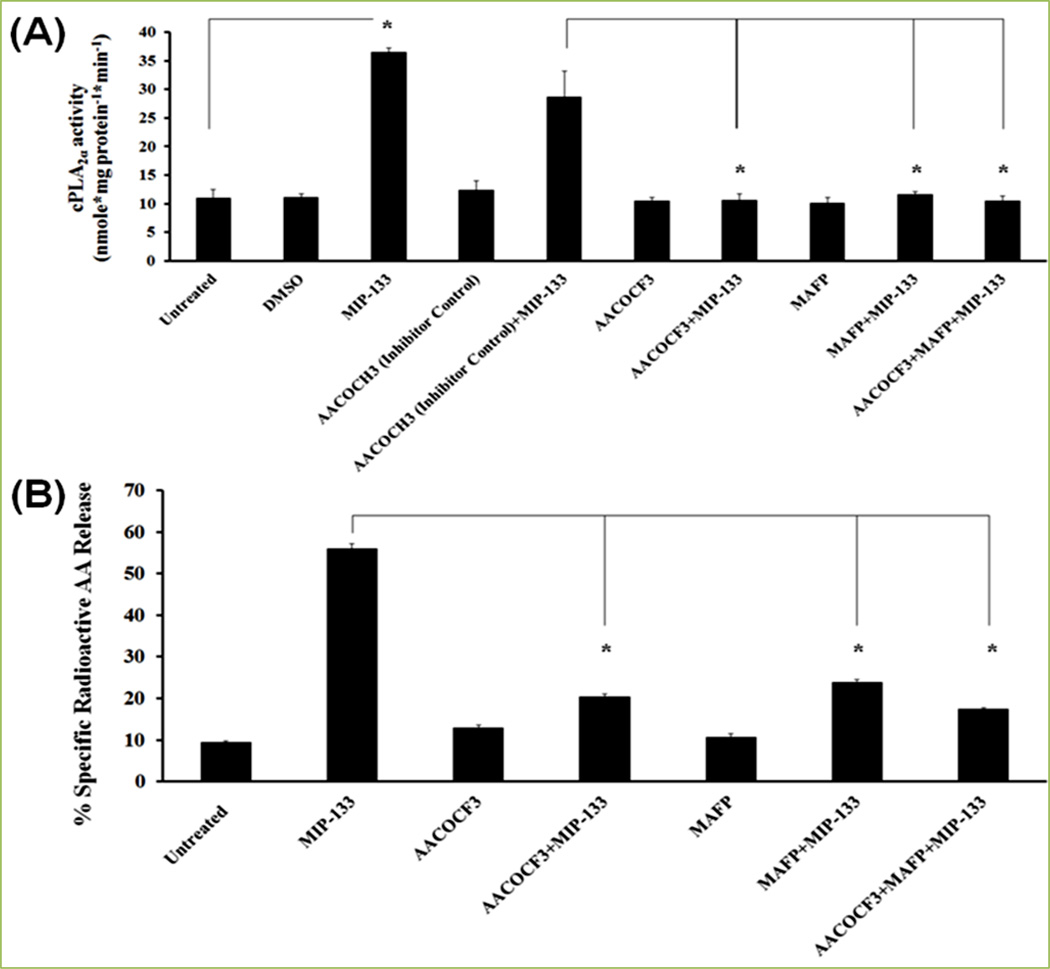
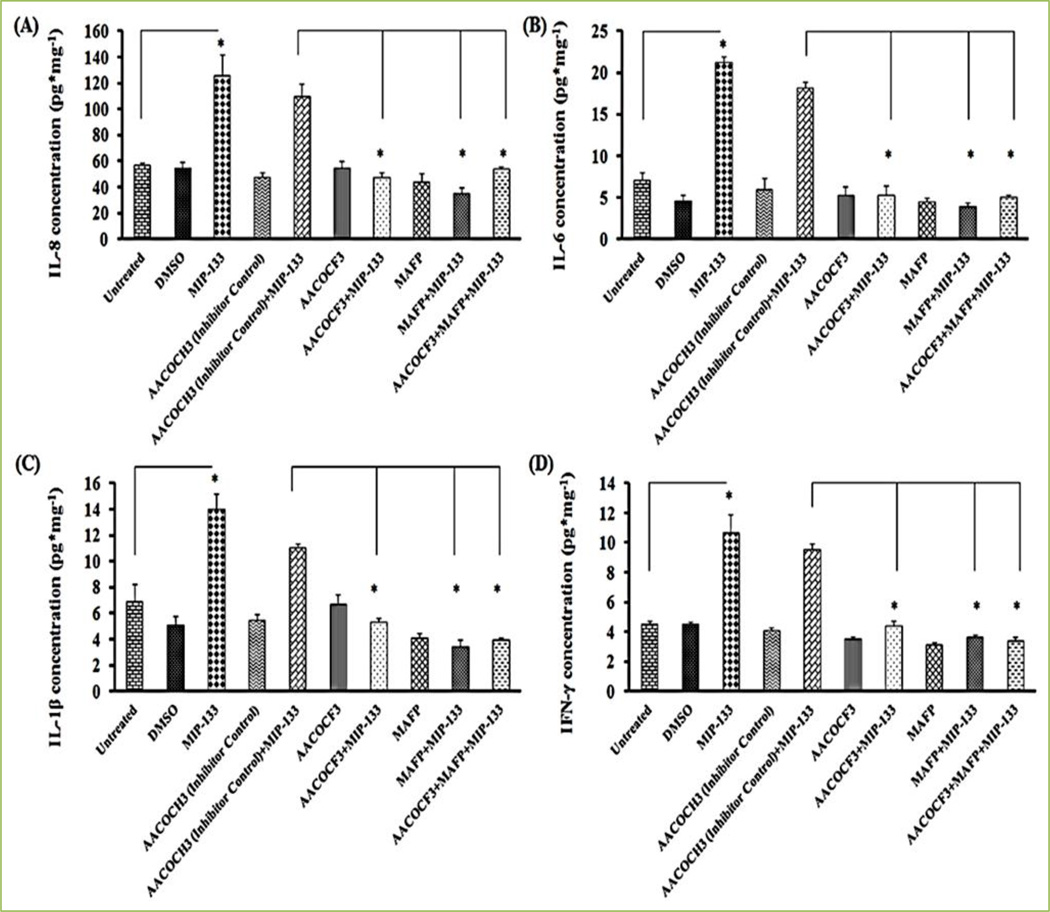
MIP-133 is a mannose-induced 133 kDa serine protease of Acanthamoeba which secretes upon interaction of Acanthamoeba with a mannosylated protein on corneal epithelial cells. Enzyme activity of cPLA2α induced by MIP-133 is significantly inhibited by specific inhibitors (AACOCF3 and MAFP) of cPLA2α [13, 14] (Fig. 6A). We confirmed the MIP-133 induced upregulation of cPLA2α by inhibition with chicken anti-MIP-133 antiserum [13]. Further to explore if cPLA2α is involved in MIP-133-induced AA secretion from corneal epithelial cells, we observed the effect of cPLA2α inhibitors (AACOCF3 and MAFP) on AA secretion. Results demonstrated that AACOCF3 and MAFP significantly diminished AA secretion stimulated by MIP-133 from HCE cells [13] (Fig. 6B). Our findings suggested that cPLA2α pathway is involved in AA release from corneal epithelial cells stimulated with MIP-133[13]. Moreover, we observed that functional activity of cPLA2α-induced AA release signaling in corneal epithelial cell-mediated proinflammatory mediators. In addition, P. aeruginosa challenged upregulation of proinflammatory cytokines/chemokines (IL-8, TNFα, IL-1β, and IL-6) gene expression have been revealed in HCE cell lines[57]. We demonstrated that the pretreatment of HCE and HCORN cells with MAFP and AACOCF3 inhibits protein production of cytokines/chemokines (IL-8, IL-1β, IL-6, IFN-γ, and CXCL2) [13, 14] (Fig. 7 and 8).
Figure 6. Effect of cPLA2α inhibitors (AACOCF3 and MAFP) on MIP-133 induced cPLA2α enzyme activity in HCE cells and arachidonic acid (AA) release from HCE cells.
HCE cells were preincubated for 1 hour with cPLA2α inhibitors (10 µM MAFP or 20 µM AACOCF3) or an inactive negative control (20 µM AACOCH3), and then incubated with or without 15 µg/mL MIP-133 for 24 hours. Induction of cPLA2α enzyme activity was examined using a cPLA2 assay kit (A). AA production by MIP-133 treatment was determined by liquid scintillation counting (B). The data are mean±SEM of three independent experiments. Asterisk indicates P value < 0.05 by unpaired Student’s t-test. Reprinted from Tripathi et al. [13] with permission from copyright holder the Association for Research in Vision and Ophthalmology (ARVO).
Figure 7. MIP-133 induces the up-regulation of proinflammatory cytokines (IL-8 (A), IL-6 (B), IL-1β (C), and IFN-γ (D) protein expression) is blocked by cPLA2α inhibitors in HCE cells.
Inhibition of cPLA2α involved pre-incubating HCE cells for 1 hour with cPLA2α inhibitors (10 µM MAFP or 20 µM AACOCF3) or an inactive control (20 µM AACOCH3), and then incubated with or without 15 µg/ml of MIP-133 for 24 hours. Cytokine production was examined by ELISA. The data are mean ± SEM of three independent experiments. Asterisk indicates P value < 0.05 by unpaired Student’s t-test. AACOCF3, MAFP, AACOCH3, and DMSO without MIP-133 did not show significant effect on CXCL2 production when compared to untreated cells. The inactive control compound AACOCH3 did not block IL-8, IL-6, IL-1β, and IFN-γ protein production induced by MIP-133. Reprinted from Tripathi et al.[13] with permission from copyright holder the Association for Research in Vision and Ophthalmology (ARVO).
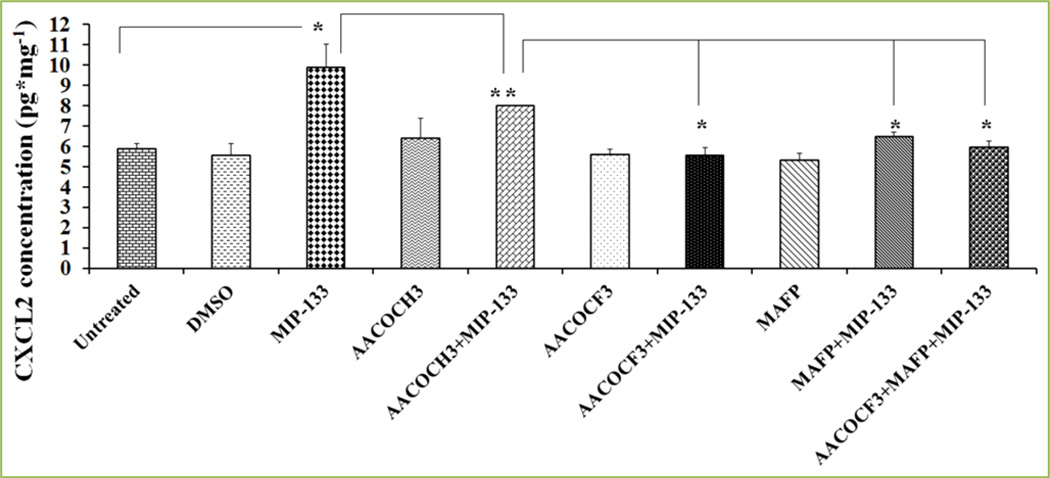
Figure 8. MIP-133 induces the up-regulation of chemokine CXCL2, and cPLA2α inhibitors diminish CXCL2 production in HCORN cells.
Inhibition of cPLA2α involved pre-incubating HCORN cells for 1 hour with cPLA2α inhibitors (20 µM AACOCF3 or 10 µM MAFP) or an inactive control compound (20 µM AACOCH3) or DMSO (5 µL solvent of AACOCF3, MAFP, and AACOCH3), and then incubated with or without 15 mg/ml of MIP-133 for 24 hours. CXCL2 production was examined by ELISA. The data shown represent mean±SEM of three independent experiments (*P<0.05). P values were obtained by unpaired Student’s t-test. AACOCF3, MAFP, AACOCH3, and DMSO without MIP-133 did not show significant effect on CXCL2 production when compared to untreated cells. The inactive control compound AACOCH3 did not block CXCL2 production induced by MIP-133. Reprinted from Tripathi et al.[14] with permission from Elsevier.
Results of our study suggested that activation of cPLA2α-AA pathway stimulated by MIP-133 is responsible for the upregulation of proinflammatory cytokines/chemokines in cornea [13, 14]. Thus, these findings indicate a novel pathogenic mechanism of Acanthamoeba keratitis mediated via cPLA2α-AA pathway.
Arachidonic acid in the accumulation of inflammatory cells
The cornea is a nonvascular that is significantly dissimilar from other tissues of eye in regard to numerous function and biological response to a wide variety of extracellular stimuli [58]. Cytosolic Ca2+-dependent PLA2α activation may have both valuable and damaging inflammatory effects on the cornea, depending on the efficacy and period of the host inflammatory response. Cytosolic Ca2+-dependent PLA2α may involve in the critical balance between eliminate the microorganism to generate a successful inflammatory response in cornea and in corneal scarring and blindness by inducing an excessive inflammatory response [58]. Leukocyte recruitment is characteristic of corneal inflammation, and these cells are known to be a source of cyclooxygenase (COX) and lipoxygenase (LOX) products. In corneal stimulation, polymorphonuclear leukocytes (PMNs) and lymphocytes can enter the corneal stroma from the vascular space within hours. These cells may become resident in a seemingly healed corneal epithelium. Mediators released by the injured corneal epithelium may provide a chemotactic signal to vascular PMNs. COX and LOX products were increased over control levels in the three corneal layers, especially in the corneal stroma, suggesting a persistence of PMNs associated with inflammation. PMNs and macrophages have been recognized in the stroma after a viral infection in the cornea. PMNs were also found in rabbit corneal stroma following complete reepithelialization after mechanical denudation [59–63].
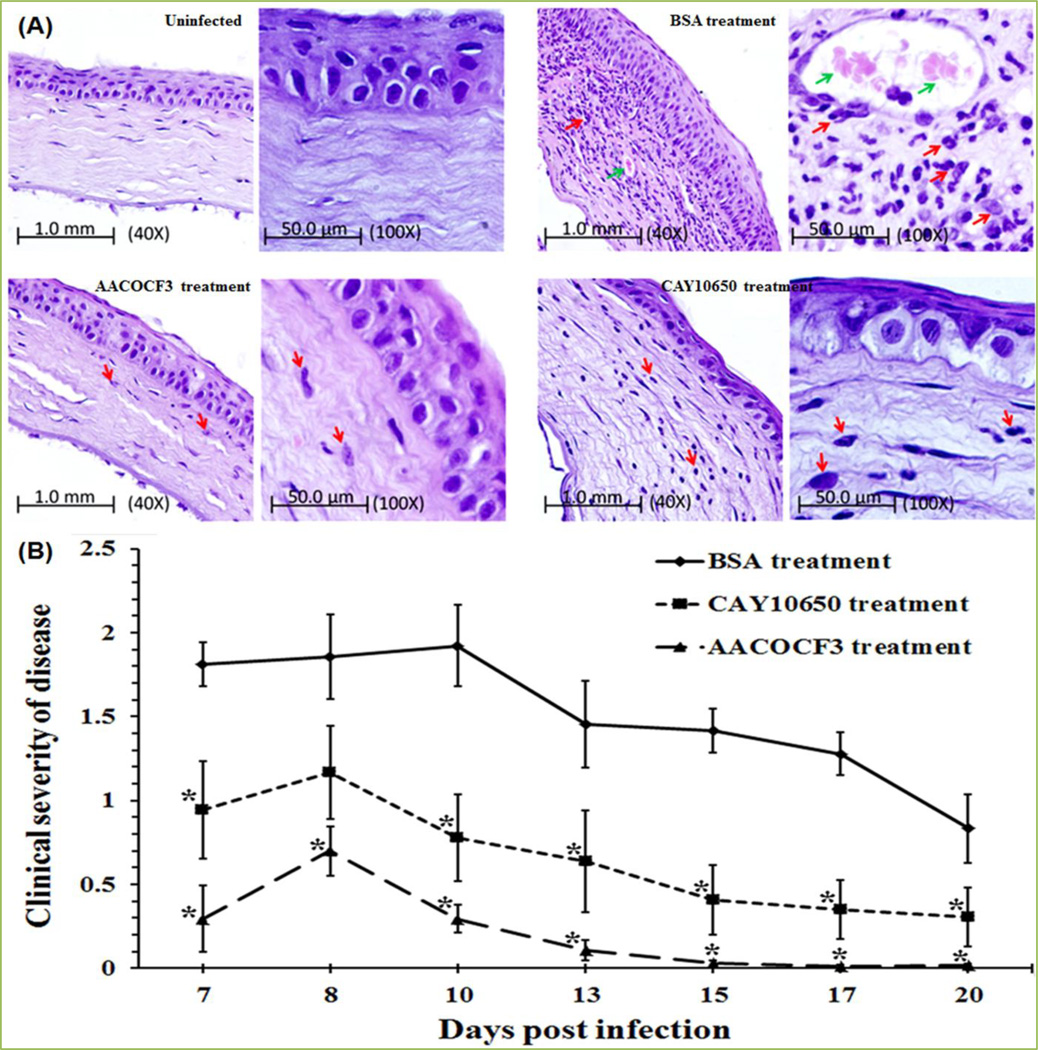
We showed that MIP-133 interacts with the corneal epithelial cells, and this interaction stimulates a rapid immune response by the production of cytokines/chemokines (IL-8, IL-6, IL-1β, and IFN-γ) which can recruit an effective host response to corneal infections [13]. Histologic features of the Chinese hamster corneas infected with Acanthamoeba castellanii and treated with cPLA2α inhibitors (AACOCF3 and CAY 10650) confirmed the efficacy of cPLA2α inhibitors treatment. The results indicated that inflammatory responses in the BSA-treated group were significantly more severe than either AACOCF3 or CAY10650-treated or untreated Chinese hamsters [14] (Fig. 9A). Treatment with the AACOCF3 and CAY10650 had an intense effect on the severity of keratitis (Fig. 9B). Moreover, Chinese hamsters treated with AACOCF3 had significantly less severe keratitis as compared with CAY10650 group. Observation of clinical severity of disease indicated that a cPLA2α inhibitor can be used as a therapeutic target in Acanthamoeba keratitis [14].
Figure 9. Therapeutic role of cPLA2α inhibitors in vivo Chinese hamsters.
cPLA2α inhibitors (AACOCF3 and CAY10650) 50 µg/5 µl was injected under the contact lens of an infected cornea three times a day for 6 days and topically from 7 to 20 days post-infection. As a control infected animals were treated with 50 µg/5µl of BSA. Lenses were removed 7 days post-infection. (A) Histopathological examination of infected cornea treated with cPLA2α inhibitor. Photomicrograph of corneas from a Chinese hamster infected with Acanthamoeba trophozoites-laden contact lenses. Animals were anesthetized and sacrificed 15 days after application of cPLA2α inhibitors. Uninfected group: Cornea of control animal; BSA treatment group: Cornea from infected animal, the corneal stroma of this group contained marked lamellar connective tissue disruption, extensive stroma swelling and neovascularized; Severe PMN cells and lymphocytes infiltration were present; AACOCF3 treatment group: Cornea from infected animal treated with AACOCF3 had very mild inflammation. Which is similar to the corneal section from control, untreated animals; CAY10650 treatment group: Cornea from animals treated with CAY10650 had very mild inflammation and few PMN cells infiltration. PMN cells (small arrows), and blood vessels (thick arrows) were present in the corneal stroma. Few inflammatory cells were also observed under the corneal endothelium from control, BSA treated animals. (B) cPLA2α inhibitor mitigates the pathogenesis of Acanthamoeba keratitis in vivo. Corneas were scored for clinical severity for the period indicated. Each graph line represents the mean±SEM of clinical severity of nine animals for the observed time points. The results shown are representative of three separate experiments. Asterisk indicates P values < 0.05 compared with infected untreated group and were analyzed by the Manne-Whitney’s U test. Reprinted from Tripathi et al. [14] with permission from Elsevier.
It has been revealed that corneal lesions are severely infiltrated with neutrophils in Acanthamoeba and Pseudomonas aeruginosa keratitis [64, 65]. Neutrophils are infiltrated to the cornea in response to the chemokine, CXCL2, which is secreted from corneal cells during infection [66, 67]. We have demonstrated that corneal infections with Acanthamoeba trophozoites stimulate CXCL2 production, and promotes the neutrophils infiltration to the infected cornea [38, 68]. We also showed that blockers of cPLA2α significantly inhibited CXCL2 production from HCORN cells [14]. The increased production of CXCL2 and its inhibition by AACOCF3 and MAFP, suggested that cPLA2α plays an important role in controlling chemokine production. Moreover, cPLA2 signaling has been shown to be involved in the expression of CXCL2 through cPLA2α-12/15-LOX pathway [69]. CXCL2 has a chemotactic effect on neutrophils and stimulates neutrophil infiltration [68]. In addition, macrophages and neutrophils are the first-line of defense in active Acanthamoeba keratitis infections; elimination of these cell types drastically changes the result of Acanthamoeba keratitis[68]. However, the signaling pathways involved in attracting neutrophils and macrophages into the infected cornea in Acanthamoeba keratitis are not completely understood.
It has been demonstrated that in the stroma, the lipoxygenase pathway resulted in an increased accumulation of 5- and 12-lipoxygenase products, which are known to be potent chemotactic agents for PMNs; these substances may play an imperative role in the initiation of PMNs migration in corneal stroma. Macrophages also appear to have a significant capacity to metabolize AA for the production of HETEs and leukotrienes, primarily 12-HETE. Stromal prostaglandins also increased early, but they reached maximal values after the injury. In acute phase of injury, TXB2 and 6-keto-PGFlα are produced during inflammation. Migration of PMNs is the major source of TXB2, whereas the increase in PGE2 and 6-keto-PGFlα suggests that other cells, as well, contribute to the synthesis of these products [70]
Recently in a clinical study of patients with bacterial keratitis, fungal keratitis, and Acanthamoeba keratitis showed a profound diminishment of the sub-basal corneal nerve plexus with increased corneal dendritic cells (DCs). DCs are the most potent antigen-presenting cells (APCs) of the body and have been demonstrated to be critical for the initiation of adaptive immune responses and for maintenance of peripheral tolerance. DCs, as well, represent the principal immune guards to the foreign world in the cornea and ocular surface [71]. Although numerous studies have comprehensively demonstrated the implication of AA metabolites in DCs activation, migration, and function [72–76]. DCs biology in cornea and involvement of lipid mediators in the DCs’ functions in ocular diseases need to be elucidated.
Recently, Biswas et al. [77] showed important findings of ocular infection with herpes simplex virus (HSV) which results in a blinding immunoinflammatory stromal keratitis lesion. They demonstrated that the inflammatory milieu and angiogenic stimuli started early after infection play a vital role in HSV-stimulated ocular lesions. COX-2-induced prostanoid synthesis played an important role in participant of this environment and the COX-2 inhibitors abrogate the cascade of events that culminate in stromal keratitis [77].
Thus, existing literatures highlighted the importance of AA in corneal immunobiology in numerous corneal inflammatory diseases; however, this knowledge is only the beginnings, not the end. Implications of AA-derived metabolites and their inhibitors in the cornea would attract the researchers to understand the pathogenesis of corneal diseases and to design the valuable approach for disease management.
Arachidonic acid (AA) in apoptosis
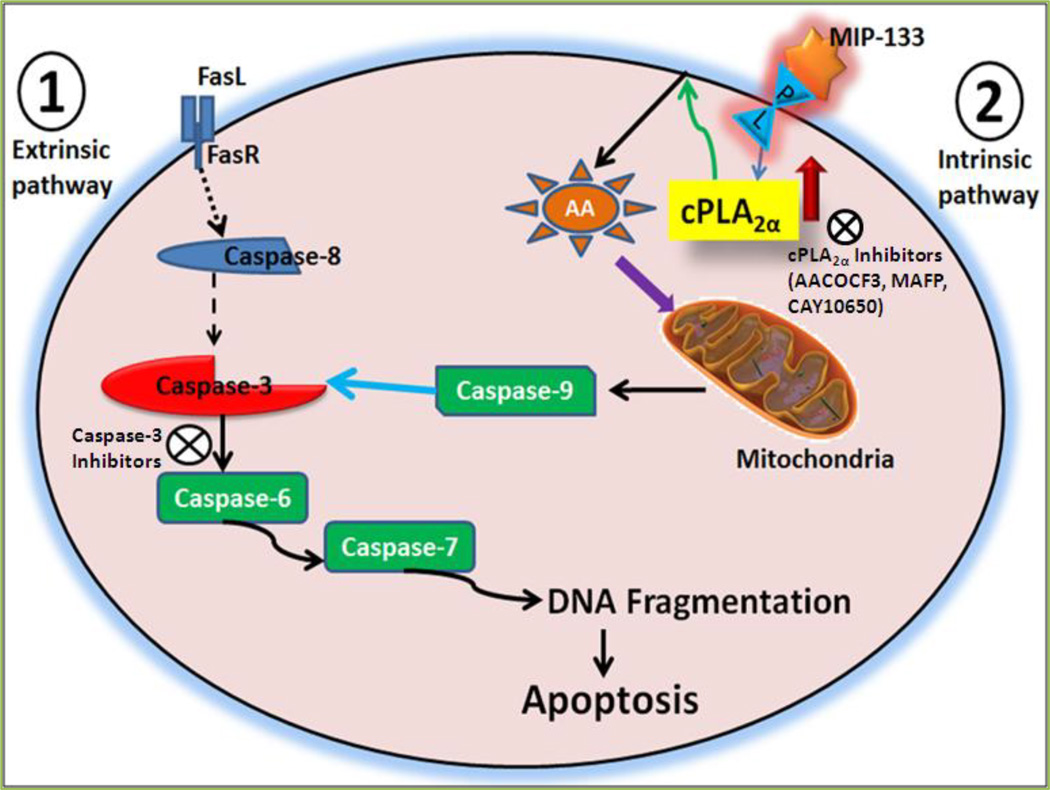
The term “apoptosis” was first introduced by Kerr and his colleagues in 1972 [78]. Apoptosis occurs via two major pathways - i) the extrinsic pathway and ii) the intrinsic pathway [79]. i) The extrinsic pathway of apoptosis is started upon the binding of an extracellular ligand to transmembrane death receptors of the tumor necrosis factor (TNF) super family. This interaction induces the assembly of the death-inducing signaling complex which then triggers initiator caspases to activate the enzymatic pathway and induces apoptotic cell death. ii) The intrinsic pathway (also known as mitochondrial pathway) is stimulated by the stimuli that lead to the permeabilization of mitochondrial outer membrane and then secrete the critical protein, cytochrome c (cyt c), from the mitochondrial intermembrane space to the cytosol to initiate and regulate caspase activation [79]. Thus, the mitochondria exhibit an important role in the mechanism of apoptosis. AA-derived eicosanoids, PGs, TX, LTs, EETs, and HETEs are the important group of lipid mediators that play a vital role in several physiological and pathophysiological processes [79].
Arachidonic acid is emerged as an imperative player in the apoptosis signaling. Nonsteroidal anti-inflammatory drugs (NSAID), which are among the few agents that can effectively prevent neoplasias [80], stimulate apoptosis via increased intracellular levels of AA, and their effects could mimic through the addition of exogenous AA [81]. It has been shown that the levels of intracellular AA could be modulated through over expression of COX-2 and fatty acyl-CoA ligase. Apoptosis was diminished by the stimulation of either enzyme, which also protected from the killing effects of AA [82]. Stimulation of apoptosis by the AA involved activation of caspase-3, a signaling mechanism that is amplified by the secretion of mitochondrial cytochrome c [83] and Smac/DIABLO [84]. Most of these eicosanoids generated from LOX, COXs, and CYP P450 pathways regulate apoptosis in the context of tumorigenesis and cancer although these eicosanoids, as well, participate in the apoptosis in non-cancer tissues [85, 86]. The PLA2 reaction is the principal step through which AA is synthesized from phospholipids [87]. It has also been shown that AA-selective Ca2+-dependent cPLA2 is essential for the cytotoxic action of TNFα, which triggers mitochondrial permeability transition pore opening by the activation of hydrolysis of phospholipid and then AA signals apoptosis via a mitochondrial effect that can be amplified by inhibition of LOX and COX [88].
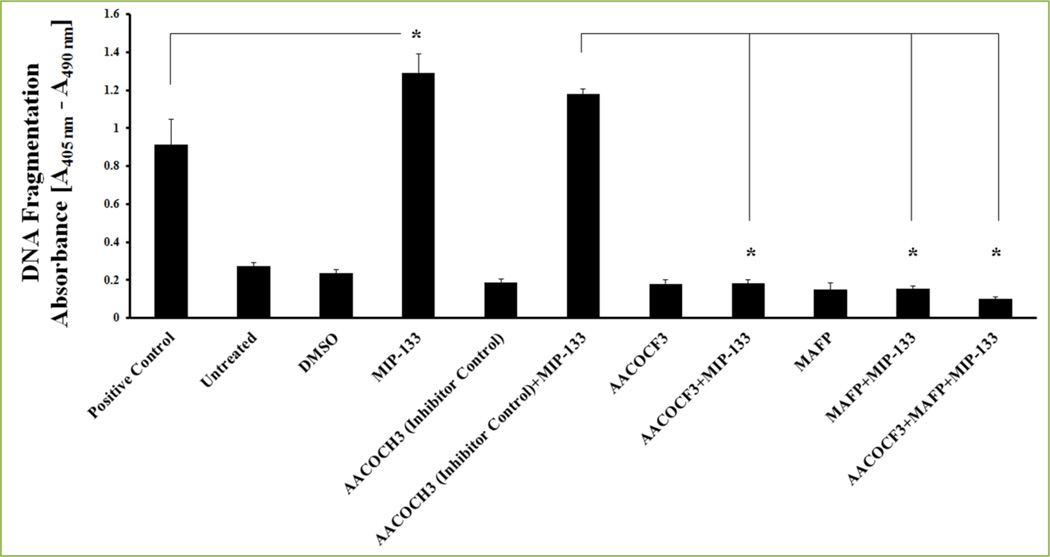
Recently, we demonstrated the implication of cPLA2α and AA role in corneal inflammation and apoptosis of corneal epithelial cells in Acanthamoeba castellanii infection [13, 14]. Acanthamoeba trophozoites’ secreted cytopathic protein, MIP-133, was known to be potent at triggering a caspase-3-dependent apoptosis signaling in corneal epithelial cells in vitro [64, 89, 90,]. We have revealed that specific inhibitors of cPLA2α enzymes (AACOCF3, MAFP, and CAY10650) and chicken anti-MIP-133 antiserum inhibit MIP-133 induced activation of cPLA2α activity and attenuate MIP-133-induced apoptosis of corneal epithelial cells [13, 14] (Fig. 10). Also, we showed that MIP-133 induced apoptosis in corneal epithelial cells of Fas receptor-deficient mice (lpr/lpr) is Fas receptor-independent [13]. MIP-133 interacts with phospholipids on HCE and HCORN cells to stimulate cPLA2α, AA release, and utilizes this cPLA2α pathway to provoke apoptosis of corneal epithelial cells [13, 14] (Fig. 11). Our findings [13, 14] are largely in concurrence with those of Kirschnek and Gulbins[91], who demonstrated an important role for PLA2 in Pseudomonas aeruginosa-stimulated apoptosis of human lung fibroblast cell line and conjunctiva epithelial cell line. They observed that AA production by activation of cPLA2 during P. aeruginosa infection exhibits an important role in the induction of host cell apoptosis [91].
Figure 10. MIP-133 induced apoptosis is blocked by cPLA2α inhibitors in HCE cells.
HCE cells were pre-incubated for 1 hour with cPLA2α inhibitors (10µM MAFP or 20µM AACOCF3) or an inactive negative control (20µM AACOCH3), and then incubated with or without 15 µg/ml MIP-133 for 24 hours. Apoptosis was examined by Cell Death Detection ELISAplus. MIP-133-induced apoptosis was confirmed by a positive control provided in the apoptosis kit. The data are mean ± SEM of three independent experiments. Asterisk indicates P value < 0.05 and were obtained by unpaired Student’s t-test. Reprinted from Tripathi et al. [13] with permission from copyright holder the Association for Research in Vision and Ophthalmology (ARVO).
Figure 11.
MIP-133 induces apoptosis of corneal epithelial cells through intrinsic apoptotic pathway[13,14].
Thus, Acanthamoeba induces apoptosis on contact-dependent mechanism to corneal epithelium which starts from MIP-133 interaction to cell membrane phospholipids of corneal epithelial cells and leads to the induction of cPLA2→AA pathway in sequential pathogenesis of Acanthamoeba keratitis [13, 14].
Future prospects
Arachidonic acid-derived metabolites has been an intensive area of lipid research for many decades. Therapeutic interventions based on the conventional AA cascades have become routine practices, such as COX inhibitors and LOX inhibitors in several diseases. The emerging evidence of AA-derived eicosanoids in corneal biology reviewed here suggests an important role of AA in modulating inflammatory cascades and apoptosis, may provide fruitful and stimulating avenue for exploring the novel approaches into the biological activities of AA-derived metabolites, which may be led to potential therapeutic interventions in the cure of corneal inflammation and infection. The complex corneal biology and the multifaceted nature of the AA metabolic pathways and complex pathogenic property of microbial pathogens might be a challenge to target efficient therapeutic remedies. Thus, researchers need to pay more attention to develop effective, safe, and “on target” pharmacological intervention to cure microbial corneal diseases.
Acknowledgments
This work was supported by Public Health Service Grant EY09756 from the National Institutes of Health.
Footnotes
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Yin H, Zhou Y, Zhu M, Hou S, Li Z, Zhong H, et al. Role of mitochondria in programmed cell death mediated by arachidonic acid-derived eicosanoids. Mitochondrion. 2013;13:209–224. doi: 10.1016/j.mito.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Khanapure SP, Garvey DS, Janero DR, Letts LG. Eicosanoids in inflammation: biosynthesis, pharmacology, and therapeutic frontiers. Curr Top Med Chem. 2007;7:311–340. doi: 10.2174/156802607779941314. [DOI] [PubMed] [Google Scholar]
- 3.Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. 2008;14:461–469. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Rossen NS, Hansen AJ, Selhuber-Unkel C, Oddershede LB. Arachidonic acid randomizes endothelial cell motion and regulates adhesion and migration. PLoS One. 2011;6:e25196. doi: 10.1371/journal.pone.0025196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piomelli D. Arachidonic acid in cell signaling. Curr Opin Cell Biol. 1993;5:274–280. doi: 10.1016/0955-0674(93)90116-8. [DOI] [PubMed] [Google Scholar]
- 6.Griswold DE, Webb E, Schwartz L, Hanna N. Arachidonic acid-induced inflammation: inhibition by dual inhibitor of arachidonic acid metabolism, SK&F 86002. Inflammation. 1987;11:189–199. doi: 10.1007/BF00916020. [DOI] [PubMed] [Google Scholar]
- 7.Kernacki KA, Berk RS. Characterization of arachidonic acid metabolism and the polymorphonuclear leukocyte response in mice infected intracorneally with Pseudomonas aeruginosa . Invest Ophthalmol Vis Sci. 1995;36:16–23. [PubMed] [Google Scholar]
- 8.Song W, Jiang R, Zhao C. Regulation of arachidonic acid in esophageal adenocarcinoma cells and tumor-infiltrating lymphocytes. Oncol Lett. 2013;5:1897–1902. doi: 10.3892/ol.2013.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scorrano L, Penzo D, Petronilli V, Pagano F, Bernardi P. Arachidonic acid causes cell death through the mitochondrial permeability transition. Implications for tumor necrosis factor-α aopototic signaling. J Biol Chem. 2001;276:12035–12040. doi: 10.1074/jbc.M010603200. [DOI] [PubMed] [Google Scholar]
- 10.Hyde CA, Missailidis S. Inhibition of arachidonic acid metabolism and its implication on cell proliferation and tumour-angiogenesis. Int Immunopharmacol. 2009;9:701–715. doi: 10.1016/j.intimp.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura M, Fujihara T, Mibu H, Hikida M. Arachidonic acid stimulates corneal epithelial migration. J Ocul Pharmacol. 1994;10:453–459. doi: 10.1089/jop.1994.10.453. [DOI] [PubMed] [Google Scholar]
- 12.Astudillo AM, Pérez-Chacón G, Balboa MA, Balsinde J. Arachidonic acid mobilization by stimuli of the innate immune response. Inmunología. 2009;28:182–192. [Google Scholar]
- 13.Tripathi T, Smith AD, Abdi M, Alizadeh H. Acanthamoeba-cytopathic protein induces apoptosis and proinflammatory cytokines in human corneal epithelial cells by cPLA2α activation. Invest Ophthalmol Vis Sci. 2012;53:7973–7982. doi: 10.1167/iovs.12-10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripathi T, Abdi M, Alizadeh H. Role of phospholipase A2 (PLA2) inhibitors in attenuating apoptosis of the corneal epithelial cells and mitigation of Acanthamoeba keratitis. Exp Eye Res. 2013;113:182–191. doi: 10.1016/j.exer.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 16.Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, et al. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci USA. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 18.Suram S, Brown GD, Ghosh M, Gordon S, Loper R, Taylor PR, et al. Regulation of cytosolic phospholipase A2 activation and cyclooxygenase 2 expression in macrophages by the β-glucan receptor. J Biol Chem. 2006;281:5506–5514. doi: 10.1074/jbc.M509824200. [DOI] [PubMed] [Google Scholar]
- 19.Kikawada E, Bonventre JV, Arm JP. Group V secretory PLA2 regulates TLR2-dependent eicosanoid generation in mouse cells through amplification of ERK and cPLA2α activation. Blood. 2007;110:561–567. doi: 10.1182/blood-2006-10-052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noor S, Goldfine H, Tucker DE, Suram S, Lenz LL, Akira S, et al. Activation of cytosolic phospholipase A2α in resident peritoneal macrophages by Listeria monocytogenes involves listeriolysin O and TLR2. J Biol Chem. 2008;283:4744–4755. doi: 10.1074/jbc.M709956200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pindado J, Balsinde J, Balboa MA. TLR3-dependent induction of nitric oxide synthase in RAW264.7 macrophage-like cells via a cytosolic phospholipase A2/cyclooxygenase-2 pathway. J Immunol. 2007;179:4821–4828. doi: 10.4049/jimmunol.179.7.4821. [DOI] [PubMed] [Google Scholar]
- 22.Aderem AA, Cohen DS, Wright SD, Cohn ZA. Bacterial lipopolysaccharides prime macrophages for enhanced release of arachidonic acid metabolites. J Exp Med. 1986;164:165–179. doi: 10.1084/jem.164.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aderem AA, Cohn ZA. Calcium ionophore synergizes with bacterial lipopolysaccharides in activating macrophage arachidonic acid metabolism. J Exp Med. 1988;167:623–631. doi: 10.1084/jem.167.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balsinde J, Dennis EA. Distinct roles in signal transduction for each of the phospholipase A2 enzymes present in P388D1 macrophages. J Biol Chem. 1996;271:6758–6765. doi: 10.1074/jbc.271.12.6758. [DOI] [PubMed] [Google Scholar]
- 25.Balboa MA, Balsinde J, Winstead MV, Tischfield JA, Dennis EA. Novel Group V phospholipase A2 involved in arachidonic acid mobilization in murine P388D1 macrophages. J Biol Chem. 1996;271:32381–32384. doi: 10.1074/jbc.271.50.32381. [DOI] [PubMed] [Google Scholar]
- 26.Balsinde J, Balboa MA, Dennis EA. Inflammatory activation of arachidonic acid signaling in murine P388D1 macrophages via sphingomyelin synthesis. J Biol Chem. 1997;272:20373–20377. doi: 10.1074/jbc.272.33.20373. [DOI] [PubMed] [Google Scholar]
- 27.Shinoara H, Balboa MA, Johson CA, Balsinde J, Dennis EA. Regulation of delayed prostaglandin production in activated P388D1 macrophages by Group IV cytosolic and Group V secretory phospholipase A2s. J Biol Chem. 1999;274:12263–12268. doi: 10.1074/jbc.274.18.12263. [DOI] [PubMed] [Google Scholar]
- 28.Balsinde J, Shinoara H, Lefkowitz LJ, Johnson CA, Balboa MA, Dennis EA. Group V phospholipase A2-dependent induction of cyclooxygenase-2 in macrophages. J Biol Chem. 1999;274:25967–25970. doi: 10.1074/jbc.274.37.25967. [DOI] [PubMed] [Google Scholar]
- 29.Shirai Y, Balsinde J, Dennis EA. Localization and functional interrelationships among cytosolic Group V, and Ca2+-independent Group VI phospholipase A2s in P388D1 macrophages using GFP/RFP constructs. Biochim Biophys Acta. 2005;1735:119–129. doi: 10.1016/j.bbalip.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Qi H-Y, Shelhamer JH. Toll-like receptor 4 signaling regulates cytosolic phospholipase A2 activation and lipid generation in lipopolysaccharide-stimulated macrophages. J Biol Chem. 2005;280:38969–38975. doi: 10.1074/jbc.M509352200. [DOI] [PubMed] [Google Scholar]
- 31.Grkovich A, Johnson CA, Buczynski MW, Dennis EA. Lipopolysaccharide-induced cyclooxygenase-2 expression in human U937 macrophages is phosphatidic acid phosphohydrolase-1-dependent. J Biol Chem. 2006;281:32978–32987. doi: 10.1074/jbc.M605935200. [DOI] [PubMed] [Google Scholar]
- 32.Grkovich A, Armando A, Quehenberger O, Dennis EA. TLR-4 mediated group IVA phospholipase A2 activation is phosphatidic acid phosphohydrolase 1 and protein kinase C dependent. Biochim Biophys Acta. 2009;1791:975–982. doi: 10.1016/j.bbalip.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hattermann K, Picard S, Borgeat M, Leclerc P, Pouliot M, Borgeat P. The Toll-like receptor 7/8-ligand resiquimod (R-848) primes human neutrophils for leukotriene B4, prostaglandin E2 and platelet-activating factor biosynthesis. FASEB J. 2007;21:1575–1585. doi: 10.1096/fj.06-7457com. [DOI] [PubMed] [Google Scholar]
- 34.Lee S-H, Lee J-G, Kim J-R, Baek S-H. Toll-like receptor 9-mediated cytosolic phospholipase A2 activation regulates expression of inducible nitric oxide synthase. Biochem Biophys Res Commun. 2007;364:996–1001. doi: 10.1016/j.bbrc.2007.10.111. [DOI] [PubMed] [Google Scholar]
- 35.Lee J-G, Lee S-H, Park D-W, Lee S-H, Yoon H-S, Chin B-R, et al. Toll-like receptor 9-stimulated monocyte chemoattractant protein-1 is mediated via JNK-cytosolic phospholipase A2-ROS signaling. Cell Signal. 2008;20:105–111. doi: 10.1016/j.cellsig.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Buczynski MW, Stephens DL, Bowers-Gentry RC, Grkovich A, Deems RA, Dennis EA. TLR-4 and sustained calcium agonists synergistically produce eicosanoids independent of protein synthesis in RAW264.7 cells. J Biol Chem. 2007;282:22834–22847. doi: 10.1074/jbc.M701831200. [DOI] [PubMed] [Google Scholar]
- 37.Ruipérez V, Astudillo AM, Balboa MA, Balsinde J. Coordinate regulation of TLR-mediated arachidonic acid mobilization in macrophages by Group IVA and Group V phospholipase A2s. J Immunol. 2009;182:3877–3883. doi: 10.4049/jimmunol.0804003. [DOI] [PubMed] [Google Scholar]
- 38.Alizadeh H, Tripathi T, Abdi M, Smith AD. Pathogenic strains of Acanthamoeba are recognized by TLR4 and initiated inflammatory responses in the cornea. PLoS One. 2014;9:e92375. doi: 10.1371/journal.pone.0092375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulkarni PS, Fleisher L, Srinivasan BD. The synthesis of cyclooxygenase products in ocular tissues of various species. Curr Eye Res. 1984;3:447–452. doi: 10.3109/02713688408997232. [DOI] [PubMed] [Google Scholar]
- 40.Kulkarni P, Rodriguez AV, Srinivasan BD. Human anterior uvea synthesizes lipoxygenase products from arachidonic acid. Invest Ophthalmol Vis Sci. 1984;25:221–223. [PubMed] [Google Scholar]
- 41.Bito LZ, Draga A, Blanco J, Camras CB. Long-term maintenance of reduced intraocular pressure by daily or twice daily topical application of prostaglandins to cat or rhesus monkey eyes. Invest Ophthalmol Vis Sci. 1983;24:312–319. [PubMed] [Google Scholar]
- 42.Stjernschantz J, Sherk T, Borget P, Sears M. Intraocular effects of lipoxygenase pathway products in arachidonic acid metabolism. Acta Ophthalmol (Copenh) 1984;62:104–111. doi: 10.1111/j.1755-3768.1984.tb06763.x. [DOI] [PubMed] [Google Scholar]
- 43.Taylor L, Menconi M, Leibowitz HM, Polgar P. The effect of ascorbate, hydroperoxides, and bradykinin on prostaglandin production by corneal and lens cells. Invest Ophthalmol Vis Sci. 1982;23:378–382. [PubMed] [Google Scholar]
- 44.Bazan HE, Birkle DL, Beuerman R, Bazan NG. Cryogenic lesion alters the metabolism of arachidonic acid in rabbit cornea layers. Invest Ophthalmol Vis Sci. 1985;26:474–480. [PubMed] [Google Scholar]
- 45.Hurst JS, Balazy M, Bazan HE, Bazan NG. The epithelium, endothelium, and stroma of the rabbit cornea generate (12S)-hydroxyeicosatetraenoic acid as the main lipoxygenase metabolite in response to injury. J Biol Chem. 1991;266:6726–6730. [PubMed] [Google Scholar]
- 46.Gupta AG, Hirakata A, Proia AD. Effect of inhibitors of arachidonic acid metabolism on corneal reepithelialization in the rat. Exp Eye Res. 1993;56:701–708. doi: 10.1006/exer.1993.1087. [DOI] [PubMed] [Google Scholar]
- 47.Stoltz RA, Conners MS, Dunn MW, Schwartzman ML. Effect of metabolic inhibitors on arachidonic acid metabolism in the corneal epithelium: evidence for cytochrome P450-mediated reactions. J Ocul Pharmacol. 1994;10:307–317. doi: 10.1089/jop.1994.10.307. [DOI] [PubMed] [Google Scholar]
- 48.McGiff JC. Cytochrome P-450 metabolism of arachidonic acid. Annu Rev Pharmacol Toxicol. 1991;31:339–369. doi: 10.1146/annurev.pa.31.040191.002011. [DOI] [PubMed] [Google Scholar]
- 49.Sellers RS, Silverman L, Khan KNM. Cyclooxygenase-2 Expression in the Cornea of Dogs with Keratitis. Vet Pathol. 2004;41:116–121. doi: 10.1354/vp.41-2-116. [DOI] [PubMed] [Google Scholar]
- 50.Takahira M, Sakurada N, Segawa Y, Shirao Y. Two types of K+ currents modulated by arachidonic acid in bovine corneal epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:1847–1854. [PubMed] [Google Scholar]
- 51.Burke JE, Dennis ED. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50:S237–S242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taketo MM, Sonoshita M. Phospholipase A2 and apoptosis. Biochim Biophys Acta. 2002;1585:72–76. doi: 10.1016/s1388-1981(02)00326-8. [DOI] [PubMed] [Google Scholar]
- 53.Hirabayashi T, Shimizu T. Localization and regulation of cytosolic phospholipase A(2) Biochim Biophys Acta. 2000;1488:124–138. doi: 10.1016/s1388-1981(00)00115-3. [DOI] [PubMed] [Google Scholar]
- 54.Hefner Y, Borsch-Haubold AG, Murakami M, Wilde JI, Pasquet S, Schieltz D, et al. Serine 727 phosphorylation and activation of cytosolic phospholipase A2 by MNK1-related protein kinases. J Biol Chem. 2000;275:37542–37551. doi: 10.1074/jbc.M003395200. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh M, Loper R, Ghomashchi F, Tucker DE, Bonventre JV, Gelb MH, et al. Function, Activity, and Membrane Targeting of Cytosolic Phospholipase A2ζ in mouse lung fibroblasts. J Biol Chem. 2007;282:11676–11686. doi: 10.1074/jbc.M608458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Kolko M. Phospholipases A2 in ocular homeostasis and diseases. Biochimie. 2010;92:611–619. doi: 10.1016/j.biochi.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Xue ML, Thakur A, Lutze-Mann L, Willcox MD. Pro-inflammatory cytokine/chemokine gene expression in human corneal epithelial cells colonized by Pseudomonas aeruginosa . Clin Experiment Ophthalmol. 2000;28:197–200. doi: 10.1046/j.1442-9071.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 58.Song PI, Abraham TA, Park Y, Zivony AS, Harten B, Edelhauser HF, et al. The expression of functional LPS receptor proteins CD14 and toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 2001;42:2867–2877. [PubMed] [Google Scholar]
- 59.Srinivasan BD, Kulkarni PS, Eakins KE. Characterization of chemotactic factors in corneal wound healing. Adv Prostaglandin Thromboxane Res. 1980;7:861–864. [PubMed] [Google Scholar]
- 60.Higgs GA, Moncada S, Salmon JA, Seager K. The source of thromboxane and prostaglandins in experimental inflammation. Br J Pharmacol. 1983;79:863–868. doi: 10.1111/j.1476-5381.1983.tb10530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Metcalf JF, Reichert RW. Histological and electron microscopic studies of experimental herpetic keratitis in the rabbit. Invest Ophthalmol Vis Sci. 1979;18:1123–1138. [PubMed] [Google Scholar]
- 62.Pfister RR. The healing of corneal epithelial abrasions in the rabbit: a scanning electron microscope study. Invest Ophthalmol. 1975;14:648–661. [PubMed] [Google Scholar]
- 63.Srinivasan BD, Worgul BV, Iwamoto T, Eakins KE. The reepithelialization of rabbit cornea following partial and complete epithelial denudation. Exp Eye Res. 1977;25:343–351. doi: 10.1016/0014-4835(77)90101-4. [DOI] [PubMed] [Google Scholar]
- 64.Clarke DW, Niederkorn JY. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol. 2006;22:175–180. doi: 10.1016/j.pt.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Kernacki KA, Barrett RP, McClellan SA, Hazlett LD. Aging PMN response to P. aeruginosa infection. Invest Ophthalmol Vis Sci. 2000;41:3019–3025. [PubMed] [Google Scholar]
- 66.Kernacki KA, Barrett RP, Hobden JA, Hazlett LD. Macrophage inflammatory protein-2 is a mediator of polymorphonuclear neutrophil influx in ocular bacterial infection. J Immunol. 2000;164:1037–1045. doi: 10.4049/jimmunol.164.2.1037. [DOI] [PubMed] [Google Scholar]
- 67.Kwon B, Hazlett LD. Association of CD4+ T cell-dependent keratitis with genetic susceptibility to Pseudomonas aeruginosa keratitis. J Immunol. 1997;159:6283–6290. [PubMed] [Google Scholar]
- 68.Hurt M, Apte S, Leher H, Howard K, Niederkorn J, Alizadeh H. Exacerbation of Acanthamoeba keratitis in animals treated with anti-macrophage inflammatory protein 2 or antineutrophil antibodies. Infect Immun. 2001;69:2988–2995. doi: 10.1128/IAI.69.5.2988-2995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuwata H, Nonaka T, Murakami M, Kudo I. Search of factors that intermediate cytokine-induced group IIA phospholipase A2 expression through the cytosolic phospholipase A2- and 12/15-lipoxygenase-dependent pathway. J Biol Chem. 2005;280:25830–25839. doi: 10.1074/jbc.M500168200. [DOI] [PubMed] [Google Scholar]
- 70.Bazan HE, Birkle DL, Beuerman R, Bazan NG. Cryogenic lesion alters the metabolism of arachidonic acid in rabbit cornea layers. Invest Ophthalmol Vis Sci. 1985;26:474–480. [PubMed] [Google Scholar]
- 71.Cruzat A, Witkin D, Baniasadi N, Zheng L, Ciolino JB, Jurkunas UV, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52:5136–5143. doi: 10.1167/iovs.10-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zimpfer U, Dichmann S, Termeer CC, Simon JC, Schröder JM, Norgauer J. Human dendritic cells are a physiological source of the chemotactic arachidonic acid metabolite 5-oxo-eicosatetraenoic acid. Inflamm Res. 2000;49:633–638. doi: 10.1007/s000110050641. [DOI] [PubMed] [Google Scholar]
- 73.Harizi H, Gualde N. Eicosanoids: an emerging role in dendritic cell biology. Arch Immunol Ther Exp (Warsz) 2004;52:1–5. [PubMed] [Google Scholar]
- 74.Zeyda M, Säemann MD, Stuhlmeier KM, Mascher DG, Nowotny PN, Zlabinger GJ, et al. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-κB activation. J Biol Chem. 2005;280:14293–14301. doi: 10.1074/jbc.M410000200. [DOI] [PubMed] [Google Scholar]
- 75.Valera I, Fernández N, Trinidad AG, Alonso S, Brown GD, Alonso A, et al. Costimulation of dectin-1 and DC-SIGN triggers the arachidonic acid cascade in human monocyte-derived dendritic cells. J Immunol. 2008;180:5727–5736. doi: 10.4049/jimmunol.180.8.5727. [DOI] [PubMed] [Google Scholar]
- 76.Yen JH, Khayrullina T, Ganea D. PGE2-induced metalloproteinase-9 is essential for dendritic cell migration. Blood. 2008;111:260–270. doi: 10.1182/blood-2007-05-090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Biswas PS, Banerjee K, Kim B, Kinchington PR, Rouse BT. Role of inflammatory cytokine-induced cyclooxygenase 2 in the ocular immunopathologic disease herpetic stromal keratitis. J Virol. 2005;79:10589–10600. doi: 10.1128/JVI.79.16.10589-10600.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kerr JFR. Shrinkage necrosis: a distinct mode of cellular death. J Pathol. 1971;105:13–20. doi: 10.1002/path.1711050103. [DOI] [PubMed] [Google Scholar]
- 79.Yin H, Zhou Y, Zhu M, Hou S, Li Z, Zhong H, et al. Role of mitochondria in programmed cell death mediated by arachidonic acid-derived eicosanoids. Mitochondrion. 2013;13:209–224. doi: 10.1016/j.mito.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 80.Baron JA, Sandler RS. Nonsteroidal anti-inflammatory drugs and cancer prevention. Annu Rev Med. 2000;51:511–523. doi: 10.1146/annurev.med.51.1.511. [DOI] [PubMed] [Google Scholar]
- 81.Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci USA. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cao Y, Pearman AT, Zimmerman GA, McIntyre TM, Prescott SM. Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci USA. 2000;97:11280–11285. doi: 10.1073/pnas.200367597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 84.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 85.Claria J. Regulation of cell proliferation and apoptosis by bioactive lipid mediators. Recent Pat Anticancer Drug Discov. 2006;1:369–382. doi: 10.2174/157489206778776961. [DOI] [PubMed] [Google Scholar]
- 86.de Moraes E, Dar NA, de Moura Gallo CV, Hainaut P. Cross-talks between cyclooxygenase-2 and tumor suppressor protein p53: balancing life and death during inflammatory stress and carcinogenesis. Int J Cancer. 2007;121:929–937. doi: 10.1002/ijc.22899. [DOI] [PubMed] [Google Scholar]
- 87.Caro AA, Cederbaum AI. Role of cytochrome P450 in phospholipase A2- and arachidonic acid-mediated cytotoxicity. Free Radic Biol Med. 2006;40:364–375. doi: 10.1016/j.freeradbiomed.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 88.Penzo D, Petronilli V, Angelin A, Cusan C, Colonna R, Scorrano L, et al. Arachidonic acid released by phospholipase A2 activation triggers Ca2+-dependent apoptosis through the mitochondrial pathway. J Biol Chem. 2004;279:25219–25225. doi: 10.1074/jbc.M310381200. [DOI] [PubMed] [Google Scholar]
- 89.Leher H, Silvany R, Alizadeh H, Huang J, Niederkorn JY. Mannose induces the release of cytopathic factors from Acanthamoeba castellanii . Infect Immun. 1998;66:5–10. doi: 10.1128/iai.66.1.5-10.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hurt M, Neelam S, Niederkorn J, Alizadeh H. Pathogenic Acanthamoeba spp secrete a mannose-induced cytolytic protein that correlates with the ability to cause disease. Infect Immun. 2003;71:6243–6255. doi: 10.1128/IAI.71.11.6243-6255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kirschnek S, Gulbins E. Phospholipase A2 functions in Pseudomonas aeruginosa-induced apoptosis. Infect Immun. 2006;74:850–860. doi: 10.1128/IAI.74.2.850-860.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]