Abstract
Peripheral neuropathic pain, typified by the development of spontaneous pain or pain hypersensitivity following injury to the peripheral nervous system, is common, greatly impairs quality of life, and is inadequately treated with available drugs. Maladaptive changes in chloride homeostasis due to a decrease in the functional expression of the potassium-chloride cotransporter KCC2 in spinal cord dorsal horn neurons are a major contributor to the central disinhibition of γ-aminobutyric acid type A receptor– and glycine receptor–mediated signaling that characterizes neuropathic pain. A compelling novel analgesic strategy is to restore spinal ionotropic inhibition by enhancing KCC2-mediated chloride extrusion. We review the data on which this theory of alternative analgesia is based, discuss recent high-throughput screens that have searched for small-molecule activators of KCC2, and propose other strategies of KCC2 activation based on recent developments in the basic understanding of KCC2’s functional regulation. Exploiting the chloride-dependent functional plasticity of the γ-aminobutyric acid and glycinergic system by targeting KCC2 may be a tenable method of restoring ionotropic inhibition not only in neuropathic pain but also in other “hyperexcitable” diseases of the nervous system such as seizures and spasticity.
Several neurological disorders are characterized by excessive activity of neuronal circuits due to a loss of the hyperpolarizing action of γ-aminobutyric acid (GABA), the principal inhibitory amino acid neurotransmitter in the mammalian central nervous system, including a number of seizure disorders (such as temporal lobe epilepsy, neonatal seizures, and perilesional seizures) and neuropathic pain.1 Peripheral neuropathic pain is a prototypic disorder featuring so-called GABAergic disinhibition—in this case, within the dorsal horn of the spinal cord.2 Neuropathic pain is a common disease, greatly impairs function and quality of life, and places a high economic burden on society.3 Following peripheral nerve injury, maladaptive neuronal plasticity occurring anywhere along the nociceptive pathway in the peripheral and central nervous systems can alter signal processing so that pain is felt in the absence of a stimulus, and responses to innocuous stimuli (allodynia) and/or noxious stimuli (hyperalgesia) are enhanced. Chronic neuropathic pain constitutes a major area of unmet need in clinical medicine, as symptoms in most patients are recalcitrant to existing analgesics.3 New therapeutic strategies specifically based on an improved understanding of disease pathogenesis at the molecular level are needed. Currently, GABAergic disinhibition is recognized to be due not only to a loss of inhibitory GABAergic interneurons but also to impaired neuronal chloride (Cl−) homeostasis of postsynaptic secondary neurons in the dorsal horn secondary to decreased functional expression of the potassium (K+)–Cl− cotransporter KCC2. In turn, the recent discovery of compounds that indirectly restore GABA inhibition via KCC2 activation has set the stage for a new generation of “indirect ionotropic analgesics.”
Neuropathic Pain: A Major Problem of Unmet Clinical Need
Neuropathic pain arises from persistent pathological changes in neurons anywhere in the nociceptive pathway that lower the threshold for activation. Peripheral nerves can become sensitized by changes in the expression and/or activity of ion channels that alter intrinsic membrane excitability (eg, TRESK, hyperpolarization-activated cyclic nucleotide-gated channels, and voltage-gated sodium [Na+] channels). Altered processing of nociception in the central nervous system, ie, “central sensitization,” is also important3 (Figure 1). The loss of inhibitory GABAergic and glycinergic signaling within the dorsal horn is critical in this process, amplifying the response to incoming stimuli.
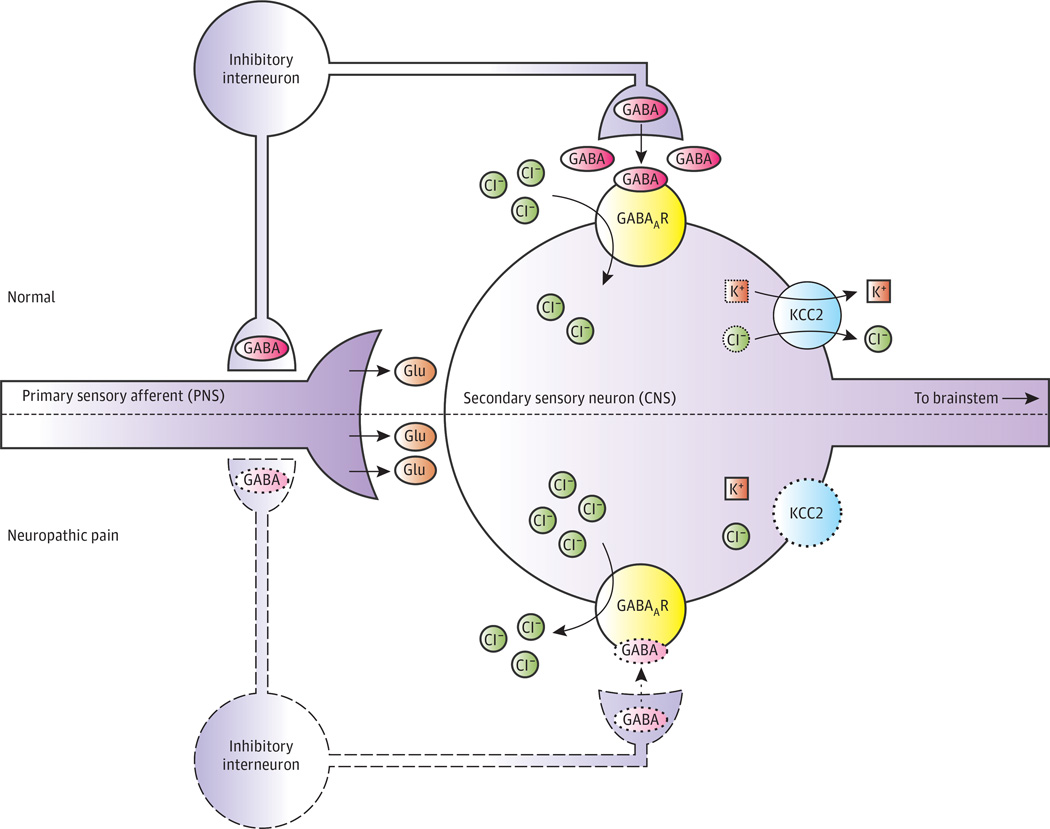
Figure 1. Dysfunction of γ-Aminobutyric Acid (GABA)–ergic Signaling in the Superficial Dorsal Horn in Neuropathic Pain.
In normal function, nociceptive fibers innervate peripheral tissues and form excitatory (glutamatergic) synapses onto secondary sensory neurons in superficial laminae (I and II) of the dorsal horn. Within the dorsal horn, GABAergic interneurons organized in polysynaptic translaminar networks regulate nociceptive signals by inhibiting primary and secondary neurons. Activity of KCC2 in sensory neuronsmaintains a low intracellular chloride (Cl−) concentration. Consequently, GABA type A receptor (GABAAR) activation by GABA released from interneurons results in Cl− influx and neuronal hyperpolarization. Neuropathic pain is characterized by dysregulation of inhibitory networks. Some inhibitory interneurons undergo apoptosis, and GABA synthesis and release by interneurons decrease. Furthermore, KCC2 activity is significantly reduced, resulting in the accumulation of Cl− within neurons. Thus, GABAAR activation results in reduced hyperpolarization and may even result in depolarization (excitation). Together, these effects result in disinhibition of primary afferent fibers and fewer inhibitory postsynaptic currents, leading to hyperalgesia and allodynia. CNS indicates central nervous system; Glu, glutamate; K+, potassium; and PNS, peripheral nervous system. Adapted with permission from Elsevier.4
Neuropathic pain presents a major clinical challenge. Etiological heterogeneity, variation in genetic susceptibility, and environmental factors make it difficult to predict which patients will develop neuropathic pain after injury and how particular patients will respond to specific drugs. An incomplete understanding of the molecular mechanisms in neuropathic pain has hindered the development of targeted intervention. Current treatments aim to inhibit neuronal excitability (eg, anticonvulsants, Na+ channel inhibitors), activate the endogenous opioid system (eg, morphine), antagonize enzymes responsible for pain fiber sensitization (eg, nonsteroidal anti-inflammatory drugs), or stimulate descending pain modulation systems (eg, dual uptake inhibitors). However, these drugs have significant adverse effects and only about 30% of patients are adequately treated.5 Furthermore, neuropathic pain represents a tremendous health care resource burden; in the United States, patients with peripheral neuropathic pain have about 3-fold greater annual health care expenditures than matched controls.6 Thus, there is great need for more effective ways to treat neuropathic pain.
Spinal GABA/Glycinergic System, Which Gates Nociceptive Input, Is a Key Molecular Substrate of Neuropathic Pain
In the spinal cord, GABAergic and glycinergic interneurons synapse with both presynaptic (primary sensory) and postsynaptic (secondary) dorsal horn neurons to modulate afferent input. In rodents, pharmacological blockade of GABA type A (GABAA) and glycine receptors causes allodynia and hyperalgesia, indicating that these neurogenic pain states can be unmasked even without direct nerve damage, thus emphasizing the importance of constitutive activity in inhibitory interneurons in maintaining normal sensitivity.7 Furthermore, GABAA receptor (GABAAR) inhibition enhances polysynaptic excitatory transmission to the superficial dorsal horn, suggesting the importance of GABAergic regulation of polysynaptic circuits.8 Thus, inhibitory interneurons “gate” nociceptive input by modulating the activation produced by peripheral nociceptor input in dorsal horn neurons.
In peripheral neuropathic pain, there is a decrease in inhibitory signaling from GABAergic interneurons, which dysregulates nociceptive gating (Figure 1). There is a significant postinjury decrease in GABAAR-mediated, primary afferent-evoked inhibitory postsynaptic currents in superficial laminae of the dorsal horn.2 Immunohistochemical staining reveals significantly reduced levels of GABA in laminae I through III early in the development of neuropathic pain, which is followed by dysfunctional GABA release and impaired GABAAR-mediated inhibition in later stages.9 There is also evidence for some apoptotic cell loss of GABAergic dorsal horn neurons triggered by primary afferent glutamatergic signaling.10 Application of the GABAAR agonist muscimol to the spinal cord prevents thermal hyperalgesia following peripheral nerve injury,11 and transplantation of GABAergic neuron precursors into the dorsal horn reduces neuropathic pain.12 Apart from loss of GABAergic cells and reduction in GABA release, GABAergic inhibition is reduced through a number of other mechanisms; one of these is loss of KCC2 functional expression, which appears to play a role in driving GABAergic disinhibition by collapsing the Cl− gradient that is required for GABAAR-mediated hyperpolarization (discussed later).1
The importance of inhibitory signaling in the dorsal horn and its loss in neuropathic pain states suggests the potential therapeutic effectiveness of specifically targeting the GABAergic system. However, existing GABAAR modulators, such as benzodiazepines, or GABABR agonists are rarely used for the treatment of neuropathic pain because of their narrow therapeutic window and adverse effects such as sedation or motor impairment. Receptor subtype–specific GABA agonists might allow analgesia without adverse effects, but the complexity of GABA receptor subunit localization and composition makes this challenging, and no such drugs are yet available. Analternative approach would be to correct abnormal Cl− gradients that occur in the dorsal horn in neuropathic pain, which contribute to central disinhibition, by targetingKCC2 to reduce this disinhibition or actually restore inhibition.
KCC2-Dependent GABA Receptor and Glycine Receptor Functional Plasticity
The GABAARs are ligand-gated, Cl−-permeable ion channels that can trigger a continuum of responses when activated, ranging from membrane depolarization and excitation to hyperpolarization and inhibition, depending on the intracellular Cl− concentration ([Cl−]i)of the postsynaptic neurons that express these receptors. When a Cl−-selective channel such as GABAAR opens, it pulls the neuron’s membrane potential toward the Cl− equilibrium potential (ECl). When[Cl−]i is high, ECl is more positive (for [Cl−]i = 30 mM, [Cl−]o = 110 mM and ECl = −35 mV) than when [Cl−]i is low(for[Cl−]i = 5 mM, [Cl−]o = 110 mM and ECl = −83 mV). The [Cl−]i is dynamically regulated by the combined activities of plasmalemmal Cl− channels and transporters, endowing GABAergic neurons with a remarkable functional plasticity. The K+-Cl− cotransporter KCC2 is critical for proper neuronal Cl− homeostasis and consequently GABA signaling1 (Figure 2). The low [Cl−]i present in mature neurons leads to hyperpolarizing inhibitory postsynaptic potentials mediated by GABAARs and is largely achieved viaKCC2-mediated Cl− extrusion. Because the firing threshold is set by voltage-gated Na+ channels (roughly −60 mV), GABAAR activation can be excitatory or inhibitory depending on intraneuronal [Cl−]i. Thus, KCC2 acts as a slider control of neuronal excitability by varying ECl.
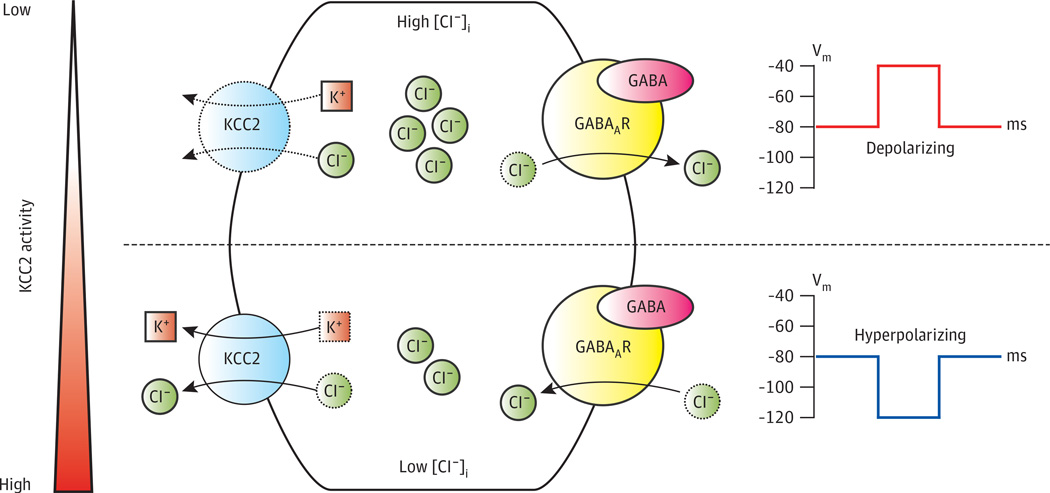
Figure 2. Potassium-Chloride Cotransporter KCC2 as a Slider Control of Neuronal Excitability.
γ-Aminobutyric acid (GABA) type A receptors (GABAARs) are ligand-gated chloride (Cl−) channels whose effect on membrane potential (Vm) depends on intracellular Cl− concentration ([Cl−]i). When GABAAR channels are opened, the Vm is pulled toward the Cl− equilibrium potential (ECl), which is determined by [Cl−]i and the extracellular Cl− concentration ([Cl−]e), the latter of which remains relatively constant. The potassium [K+]–Cl− cotransporter KCC2 is the major Cl− efflux mechanism of neurons. Thus, activity of KCC2 is a major determinant of [Cl−]i and, consequently, the effect of GABAAR activation on Vm. In conditions of low KCC2 activity, such as early in development or in certain neuropathic pain states, Cl– influx mechanisms (eg, sodium-K+–Cl– cotransporter 1 [NKCC1], not shown) outweigh KCC2-mediated Cl– efflux, resulting in a high [Cl−]i and subsequently a more depolarized ECl. Activation of GABAARs depolarizes the cell. Increasing KCC2 activity or expression lowers [Cl−]i and hyperpolarizes ECl. In conditions of high KCC2 expression and activity, such as in healthy, mature neurons, KCC2-mediated efflux maintains low [Cl−]i and hyperpolarized ECl such that GABAAR activation results in neuronal hyperpolarization.
Decreased Functional Expression of KCC2, Impairing GABAergic Inhibition, Drives Neuropathic Pain
Loss of normal GABAergic inhibition following peripheral nerve injury is caused in part by a pathological decrease in KCC2 activity in superficial dorsal horn neurons.1 The consequent increase in neuronal [Cl−]i attenuates GABA-induced hyperpolarization and in extreme cases renders it depolarizing and even excitatory, which increases transmission in nociceptive lamina I and II neurons that project to the thalamus. In naive rats, pharmacological blockade or genetic knockdown of KCC2 causes behavioral changes indicative of allodynia and hyperalgesia, revealing that inhibition of KCC2 activity is sufficient to cause pain.13 In rats sustaining peripheral nerve injury, there is a rapid decrease in KCC2 activity due to proteolytic cleavage of the KCC2 polypeptide.14 This decrease in KCC2 activity occurs in a wide variety of pathological pain states. Peripheral nerve injury causes KCC2 downregulation, depolarization of ECl, and allodynia.15 Also, KCC2 is downregulated following peripheral inflammation16 and in morphine-induced hyperalgesia.17 Collectively, these data show that impaired KCC2 activity is likely to be an important feature of multiple chronic pain syndromes.
Toward a Small-Molecule KCC2 Enhancer to Restore GABAergic Inhibition for Analgesia
Given its decreased activity in a number of pathological pain states, a positive modulator (activator) of KCC2 might provide effective therapy by decreasing neuronal [Cl−]i and restoring GABAergic inhibition where it is reduced (Figure 3). Targeting KCC2 to restore GABAergic inhibition would not affect neuronal excitability directly but would instead modulate the effectiveness of endogenous GABAergic signaling, which could potentially yield increased specificity and a wider therapeutic window compared with GABA agonists. Developing a direct pharmacological agonist of KCC2, however, has proven difficult owing to a lack of data regarding the transporter’s structure and limited knowledge of its transcriptional and posttranslational regulation. Furthermore, developing high-throughput screening (HTS) assays for KCC2 has been technically challenging because KCC2 cotransport is electroneutral.
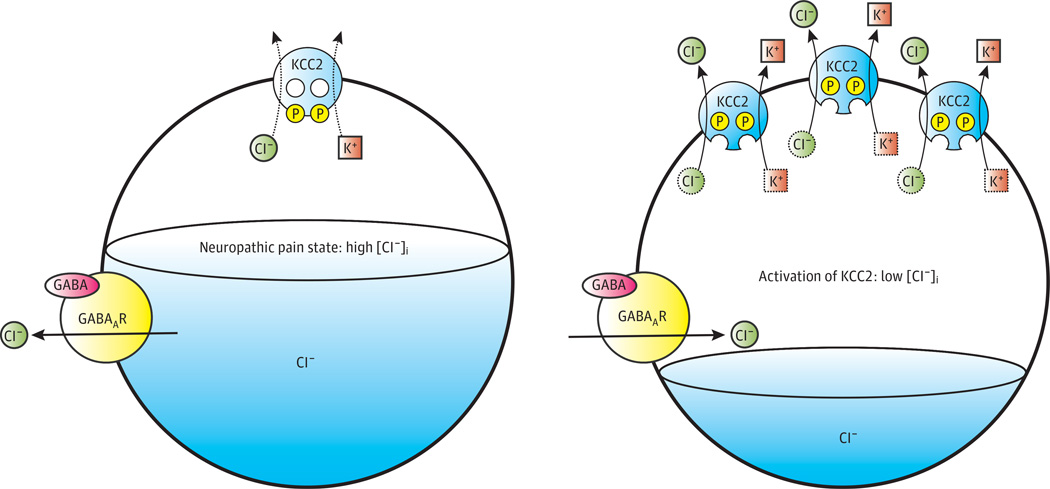
Figure 3. Potassium-Chloride Cotransporter KCC2 Activation as a Potential Therapeutic Strategy for Neuropathic Pain.
Functional downregulation of KCC2 activity is a major mechanism of spinal disinhibition and the development of neuropathic pain. The potassium [K+]–chloride (Cl−) cotransporter KCC2 uses the favorable outwardly directed electrochemical gradient of K+ across the plasma membrane to extrude Cl− from neurons. Low intraneuronal Cl– drives Cl– influx and membrane hyperpolarization when γ-aminobutyric acid (GABA) binds to Cl−-permeable GABA type A receptors (GABAARs). In several pathogenic pain states (and in other neurological diseases such as epilepsy and spasticity), the functional expression of KCC2 activity is decreased and the intracellular Cl− concentration ([Cl−]i) increases. As a result, GABAAR activation fails to hyperpolarize cells and instead can depolarize and even excite neurons. Pharmacological enhancement of KCC2 activity, which could be achieved by increasing the intrinsic activity of transporters already at the cell surface or by promoting the increased insertion or decreased retrieval of transporters to and from the cell surface, respectively, would be expected to lower neuronal Cl− levels and restore GABAergic inhibition of neurons in the nociceptive pathway. Endogenous regulators specific for KCC2 activity (eg, kinases, phosphatases, trafficking machinery, and/or degradation enzymes) are prime potential targets for therapeutic intervention. P indicates phosphorylation. Adapted with permission from Macmillan Publishers Ltd.18
Several groups have recently invented innovative HTS assays to search for KCC2 modulators. Delpire et al19 developed an assay using a thallium-sensitive, fluorescence-based ion flux indicator to screen a library of 234 000 small molecules for cell-permeable, potent, and specific modulators of KCC2 activity. Zhang et al20 validated and extended this thallium-based fluorescence HTS strategy by adding low levels of bumetanide to the assay system to inhibit background Na+-K+-Cl− cotransport. While these studies failed to identify a bona fide KCC2 activator, they established that HTS might be useful for finding small-molecule modulators of KCC2 activity with in vivo activity. Indeed, Austin and Delpire21 identified a specific KCC2 inhibitor (D4) that, when intrathecally injected into mice, decreased heat-evoked withdrawal latency.
A recent study by Gagnon et al22 used a different HTS assay involving the ratiometric fluorescent Cl− sensor Chlomeleon to identify compounds that reduced cellular [Cl−]i by activating KCC2-mediated Cl− extrusion in a neuroblastoma-glioma hybrid cell line with low baseline functional membrane expression of endogenous KCC2, which mimics disease models of GABAergic disinhibition. After screening 92 500 small molecules, they identified 1 KCC2-selective analogue (CLP257) in the arylmethylidine family of compounds that restored KCC2-mediated Cl− extrusion in spinal neurons from cord slices derived from a rat model of neuropathic pain. In these neurons, peripheral nerve injury resulted in a significant depolarization of the GABAA reversal potential. This depolarization was reversed (hyperpolarized) by application of CLP257, and the effect of CLP257 was blocked by the KCC2 antagonist VU0240551 (discovered by Delpire and colleagues [discussed earlier]), indicating that CLP257 restores neuronal Cl− extrusion capacity through KCC2. Mechanistically, CLP257 rescuedKCC2 activity by apparently modifying the posttranslational plasmalemmal turnover of transporters, increasing KCC2 surface expression. Impressively, administration of CLP257 in vivo also normalized stimulus-evoked responses in spinal nociceptive pathways previously sensitized by nerve injury, and it alleviated hypersensitivity in a rat model of neuropathic pain. An oral CLP257 carbamate prodrug (CLP290), synthesized because CLP257 undergoes rapid glucuronidation into an inactive metabolite, was found to be nontoxic and have analgesic effects equivalent to those of pregabalin but without its associated motor impairment at high doses.
Conclusions
Accumulation of intracellular Cl− due to adecrease in KCC2 activity that follows insult to nerve fibers clearly plays a major role in neuropathic pain by depolarizing ECl and disrupting GABAergic inhibition. Activation of KCC2 is a potential therapeutic strategy. Recently, a few groups have developed innovative HTS assays for compounds that modulate KCC2 activity, and they show early promise in identifying small-molecule activators of KCC2. These results are encouraging but require validation by other investigators and in multiple models of neuropathic pain, in addition to more detailed analyses of adverse effects and toxicology profiles. Nonetheless, these findings emphasize the validity of targeting Cl− derangements affecting GABA activity in peripheral neuropathic pain in the development of new therapeutic approaches. Other strategies of pharmacotherapeutic KCC2 activation are also worth exploring, including the targeting of upstream inhibitory regulatory kinases, because not all impairments of KCC2 result from derangements in transporter trafficking or degradation and intrinsic KCC2 activity can be robustly modulated by phosphorylation.23,24 Importantly, because impaired KCC2-mediated Cl− extrusion resulting in GABA depolarization or excitation has been documented in animal models of epilepsy (including temporal lobe epilepsy, neonatal seizures, and perilesional subtypes), psychiatric disease (eg, anxiety, schizophrenia, and autism), and spasticity following spinal cord injury,1 research efforts aimed at modulating Cl− extrusion via KCC2 potentiation might bear fruit for other debilitating neurological conditions that share the common underlying molecular pathogenesis of deranged Cl− homeostasis.
Acknowledgments
Funding/Support: This work was supported by the National Institutes of Health (Drs Kahle, Clapham, and Woolf), the Howard Hughes Medical Institute (Dr Clapham), and the Manton Center for Orphan Disease Research (Drs Kahle and Clapham).
Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Kahle had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kahle, Woolf.
Acquisition of data: Kahle, Khanna.
Analysis and interpretation of data: Kahle, Khanna, Clapham.
Drafting of the manuscript: Kahle, Khanna, Woolf.
Critical revision of the manuscript for important intellectual content: Kahle, Clapham, Woolf.
Study supervision: Clapham.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Kahle KT, Staley KJ, Nahed BV, et al. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4(9):490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- 2.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22(15):6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73(4):638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gwak YS, Hulsebosch CE. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology. 2011;60(5):799–808. doi: 10.1016/j.neuropharm.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Mann R, Schaefer C, Sadosky A, et al. Burden of spinal cord injury-related neuropathic pain in the United States: retrospective chart review and cross-sectional survey. Spinal Cord. 2013;51(7):564–570. doi: 10.1038/sc.2013.34. [DOI] [PubMed] [Google Scholar]
- 7.Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72(1):169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- 8.Baba H, Ji RR, Kohno T, et al. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24(3):818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 9.Janssen SP, Truin M, VanKleef M, Joosten EA. Differential GABAergic disinhibition during the development of painful peripheral neuropathy. Neuroscience. 2011;184:183–194. doi: 10.1016/j.neuroscience.2011.03.060. [DOI] [PubMed] [Google Scholar]
- 10.Scholz J, Broom DC, Youn DH, et al. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25(32):7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miletic G, Draganic P, Pankratz MT, Miletic V. Muscimol prevents long-lasting potentiation of dorsal horn field potentials in rats with chronic constriction injury exhibiting decreased levels of the GABA transporter GAT-1. Pain. 2003;105(1–2):347–353. doi: 10.1016/s0304-3959(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 12.Bráz JM, Sharif-Naeini R, Vogt D, et al. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74(4):663–675. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coull JA, Boudreau D, Bachand K, et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424(6951):938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 14.Zhou HY, Chen SR, Byun HS, et al. N-methyl-D-aspartate receptor- and calpain-mediated proteolytic cleavage of K+-Cl− cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. J Biol Chem. 2012;287(40):33853–33864. doi: 10.1074/jbc.M112.395830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coull JA, Beggs S, Boudreau D, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Liu LY, Xu TL. Reduced potassium-chloride co-transporter expression in spinal cord dorsal horn neurons contributes to inflammatory pain hypersensitivity in rats. Neuroscience. 2008;152(2):502–510. doi: 10.1016/j.neuroscience.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 17.Ferrini F, Trang T, Mattioli TA, et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl homeostasis. Nat Neurosci. 2013;16(2):183–192. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miles R. Neurobiology: a homeostatic switch. Nature. 1999;397(6716):215–216. doi: 10.1038/16604. [DOI] [PubMed] [Google Scholar]
- 19.Delpire E, Baranczak A, Waterson AG, et al. Further optimization of the K-Cl cotransporter KCC2 antagonist ML077: development of a highly selective and more potent in vitro probe. Bioorg Med Chem Lett. 2012;22(14):4532–4535. doi: 10.1016/j.bmcl.2012.05.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang D, Gopalakrishnan SM, Freiberg G, Surowy CS. A thallium transport FLIPR-based assay for the identification of KCC2-positivemodulators. J Biomol Screen. 2010;15(2):177–184. doi: 10.1177/1087057109355708. [DOI] [PubMed] [Google Scholar]
- 21.Austin TM, Delpire E. InhibitionofKCC2in mouse spinal cord neurons leads to hypersensitivity to thermal stimulation. Anesth Analg. 2011;113(6):1509–1515. doi: 10.1213/ANE.0b013e31822e0a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagnon M, Bergeron MJ, Lavertu G, et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med. 2013;19(11):1524–1528. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahle KT, Deeb TZ, Puskarjov M, et al. Modulation of neuronal activity by phosphorylation of the K-Cl cotransporter KCC2. Trends Neurosci. 2013;36(12):726–737. doi: 10.1016/j.tins.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Los Heros P, Alessi DR, Gourlay R, et al. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+-Cl− co-transporters. Biochem J. doi: 10.1042/BJ20131478. [published online January 7, 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]