Abstract
Adult work shows differences in emotional processing influenced by sexes of both the viewer and expresser of facial expressions. We investigated this in 120 healthy youths (57 boys; 10–17 years old) randomly assigned to fear conditioning and extinction tasks using either neutral male or female faces as the conditioned threat and safety cues, and a fearful face paired with a shrieking scream as the unconditioned stimulus. Fear ratings and skin conductance responses (SCRs) were assessed. Male faces triggered increased fear ratings in all participants during conditioning and extinction. Greater differential SCRs were observed in boys viewing male faces and in girls viewing female faces during conditioning. During extinction, differential SCR findings remained significant in boys viewing male faces. Our findings demonstrate how sex of participant and sex of target interact to shape fear responses in youths, and how the type of measure may lead to distinct profiles of fear responses.
Keywords: Sex, Gender, Youth, Fear conditioning, Fear extinction, Skin conductance
1. Introduction
A wealth of work performed in adults reports that sex may influence emotional processing. Not only are there differences in the way men and women recognize and process emotions, there are also differences in the way the sex of facial targets used in tasks may modulate the analysis of emotional cues (Kret & De Gelder, 2012; Kring & Gordon, 1998; Pattwell, Lee, & Casey, 2013). Little is known about the influence sexes of participants and targets may have on emotional processing in younger population. In the current study, we aim at better understanding the influence of sex of participant and sex of target on fear conditioning and extinction in youths, as these tasks are often used to study emotions and emotion-related brain function in healthy and psychiatric paediatric populations.
Fear conditioning refers to the process by which a neutral stimulus is paired with an aversive unconditional stimulus (US; e.g. electric shocks), becoming a conditioned stimulus (CS+) eliciting a conditioned fear response when presented independently of the US. In humans, this response is usually measured with subjective fear ratings and/or skin conductance responses (SCR) (Lissek et al., 2005). Fear extinction, in comparison, refers to the diminution of the fear response after repeated presentation of the CS+ without the US. In discrimination fear conditioning and extinction, fear responses to the CS+ are compared to fear responses to a CS never paired with the US (CS−), which serves as a safety signal.
When using such classical fear conditioning and extinction paradigms in rodents, males show greater fear conditioning and more resistance to fear extinction than females, differences that emerge around puberty, presumably due to effects of sex hormones (Dalla & Shors, 2009). In human youths, no prior work examined sex differences during fear conditioning and extinction; however, tasks using intrinsically evocative faces were employed. Findings from this work report mixed observations. An important amount of studies demonstrated that female children and adolescents are more negatively aroused by threatening faces, as well as faster and more accurate in labelling and recognizing these cues, compared to male children and adolescents (Lee et al., 2013; see reviews by Kret & De Gelder, 2012; McClure, 2000). Some other work, however, did not observe sex differences in emotional processing, neither in child nor adolescent samples (De Sonneville et al., 2002; Herba, Landau, Russell, Ecker, & Phillips, 2006; Kret & De Gelder, 2012; McClure, 2000; Thomas, De Bellis, Graham, & LaBar, 2007; Vicari, Reilly, Pasqualetti, Vizzotto, & Caltagirone, 2000). In adults, conflicting findings are also observed. Women are shown to rate the CS+ and the US as more distressing and unpleasant than men during fear conditioning and extinction (Forsyth & Eifert, 1998; Kelly & Forsyth, 2007). Increased fear ratings of pain during movement-related conditioning are also observed in women relative to men (Meulders, Vansteenwegen, & Vlaeyen, 2012). When using physiological responses (SCRs, brain activation), however, men have often been reported as more physiologically reactive during the processing of threatening stimuli – especially male facial cues –compared to women. These findings were interpreted in an evolutionary perspective, with men prepping defence responses towards other threatening rival males, in relation with reproduction and survival (Kret & De Gelder, 2012; Milad et al., 2006). Regarding fear conditioning specifically, physiological data are less clear-cut as some findings show greater SCRs to facial threat cues in men relative to women (Dimberg & Öhman, 1996), whereas other work report similar SCRs to facial threat cues in both men and women (Kret & De Gelder, 2012; Navarrete et al., 2009).
Concerning the influence sex of facial targets may have on emotional processing, threatening male facial expressions (anger, fear) have consistently been demonstrated to activate greater fearful responses than threatening female facial expressions. This was observed in both youths and adults (Aguado, Garcia-Gutierrez, & Serrano-Pedraza, 2009; Becker, Kenrick, Neuberg, Blackwell, & Smith, 2007; Egger et al., 2011; Goos & Silverman, 2002; Hess, Blairy, & Kleck, 1997; Navarrete et al., 2009; Seidel, Habel, Kirschner, Gur, & Derntl, 2010).
In the current study, we aimed at examining, firstly, the influence of sex of participants, and secondly, the influence of sex of target, on fear learning and extinction in boys and girls aged 10–17 years old. To reach this goal, we used a discrimination fear conditioning and extinction paradigm recently developed by Lau and collaborators (Lau et al., 2008, 2011). This unique paradigm uses a paediatrically safe US shown to be as efficient as the US usually employed in animals and adults, electric shocks, which may not be used in youths due to ethical considerations. Head shots of two different actresses constitute the CS+ and CS− (neutral facial expressions) and the US is constituted of the CS+ actress’s picture depicting a fearful facial expression, which is simultaneously presented with a shrieking female scream. Hence, here, we capitalized on the intrinsic aversiveness of witnessing fear in others. With this task, Lau and collaborators were successful in triggering fear acquisition and extinction, as measured through fear ratings and SCRs in healthy and anxious youths (Lau et al., 2008, 2011). However, the influence of sexes of participants and targets was not measured in these previous studies.
Taking these two variables into account, and based on the above mentioned findings (especially those concerning human youths), we hypothesized that during conditioning, (1) boys and girls would show differential learning, manifested as greater fear evoked by the CS+ vs. CS−, (2) girls would show greater overall fear responses (CS+ and CS−) compared to boys, and (3) male fearful facial expressions would trigger greater fear responses in both sexes compared to female faces. During extinction, both boys and girls should extinguish fear, with levels of fear responses being similar for both the CS+ and CS−. Overall fear responses (CS+ and CS−) should remain higher in girls compared to boys, and for male faces relative to female faces in both sexes.
2. Method
2.1. Participants
A total of 120 healthy participants completed the study. Participants ranged in age from 10 to 17 years. Exclusion criteria for participation in the study were any type of past or present mental disorders, medical illness and use of medication as assessed by self-report in youths and one of their parents. Subjects were recruited in community centres (e.g., libraries, day camps) as well as schools of the Montreal greater area using flyers. The study protocol was approved by the Research Ethics Boards of the CHU Ste-Justine, Montreal, Canada. Participants and their parents gave informed assent and consent, respectively, and youths were compensated for their participation. Of the initial 120 participants recruited, two abandoned before completing the study, and data for one participant were lost due to technical problems. Hence, analyses were carried on 117 youths, 56 boys (mean age = 14.05 ± 2.11) and 61 girls (mean age = 13.77 ± 1.93).
2.2. Experimental design
The paradigm lasted 17 min and comprised two phases: a fear conditioning phase and a fear extinction phase (Lau et al., 2008, 2011). During each phase, participants saw head shots of individuals presenting neutral emotional expressions. These photos were selected from the NimStim Set of Facial Expressions (Tottenham et al., 2009). One individual was randomly selected to serve as the conditioned stimulus (CS+) for each participant, whereas the other served as the CS− (safety signal). During conditioning, the CS+ was paired with the US on 50% of trials. Because fear conditioning is a process inducing a fear response that tends to naturally decrease over time, a partial reinforcement contingency ratio was used to prevent habituation to the US (Mackintosh, 1974). The US was constituted of the photo of the same actor/actress selected for the CS+, but depicting a fearful expression and presented simultaneously with a 90 dB shrieking male or female scream. Participants were not aware of the CS+ – US association prior to the experiment. The other actor/actress served as a conditioned stimulus unpaired (CS−) with the aversive US. In the present study, 49 participants (24 boys, 25 girls) saw photos of males posing as the CS+ and CS−, while 68 participants (32 boys, 36 girls) saw photos of females posing as the CS+ and CS−. Four groups were constituted: Group 1 – boys viewing male facial expressions; Group 2 – girls viewing male facial expressions; Group 3 – boys viewing female facial expressions; Group 4 – girls viewing female facial expressions. During extinction, task procedures were identical to that of the conditioning session except that no US were presented, and only 14 CS+ unpaired and 14 CS− were shown.
Fear ratings and SCRs served as the behavioural dependent measures, i.e., the fear responses to the CS+ and CS− during conditioning and extinction. Fear ratings were performed during each presentation of the photographs (CS+ before apparition of US, CS−), in both the conditioning and extinction phases (Fig. 1). Participants were asked to indicate on a 5-point Likert scale the degree to which they felt afraid when viewing the actor/actress in the CS+ and CS− photos (Are you afraid?; 1 = not at all, 5 = extremely). Fear ratings were recorded with a right hand-held button response box developed to allow for a graded range of responses (Current designs, Philadelphia).
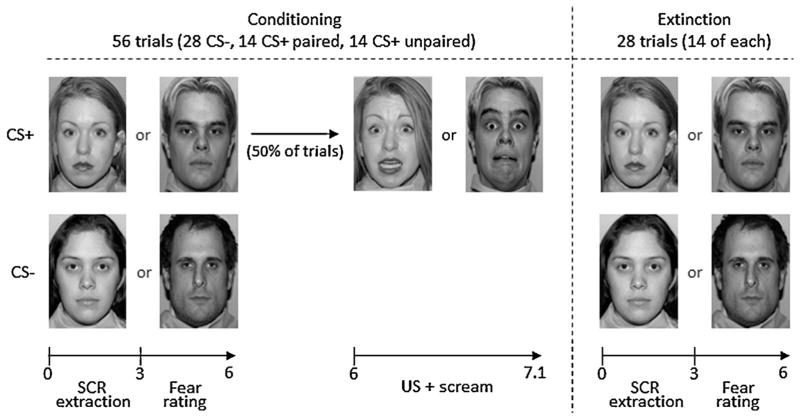
Fig. 1.
A schematic depiction of the fear conditioning and extinction tasks using female and male facial cues. CS+: conditioned stimulus, CS−: safety cue, US: unconditioned stimulus.
Overall, 84 stimuli were presented (Fig. 1). Conditioning trials (n = 56) comprised one of the three events: CS+ paired (n = 14), CS+ unpaired (n = 14) or CS− stimuli (n = 28). The CS+ paired events consisted of the presentation of a neutral face stimulus (3-s), a rating response (3-s), and a fearful face stimulus (1.1-s) paired with the auditory stimulus (1-s). The CS+ unpaired and CS− events consisted of the presentation of the neutral face stimulus (3-s), followed by the rating response (3-s). Events were presented for durations of 6 (CS+-unpaired and CS−) or 7.1 (CS+-US-paired) s with inter-stimulus intervals of 3, 4, 5, 6, 8, 10 or 12 s. During extinction, 14 CS+ unpaired (3 s – face presentation, 3 s – rating response) and 14 CS− (3 s – face presentation, 3 s – rating response) were shown. Trials were presented in a pseudorandom order and the assignment of actors or actresses (either blond or brown hair) to CS-type (CS+, CS−) was counterbalanced across participants.
Before testing, participants were familiarized with the discrimination conditioning and extinction tasks to ensure understanding of picture rating. The pictures presented during the practice session were different from the ones used during the actual fear conditioning and extinction paradigm to prevent habituation to the CS+, US and CS−. Before practice and testing sessions, participants were told they would see two different images and hear sounds, but no details were given on the images or sounds. Visual and auditory stimuli were presented through a laptop computer using E-Prime software (PST, Inc., Pittsburgh, PA) and headphones were placed on the ears of the participants.
Following completion of the conditioning and extinction tasks, photos of the actors or actresses used for the CS+ and CS−, and depicting a neutral facial expression, were presented again to participants, who were asked to rate their fear levels on the 5-point Likert scale one last time. During this post-experiment interview, participants were also debriefed and asked about their contingency awareness of the CS–US relationship. Specifically, youths were asked if the blond- and/or brown-haired actor/actress screamed. Contingency awareness (1 = yes, 0 = no) was granted if participants correctly identified which actor/actress had been paired with the scream (CS+), and which represented the safety signal (CS−).
2.3. Physiological measurements
Skin conductance, an index of sympathetic nervous system activity, was used to measure physiological responses to the fear-related (CS+) and safe (CS−) cues during fear conditioning and extinction. Skin conductance responses were recorded using non-invasive procedures, i.e., two 10-mm EDA isotonic gel radio-translucent electrodes placed on the plantar surface of the right foot of participants. Collection and preprocessing of the SCR data were performed according to Dubé et al. (2009). Hence, physiological data were amplified, digitized, and recorded at 1000 Hz using a computerized data acquisition system (MP150-BIOPAC System), and SCR analyses were performed using Acknowledge Analysis Software (version 4.2 BIOPAC). Preprocessing of the data included 500 ms mean smoothing, 1 s delay signal subtraction, and replacement of negative values by 0 (Dubé et al., 2009). The area under the differential curve was extracted for a 3 s-window following cue onset, delayed by 1–3 s to account for the latency of the SCR, for each stimulus presented (CS+ and CS−) in every participant. This index, reflecting the amplitude of the SCR, is highly sensitive to rapid increases in phasic skin conductance (positive slope; Dawson, Schell, & Filion, 2000). The extracted area under the differential curve was limited to the first 3 s following cue onset in order to avoid contamination with skin conductance activity triggered by the motor response performed during stimulus rating, which occurred in the last 3 s-segment of each stimulus presentation (cf. Fig. 1).
2.3.1. Primary analysis
Because high variability characterizes SCRs from one event to the other in each participant, amplitude of the SCRs was standardized within each subject, for both the conditioning and extinction phases, using Z transformations. Means were calculated over SCRs during both the CS+ and CS− events, separately for the conditioning and extinction phases. This allowed for statistical analysis comparing SCRs to the CS+ vs. CS− within each group, during conditioning as well as extinction.
2.3.2. Secondary analysis
Because absence of differential conditioning (CS+ > CS−) in two of the groups (boys viewing female faces and girls viewing male faces) was observed (cf. Section 3), we proceeded in calculating the number of significant SCRs to both the CS+ and CS− in each participant of all four groups. This was done in order to determine if similar levels of physiological reactivity were triggered by the stimuli (CS+ and CS−), or if the absence of differential conditioning in these two groups was due to an absence of physiological reactivity and thus, of fear learning. A participant’s SCRs were considered significant if they were two times larger (and thus, presumably in reaction to the CS+ and CS− events) than his “noise-level” (i.e., non-significant) SCRs. The noise-level SCR was determined based on the rest period occurring before the onset of the conditioning task. Specifically, within the 6 s-segment before the end of the rest period, the amplitude of the SCR was extracted for a 3 s-window, in each participant. The significant SCRs, thus personalized for every youth, were coded 1, and noise-level (non-significant) SCRs were coded 0. For every participant, the sum of significant SCRs was calculated separately for the CS+ and CS− events, and separately for the conditioning and extinction phases.
2.4. Data analyses
Demographic, behavioural and physiological data analyses were performed using SPSS 18.0 (SPSS Inc., Chicago, IL).
2.4.1. Demographic characteristic data
Demographic characteristic data of participants met sphericity and normality assumptions in the four groups. To investigate whether an equal number of male and female facial expressions were viewed by both boys and girls, and to determine if an equal number of boys and girls were evaluated, chi-squares for quantitative measures were used. Age of participants was compared between the four groups using a one-way analysis of variance (ANOVA).
2.4.2. Behavioural and physiological measures
Sphericity was met for both the fear ratings and SCRs, but normality was met only for the SCRs. Subjective fear rating data were therefore log transformed. Subjective fear ratings, SCR amplitude data and number of significant SCRs were analysed in distinct ANOVAs, and conditioning and extinction phases were analysed separately. Four-way repeated-measures ANOVAs with sex of participants (boys vs. girls) and sex of target (male vs. female faces) as between-subjects factors, and CS-type (CS+, CS−) and time of cue presentation (early vs. late; for conditioning, early: 14 first cues, late: 14 last cues; for extinction, early: 7 first cues, late: 7 last cues) as the within-subjects factor were conducted on the dependent variables subjective fear ratings and SCRs. For the number of significant SCR data, the pattern of results was not affected by the factor time of cue presentation, in both the conditioning and extinction phases. Therefore, the results presented are based on ANOVAs with two between-subjects factors (sex of participants, sex of target) and one within-subjects factor (CS-type). Post hoc comparisons performed on significant ANOVA findings were done using Tukey group comparisons test at an alpha level of 0.05.
3. Results
3.1. Demographic characteristics
The final sample consisted of 117 youths: 24 boys viewing male faces (mean age = 13.71 ± 2.26), 25 girls viewing male faces (mean age = 13.96 ± 2.07), 32 boys viewing female faces (mean age = 14.31 ± 2.01), 36 girls viewing female faces (mean age = 13.64 ± 1.84). An equal number of male and female facial expressions were seen by boys, as well as by girls (χ2 = .04, p = .83). Groups did not differ in terms of age (F3,116 = 0.72, p = .54).
3.2. Behavioural and physiological measures
Fear ratings and SCRs were unrelated (all rs < .19, all ps > .05). Means and standard deviations of fear ratings and SCRs to the CS+ and CS− during conditioning and extinction are displayed in Table 1.
Table 1.
Means and standard deviations of fear ratings and skin conductance responses to the CS+ and CS− during conditioning and extinction.
| Variable | Conditioning
|
Extinction
|
||||||
|---|---|---|---|---|---|---|---|---|
| CS+ | CS− | CS+ | CS− | |||||
| Ratings, mean (SD) | ||||||||
| Male faces | ||||||||
| Early | ||||||||
| Boys | 2.54 | (0.92) | 2.06 | (0.71) | 2.17 | (1.03) | 1.38 | (0.51) |
| Girls | 3.11 | (1.10) | 2.20 | (0.76) | 2.65 | (1.25) | 1.61 | (0.68) |
| Late | ||||||||
| Boys | 2.53 | (1.30) | 1.61 | (0.55) | 1.79 | (0.83) | 1.30 | (0.42) |
| Girls | 2.98 | (1.16) | 1.79 | (0.66) | 2.43 | (1.21) | 1.55 | (0.55) |
| Mean | ||||||||
| Boys | 2.54 | (1.06) | 1.83 | (0.59) | 1.98 | (0.90) | 1.34 | (0.44) |
| Girls | 3.04 | (1.11) | 1.99 | (0.68) | 2.54 | (1.21) | 1.58 | (0.55) |
| Female faces | ||||||||
| Early | ||||||||
| Boys | 2.07 | (0.94) | 1.66 | (0.73) | 1.90 | (1.12) | 1.26 | (0.50) |
| Girls | 2.22 | (1.05) | 1.86 | (0.95) | 1.76 | (0.90) | 1.58 | (0.82) |
| Late | ||||||||
| Boys | 2.00 | (1.14) | 1.32 | (0.51) | 1.60 | (0.88) | 1.33 | (0.60) |
| Girls | 1.97 | (0.92) | 1.63 | (0.94) | 1.55 | (0.71) | 1.39 | (0.56) |
| Mean | ||||||||
| Boys | 2.03 | (1.01) | 1.49 | (0.58) | 1.75 | (0.97) | 1.30 | (0.53) |
| Girls | 2.09 | (0.97) | 1.75 | (0.92) | 1.66 | (0.79) | 1.48 | (0.67) |
| SCR, mean (SD) | ||||||||
| Male faces | ||||||||
| Early | ||||||||
| Boys | .30 | (.24) | −.07 | (.28) | .17 | (.45) | −.09 | (.26) |
| Girls | .13 | (.34) | .11 | (.28) | −.001 | (.36) | −.006 | (.34) |
| Late | ||||||||
| Boys | −.02 | (.24) | −.22 | (.23) | .007 | (.34) | −.09 | (.36) |
| Girls | −.11 | (.24) | −.12 | (.27) | −.12 | (.22) | .12 | (.28) |
| Mean | ||||||||
| Boys | .14 | (.17) | −.14 | (.17) | .09 | (.20) | −.09 | (.20) |
| Girls | .007 | (.20) | −.007 | (.20) | −.06 | (.17) | .06 | (.17) |
| Female faces | ||||||||
| Early | ||||||||
| Boys | .14 | (.32) | .08 | (.33) | −.04 | (.35) | .11 | (.37) |
| Girls | .21 | (.32) | .02 | (.31) | .08 | (.36) | −.02 | (.43) |
| Late | ||||||||
| Boys | −.11 | (.26) | −.10 | (.26) | −.006 | (.36) | −.06 | (.31) |
| Girls | −.08 | (.26) | −.15 | (.23) | −.01 | (.37) | −.09 | (.33) |
| Mean | ||||||||
| Boys | .01 | (.15) | −.01 | (.15) | −.02 | (.19) | .02 | (.13) |
| Girls | .07 | (.18) | −.07 | (.18) | .04 | (.24) | −.04 | (.24) |
Note: SD = standard deviation.
3.2.1. Fear ratings
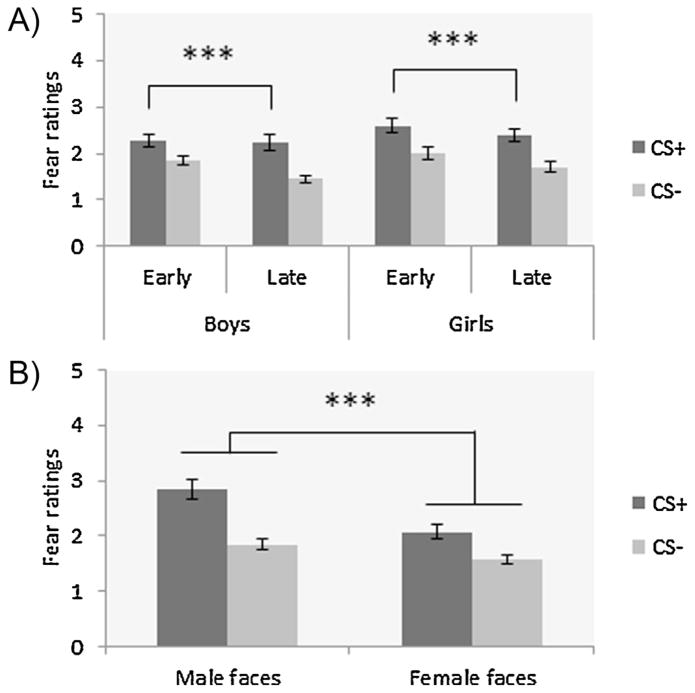
Eight participants presented multivariate outlier data to both the CS+ and CS− (unusual pattern of responses – i.e., much higher scores – compared to the mean CS+ and CS− scores of their respective group; as per Achim, 2012), and were rejected from the subjective fear ratings analysis; hence, analyses were performed on 109 participants. During conditioning, there were significant main effects of CS-type (F1,105 = 84.00, p < .001; η2 = .44) and time of cue presentation (F1,105 = 55.00, p < .001; η2 = .34), which were subsumed by a significant two-way interaction of CS-type × time of cue presentation (F1,105 = 16.09, p < .001; η2 = .13). Post hoc analyses showed that subjective fear ratings were higher for the CS+ during early compared to late conditioning (p < .001; Fig. 2A). No early vs. late differences in CS− ratings were observed (p > .05). The ANOVA also revealed a significant main effect for sex of target, with greater fear ratings to the male faces compared to female faces (F1,105 = 11.67, p = .001; η2 = .10; Fig. 2B). No main effect of sex of participants (F1,105 = 2.40, p = .12), and no other two- or three-way interactions (all Fs1,105 < 3.25; all ps > .07) were observed.
Fig. 2.
Mean fear ratings during early and late conditioning for the CS+ and CS− in all groups; (A) greater fear ratings during early vs. late conditioning for the CS+ (p < .001); (B) greater fear ratings for male faces compared to female faces (p = .001). ***p ≤ .001.
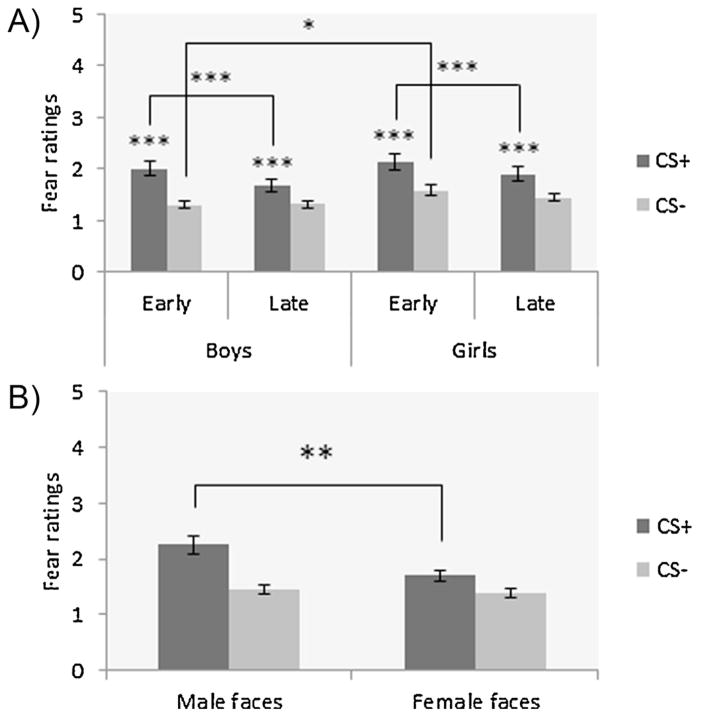
During extinction, there were main effects for CS-type (F1,105 = 61.65, p < .001, η2 = .37) and time of cue presentation (F1,105 = 23.47, p < .001; η2 = .18), as well as a CS-type × time of cue presentation interaction (F1,105 = 16.17, p < .001; η2 = .13), which were subsumed by a CS-type × time of cue presentation × sex of participants interaction (F1,105 = 4.68, p = .03; η2 = .04). Summarized post hoc findings show greater differential fear learning (CS+ vs. CS−) for ratings during early and late extinction in both boys and girls (ps < .001). Additionally, in early compared to late extinction, fear ratings were more elevated in both sexes for the CS+ condition (ps ≤ .001), but more elevated in girls relative to boys for the CS− condition (p = .03; Fig. 3A). The ANOVA also revealed a main effect of sex of target (F1,105 = 5.79, p = .02; η2 = .05), which was subsumed by a CS-type × sex of target interaction (F1,105 = 10.43, p = .002, η2 = .09). Post hoc analyses showed that the CS+ triggered greater fear ratings for male faces compared to female faces in both boys and girls (p = .002; Fig. 3B). No other main effect or two- or three-way interactions were found (all Fs1,105 < 3.6; all ps > .06).
Fig. 3.
Mean fear ratings during early and late extinction for CS+ and CS− in all groups; (a) resistance to fear extinction as demonstrated by greater ratings to CS+ compared to CS− during early and late extinction for both boys and girls (all ps ≤ .001); greater fear ratings to CS+ in early vs. late extinction for both boys and girls (all ps = .001); greater fear ratings to CS− for girls relative to boys during early extinction (p = .03); (B) greater fear ratings for male faces vs. female faces in the CS+ condition, in both boys and girls (p = .002). *p < .05, **p < .01, ***p < .001.
3.2.2. Skin conductance responses
Five participants were excluded from the SCR analyses because they showed no SCRs or because of bad quality data (e.g. noise); hence analyses were carried on 112 participants.
SCR amplitude during conditioning
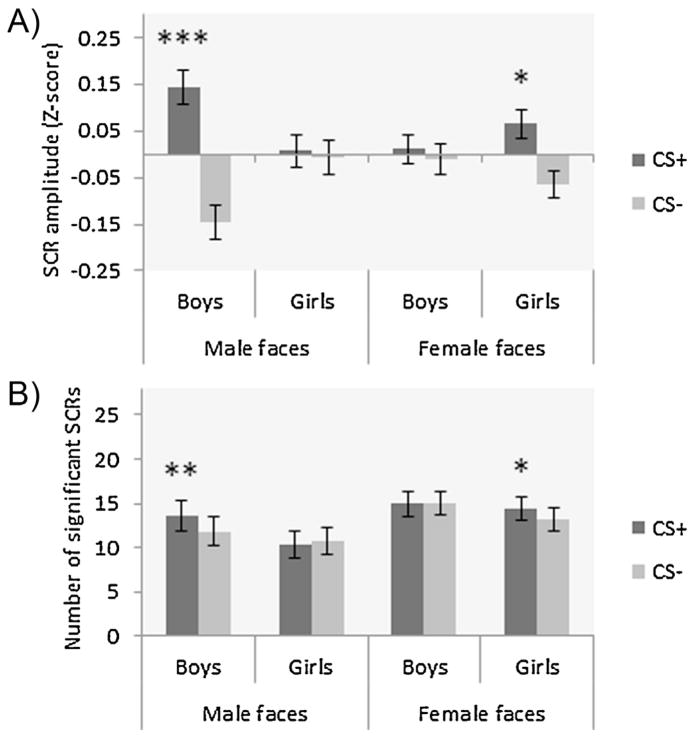
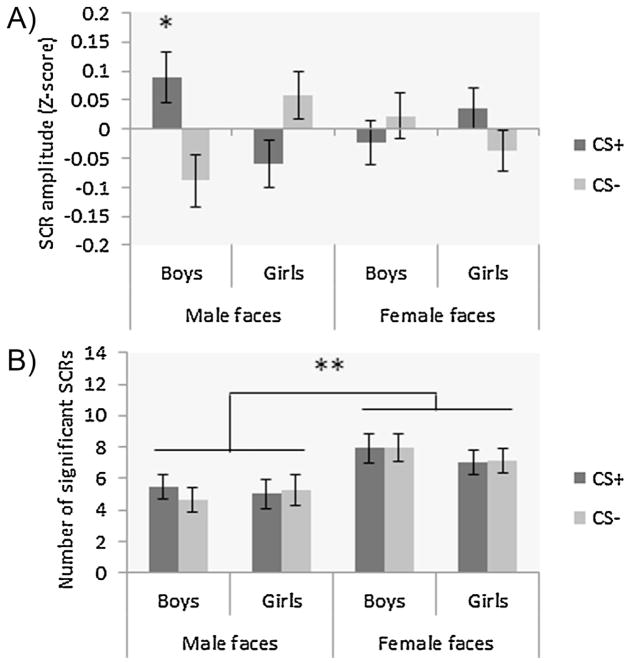
During conditioning, we observed a main effect of time of cue presentation, with greater SCRs observed during early relative to late conditioning (F1,108 = 46.79, p < .001; η2 = .30). The ANOVA also revealed a main effect of CS-type (F1,108 = 11.52, p = .001; η2 = .10), which was subsumed by a CS-type × sex of participant × sex of target interaction (F1,108 = 8.10, p = .005, η2 = .07; Fig. 4A). Post hoc analyses showed greater SCRs triggered by the CS+ vs. CS− in boys viewing male faces (p < .001), and in girls viewing female faces (p = .03). No other main effects, or two- or three-way interactions were found (all Fs1,108 < 3.43; all ps > .07).
Fig. 4.
Mean skin conductance responses during conditioning for CS+ and CS− in all groups; (A) greater differential fear conditioning (CS+ > CS−) in boys viewing male faces (p < .001) and in girls viewing female faces (p = .03), (B) greater number of significant SCRs for the CS+ relative to the CS− in boys viewing male faces (p = .01) and in girls viewing female faces (p = .05). *p < .05, **p < .01, ***p < .001.
Number of SCRs during conditioning
No significant main effects were observed during conditioning (Fs1,108 < 3.72, p > .05). However, a CS-type × sex of participants × sex of target was observed (F1,108 = 7.50, p = .007; η2 = .07; Fig. 4B). Post hoc analyses showed that there was a greater number of SCRs for the CS+ compared to the CS− in boys viewing male faces (p = .01), and in girls viewing female faces (p = .05). As can be seen in Fig. 4B, there were SCRs to the CS+ and CS− in boys viewing female faces and in girls viewing male faces, however this reactivity was of similar level for both stimuli. Thus, the observed absence of differential learning in the SCR amplitude data is not due to failed fear conditioning, but to comparable SCRs to both events. No other two- or three-way interactions were found (all Fs1,108 < .55; all ps > .46).
SCR amplitude during extinction
During extinction, no significant main effects were observed (all Fs1,108 < 2.40, all ps > .13). However, a CS-type × sex of participants × sex of target interaction was found (F1,108 = 6.78, p = .01; η2 = .06). Post hoc analyses showed greater SCRs triggered by the CS+ vs. CS− for male faces in boys (p = .05; Fig. 5A). We also observed a CS-type × time of cue presentation × sex of target interaction (F1,108 = 4.23, p = .04; η2 = .04); however, no CS-type differences (CS+ vs. CS−) were observed in any of the groups (all ps > .14). No other two- or three-way interactions were found (all Fs1,108 < 1.25, all ps > .27).
Fig. 5.
Mean skin conductance responses during extinction for CS+ and CS− in all groups; (A) greater SCRs to CS+ vs. CS− for male faces in boys (p = .05). (B) greater number of significant SCRs for female faces compared to male faces (p = .006). *p < .05, **p < .01.
Number of SCRs during extinction
We observed a main effect of sex of target during extinction, with a greater number of SCRs observed for female faces compared to male faces (F1,108 = 7.87, p = .006; η2 = .07; Fig. 5B). No other main effects or two- or three-way interactions were found (all Fs1,108 < 2.22; all ps > .14).
3.2.3. Post-experiment questionnaire
Over 95% of participants showed contingency awareness of the CS–US relationship. The chi-squared analysis of participants showing correct vs. incorrect contingency awareness did not differ across groups (χ2 = 1.23, p = .05). Moreover, excluding data of the 3 unaware participants did not affect the pattern of results for the fear ratings or SCRs during conditioning and extinction. Ratings obtained with the post-experiment questionnaire (cf. means and standard deviations in Table 2) led to similar conclusions as those observed with ratings collected during the task, i.e., greater fear levels manifested to the CS+ vs. CS− (F1,100 = 130.72, p < .001; η2 = .57; as observed during both conditioning and extinction), and greater fear levels triggered by male faces relative to female faces (F1,100 = 7.28, p = .008; η2 = .07; as observed during conditioning and extinction). Moreover, a CS-type × sex of target interaction was found (F1,100 = 5.32, p = .02, η2 = .05), showing greater fear ratings triggered by the CS+ for male faces compared to female faces in both boys and girls (p = .003; as observed during conditioning and extinction).
Table 2.
Means and standard deviations of post-questionnaire fear ratings to the CS+ and CS−.
| Variable | Male faces
|
Female faces
|
||
|---|---|---|---|---|
| Boys (n = 23) | Girls (n = 23) | Boys (n = 29) | Girls (n = 29) | |
| Post-questionnaire, mean (SD) | ||||
| CS+ | 2.80 (1.15) | 3.15 (1.36) | 2.10 (0.99) | 2.29 (1.11) |
| CS− | 1.43 (0.59) | 1.43 (0.66) | 1.24 (0.51) | 1.40 (0.56) |
Note: SD = standard deviation.
4. Discussion
To the best of our knowledge, this is the first study to explicitly investigate the influence of sex of participants and sex of target on discrimination fear conditioning and extinction in youths. Two key findings emerge from the conditioning rating data. Firstly, greater fear ratings to the CS+ in early relative to late trials were observed in both boys and girls. Decreased fear responses in late conditioning trials are expected as habituation to threat-related cues occurs over time. Fear ratings to the CS− remained low from early to late trials, confirming that all participants correctly identified the safety cue. Secondly, fear ratings to both the CS+ and CS− during conditioning were greater for male faces compared to female faces, in both boys and girls. Two key findings also emerge regarding the physiological reactivity measured during conditioning. First, similarly to the rating findings, SCRs were greater in early relative to late trials in all groups, suggesting elevation of fear reactivity that eventually habituated over time. Secondly, differential fear learning as reflected by SCR amplitude data was characterized by an “own-sex” effect, as boys showed greater physiological reactivity to the CS+ relative to the CS− only when viewing male faces and girls, only when viewing female faces. In contrast, when conditioned with stimuli from the opposite sex, no difference was observed between CS+ and CS− in boys viewing female faces and in girls viewing male faces. This suggests that participants failed to efficiently recognize safety cues when they were depicted by facial features of the opposite sex.
During extinction, the absence of attenuation in fear ratings (i.e., CS+ > CS−) from early to late trials was observed in both boys and girls. Girls were also slow in minimizing fear ratings to the safety cues (CS−) during early trials. Moreover, as observed during conditioning, male faces triggered greater fear ratings than female faces in the CS+ condition, in both sexes. Finally, in terms of SCRs, only an “own-sex” effect reflecting greater physiological reactivity to the CS+ relative to the CS− in boys viewing male faces was maintained.
Findings from subjective fear ratings analyses show that both boys and girls reported being more afraid of male neutral facial expressions than of female neutral faces, during conditioning and extinction. This confirms adult work showing that male neutral faces are perceived as more threatening than female neutral facial expressions (Adams, Nelson, Soto, Hess, & Kleck, 2012), and that male facial expressions perceived as threatening trigger longer-lasting fear and hostile responses (Becker et al., 2007; Kret & De Gelder, 2012; Navarrete et al., 2009; Ohman, 2009; Rotteveel & Phaf, 2004). This may be explained by the physiognomy of men – heavier and lower eyebrows, angular facial features (e.g., jaw), thinner lips, larger nose – which naturally connotes greater hostility and threat than that of women (Becker et al., 2007; Hess et al., 1997). Additionally, the fact that more crime and violence are linked to men than women reinforces the stereotypic feelings of threat conveyed by male facial expressions (Becker et al., 2007; Daly & Wilson, 1994; Dimberg & Öhman, 1996; Kret & De Gelder, 2012).
Contrary to our predictions, however, girls did not show greater subjective fear ratings than boys, and this was true for both conditioning and extinction phases. Such findings are not necessarily in contradiction with the literature as conflicting results regarding sex differences in emotional processing are reported (De Sonneville et al., 2002; Herba et al., 2006; Thomas et al., 2007; Vicari et al., 2000; cf. Kret & De Gelder, 2012). The female advantage is indeed characterized as being quite modest, and could be influenced by some methodological factors (Kret & De Gelder, 2012; McCarthy & Konkle, 2005). For example, sex differences, to the advantage of females, are thought to be particularly apparent when the intensity of the emotion portrayed is maximal, as opposed to the neutral facial expressions presented in the current study (Kret & De Gelder, 2012). The female advantage is also thought to be particularly salient when using verbal instead of visuo-spatial cues (as in the current paradigm, which employed photos; Herba et al., 2006). Finally, a sex advantage in emotional processing may vary according to the wax and wane of hormonal levels, as observed during puberty or phases of girls’ menstrual cycle, and according to differences in the maturation of brain structure and function. In the present study, youths were tested in different puberty stages, phases of their menstrual cycle or states of brain maturation. Not having controlled for these aspects, it is possible that the different biological states in which were the participants at the time of testing dampened the girls’ reactivity to the fear-related cues (CS+; Guapo et al., 2009; Kret & De Gelder, 2012; Little, 2013).
Regarding physiological fear responses during conditioning, an “own-sex” effect characterized SCRs, with boys showing greater physiological reactivity to the CS+ vs. CS− for male faces and girls, for female faces. This parallels findings from other physiological studies showing greater SCRs or electroencephalogram-measured cortical activity in males processing or being conditioned to male facial features, and in females processing female facial features (Doi, Amamoto, Okishige, Kato, & Shinohara, 2010; Kret & De Gelder, 2012; Mazurski, Bond, Siddle, & Lovibond, 1996; Suyama, Hoshiyama, Shimizu, & Saito, 2008). An “own-sex” bias was also observed for neural brain activation during memory encoding, with greater right amygdala activity being triggered in men for male faces and greater left amygdala activation being triggered in women for female faces (Armony & Sergerie, 2007). Our findings, as that of the above mentioned studies, could be accounted for by early developmental socialization processes. Indeed, young adolescents tend to spend more time with same-sex mates. As proposed by previous work, this could lead to better decoding of same-sex facial expressions, and a more thorough identification of the emotional cues transmitted (Cellerino, Borghetti, & Sartucci, 2004; McClure, 2000). Such behaviour is of particular importance since a more efficient analysis of emotional cues warning of potential self-related threat, as those efficiently transmitted by individuals of one’s own-sex, may enhance chances of survival.
In contrast, when participants were conditioned with stimuli from the opposite sex, no difference was observed in SCRs between CS+ and CS−. Despite the absence of differential learning (CS+ > CS−), it is difficult to argue that conditioning failed to occur in these two groups (boys viewing female faces and girls viewing male faces). Firstly, the contingency awareness data indicate clear stimulus distinction for practically all participants in the current study (i.e., CS+ perceived as threat-related and CS−, as a safety cue). Secondly, as demonstrated by the number of significant SCRs depicted in Fig. 4B, all participants showed SCRs to both CS+ and CS−. Amplitude of SCRs was, however, similar in both conditions. Therefore, the observed equivalent increases in SCRs for both the CS+ and CS− are most probably best explained by fear generalization, which occurs when the CS− (in this study, a neutral facial expression), by being perceptually similar to the CS+ (also a neutral facial expression in this study), triggers similar or even greater fear responses than the CS+ itself (Dunsmoor, Prince, Murty, Kragel, & LaBar, 2011; Lissek et al., 2005).
Such enhanced physiological fear reactivity to faces of the “out-group” (the social group to which one does not identify, e.g., because of sex, ethnicity or social category) has often been reported, explained by difficulties in accurately discriminating facial features of the “out-group” as opposed to that of the “in-group”, especially when threatening emotions are being displayed (Aleman & Swart, 2008; Navarrete et al., 2009; Rotteveel & Phaf, 2004; van der Schalk et al., 2011). Because neutral facial expressions are ambiguous and often misinterpreted as threatening (Cellerino et al., 2004; McClure, 2000), the opposite-sex effect observed in two of our groups (boys viewing female faces, girls viewing male faces) could be due to difficulties in efficiently discriminating opposite-sex neutral facial features. This could be explained, as mentioned above, by youths tending to spend more time with same-sex friends. Additionally, youths are in a period of the lifespan during which important changes in brain development are occurring, especially in the prefrontal cortex (Blakemore, 2012; Kret & De Gelder, 2012; Lenroot & Giedd, 2010). This region being a key player in the processing and interpretation of socio-affective cues, it is possible that the less familiar opposite-sex neutral facial cues (CS+ and CS−) were both perceived as threatening. Hence, important physiological reactivity was triggered by the opposite-sex targets, with boys not efficiently discriminating the threat-related CS+ from the safe CS− when depicted by female facial features (and vice versa for girls), explaining why fear was transferred from the CS+ to the CS−, and why fear generalization occurred only in those two groups.
Regarding differences in SCRs relative to ratings during conditioning, such discrepancies are not uncommon. As reported by a wealth of data, physiological responses are unconscious, automatic reflex-like responses triggered by the brain’s amygdala, which allows for rapid processing of crudely analysed information that are transmitted by downstream connections (e.g., with the midbrain and brainstem) and the thalamus. Hence, when the information is finally processed more thoroughly by the cortex, discrepancies easily arise between the automatic physiological responses and the cognitive appraisal of the same cue (LeDoux, 2014; Ohman, Carlsson, Lundqvist, & Ingvar, 2007).
Findings from the extinction phase led to another discrepancy, as we did not observe the same attenuation of fear responses in our young participants as that usually observed in adults (see reviews in Delgado, Olsson, & Phelps, 2006; Dimberg & Öhman, 1996; Jovanovic, Nylocks, & Gamwell, 2013; Ohman, 2009; Sehlmeyer et al., 2009). Indeed, resistance to fear extinction was observed in fear ratings for male faces in both boys and girls, and in SCRs of boys who viewed male faces. Lack of fear extinction has been reported before, notably by Lau and collaborators, who used a very similar version of the task presented here, in adolescents (Haddad, Lissek, Pine, & Lau, 2011; Lau et al., 2008). Such resistance to fear extinction could be related to social desirability and participants’ impression that the CS+ commanded elevated subjective fear responses. However, though this explanation seems fitted for the cognitive fear rating data, it seems more difficult to reconcile with the observed elevations in physiological responses, which depend on automatic, reflex-like mechanisms (LeDoux, 2014; Ohman et al., 2007). Another explanation related to task methodology could be suggested. In the current study, a 50% partial reinforcement schedule was used in order to prevent habituation to the US (Mackintosh, 1974). Such schedules have been linked to slower extinction of fear responses. But again, it is unlikely that this may explain our findings as other previous adolescent work using over 50% contingency reinforcement ratios (i.e., 75–100%) also report resistance to fear extinction (Lau et al., 2008; Neumann, Waters, Westbury, & Henry, 2008; Pattwell et al., 2012, 2013).
Most likely, a developmental bias may explain the lack of fear extinction observed. Healthy adults are generally quite efficient in suppressing fear responses (diminution of CS+ fear levels to that of the CS; Delgado et al., 2006; Dimberg & Öhman, 1996; Lissek et al., 2005; Ohman, 2009), even with a 50% contingency reinforcement ratio (e.g., Barrett & Armony, 2009; Gottfried & Dolan, 2004; Phelps, Delgado, Nearing, & LeDoux, 2004). As suggested by recent developmental fear conditioning and extinction studies performed in rodents and humans (Li, Kim, & Richardson, 2012; Pattwell et al., 2012, 2013), the persistent fear responses observed during extinction in youths may be due to differences in emotion processing between youths and adults. Work on normal brain development indeed demonstrates that youths are characterized by a mature limbic lobe but an under-developed frontal cortex, whereas both structures are optimally developed in adults. This normal protracted development of frontal regions relative to limbic areas in youths may have prevented the efficient regulation of the prefrontal cortex over the amygdala, leading to blunted cognitive and physiological regulation of amygdala-dependent fear responses and lack of fear extinction (Casey et al., 2010; Gogtay & Thompson, 2010; Pattwell et al., 2013).
Finally, though SCRs amplitude to male and female faces for the extinction phase were equivalent, the number of significant SCRs was significantly greater for female faces relative to male faces in all participants (Fig. 5B). Cautious interpretation of the number of significant SCRs is required since they do not reflect the magnitude of the responses. This finding could be reconciled with the extensively investigated perception that females are more emotional, fragile and vulnerable – especially when in a threat-related context – than males, a judgement based on implicit stereotypes, and social prejudice and desirability (Fisher, 1993; Friedman & Zebrowitz, 1992). This implicit stereotyped perception may have triggered unconscious, automatic reflex-like SCRs more often in participants.
4.1. Limitations and recommendations
Our findings should be considered in light of some limitations. Firstly, we did not control for hormonal puberty and menstrual cycle variations, the possible use of oral contraception, or differences in the maturation of brain structure and function. Since sex differences related to fear learning and extinction were recently shown to be influenced by these variables (Merz, Stark, Vaitl, Tabbert, & Wolf, 2013; Merz et al., 2012; Milad et al., 2006; Zeidan et al., 2011), further studies should take these factors into account. Secondly, a more thorough investigation of emotional difficulties in participants, using more standard mood and anxiety disorders questionnaires or interviews, could have helped control for confounding emotional symptoms which may have influenced participants’ performance on our fear-related task.
5. Conclusion
Despite these limitations, this first study of the influence of sex of participants and sex of target on fear conditioning and extinction in youths suggests that important differences exist in terms of how boys and girls react to male and female threatening cues. Both boys and girls were similarly conditioned to fear, and showed resistance to fear extinction. Moreover, even though both male and female faces triggered conditioning effects, resistance to fear extinction was observed only for male faces in boys and girls. Additionally, findings also reveal that fear responses, depending on whether they were measured subjectively or objectively, lead to different perspectives as to whether cues were perceived as threatening or safe in youths. These findings underline three important points: firstly, that male and female faces do not have the same impact on fear conditioning and extinction, with female faces triggering more comparable levels of fear learning and extinction in boys and girls, compared to male faces. Secondly, that the sex of the participant may interact with the sex of the target and lead to different fear conditioning and extinction responses. Third, that findings obtained via subjective measures (e.g., ratings) do not necessarily mirror findings obtained via objective measures (e.g., SCRs), suggesting that our conscious interpretation of threat may not match our automatic physiological reactivity to the same emotionally negative cues. These conclusions underlie the importance of carefully choosing the sex of target, depending on the effects one desires to obtain, and the necessity of using both types of measures in order to obtain a more complete comprehension of fear learning and extinction in youths.
Acknowledgments
This research was supported by a grant from the Canadian Institutes for Health Research (CIHR; MOP-97983) to FSM. FSM is a recipient of the CIHR New Investigator Award and Fonds de recherche en santé du Québec (FRSQ) Junior 1 Award. MC and VLB received PhD fellowships from the CIHR and the FRSQ. SS received a PhD fellowship from the Fonds Québécois de la Recherche sur la Société et la Culture (FQRSC) and the Foundation of Stars/Research Center of the Ste-Justine University Hospital. We acknowledge Drs. Pierre Rainville and Étienne Vachon-Presseau for their help and expertise with SCR analyses. We also thank the participants for taking part in this study.
References
- Achim A. Detecting outliers in multivariate data while controlling false alarm rate. Tutorials in Quantitative Methods for Psychology. 2012;8(2):108–121. [Google Scholar]
- Adams RB, Jr, Nelson AJ, Soto JA, Hess U, Kleck RE. Emotion in the neutral face: A mechanism for impression formation? Cognition and Emotion. 2012;26(3):431–441. doi: 10.1080/02699931.2012.666502. http://dx.doi.org/10.1080/02699931.2012.666502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado L, Garcia-Gutierrez A, Serrano-Pedraza I. Symmetrical interaction of sex and expression in face classification tasks. Attention, Perception, & Psychophysics. 2009;71(1):9–25. doi: 10.3758/APP.71.1.9. 71/1/9 [pii] [DOI] [PubMed] [Google Scholar]
- Aleman A, Swart M. Sex differences in neural activation to facial expressions denoting contempt and disgust. [Randomized Controlled Trial Research Support, Non-U.S. Gov’t] PLoS ONE. 2008;3(11):e3622. doi: 10.1371/journal.pone.0003622. http://dx.doi.org/10.1371/journal.pone.0003622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony JL, Sergerie K. Own-sex effects in emotional memory for faces. Neuroscience Letters. 2007;426(1):1–5. doi: 10.1016/j.neulet.2007.08.032. http://dx.doi.org/10.1016/j.neulet.2007.08.032. pii:S0304-3940(07)00886-5. [DOI] [PubMed] [Google Scholar]
- Barrett J, Armony JL. Influence of trait anxiety on brain activity during the acquisition and extinction of aversive conditioning. [Research Support, Non-U.S. Gov’t] Psychological Medicine. 2009;39(2):255–265. doi: 10.1017/S0033291708003516. http://dx.doi.org/10.1017/S0033291708003516. [DOI] [PubMed] [Google Scholar]
- Becker DV, Kenrick DT, Neuberg SL, Blackwell KC, Smith DM. The confounded nature of angry men and happy women. Journal of Personality and Social Psychology. 2007;92(2):179–190. doi: 10.1037/0022-3514.92.2.179. http://dx.doi.org/10.1037/0022-3514.92.2.179, pii:2007-00654-002. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. Development of the social brain in adolescence. [Research Support, Non-U.S. Gov’t Review] Journal of the Royal Society of Medicine. 2012;105(3):111–116. doi: 10.1258/jrsm.2011.110221. http://dx.doi.org/10.1258/jrsm.2011.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, et al. The storm and stress of adolescence: Insights from human imaging and mouse genetics. Developmental Psychobiology. 2010;52(3):225–235. doi: 10.1002/dev.20447. http://dx.doi.org/10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellerino A, Borghetti D, Sartucci F. Sex differences in face gender recognition in humans. Brain Research Bulletin. 2004;63(6):443–449. doi: 10.1016/j.brainresbull.2004.03.010. http://dx.doi.org/10.1016/j.brainresbull.2004.03.010. pii:S0361-9230(04)00101-7. [DOI] [PubMed] [Google Scholar]
- Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiology and Behavior. 2009;97(2):229–238. doi: 10.1016/j.physbeh.2009.02.035. http://dx.doi.org/10.1016/j.physbeh.2009.02.035. pii:S0031-9384(09)00075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M, Wilson M. Evolutionary psychology of male violence. In: Routledge C, editor. Male violence. New York: J. Archer; 1994. [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Bernston GG, editors. Handbook of psychophysiology. 2. USA: Cambridge University Press; 2000. pp. 200–223. [Google Scholar]
- De Sonneville LM, Verschoor CA, Njiokiktjien C, Op het Veld V, Toorenaar N, Vranken M. Facial identity and facial emotions: Speed, accuracy, and processing strategies in children and adults. [Research Support, Non-U.S. Gov’t] Journal of Clinical and Experimental Neuropsychology. 2002;24(2):200–213. doi: 10.1076/jcen.24.2.200.989. http://dx.doi.org/10.1076/jcen.24.2.200.989. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. [Comparative Study Review] Biological Psychology. 2006;73(1):39–48. doi: 10.1016/j.biopsycho.2006.01.006. http://dx.doi.org/10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Dimberg U, Öhman A. Behold the wrath: Psychophysiological responses to facial stimuly. Motivation and Emotion. 1996;20(2) [Google Scholar]
- Doi H, Amamoto T, Okishige Y, Kato M, Shinohara K. The own-sex effect in facial expression recognition. [Research Support, Non-U.S. Gov’t] Neuroreport. 2010;21(8):564–568. doi: 10.1097/WNR.0b013e328339b61a. http://dx.doi.org/10.1097/WNR.0b013e328339b61a. [DOI] [PubMed] [Google Scholar]
- Dubé AA, Duquette M, Roy M, Lepore F, Duncan G, Rainville P. Brain activity associated with the electrodermal reactivity to acute heat pain. [Research Support, Non-U.S. Gov’t] Neuroimage. 2009;45(1):169–180. doi: 10.1016/j.neuroimage.2008.10.024. http://dx.doi.org/10.1016/j.neuroimage.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Prince SE, Murty VP, Kragel PA, LaBar KS. Neurobehavioral mechanisms of human fear generalization. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] Neuroimage. 2011;55(4):1878–1888. doi: 10.1016/j.neuroimage.2011.01.041. http://dx.doi.org/10.1016/j.neuroimage.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Pine DS, Nelson E, Leibenluft E, Ernst M, Towbin KE, et al. The NIMH Child Emotional Faces Picture Set (NIMH-ChEFS): A new set of children’s facial emotion stimuli. International Journal of Methods in Psychiatric Research. 2011;20(3):145–156. doi: 10.1002/mpr.343. http://dx.doi.org/10.1002/mpr.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AH. Sex differences in emotionality: Fact of stereotype? Feminism & Psychology. 1993;3(3):303–318. [Google Scholar]
- Forsyth JP, Eifert GH. Response intensity in content-specific fear conditioning comparing 20% versus 13% CO2 -enriched air as unconditioned stimuli. Journal of Abnormal Psychology. 1998;107(2):291–304. doi: 10.1037//0021-843x.107.2.291. [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Friedman H, Zebrowitz LA. The contribution of typical sex differences in facial maturity to sex role stereotypes. Society for Personality and Social Psychology. 1992;18:430–438. [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. [Review] Brain and Cognition. 2010;72(1):6–15. doi: 10.1016/j.bandc.2009.08.009. http://dx.doi.org/10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goos LM, Silverman I. Sex related factors in the perception of threatening facial expressions. Journal of Nonverbal Behavior. 2002;26(1) [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. [Research Support, Non-U.S. Gov’t] Nature Neuroscience. 2004;7(10):1144–1152. doi: 10.1038/nn1314. http://dx.doi.org/10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Guapo VG, Graeff FG, Zani AC, Labate CM, dos Reis RM, Del-Ben CM. Effects of sex hormonal levels and phases of the menstrual cycle in the processing of emotional faces. [Comparative Study. Research Support, Non-U.S. Gov’t] Psychoneuroendocrinology. 2009;34(7):1087–1094. doi: 10.1016/j.psyneuen.2009.02.007. http://dx.doi.org/10.1016/j.psyneuen.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Haddad AD, Lissek S, Pine DS, Lau JY. How do social fears in adolescence develop? Fear conditioning shapes attention orienting to social threat cues. Cognition and Emotion. 2011;25(6):1139–1147. doi: 10.1080/02699931.2010.524193. http://dx.doi.org/10.1080/02699931.2010.524193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herba CM, Landau S, Russell T, Ecker C, Phillips ML. The development of emotion-processing in children: Effects of age, emotion, and intensity. [Research Support, Non-U.S. Gov’t] Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47(11):1098–1106. doi: 10.1111/j.1469-7610.2006.01652.x. http://dx.doi.org/10.1111/j.1469-7610.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Hess H, Blairy S, Kleck RE. The intensity of emotional facial expressions and decoding accuracy. Journal of Nonverbal Behavior. 1997;21(4) [Google Scholar]
- Jovanovic T, Nylocks KM, Gamwell KL. Translational neuroscience measures of fear conditioning across development: Applications to high-risk children and adolescents. Biol Mood Anxiety Disord. 2013;3(1):17. doi: 10.1186/2045-5380-3-17. http://dx.doi.org/10.1186/2045-5380-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MM, Forsyth JP. Observational fear conditioning in the acquisition and extinction of attentional bias for threat: An experimental evaluation. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Emotion. 2007;7(2):324–335. doi: 10.1037/1528-3542.7.2.324. http://dx.doi.org/10.1037/1528-3542.7.2.324. [DOI] [PubMed] [Google Scholar]
- Kret ME, De Gelder B. A review on sex differences in processing emotional signals. Neuropsychologia. 2012;50(7):1211–1221. doi: 10.1016/j.neuropsychologia.2011.12.022. http://dx.doi.org/10.1016/j.neuropsychologia.2011.12.022. pii:S0028-3932(12)00002-4. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gordon AH. Sex differences in emotion: Expression, experience, and physiology. Journal of Personality and Social Psychology. 1998;74(3):686–703. doi: 10.1037//0022-3514.74.3.686. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, et al. Distinct neural signatures of threat learning in adolescents and adults. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(11):4500–4505. doi: 10.1073/pnas.1005494108. http://dx.doi.org/10.1073/pnas.1005494108, pii:1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, et al. Fear conditioning in adolescents with anxiety disorders: Results from a novel experimental paradigm. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(1):94–102. doi: 10.1097/chi.0b01e31815a5f01. http://dx.doi.org/10.1097/chi.0b01e31815a5f01. pii:S0890-8567(09)62089-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Coming to terms with fear. [Review] Proceedings of the National Academy of Sciences of the United States of America. 2014;111(8):2871–2878. doi: 10.1073/pnas.1400335111. http://dx.doi.org/10.1073/pnas.1400335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NC, Krabbendam L, White TP, Meeter M, Banaschewski T, Barker GJ, et al. Do you see what I see? Sex differences in the discrimination of facial emotions during adolescence. [Research Support, Non-U.S. Gov’t] Emotion. 2013;13(6):1030–1040. doi: 10.1037/a0033560. http://dx.doi.org/10.1037/a0033560. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. [Review] Brain and Cognition. 2010;72(1):46–55. doi: 10.1016/j.bandc.2009.10.008. http://dx.doi.org/10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kim JH, Richardson R. Differential involvement of the medial prefrontal cortex in the expression of learned fear across development. [Research Support, Non-U.S. Gov’t] Behavioral Neuroscience. 2012;126(2):217–225. doi: 10.1037/a0027151. http://dx.doi.org/10.1037/a0027151. [DOI] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, et al. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behaviour Research and Therapy. 2005;43(11):1391–1424. doi: 10.1016/j.brat.2004.10.007. http://dx.doi.org/10.1016/j.brat.2004.10.007. pii:S0005-7967(04)00251-7. [DOI] [PubMed] [Google Scholar]
- Little AC. The influence of steroid sex hormones on the cognitive and emotional processing of visual stimuli in humans. [Review] Frontiers in Neuroendocrinology. 2013;34(4):315–328. doi: 10.1016/j.yfrne.2013.07.009. http://dx.doi.org/10.1016/j.yfrne.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. The psychology of animal learning. London: Academic Press; 1974. [Google Scholar]
- Mazurski EJ, Bond NW, Siddle DA, Lovibond PF. Conditioning with facial expressions of emotion: Effects of CS sex and age. Psychophysiology. 1996;33(4):416–425. doi: 10.1111/j.1469-8986.1996.tb01067.x. [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Konkle AT. When is a sex difference not a sex difference? [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, P.H.S. Review] Frontiers in Neuroendocrinology. 2005;26(2):85–102. doi: 10.1016/j.yfrne.2005.06.001. http://dx.doi.org/10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- McClure EB. A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. [Meta-Analysis] Psychological Bulletin. 2000;126(3):424–453. doi: 10.1037/0033-2909.126.3.424. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Stark R, Vaitl D, Tabbert K, Wolf OT. Stress hormones are associated with the neuronal correlates of instructed fear conditioning. Biological Psychology. 2013;92(1):82–89. doi: 10.1016/j.biopsycho.2012.02.017. http://dx.doi.org/10.1016/j.biopsycho.2012.02.017. pii:S0301-0511(12)00039-7. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, et al. Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Hormones and Behavior. 2012;62(4):531–538. doi: 10.1016/j.yhbeh.2012.09.001. http://dx.doi.org/10.1016/j.yhbeh.2012.09.001. pii:S0018-506X(12)00208-5. [DOI] [PubMed] [Google Scholar]
- Meulders A, Vansteenwegen D, Vlaeyen JW. Women, but not men, report increasingly more pain during repeated (un)predictable painful electrocutaneous stimulation: Evidence for mediation by fear of pain. [Research Support, Non-U.S. Gov’t] Pain. 2012;153(5):1030–1041. doi: 10.1016/j.pain.2012.02.005. http://dx.doi.org/10.1016/j.pain.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, et al. Fear conditioning and extinction: Influence of sex and menstrual cycle in healthy humans. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Behavioral Neuroscience. 2006;120(6):1196–1203. doi: 10.1037/0735-7044.120.5.1196. http://dx.doi.org/10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- Navarrete CD, Olsson A, Ho AK, Mendes WB, Thomsen L, Sidanius J. Fear extinction to an out-group face: The role of target gender. [Research Support, U.S. Gov’t, Non-P.H.S.] Psychological Science. 2009;20(2):155–158. doi: 10.1111/j.1467-9280.2009.02273.x. http://dx.doi.org/10.1111/j.1467-9280.2009.02273.x. [DOI] [PubMed] [Google Scholar]
- Neumann DL, Waters AM, Westbury HR, Henry J. The use of an unpleasant sound unconditional stimulus in an aversive conditioning procedure with 8- to 11-year-old children. [Research Support, Non-U.S. Gov’t] Biological Psychology. 2008;79(3):337–342. doi: 10.1016/j.biopsycho.2008.08.005. http://dx.doi.org/10.1016/j.biopsycho.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Ohman A. Of snakes and faces: An evolutionary perspective on the psychology of fear. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Scandinavian Journal of Psychology. 2009;50(6):543–552. doi: 10.1111/j.1467-9450.2009.00784.x. http://dx.doi.org/10.1111/j.1467-9450.2009.00784.x. [DOI] [PubMed] [Google Scholar]
- Ohman A, Carlsson K, Lundqvist D, Ingvar M. On the unconscious subcortical origin of human fear. [Research Support, Non-U.S. Gov’t Review] Physiology and Behavior. 2007;92(1–2):180–185. doi: 10.1016/j.physbeh.2007.05.057. http://dx.doi.org/10.1016/j.physbeh.2007.05.057. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, et al. Altered fear learning across development in both mouse and human. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Proceedings of the National Academy of Sciences of the United States of America. 2012;109(40):16318–16323. doi: 10.1073/pnas.1206834109. http://dx.doi.org/10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Lee FS, Casey BJ. Fear learning and memory across adolescent development: Hormones and behavior special issue: Puberty and adolescence. Hormones and Behavior. 2013;64(2):380–389. doi: 10.1016/j.yhbeh.2013.01.016. http://dx.doi.org/10.1016/j.yhbeh.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. [Comparative Study Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. http://dx.doi.org/10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Rotteveel M, Phaf RH. Automatic affective evaluation does not automatically predispose for arm flexion and extension. Emotion. 2004;4(2):156–172. doi: 10.1037/1528-3542.4.2.156. http://dx.doi.org/10.1037/1528-3542.4.2.156. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human fear conditioning and extinction in neuroimaging: A systematic review. [Research Support, Non-U.S. Gov’t Review] PLoS ONE. 2009;4(6):e5865. doi: 10.1371/journal.pone.0005865. http://dx.doi.org/10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel EM, Habel U, Kirschner M, Gur RC, Derntl B. The impact of facial emotional expressions on behavioral tendencies in women and men. Journal of Experimental Psychology: Human Perception and Performance. 2010;36(2):500–507. doi: 10.1037/a0018169. http://dx.doi.org/10.1037/a0018169, pii:2010-06263-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama N, Hoshiyama M, Shimizu H, Saito H. Event-related potentials for gender discrimination: An examination between differences in gender discrimination between males and females. [Comparative Study] International Journal of Neuroscience. 2008;118(9):1227–1237. doi: 10.1080/00207450601047176. http://dx.doi.org/10.1080/00207450601047176. [DOI] [PubMed] [Google Scholar]
- Thomas LA, De Bellis MD, Graham R, LaBar KS. Development of emotional facial recognition in late childhood and adolescence. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] Developmental Science. 2007;10(5):547–558. doi: 10.1111/j.1467-7687.2007.00614.x. http://dx.doi.org/10.1111/j.1467-7687.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: Judgments from untrained research participants. [Research Support, Non-U.S. Gov’t] Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. http://dx.doi.org/10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schalk J, Fischer A, Doosje B, Wigboldus D, Hawk S, Rotteveel M, et al. Convergent and divergent responses to emotional displays of ingroup and outgroup. [Research Support, Non-U.S. Gov’t] Emotion. 2011;11(2):286–298. doi: 10.1037/a0022582. http://dx.doi.org/10.1037/a0022582. [DOI] [PubMed] [Google Scholar]
- Vicari S, Reilly JS, Pasqualetti P, Vizzotto A, Caltagirone C. Recognition of facial expressions of emotions in school-age children: The intersection of perceptual and semantic categories. Acta Paediatrica. 2000;89(7):836–845. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological Psychiatry. 2011;70(10):920–927. doi: 10.1016/j.biopsych.2011.05.016. http://dx.doi.org/10.1016/j.biopsych.2011.05.016. pii:S0006-3223(11)00545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]