Abstract
Purpose of review
The global significance of cryptosporidiosis is widespread and far-reaching. In this review, we present recent data about strain diversity and the burden of disease, along with developments in therapeutic and preventative strategies.
Recent findings
Cryptosporidium is an emerging pathogen that disproportionately affects children in developing countries and immunocompromised individuals. Without a diagnostic tool amenable for use in developing countries the burden of infection and its relationship to growth faltering, malnutrition, and diarrheal mortality remain underappreciated. Disease incidence is also increasing in industrialized countries largely as a result of outbreaks in recreational water facilities. Advances in molecular methods, including subtyping analysis, have yielded new insights into the epidemiology of cryptosporidiosis. However, without practical point-of-care diagnostics, an effective treatment for immunocompromised patients, and a promising vaccine candidate, the ability to reduce the burden of disease in the near future is limited. This is compounded by inadequate coverage with antiretroviral therapy in developing countries, the only current means of managing HIV-infected patients with cryptosporidiosis.
Summary
Cryptosporidiosis is one of the most important diarrheal pathogens affecting people worldwide. Effective methods to control and treat cryptosporidiosis among high risk groups present an ongoing problem in need of attention.
Keywords: cryptosporidiosis, review, malnutrition, HIV, diarrhea
Introduction
Cryptosporidium is an Apicomplexan oocyst-forming protozoan, first recognized as a causative agent of gastroenteritis in 1976 (1). It is one of the most common human enteropathogens worldwide, with young children living in developing countries and persons with HIV/AIDS experiencing more frequent and more severe illness sometimes complicated by malnutrition and long term impairment of physical fitness. Reported cases in industrialized countries are also rising due to the leading role that Cryptosporidium plays as a cause of waterborne outbreaks. However, the true global burden of cryptosporidiosis is not known, owing at least in part to the lack of simple, inexpensive diagnostic tools, underappreciation of the frequency and severity of disease in immunocompetent patients, and difficulties quantifying the impact of an infection that causes an acute illness with long term sequelae.
This report reviews recent literature that expands our knowledge of the burden of cryptosporidiosis as a global pathogen, and the new insights provided by an expanding armamentarium of molecular diagnostics. Areas that might be addressed in the future to decrease the morbidity and mortality associated with this infection are discussed.
The Organism
During the last decade, the development of molecular typing techniques such as PCR-RFLP analysis of the small subunit rRNA-gene has distinguished ~20 species of Cryptosporidium and shown that C. hominis (found mainly in humans) and C. parvum (found commonly in humans and bovines) are the major species infecting humans. Occasionally, other species infect people, including C. meleagridis, C. felis, C. canis, C. andersoni, C. suis, C. baylei, and C. muris (2, 3). C. hominis and C. parvum can be subtyped by sequencing the 60 kDA glycoprotein (gp60, also called gp40/15), a surface antigen involved in parasite attachment and invasion (4). Sequence differences in the non-repeat regions are used to distinguish subtype families (designated Ia, Ib, etc. for C. hominis and IIa, IIb, etc. for C. parvum). Within each family, subtypes are based on the number of trinucleotide tandem repeats at the 5’ (gp40) end of the gene. Subtypes Ib, IIa, IIc, and IId are among those most commonly observed in humans (5). Greater genetic diversity is seen in developing countries, particularly in rural settings (6), and distinct populations can arise because of geographic segregation (7–11). Subtyping provides useful information regarding source of transmission (e.g., zoonotic versus anthroponotic); however, the correlation between subtype and clinical manifestations and the existence of homotypic and heterotypic subtype immunity are among the issues that require further exploration (4).
Epidemiology
Cryptosporidium is associated with diarrheal disease in all regions of the world with the exception of Antartica, but is most common in less developed countries (12). The distribution of C. hominis and C. parvum in humans varies by geographic region. C. hominis tends to predominate in most parts of the world, especially in developing countries, while C. parvum is more frequent in the Middle East and both species are common in the Europe (4).
Cryptosporidium is transmitted primarily by the fecal-oral route either by direct contact with an infected human or animal or indirectly via contaminated food or water. Contamination of crops, other agricultural products, and surface water with feces from cattle and other livestock is another important mechanism of zoonotic transmission. Several intrinsic properties of this parasite help to explain its epidemiologic behavior. For one, oocysts are infectious immediately upon being excreted in feces, are shed in high numbers (up to 109 per stool), and can be passed for as long as 2 months after cessation of diarrhea (13, 14). Secondly, the infectious dose is low, with as few as 9–10 oocysts of certain C. parvum and C. hominis strains producing symptoms in healthy volunteers (15–17). Third, oocysts can remain infectious in the environment for at least 6 months if kept moist, resist disinfection (including chlorination (18)), and survive in properly chlorinated recreational water venues for >10 days (19). Fourth, the protracted incubation period (average 7 days, range 1–30 days) allows transmission to continue for days before an outbreak is recognized by public health authorities (14). Finally, age-related decreases in disease incidence suggest that immunity induced by prior exposure is protective (20); thus, those lacking protective immunity (e.g., children in endemic settings and immunocompromised persons) are most at risk. With this in mind, one can appreciate how Cryptosporidium spreads proficiently in settings without adequate sanitation and hygiene in both developed and developing countries (21). A good example of its remarkable transmissibility is a statewide outbreak in the United States during May to December 2007 which involved 5,697 cases and ~450 contaminated recreational water venues. As the outbreak propagated, secondary transmission from ill contacts became increasingly important, eventually outweighing recreational water exposure as a risk factor (19).
In the United States, cryptosporidiosis is widespread geographically, occurs more commonly during the warm, rainy months, and has a bimodal age distribution, with the greatest number of reported cases occurring among children 1–9 year and among adults aged 25–39 years (18). Risk factors associated with sporadic infection include contact with ill persons and cattle, travel abroad (18), and anal intercourse among homosexuals (22). Outbreaks in child care centers are also common, and can result in spread to the community (23).
In developing countries, incidence of disease peaks in young children, who are often infected by the age of two years (12, 24, 25). Exclusive breastfeeding during the first 3 months of life, and partial breast feeding (compared to no breastfeeding) thereafter, appears to afford some protection (26). Peaks usually occur during warm rainy months (27).
Clinical manifestations
A wide spectrum of disease severity is seen, influenced by the age, nutritional, and immune status of the host and possibly by the infecting species and subtype (28). Many infections are asymptomatic or mild and self-limited and often go unrecognized. The cardinal symptom is diarrhea, which is typically watery, and accompanied by abdominal cramps, fatigue, nausea, and anorexia. Fever and vomiting may occur. Diarrhea tends to persist longer (median of 5 to 10 days) than that seen with other etiologies and may relapse. In industrialized countries, most cases are immunocompetent adults who experience a self-limited illness. Among children in developing countries, the diarrhea often lasts for 14 days or longer (29), making Cryptosporidium one of the most important causes of persistent diarrhea in this population. Several prospective studies have examined the complex bidirectional relationship between malnutrition and both symptomatic and asymptomatic Cryptosporidium infection in infants and children (30–34). Malnutrition is a risk factor for both diarrhea and prolonged diarrhea caused by Cryptosporidium, with significantly higher rates of infection in malnourished children controlling for HIV status (34–37). Moreover, cohort studies have demonstrated that a single episode during infancy, even if asymptomatic, can lead to growth faltering that persists for months (32, 33). Long term follow-up also suggests an association with poor physical fitness, as children who had cryptosporidiosis during the first 2 years of life had Harvard Step Test fitness scores that were 10% lower than children who did not, when measured 4–7 years later (38). Given the magnitude of this effect, even in a small study, these results warrant further exploration. Cryptosporidiosis is also an independent risk factor of childhood mortality (33, 35, 39, 40).
In persons with HIV/AIDS, it is not until the CD4 count falls below ~100 cells/ mm3 that the risk increases for severe, unrelenting disease accompanied by malabsorption, weight loss, and high case fatality (5, 41), although asymptomatic or mild infection can occur even in this group (42). Symptoms can be ameliorated and mortality rates diminished with immune reconstitution following antiretroviral therapy (43). In developing countries where most HIV-infected people lack access to antiretroviral therapy, the burden of severe cryptosporididosis remains high (8, 18, 44, 45).
Extra-intestinal manifestations of Cryptosporidium infection are seen. Biliary tract disease, including acalculous cholecystitis, pancreatitis, cholangitis, and stricture formation, is a well-documented complication in severely immunocompromised patients and carries a poor prognosis (46). Respiratory cryptosporidiosis has been described, most often in children (47). Infection is often asymptomatic, but may manifest as pulmonary infiltrates and respiratory distress.
Several studies suggest that C. hominis produces more severe disease than C. parvum (48, 49). Evidence for a possible correlation between subtype and clinical manifestations is accumulating. In a birth cohort of children from Lima, Peru C. hominis subtype family Ib was associated with nausea, vomiting and malaise, whereas C. hominis subtype family Ia, Id and Ie and the other species were not (28). Risk factors such as hygiene practices, presence of animals and economic variables were not associated with specific genotypes and subtypes. In a case series of nine HIV-infected patients from Italy, the four patients with the most severe disease, all who had a CD4+ T lymphocyte count <50 cells/mm3, harbored C. parvum subtypes within the family IIc (50). Larger studies are needed to validate these observations.
Diagnosis
The diagnosis of cryptosporidiosis is usually made by detection of oocysts, oocyst antigens, or oocyst DNA in stool specimens (Table 1)(51–54), although histologic examination of intestinal biopsies is also possible (12). Recent advances have produced high performing diagnostic tools; however, the expense and requirement for technical expertise have limited their use in resource-poor settings. The most commonly used method continues to be microscopic examination of stool (preferably preserved with formalin and concentrated) to detect this small (4–6 µm) coccidion parasite; however, microscopy requires a skilled operator. Identification is enhanced using modified acid fast staining, but the detection limit is only 50,000 to 500,000 cysts per gram of stool (55). Sensitivity of microscopy is further improved by with the use of fluorescent or immunofluorescent stains.
Table 1.
Sensitivity and specificity of Cryptosporidium assays*
| Method | Sensitivity (%) |
Specificity (%) |
Comments | Ref |
|---|---|---|---|---|
| Microscopic visualization of oocysts | ||||
| AF | 37–84 | 99–100 | Least expensive, reagents widely available but insensitive, time consuming. | (51) (52) (53) (54) |
| FS | 92 | 100 | More sensitive and less time consuming than AF, requires fluorescent microscope | (52) |
| DFA | 97 | 100 | More sensitive than EIA and FS but more laborious than EIA, requires fluorescent microscope | (52) |
| Antigen detection | ||||
| EIA | 91–94 | 92–100 | Relatively simple to perform, no need for skilled microscopist; some kits also detect Entamoeba and/ or Giardia so confirmatory tests may be required | (51) (52) |
| IC | 83–85 | 100 | Simple to perform with quick turnaround time but less sensitive than EIA | (52) (54) |
| Molecular tests | ||||
| PCR | 100 | 100 | Best performance, can differentiate species, most costly and time consuming | (51) (52) (53) (54) |
Based on studies that either used PCR as the gold standard, or that included PCR as part of the gold standard if the sensitivity and specificity of PCR was 100%
AF = modified acid fast stain, FS = fluorescence stain (auromine phenol), DFA = direct immunofluorescence stain, EIA = enzyme immunoassay, IC = immunochoromatography, PCR = polymerase chain reaction.
Many clinicians are not aware that superior methods for diagnosing cryptosporidiosis are available (56), such as enzyme immunoassays (EIA). EIA is relatively simple to perform while achieving high levels of sensitivity and specificity (51, 52, 57). Antigen tests that simultaneously detect multiple parasitic enteropathogens with high accuracy have been validated internationally, are moderately simple to perform, and can be tested in batches (58). However, a confirmatory test to distinguish the offending agent may be required. Immunochromatography, which can be performed in minutes, has high specificity, but only moderate sensitivity (52). Extremely sensitive PCR methods are available in reference laboratories and may prove useful for the diagnosis of Cryptosporidium infection in the future (59, 60).
Burden of disease in developing countries
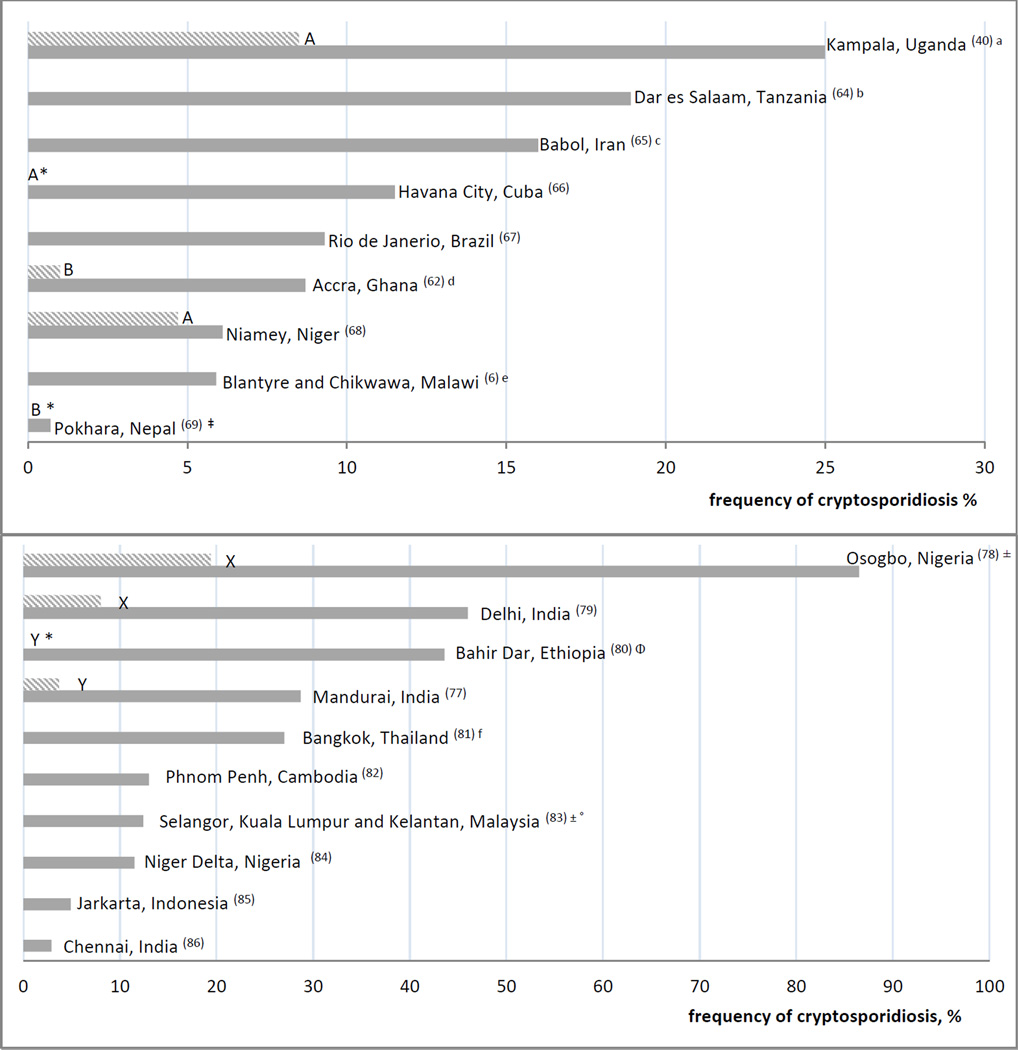
The greatest burden of cryptosporidiosis occurs among children living in developing countries. However, the burden is difficult to quantify as estimates vary widely, even among studies from the same geographic region, as a result of differences in study design, sample size, age range, HIV status, severity of disease and sensitivity of diagnostic methods used (5). For example, Cryptosporidium was detected by modified acid fast staining of fecal smears in only 2% of children less than five years with diarrhea attending health centers in Accra, Ghana (61), compared with 8.7% of a similar population from Accra using real time PCR of fecal DNA (62). The impact of study design on disease burden estimates was demonstrated in a recent review of studies from sub-Saharan Africa that used health care-seeking behavior as a proxy for more severe illness, and found higher rates of cryptosporidiosis among children recruited in hospital-based studies (14.6–22.2%) compared to community-based studies (7.5–12.5%) (39). Studies of diarrheal disease do not reflect the rate of infection in asymptomatic children, which can be high (27, 63), and negatively impact a child’s growth. Figure 1a (6, 40, 62, 64–69) shows the results of recent studies demonstrating the wide, but consistently high prevalence rates of cryptosporidiosis in young children in developing countries seeking hospital care.
Figure 1.
a: Hospital based cryptosporidiosis prevalence rates among children under 6 years with diarrhea in developing countries**
** HIV status was unknown or unreported in these studies; unless otherwise noted, primary detection of cryptosporidiosis was by microscopy with acid fast staining
a Detection by PCR
b: Urban Hospital and HIV clinic based cryptosporidiosis frequency rates among HIV-infected adult patients with diarrhea in developing countries***
*** Unless otherwise noted, primary detection of cryptosporidiosis was by microscopy with acid fast staining, ± = pediatric patients included, Φ = only 80% of HIV-infected patients in this study had diarrhea, X = HIV-infected controls without diarrhea, Y = HIV-negative controls, frequency of cryptosporidiosis was 0, Z = HIV-negative controls with diarrhea, • Studies above line included controls, IF = immunofluorescent staining, frequency 18.4% with acid fast staining
Seroprevalence studies have been useful in providing evidence that infection is more widespread than appreciated (70). Seropositive rates of 17–54% were found in the United States, reaching 70% among children living near the Mexican border. Rates were somewhat higher in Southern and Eastern Europe (33–88%) and in some developing countries (64–94%) (71–74). An increased frequency and severity of cryptosporidiosis in AIDS-associated diarrhea among adults and children in developing countries is well-documented (75), although, prevalence estimates vary considerably from study-to-study as with HIV-uninfected populations (76, 77). The burgeoning HIV epidemic in sub-Saharan Africa has undoubtedly enhanced the burden. Figure 1b (77–86) depicts recent prevalence estimates of cryptosporidiosis among HIV populations in developing countries.
Valuable information is forthcoming from the Global Enteric Multi-Center Study (GEMS) regarding the attributable burden of a comprehensive panel of enteric pathogens in children under 5 years of age living in developing countries in South Asia and sub-Saharan Africa with moderate to severe diarrhea. Preliminary results using a standard EIA at all sites indicate that Cryptosporidium is one of the most important pathogens.
Cryptosporidiosis in Industrialized countries
Although Cryptosporidium is an uncommon cause of acute sporadic diarrhea in industrialized countries, it is a leading cause of waterborne outbreaks (87, 88). The 1993 outbreak of cryptosporidiosis in Milwaukee, Wisconsin which affected over 400,000 people using the municipal water supply during a 2 month period is probably the best known example. The cost of outbreak-associated illness was estimated to be over 96 million US dollars (89).
In the United States cryptosporidiosis is a notifiable disease and was implicated in 60 (44.8%) of the 134 recreational water-associated outbreaks reported for 2007–2008, making it the most commonly identified pathogen in this setting (90). About 10,500 cases were reported in 2008, part of an overall increasing trend (18). Similarly, review of all published protozoan outbreaks that occurred worldwide between 2004–2010 showed that Cryptosporidium accounted for the majority (60%) of outbreaks, with most reports coming from Australia, North America and Europe (91). Many outbreaks undoubtedly go unrecognized, even in countries with established surveillance, and many sporadic cases actually may be part of an unrecognized outbreak.
With the wide spread availability of antiretroviral therapy in the United States, the incidence of cryptosporidiosis has decreased among people living with HIV/AIDS (44). The increasing number of transplant recipients and those receiving immunosuppressive drugs may contribute significantly to the burden in the future (92, 93).
Treatment
The thiazole compound, nitazoxanide is the only drug FDA approved for the treatment of cryptosporidiosis. Nitazoxanide has been shown to improve both clinical and microbiological cure rates and decrease the duration and severity of symptoms in immunocompetent patients. Diarrhea was resolved in 80% of adults and children within 7 days of being randomized to receive a 3 day course of nitazoxanide compared to only 41% of those randomized to placebo. Elimination of oocysts shedding occurred in 75% of nitazoxanide recipients compared to 20% of those receiving placebo (94). Several other thiazoles have been shown to have in vitro activity against C. parvum and may serve as candidates for future drug development (95). Conversely, nitazoxanide is ineffective in HIV-infected patients (96), even when high doses and prolonged treatment are used (96). Paromomycin, a nonabsorbable aminoglycoside with some activity against Cryptosporidium in immunocompetent people, is also not curative in HIV/ AIDS patients (41). Resolution of symptoms relies on restoration of immune status using combination antiretroviral therapy. Combination anti-parasitic and antiretroviral therapy, especially with a protease inhibitor based therapy which seems to have some additional anti-parasitic activity, seems to benefit patients with cryptosporidiosis (41).
Nitazoxanide is used uncommonly in developing countries. In Peru, children receiving empiric nitazoxanide had a shorter duration of diarrhea associated with multiple etiologies compared with those receiving placebo (97). This benefit was also seen in the large group of patients with no identified enteropathogen. No cases of cryptosporidiosis were detected, but diagnosis relied upon stool microscopy, which may have lacked sufficient sensitivity to detect the organism.
Prevention
No effective vaccine is available to prevent cryptosporidiosis. Evidence that vaccination might be an effective preventive strategy comes from observations of age-related declines in infection among children from developing countries (which presumably reflects acquisition of immunity), and human challenge studies showing protection associated with previous exposure (20). Efforts to develop vaccine are limited by insufficient understanding of the immune responses mediating protection. The surface-associated immunodominant antigens (gp15, gp40, p27) present on the invasive stage of the organism is one target of interest (98, 99). Antibody to the p23 antigen is observed in children with cryptosporidiosis compared to those with non-cryptosporidial diarrhea and the p23 sequence appeared to be relatively conserved among infection species and subtype families, making it another promising vaccine antigen (100). Antigens associated with the intracellular and sexual stages are also of interest and incorporation of multiple antigens into a vaccine may eventually be required (101). A recently developed reproducible model of cryptosporidiosis in weaned mice may provide a useful tool for vaccine development (102). Passive and other novel immunotherapies are also being explored [98,99].
Conclusion
Cryptosporidiosis is one of the most significant enteropathogens worldwide. Advances in molecular epidemiology have improved our knowledge about strain diversity. However, prevention is difficult, given the organism’s high infectivity, robustness, and resistance to disinfection, highlighting the need for improved therapeutics particularly for immunocompromised individuals, and a safe and effective vaccine.
Key points.
Cryptosporidium spp. is one of the most common and virulent enteropathogens in humans
Malnourished children and those living with HIV/ AIDS in developing countries bear the brunt of the burden associated with this disease
Advances in molecular epidemiology will expand our understanding of the epidemiology of this infection
Anti-parasitic therapies are ineffective in HIV/ AIDS patients
Prevention is difficult, given the organism’s high infectivity, robustness, and resistance to disinfection highlighting the need for a safe and effective vaccine
Acknowledgments
Disclosures:
Debbie-Ann Shirley receives research support through the NIH grant 2 T32 AI07524-15.
Karen Kotloff receives research support in part from the Bill and Melinda Gates Foundation and the National Institutes of Health.
Contributor Information
Debbie-Ann T. Shirley, Division of Infectious Diseases and Tropical Pediatrics, Department of Pediatrics, Center for Vaccine Development, University of Maryland School of Medicine, 685 W. Baltimore Street, HSF 480, Baltimore, MD 21201, Tel. 410/706-5328, dashirley@medicine.umaryland.edu.
Shannon N. Moonah, Division of Infectious Diseases, Department of Medicine, University of Virginia Health System, 345 Crispell Drive, P.O. Box 801340, Charlottesville, VA 22908, Tel. 434/924-5621, sm5fe@virginia.edu.
Karen L. Kotloff, Division of Infectious Diseases and Tropical Pediatrics, Department of Pediatrics, Center for Vaccine Development, University of Maryland School of Medicine, 685 W. Baltimore Street, HSF 480, Baltimore, Maryland 21201, Tel. 410/706-5328, Fax. 410/706-6205, kkotloff@medicine.umaryland.edu.
References
Recommended reading
• of special interest
•• of outstanding interest
- 1.Navin TR, Juranek DD. Cryptosporidiosis: clinical, epidemiologic, and parasitologic review. Rev Infect Dis. 1984 May-Jun;6(3):313–327. doi: 10.1093/clinids/6.3.313. [DOI] [PubMed] [Google Scholar]
- 2.Chappell CL, Okhuysen PC, Langer-Curry RC, Akiyoshi DE, Widmer G, Tzipori S. Cryptosporidium meleagridis: infectivity in healthy adult volunteers. Am J Trop Med Hyg. 2011 Aug;85(2):238–242. doi: 10.4269/ajtmh.2011.10-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson G, Elwin K, Chalmers RM. Unusual Cryptosporidium genotypes in human cases of diarrhea. Emerg Infect Dis. 2008 Nov;14(11):1800–1802. doi: 10.3201/eid1411.080239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol. 2010 Jan;124(1):80–89. doi: 10.1016/j.exppara.2009.03.018. • The author summarizes important recent developments derived from molecular epidemiologic studies of Cryptosporidium.
- 5. O'Connor RM, Shaffie R, Kang G, Ward HD. Cryptosporidiosis in patients with HIV/AIDS. AIDS. 2011 Mar 13;25(5):549–560. doi: 10.1097/QAD.0b013e3283437e88. • The authors provide an updated review of the epidemiology, clinical features and treatment of Cryptosporidiosis in people living with HIV/ AIDS and highlight important features of the immune response to cryptosporidiosis.
- 6.Morse TD, Nichols RA, Grimason AM, Campbell BM, Tembo KC, Smith HV. Incidence of cryptosporidiosis species in paediatric patients in Malawi. Epidemiol Infect. 2007 Nov;135(8):1307–1315. doi: 10.1017/S0950268806007758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatei W, Barrett D, Lindo JF, Eldemire-Shearer D, Cama V, Xiao L. Unique Cryptosporidium population in HIV-infected persons, Jamaica. Emerg Infect Dis. 2008 May;14(5):841–843. doi: 10.3201/eid1405.071277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iqbal A, Lim YA, Surin J, Sim BL. High diversity of Cryptosporidium subgenotypes identified in Malaysian HIV/AIDS individuals targeting gp60 gene. PLoS One. 2012;7(2):e31139. doi: 10.1371/journal.pone.0031139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muthusamy D, Rao SS, Ramani S, Monica B, Banerjee I, Abraham OC, et al. Multilocus genotyping of Cryptosporidium sp isolates from human immunodeficiency virus-infected individuals in South India. J Clin Microbiol. 2006 Feb;44(2):632–634. doi: 10.1128/JCM.44.2.632-634.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cama VA, Ross JM, Crawford S, Kawai V, Chavez-Valdez R, Vargas D, et al. Differences in clinical manifestations among Cryptosporidium species and subtypes in HIV-infected persons. J Infect Dis. 2007 Sep 1;196(5):684–691. doi: 10.1086/519842. [DOI] [PubMed] [Google Scholar]
- 11.Hira KG, Mackay MR, Hempstead AD, Ahmed S, Karim MM, O'Connor RM, et al. Genetic diversity of Cryptosporidium spp. from Bangladeshi children. J Clin Microbiol. 2011 Jun;49(6):2307–2310. doi: 10.1128/JCM.00164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Current WL, Garcia LS. Cryptosporidiosis. Clin Microbiol Rev. 1991 Jul;4(3):325–358. doi: 10.1128/cmr.4.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chappell CL, Okhuysen PC, Sterling CR, DuPont HL. Cryptosporidium parvum: intensity of infection and oocyst excretion patterns in healthy volunteers. J Infect Dis. 1996 Jan;173(1):232–236. doi: 10.1093/infdis/173.1.232. [DOI] [PubMed] [Google Scholar]
- 14.Jokipii L, Jokipii AM. Timing of symptoms and oocyst excretion in human cryptosporidiosis. N Engl J Med. 1986 Dec 25;315(26):1643–1647. doi: 10.1056/NEJM198612253152604. [DOI] [PubMed] [Google Scholar]
- 15.DuPont HL, Chappell CL, Sterling CR, Okhuysen PC, Rose JB, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995 Mar 30;332(13):855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 16.Okhuysen PC, Chappell CL, Crabb JH, Sterling CR, DuPont HL. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J Infect Dis. 1999 Oct;180(4):1275–1281. doi: 10.1086/315033. [DOI] [PubMed] [Google Scholar]
- 17.Chappell CL, Okhuysen PC, Langer-Curry R, Widmer G, Akiyoshi DE, Tanriverdi S, et al. Cryptosporidium hominis: experimental challenge of healthy adults. Am J Trop Med Hyg. 2006 Nov;75(5):851–857. [PubMed] [Google Scholar]
- 18.Yoder JS, Harral C, Beach MJ. Cryptosporidiosis surveillance - United States, 2006–2008. MMWR Surveill Summ. 2010 Jun 11;59(6):1–14. [PubMed] [Google Scholar]
- 19.Communitywide cryptosporidiosis outbreak--Utah, 2007. MMWR Morb Mortal Wkly Rep. 2008 Sep 12;57(36):989–993. [PubMed] [Google Scholar]
- 20.Chappell CL, Okhuysen PC, Sterling CR, Wang C, Jakubowski W, Dupont HL. Infectivity of Cryptosporidium parvum in healthy adults with pre-existing anti-C. parvum serum immunoglobulin G. Am J Trop Med Hyg. 1999 Jan;60(1):157–164. doi: 10.4269/ajtmh.1999.60.157. [DOI] [PubMed] [Google Scholar]
- 21.Heijbel H, Slaine K, Seigel B, Wall P, McNabb SJ, Gibbons W, et al. Outbreak of diarrhea in a day care center with spread to household members: the role of Cryptosporidium. Pediatr Infect Dis J. 1987 Jun;6(6):532–535. doi: 10.1097/00006454-198706000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Sorvillo FJ, Lieb LE, Kerndt PR, Ash LR. Epidemiology of cryptosporidiosis among persons with acquired immunodeficiency syndrome in Los Angeles County. Am J Trop Med Hyg. 1994 Sep;51(3):326–331. doi: 10.4269/ajtmh.1994.51.326. [DOI] [PubMed] [Google Scholar]
- 23.Vandenberg O, Robberecht F, Dauby N, Moens C, Talabani H, Dupont E, et al. Management of a Cryptosporidium hominis outbreak in a day-care center. Pediatr Infect Dis J. 2012 Jan;31(1):10–15. doi: 10.1097/INF.0b013e318235ab64. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg EB, Mendoza CE, Glass R, Arana B, Lopez MB, Mejia M, et al. Prevalence of infection with waterborne pathogens: a seroepidemiologic study in children 6–36 months old in San Juan Sacatepequez, Guatemala. Am J Trop Med Hyg. 2004 Jan;70(1):83–88. [PubMed] [Google Scholar]
- 25.Valentiner-Branth P, Steinsland H, Fischer TK, Perch M, Scheutz F, Dias F, et al. Cohort study of Guinean children: incidence, pathogenicity, conferred protection, and attributable risk for enteropathogens during the first 2 years of life. J Clin Microbiol. 2003 Sep;41(9):4238–4245. doi: 10.1128/JCM.41.9.4238-4245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilenko N, Ghosh R, Levy A, Deckelbaum RJ, Fraser D. Partial breastfeeding protects Bedouin infants from infection and morbidity: prospective cohort study. Asia Pac J Clin Nutr. 2008;17(2):243–249. [PubMed] [Google Scholar]
- 27.Siwila J, Phiri IG, Enemark HL, Nchito M, Olsen A. Intestinal helminths and protozoa in children in pre-schools in Kafue district, Zambia. Trans R Soc Trop Med Hyg. 2010 Feb;104(2):122–128. doi: 10.1016/j.trstmh.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Cama VA, Bern C, Roberts J, Cabrera L, Sterling CR, Ortega Y, et al. Cryptosporidium species and subtypes and clinical manifestations in children, Peru. Emerg Infect Dis. 2008 Oct;14(10):1567–1574. doi: 10.3201/eid1410.071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochoa TJ, Salazar-Lindo E, Cleary TG. Management of children with infection-associated persistent diarrhea. Semin Pediatr Infect Dis. 2004 Oct;15(4):229–236. doi: 10.1053/j.spid.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Duggal P, Haque R, Roy S, Mondal D, Sack RB, Farr BM, et al. Influence of human leukocyte antigen class II alleles on susceptibility to Entamoeba histolytica infection in Bangladeshi children. J Infect Dis. 2004 Feb 1;189(3):520–526. doi: 10.1086/381272. [DOI] [PubMed] [Google Scholar]
- 31.Agnew DG, Lima AA, Newman RD, Wuhib T, Moore RD, Guerrant RL, et al. Cryptosporidiosis in northeastern Brazilian children: association with increased diarrhea morbidity. J Infect Dis. 1998 Mar;177(3):754–760. doi: 10.1086/514247. [DOI] [PubMed] [Google Scholar]
- 32.Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am J Epidemiol. 1998 Sep 1;148(5):497–506. doi: 10.1093/oxfordjournals.aje.a009675. [DOI] [PubMed] [Google Scholar]
- 33.Molbak K, Andersen M, Aaby P, Hojlyng N, Jakobsen M, Sodemann M, et al. Cryptosporidium infection in infancy as a cause of malnutrition: a community study from Guinea-Bissau, west Africa. Am J Clin Nutr. 1997 Jan;65(1):149–152. doi: 10.1093/ajcn/65.1.149. [DOI] [PubMed] [Google Scholar]
- 34.Moore SR, Lima NL, Soares AM, Oria RB, Pinkerton RC, Barrett LJ, et al. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. 2010 Oct;139(4):1156–1164. doi: 10.1053/j.gastro.2010.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amadi B, Kelly P, Mwiya M, Mulwazi E, Sianongo S, Changwe F, et al. Intestinal and systemic infection, HIV, and mortality in Zambian children with persistent diarrhea and malnutrition. J Pediatr Gastroenterol Nutr. 2001 May;32(5):550–554. doi: 10.1097/00005176-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Haque R, Mondal D, Karim A, Molla IH, Rahim A, Faruque AS, et al. Prospective case-control study of the association between common enteric protozoal parasites and diarrhea in Bangladesh. Clin Infect Dis. 2009 May 1;48(9):1191–1197. doi: 10.1086/597580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mondal D, Minak J, Alam M, Liu Y, Dai J, Korpe P, et al. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis. 2012 Jan 15;54(2):185–192. doi: 10.1093/cid/cir807. •• The authors prospectively followed a birth cohort of infants born in an urban slum in Bangladesh in order to further examine the complex relationship between malnutrition and enteric infections. The enteric protozoa were found to be important causes of diarrhea in infants, cryptosporidiosis being the 5th most common pathogen isolated from diarrheal stools.
- 38.Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function fourseven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg. 1999 Nov;61(5):707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- 39.Mor SM, Tzipori S. Cryptosporidiosis in children in Sub-Saharan Africa: a lingering challenge. Clin Infect Dis. 2008 Oct 1;47(7):915–921. doi: 10.1086/591539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tumwine JK, Kekitiinwa A, Nabukeera N, Akiyoshi DE, Rich SM, Widmer G, et al. Cryptosporidium parvum in children with diarrhea in Mulago Hospital, Kampala, Uganda. Am J Trop Med Hyg. 2003 Jun;68(6):710–715. [PubMed] [Google Scholar]
- 41.Cabada MM, White AC., Jr Treatment of cryptosporidiosis: do we know what we think we know? Curr Opin Infect Dis. 2010 Oct;23(5):494–499. doi: 10.1097/QCO.0b013e32833de052. [DOI] [PubMed] [Google Scholar]
- 42.Bern C, Kawai V, Vargas D, Rabke-Verani J, Williamson J, Chavez-Valdez R, et al. The epidemiology of intestinal microsporidiosis in patients with HIV/AIDS in Lima, Peru. J Infect Dis. 2005 May 15;191(10):1658–1664. doi: 10.1086/429674. [DOI] [PubMed] [Google Scholar]
- 43.Brady MT, Oleske JM, Williams PL, Elgie C, Mofenson LM, Dankner WM, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010 Jan;53(1):86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, Lindegren ML, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000 Apr;30(Suppl 1):S5–S14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 45.Nations U. New York: United Nations; 2011. [cited 2011 May 17]. Available from: http://unstats.un.org/unsd/mdg/Resources/Static/Products/Progress2011/11-31339%20(E)%20MDG%20Report%202011_Book%20LR.pdf. [Google Scholar]
- 46.Denkinger CM, Harigopal P, Ruiz P, Dowdy LM. Cryptosporidium parvum-associated sclerosing cholangitis in a liver transplant patient. Transpl Infect Dis. 2008 Apr;10(2):133–136. doi: 10.1111/j.1399-3062.2007.00245.x. [DOI] [PubMed] [Google Scholar]
- 47.Mor SM, Tumwine JK, Ndeezi G, Srinivasan MG, Kaddu-Mulindwa DH, Tzipori S, et al. Respiratory cryptosporidiosis in HIV-seronegative children in Uganda: potential for respiratory transmission. Clin Infect Dis. 2010 May 15;50(10):1366–1372. doi: 10.1086/652140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bushen OY, Kohli A, Pinkerton RC, Dupnik K, Newman RD, Sears CL, et al. Heavy cryptosporidial infections in children in northeast Brazil: comparison of Cryptosporidium hominis and Cryptosporidium parvum. Trans R Soc Trop Med Hyg. 2007 Apr;101(4):378–384. doi: 10.1016/j.trstmh.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Hunter PR, Hughes S, Woodhouse S, Raj N, Syed Q, Chalmers RM, et al. Health sequelae of human cryptosporidiosis in immunocompetent patients. Clin Infect Dis. 2004 Aug 15;39(4):504–510. doi: 10.1086/422649. [DOI] [PubMed] [Google Scholar]
- 50.Del Chierico F, Onori M, Di Bella S, Bordi E, Petrosillo N, Menichella D, et al. Cases of cryptosporidiosis co-infections in AIDS patients: a correlation between clinical presentation and GP60 subgenotype lineages from aged formalin-fixed stool samples. Ann Trop Med Parasitol. 2011 Jul;105(5):339–349. doi: 10.1179/1364859411Y.0000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaushik K, Khurana S, Wanchu A, Malla N. Evaluation of staining techniques, antigen detection and nested PCR for the diagnosis of cryptosporidiosis in HIV seropositive and seronegative patients. Acta Trop. 2008 Jul;107(1):1–7. doi: 10.1016/j.actatropica.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 52. Chalmers RM, Campbell BM, Crouch N, Charlett A, Davies AP. Comparison of diagnostic sensitivity and specificity of seven Cryptosporidium assays used in the UK. J Med Microbiol. 2011 Nov;60(Pt 11):1598–1604. doi: 10.1099/jmm.0.034181-0. • The authors compare the diagnostic sensitivity and specificity of several disgnostic assays with PCR. The high sensitivity of immunofluorescence (97.4%) was demonstrated. Enzyme immunoassay also demonstrated good sensitivity (91.4–93.4%), and as a relatively simple test can be included in diagnostic algorithms, pending local validation.
- 53.Morgan UM, Pallant L, Dwyer BW, Forbes DA, Rich G, Thompson RC. Comparison of PCR and microscopy for detection of Cryptosporidium parvum in human fecal specimens: clinical trial. J Clin Microbiol. 1998 Apr;36(4):995–998. doi: 10.1128/jcm.36.4.995-998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calderaro A, Montecchini S, Gorrini C, Dettori G, Chezzi C. Similar diagnostic performances of antigen detection and nucleic acid detection of Cryptosporidium spp. in a low-prevalence setting. Diagn Microbiol Infect Dis. 2011 May;70(1):72–77. doi: 10.1016/j.diagmicrobio.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 55.Weber R, Bryan RT, Bishop HS, Wahlquist SP, Sullivan JJ, Juranek DD. Threshold of detection of Cryptosporidium oocysts in human stool specimens: evidence for low sensitivity of current diagnostic methods. J Clin Microbiol. 1991 Jul;29(7):1323–1327. doi: 10.1128/jcm.29.7.1323-1327.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polage CR, Stoddard GJ, Rolfs RT, Petti CA. Physician use of parasite tests in the United States from 1997 to 2006 and in a Utah Cryptosporidium outbreak in 2007. J Clin Microbiol. 2011 Feb;49(2):591–596. doi: 10.1128/JCM.01806-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.False-positive laboratory tests for Cryptosporidium involving an enzyme-linked immunosorbent assay--United States, November 1997-March 1998. MMWR Morb Mortal Wkly Rep. 1999 Jan 15;48(1):4–8. [PubMed] [Google Scholar]
- 58.Christy NC, Hencke JD, Escueta-De Cadiz A, Nazib F, von Thien H, Yagita K, et al. Multisite Performance Evaluation of an Enzyme-Linked Immunosorbent Assay for Detection of Giardia, Cryptosporidium, and Entamoeba histolytica Antigens in Human Stool. J Clin Microbiol. 2012 May;50(5):1762–1763. doi: 10.1128/JCM.06483-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taniuchi M, Verweij JJ, Noor Z, Sobuz SU, Lieshout L, Petri WA, Jr, et al. High throughput multiplex PCR and probe-based detection with Luminex beads for seven intestinal parasites. Am J Trop Med Hyg. 2011 Feb;84(2):332–337. doi: 10.4269/ajtmh.2011.10-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jex AR, Stanley KK, Lo W, Littman R, Verweij JJ, Campbell BE, et al. Detection of diarrhoeal pathogens in human faeces using an automated, robotic platform. Mol Cell Probes. 2012 Feb;26(1):11–15. doi: 10.1016/j.mcp.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Binka E, Vermund SH, Armah GE. Rotavirus diarrhea among children less than 5 years of age in urban Ghana. Pediatr Infect Dis J. 2011 Aug;30(8):716–718. doi: 10.1097/INF.0b013e318223bd85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Opintan JA, Newman MJ, Ayeh-Kumi PF, Affrim R, Gepi-Attee R, Sevilleja JE, et al. Pediatric diarrhea in southern Ghana: etiology and association with intestinal inflammation and malnutrition. Am J Trop Med Hyg. 2010 Oct;83(4):936–943. doi: 10.4269/ajtmh.2010.09-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siwila J, Phiri IG, Enemark HL, Nchito M, Olsen A. Seasonal prevalence and incidence of Cryptosporidium spp. and Giardia duodenalis and associated diarrhoea in children attending pre-school in Kafue, Zambia. Trans R Soc Trop Med Hyg. 2011 Feb;105(2):102–108. doi: 10.1016/j.trstmh.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Moyo SJ, Gro N, Matee MI, Kitundu J, Myrmel H, Mylvaganam H, et al. Age specific aetiological agents of diarrhoea in hospitalized children aged less than five years in Dar es Salaam, Tanzania. BMC Pediatr. 2011;11:19. doi: 10.1186/1471-2431-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ranjbar-Bahadori S, Sangsefidi H, Shemshadi B, Kashefinejad M. Cryptosporidiosis and its potential risk factors in children and calves in Babol, north of Iran. Trop Biomed. 2011 Apr;28(1):125–131. [PubMed] [Google Scholar]
- 66.Nunez FA, Gonzalez OM, Gonzalez I, Escobedo AA, Cordovi RA. Intestinal coccidia in Cuban pediatric patients with diarrhea. Mem Inst Oswaldo Cruz. 2003 Jun;98(4):539–542. doi: 10.1590/s0074-02762003000400021. [DOI] [PubMed] [Google Scholar]
- 67.Carvalho-Costa FA, Goncalves AQ, Lassance SL, de Albuquerque CP, Leite JP, Boia MN. Detection of Cryptosporidium spp and other intestinal parasites in children with acute diarrhea and severe dehydration in Rio de Janeiro. Rev Soc Bras Med Trop. 2007 May-Jun;40(3):346–348. doi: 10.1590/s0037-86822007000300020. [DOI] [PubMed] [Google Scholar]
- 68.Gay-Andrieu E, Adehossi E, Illa H, Garba Ben A, Kourna H, Boureima H. Prevalence of cryptosporidiosis in pediatric hospital patients in Niamey, Niger. Bull Soc Pathol Exot. 2007 Aug;100(3):193–196. [PubMed] [Google Scholar]
- 69.Mukhopadhyay C, Wilson G, Pradhan D, Shivananda PG. Intestinal protozoal infestation profile in persistent diarrhea in children below age 5 years in western Nepal. Southeast Asian J Trop Med Public Health. 2007 Jan;38(1):13–19. [PubMed] [Google Scholar]
- 70.McDonald AC, Mac Kenzie WR, Addiss DG, Gradus MS, Linke G, Zembrowski E, et al. Cryptosporidium parvum-specific antibody responses among children residing in Milwaukee during the 1993 waterborne outbreak. J Infect Dis. 2001 May 1;183(9):1373–1379. doi: 10.1086/319862. [DOI] [PubMed] [Google Scholar]
- 71.Collinet-Adler S, Ward HD. Cryptosporidiosis: environmental, therapeutic, and preventive challenges. Eur J Clin Microbiol Infect Dis. 2010 Aug;29(8):927–935. doi: 10.1007/s10096-010-0960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuhls TL, Mosier DA, Crawford DL, Griffis J. Seroprevalence of cryptosporidial antibodies during infancy, childhood, and adolescence. Clin Infect Dis. 1994 May;18(5):731–735. doi: 10.1093/clinids/18.5.731. [DOI] [PubMed] [Google Scholar]
- 73.Robin G, Fraser D, Orr N, Sela T, Slepon R, Ambar R, et al. Cryptosporidium infection in Bedouin infants assessed by prospective evaluation of anticryptosporidial antibodies and stool examination. Am J Epidemiol. 2001 Jan 15;153(2):194–201. doi: 10.1093/aje/153.2.194. [DOI] [PubMed] [Google Scholar]
- 74.Leach CT, Koo FC, Kuhls TL, Hilsenbeck SG, Jenson HB. Prevalence of Cryptosporidium parvum infection in children along the Texas-Mexico border and associated risk factors. Am J Trop Med Hyg. 2000 May;62(5):656–661. doi: 10.4269/ajtmh.2000.62.656. [DOI] [PubMed] [Google Scholar]
- 75.Tumwine JK, Kekitiinwa A, Bakeera-Kitaka S, Ndeezi G, Downing R, Feng X, et al. Cryptosporidiosis and microsporidiosis in ugandan children with persistent diarrhea with and without concurrent infection with the human immunodeficiency virus. Am J Trop Med Hyg. 2005 Nov;73(5):921–925. [PubMed] [Google Scholar]
- 76.Hunter PR, Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev. 2002 Jan;15(1):145–154. doi: 10.1128/CMR.15.1.145-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramakrishnan K, Shenbagarathai R, Uma A, Kavitha K, Rajendran R, Thirumalaikolundusubramanian P. Prevalence of intestinal parasitic infestation in HIV/AIDS patients with diarrhea in Madurai City, South India. Jpn J Infect Dis. 2007 Jul;60(4):209–210. [PubMed] [Google Scholar]
- 78.Ojurongbe O, Raji OA, Akindele AA, Kareem MI, Adefioye OA, Adeyeba AO. Cryptosporidium and other enteric parasitic infections in HIV-seropositive individuals with and without diarrhoea in Osogbo, Nigeria. Br J Biomed Sci. 2011;68(2):75–78. doi: 10.1080/09674845.2011.11730327. [DOI] [PubMed] [Google Scholar]
- 79.Dwivedi KK, Prasad G, Saini S, Mahajan S, Lal S, Baveja UK. Enteric opportunistic parasites among HIV infected individuals: associated risk factors and immune status. Jpn J Infect Dis. 2007 May;60(2–3):76–81. [PubMed] [Google Scholar]
- 80.Alemu A, Shiferaw Y, Getnet G, Yalew A, Addis Z. Opportunistic and other intestinal parasites among HIV/AIDS patients attending Gambi higher clinic in Bahir Dar city, North West Ethiopia. Asian Pac J Trop Med. 2011 Aug;4(8):661–665. doi: 10.1016/S1995-7645(11)60168-5. [DOI] [PubMed] [Google Scholar]
- 81.Srisuphanunt M, Saksirisampant W, Karanis P. Prevalence and genotyping of Cryptosporidium isolated from HIV/AIDS patients in urban areas of Thailand. Ann Trop Med Parasitol. 2011 Sep;105(6):463–468. doi: 10.1179/1364859411Y.0000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kong BN, Harwell JI, Suos P, Lynen L, Mohiuddin S, Reinert S, et al. Opportunistic infections and HIV clinical disease stage among patients presenting for care in Phnom Penh, Cambodia. Southeast Asian J Trop Med Public Health. 2007 Jan;38(1):62–68. [PubMed] [Google Scholar]
- 83.Asma I, Johari S, Sim BL, Lim YA. How common is intestinal parasitism in HIV-infected patients in Malaysia? Trop Biomed. 2011 Aug;28(2):400–410. [PubMed] [Google Scholar]
- 84.Erhabor O, Obunge O, Awah I. Cryptosporidiosis among HIV-infected persons in the Niger Delta of Nigeria. Niger J Med. 2011 Jul-Sep;20(3):372–375. [PubMed] [Google Scholar]
- 85.Kurniawan A, Karyadi T, Dwintasari SW, Sari IP, Yunihastuti E, Djauzi S, et al. Intestinal parasitic infections in HIV/AIDS patients presenting with diarrhoea in Jakarta, Indonesia. Trans R Soc Trop Med Hyg. 2009 Sep;103(9):892–898. doi: 10.1016/j.trstmh.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 86.Vignesh R, Balakrishnan P, Shankar EM, Murugavel KG, Hanas S, Cecelia AJ, et al. High proportion of isosporiasis among HIV-infected patients with diarrhea in southern India. Am J Trop Med Hyg. 2007 Nov;77(5):823–824. [PubMed] [Google Scholar]
- 87.Bresee JS, Marcus R, Venezia RA, Keene WE, Morse D, Thanassi M, et al. The Etiology of Severe Acute Gastroenteritis Among Adults Visiting Emergency Departments in the United States. J Infect Dis. 2012 May;205(9):1374–1381. doi: 10.1093/infdis/jis206. [DOI] [PubMed] [Google Scholar]
- 88.Kotloff KL, Wasserman SS, Steciak JY, Tall BD, Losonsky GA, Nair P, et al. Acute diarrhea in Baltimore children attending an outpatient clinic. Pediatr Infect Dis J. 1988 Nov;7(11):753–759. doi: 10.1097/00006454-198811000-00002. [DOI] [PubMed] [Google Scholar]
- 89.Corso PS, Kramer MH, Blair KA, Addiss DG, Davis JP, Haddix AC. Cost of illness in the 1993 waterborne Cryptosporidium outbreak, Milwaukee, Wisconsin. Emerg Infect Dis. 2003 Apr;9(4):426–431. doi: 10.3201/eid0904.020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hlavsa MC, Roberts VA, Anderson AR, Hill VR, Kahler AM, Orr M, et al. Surveillance for waterborne disease outbreaks and other health events associated with recreational water --- United States, 2007–2008. MMWR Surveill Summ. 2011 Sep 23;60(12):1–32. [PubMed] [Google Scholar]
- 91.Baldursson S, Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2004–2010. Water Res. 2011 Dec 15;45(20):6603–6614. doi: 10.1016/j.watres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 92.Desoubeaux G, Caumont C, Passot C, Dartigeas C, Bailly E, Chandenier J, et al. Two cases of opportunistic parasite infections in patients receiving alemtuzumab. J Clin Pathol. 2012 Jan;65(1):92–95. doi: 10.1136/jclinpath-2011-200403. [DOI] [PubMed] [Google Scholar]
- 93.Bonatti H, Barroso IiLF, Sawyer RG, Kotton CN, Sifri CD. Cryptosporidium enteritis in solid organ transplant recipients: multicenter retrospective evaluation of 10 cases reveals an association with elevated tacrolimus concentrations. Transpl Infect Dis. 2012 Feb 19; doi: 10.1111/j.1399-3062.2012.00719.x. [DOI] [PubMed] [Google Scholar]
- 94.Rossignol JF, Ayoub A, Ayers MS. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of Nitazoxanide. J Infect Dis. 2001 Jul 1;184(1):103–106. doi: 10.1086/321008. [DOI] [PubMed] [Google Scholar]
- 95.Gargala G, Le Goff L, Ballet JJ, Favennec L, Stachulski AV, Rossignol JF. Evaluation of new thiazolide/thiadiazolide derivatives reveals nitro group-independent efficacy against in vitro development of Cryptosporidium parvum. Antimicrob Agents Chemother. 2010 Mar;54(3):1315–1318. doi: 10.1128/AAC.00614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amadi B, Mwiya M, Sianongo S, Payne L, Watuka A, Katubulushi M, et al. High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: a randomised controlled trial. BMC Infect Dis. 2009;9:195. doi: 10.1186/1471-2334-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rossignol JF, Lopez-Chegne N, Julcamoro LM, Carrion ME, Bardin MC. Nitazoxanide for the empiric treatment of pediatric infectious diarrhea. Trans R Soc Trop Med Hyg. 2012 Mar;106(3):167–173. doi: 10.1016/j.trstmh.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 98.McDonald V. Cryptosporidiosis: host immune responses and the prospects for effective immunotherapies. Expert Rev Anti Infect Ther. 2011 Nov;9(11):1077–1086. doi: 10.1586/eri.11.123. [DOI] [PubMed] [Google Scholar]
- 99.Boulter-Bitzer JI, Lee H, Trevors JT. Molecular targets for detection and immunotherapy in Cryptosporidium parvum. Biotechnol Adv. 2007 Jan-Feb;25(1):13–44. doi: 10.1016/j.biotechadv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 100.Borad AJ, Allison GM, Wang D, Ahmed S, Karim MM, Kane AV, et al. Systemic antibody responses to the immunodominant p23 antigen and p23 polymorphisms in children with cryptosporidiosis in Bangladesh. Am J Trop Med Hyg. 2012 Feb;86(2):214–222. doi: 10.4269/ajtmh.2012.11-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mead JR. Challenges and prospects for a Cryptosporidium vaccine. Future Microbiol. 2010 Mar;5(3):335–337. doi: 10.2217/fmb.09.115. [DOI] [PubMed] [Google Scholar]
- 102.Costa LB, Noronha FJ, Roche JK, Sevilleja JE, Warren CA, Oria R, et al. Novel in vitro and in vivo models and potential new therapeutics to break the vicious cycle of cryptosporidium infection and malnutrition. J Infect Dis. 2012 May;205(9):1464–1471. doi: 10.1093/infdis/jis216. [DOI] [PMC free article] [PubMed] [Google Scholar]