Abstract
Seed inoculation with bacterial consortium was found to increase legume yield, providing a higher growth than the standard nitrogen treatment methods. Alfalfa plants were inoculated by mono- and binary compositions of nitrogen-fixing microorganisms. Their physiological and biochemical properties were estimated. Inoculation by microbial consortium of Sinorhizobium meliloti T17 together with a new cyanobacterial isolate Nostoc PTV was more efficient than the single-rhizobium strain inoculation. This treatment provides an intensification of the processes of biological nitrogen fixation by rhizobia bacteria in the root nodules and an intensification of plant photosynthesis. Inoculation by bacterial consortium stimulates growth of plant mass and rhizogenesis and leads to increased productivity of alfalfa and to improving the amino acid composition of plant leaves. The full nucleotide sequence of the rRNA gene cluster and partial sequence of the dinitrogenase reductase (nifH) gene of Nostoc PTV were deposited to GenBank (JQ259185.1, JQ259186.1). Comparison of these gene sequences of Nostoc PTV with all sequences present at the GenBank shows that this cyanobacterial strain does not have 100% identity with any organisms investigated previously. Phylogenetic analysis showed that this cyanobacterium clustered with high credibility values with Nostoc muscorum.
1. Introduction
Continuous anthropogenic impact on the environment of different chemicals, fertilizers, herbicides, plant protection from pests and diseases, plant growth regulators, and so forth that are used in agriculture, makes it necessary to develop an alternative to agricultural production, which would be based on the use of cost-effective and environmentally friendly systems for land application of fertilizers and plant protection. An important role in this respect is given to the maximum use of the soil microflora.
In many countries around the world, studies and implementation of the compositions consisting of symbiotic and free-living nitrogen-fixing microorganisms have started to increase productivity of crops. Among the wide range of diazotrophic microorganisms cyanobacteria are the most versatile for biochemical potential, since they do not need to be provided with soil organic substances for nitrogen fixation unlike heterotrophic nitrogen-fixing microorganisms.
Positive ecological role of cyanobacteria in the soil as nitrogen-fixing bacteria which participate in deposition of organic matter is well known now, and besides they are the centers of microcosms as autotrophic organisms with amazing abilities for symbiotrophic relations [1, 2]. The last property of cyanobacteria is particularly interesting in case of using the consortia of microorganisms in biotechnology instead of monocultures [3]. In nature, cyanobacteria are never found in the form of cell populations of one species. They are in a close relationship with the microbial community, located in the mucus of the surrounding cells. Research in this field has shown that the composition of satellite cyanobacteria is very labile and it depends on changes in habitat conditions. Axenic cultures of cyanobacteria exist only in the laboratories. In nature, they form a community and, being the edificators of microbial communities, cyanobacteria can change the microbial composition [4]. It allows the constructing of artificial microbial consortium. Nitrogen-fixing activity (NFA) of soil compositions of diazotrophic microorganisms can be an effective way to supply the crop by environmentally friendly biological nitrogen. Use of this approach requires in-depth study of the relationship between bacteria, cyanobacteria, and plants, as well as compatibility of microorganisms-partners in created artificial associations. It is important to perform the screening of the most suitable strains of microorganisms, to create conditions for the effective functioning of these symbiotic consortia. It is necessary to select an optimal quantitative ratio of microorganisms and methods of their implantation into the rhizosphere.
The goals of this research were the study of the effect of artificial stable microbial consortium based on nitrogen-fixing cyanobacterium Nostoc PTV and Tn5-mutant of nodule bacteria Sinorhizobium meliloti T17, on the physiological and biochemical characteristics of growth and development of alfalfa, and, finally, on its yield and product quality and the molecular typing and phylogenetic analysis of this new cyanobacterial isolate Nostoc PTV.
2. Material and Methods
2.1. Organisms and Growth Conditions
Plant alfalfa Medicago sativa (L.) sort of Jaroslavna obtained from the NSC Institute of Agriculture of National Academy of Agrarian Sciences of Ukraine has been used in the experiments. For the inoculation of alfalfa seeds we used the strain of nodule bacteria Sinorhizobium (Rhizobium) meliloti T17 (patent of Ukraine number 55432) from the collection of nitrogen-fixing microorganisms of the Institute of Plant Physiology and Genetics, National Academy of Sciences of Ukraine (Kyiv) [6]. The strain of S. meliloti T17 was obtained as a result of intergeneric conjugation of Escherichia coli S17-1 (pSUP2021::Tn5) and S. meliloti 425a on agar medium TY (tryptone/yeast extract) as described in [7] and it was selected for improved symbiotic properties. To create the binary composition of nitrogen-fixing microorganisms the culture of cyanobacterium Nostoc PTV (from the collection of the Institute of Hydrobiology, National Academy of Sciences of Ukraine) was used. Cyanobacterium was grown on Fitzgerald medium with the modification by Zehnder and Gorham [8] in Erlenmeyer flask at 22°C ± 2°C and illumination of 2500 lux until the stationary growth phase. The concentration of chlorophyll (Chl) in cyanobacterial cells was determined by differential fluorometry (Fluorometer FL300 3M, Russia) [9]. The binary composition was prepared by mixing the bacterial suspensions consisting of nodule bacteria (1 × 109 cells/mL) and cyanobacteria (Chl, mg/L = 1506,6 ± 13,4, ΔF = 0,088) in the ratio 1 : 1. In parallel, the viability of cyanobacterial cells was determined by the difference of fluorescence intensity (ΔF) before and after the addition of simazine, the inhibitor of cells photosynthetic electron transport [10, 11].
Investigations were carried out in the model experiments in a growth area of Institute of Plant Physiology and Genetics with natural light and humidity of the substrate 60% of full capacity. Plastic containers with 10 kg of sand were used in experiments. 12 alfalfa plants were grown in each container. Containers were preliminarily sterilized with 20% solution of H2O2. Washed river sand with the mineral nutrient mixture of Gelrigel [12] containing the “start” of nitrogen (177 mg of Ca(NO3)2 × 4H2O per 1 kg of sand) was used as a substrate. This amount of nitrogen represents one-quarter of the normal nitrogen supply. Before sowing the seeds were sterilized with concentrated sulfuric acid for 5 minutes, and then they were washed in running water for 1 h. The treatment of seeds by microorganism compositions was continuing during 1 h.
The controls in the experiments were the samples of seeds treated by monoculture of T17 S. meliloti or only by N. PTV. We used samples of alfalfa seeds moisturized with tap water as an additional “absolute” control. Experiments were performed in seven replications. Plants for analysis were selected in phases of stem (32nd day of emergence), budding (40th day), and flowering (50th day).
2.2. Measurements of Nitrogen Activity, Pigments Content, and Efficiency of Photosynthesis
Nitrogen-fixing (nitrogenase) activity was determined by the level of activity of root nodules by acetylene method and expressed as micromoles of ethylene formed by nodules per plant for 1 h [13]. The gas mixture was analyzed by gas chromatography of Agilent Technologies 6855 Network GC System (USA). The measurements were performed in five replications.
The content of the photosynthetic pigments in leaves of alfalfa plants was determined by the Wellburn method [14]. Pigments were extracted with dimethyl sulfoxide (0.1 g vegetable material was treated in 10 mL DMSO) of leaf cut for 3 h at +67°C until complete extraction. The absorbance of the solution was measured by spectrophotometer Smart Spec Plus (BioRad, USA) at 665 and 649 nm in a 1 cm cuvette. Leaves were collected from the middle tiers of the five randomized plants of the same version. Measurements were performed in triplicate.
The net assimilation rate of shoots was determined in controlled environment with installation built on base of the photoacoustic infrared gas analyzer GIAM-5 M (Russia), which was connected by differential circuit. Container with plants was placed in sealed plexiglass chamber of 50 liters through which air was blown at rate of 15 L/min. At the outlet of chamber 1 L/min of air was taken to the gas analyzer, and the remaining air was discharged into atmosphere. The chamber was irradiated with light by the lamp CG-2000 through a water filter. The illumination on the substrate level was 250 W/m2; temperature was 25° ± 2°C. After the adaptation of plants to the conditions of measurement (30–40 min after closing the chamber), the rate of absorption of CO2 by plants was recorded (it is an apparent photosynthesis). After this, shoots of plants were cut at the substrate level and respiration of soil with roots were measured. Net assimilation rate was calculated as sum of apparent photosynthesis and respiration. Calculations of gas exchange parameters were performed according to the standard procedure [15].
The protein content was determined in leaves of alfalfa plants in the budding stage by Lowry method [16]. Qualitative and quantitative composition of amino acids was determined by liquid-ion exchange column chromatography with the use of automatic analyzer T339 (Czech) on the basis of ninhydrin detection method [17].
2.3. Plant Stress Resistance Determination
In order to study the effect of mono- and binary inoculation on plant resistance, the basic parameters of the stress state of alfalfa were determined. Plants in the budding stage were treated with herbicide diquat (100 pmol), which was used as a stress factor. Sampling was carried out after 30 minutes, 60 minutes, and 24 hours of diquat action on plants. Specific changes in the composition of the components of a lipid-pigment complex and antioxidant system were studied in photosynthetic tissues of alfalfa.
Intensity of lipid peroxidation (LPO) was evaluated by the number of end-products of lipid oxidation based on the reaction with 2-thiobarbituric acid (TBA) [18]. The activity of antioxidant systems is determined by the activity of superoxide dismutase (SOD) [19].
A statistical analysis of the experimental data was performed by standard methods, involving a package of special statistical functions of Microsoft Excel. Probability of differences between the variants was assessed by t-test and a significance level of P < 0,05.
2.4. Scanning Electron Microscopy (SEM)
Cyanobacterial samples were fixed as described above and dehydrated through an ethanol series, with an overnight exposure in absolute acetone followed by critical-point drying in a Dryer HCP-2 (Hitachi, Japan), coated with Au-Pd alloy in an IB-3 Ion Coater (Eiko, Japan) and examined with a JSM-6380LA scanning electron microscope (JEOL, Japan).
2.5. DNA Isolation and PCR Amplification
For molecular typing cyanobacterial genomic DNA was isolated according to [20] and synthetic oligonucleotides (“Synthol,” Moscow, Russia) have been used as cyanobacterial primers for 16S–23S rRNA PCR, according to [21]. As a second molecular marker the nifH gene has been used with corresponding PCR primers [22]. PCR for 16S–23S rRNA gene cluster was carried out on a Tercik DNA amplifier (DNA Technology, Russia) by using DreamTaq PCR Master Mix (Fermentas, EU), under the following conditions: 1 cycle at 94°C for 10 min, 25 cycles at 94°C for 45 sec, 54°C for 45 sec, 68°C for 2 min, 1 cycle at 68°C for 7 min, and a final soak step at 4°C. PCR for partial nifH gene was performed under the following conditions: 1 cycle at 94°C for 4 min, 25 cycles at 94°C for 30 sec, 54°C for 30 sec, 68°C for 30 sec, 1 cycle at 68°C for 7 min, and a final soak step at 4°C. PCR products were resolved in 1.5% agarose gel containing ethidium bromide at 5 microgram mL−1.
2.6. Cloning and Sequencing of PCR Products
DNA fragments obtained during PCR were cloned with CloneJet PCR Cloning Kit # K1231 (Fermentas, EU). Transformation of competent XL-1 cells of Escherichia coli and plasmid purification were performed according to [23]. DNA sequencing was performed with ABI PRISM BigDye Terminator version 3.1 at the Applied Biosystems 3730 DNA Analyzer (Center for Collective Use “Genome”). Sequences were edited and assembled with Bioedit (Invitrogen, Carlsbad, CA). The full nucleotide sequence of the rRNA gene cluster of cyanobacterium Nostoc PTV and a part of the nifH gene were accomplished and deposited to GenBank under accession numbers JQ259185.1 and JQ259186.1.
2.7. Phylogenetic Analysis
Search of the nucleotide sequences in the database GenBank, homologous to the sequenced genes of studied species of cyanobacteria, was performed using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome) with the option: the least degree of similarity (Minimum Identity). The sequences of selected species were aligned using the algorithm Muscle (MEGA 6.0) [24]. Phylogenetic reconstructions were performed using Bayesian inference (MrBayes version 3.1.2) [25] with the preselection of an adequate model of nucleotide substitutions (MEGA 6.0).
2.8. Mating and Conjugal Transfer of Plasmid DNA
Transformations of Nostoc PTV through triparental conjugations followed published protocols [26] with minor modifications. Standard bacterial mating involved the cyanobacterial strain Nostoc PTV and E. coli strains (DH10B) that harbored the following three plasmids: (i) the conjugal plasmid pRL443 [27], (ii) the “helper” plasmid pRL623, [27], and (iii) the cargo plasmid pRL692 that carries the mobile element Tn5-692 [28]. E. coli strains were grown in 3 mL LB with the appropriate antibiotic(s) and incubated at 37°C overnight. Cells of E. coli were diluted 1 : 20 and were grown for 1.5–2 h at 37°C. Then E. coli cells were harvested from 1 mL of each E. coli culture by centrifugation and resuspended in 1 mL fresh LB. This step was repeated twice to wash the cells. After the third centrifugation, the cells were resuspended in 200 mL BG-11. Five milliliters of a growing Nostoc PTV culture was harvested by centrifugation at low speed (4000 g) and resuspended in 1 mL BG-11. Then the filaments were fragmented in a water bath sonicator for 2 to 5 min so that more than half of the filaments were shorter than 5 cells. The cyanobacterial cells were collected by centrifugation for 2 min and resuspended in 1 mL BG-11. The cargo strain, the conjugal strain (for triparental mating), and Nostoc PTV were combined, pelleted by centrifugation, and finally resuspended in 200 mL BG-11. The conjugation mixture was incubated for about 1 h in low light at 28°C. Then the cells were spread on sterile nitrocellulose filters laid on BG11+ 5% (vol/vol) LB agar plates (mating plates). The mating plates were incubated without antibiotic selection for 18 to 24 h in low light at 28°C, and then the filters were transferred to BG-11 for 24 h and then to BG-11 agar with 10 μg/mL Spectinomycin (Sp10) and 2 μg/mL Streptomycin (Sm2). After incubation for 8 to 12 days, isolated antibiotic-resistant transconjugant colonies were patched on fresh selective BG-11 plates.
3. Results and Discussion
3.1. Effect of Microbial Inoculation on Plant Growth and Productivity
Earlier in the laboratory study of pure cultures of N. PTV and S. meliloti [29], we found stimulation of cell growth area of nodule bacteria around the colonies of cyanobacterium N. PTV on the surface of the agar medium. Our results are consistent with the literature data, since it is known that cyanobacteria are producers of a wide range of biologically active substances, which include a group of growth-stimulating compounds, analogues of phytohormone [30].
In our previous study we have tested different associations of nitrogen-fixing microorganisms in the rhizosphere of alfalfa [29]. The most effective bacterial consortium included cyanobacterium Nostoc PTV and Tn5-mutants of nodule bacteria S. meliloti. Usage of the optimal proportions of components in the inoculation mixtures promotes the absence of antagonism between microorganisms and provides the stimulating effect of these consortia on various physiological and biochemical features of alfalfa plants.
In this study the possibility of the formation of artificial stable microbial consortium based on nitrogen-fixing cyanobacterium N. PTV (Figure 1) and one Tn5-mutant of nodule bacteria S. meliloti T17 was investigated. In pot experiments it was revealed that inoculation of alfalfa by binary mixture of S. meliloti T17 + N. PTV has a stimulating effect on the growth of the vegetative mass of plants (Table 1). The increase of above-ground plant mass after application of the consortium S. meliloti T17 + N. PTV in the phase of stem was 15.4% compared with rhizobial T17 monoinoculation and 21% compared with N. PTV monoinoculation, respectively. The growth of above-ground alfalfa plant mass was 13.8% and 10.4%, after application of the S. meliloti T17 + N. PTV consortium in the budding stage, and 5% and 13% in the beginning of flowering, in comparison with monoinoculations, correspondingly. It is known that cells of nitrogen-fixing cyanobacteria produce polypeptides, amino acids, polysaccharides, and vitamins. Due to this diverse biochemical activity in the mucous environment of cyanobacterial cells favorable conditions for growth and reproduction of other microorganisms were created. Perhaps, it could promote a more active cell proliferation of nodule bacteria T17 associated with cyanobacteria in the root zone of alfalfa and contribute to formation of efficient Rhizobium-legume symbiosis.
Figure 1.
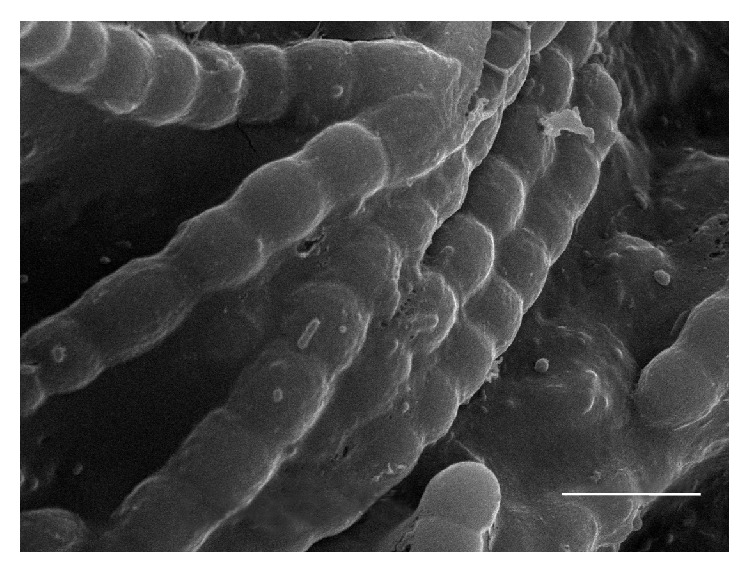
A SEM image of the N. PTV cells. Scale bar: 5 μm.
Table 1.
Dynamics of accumulation of vegetative mass of alfalfa inoculated by mono- and binary suspensions of diazotrophic microorganisms.
| Inoculants | Phase of plant development | |||||
|---|---|---|---|---|---|---|
| Stooling | Budding | Flowering | ||||
| Above-ground mass (g/plant) |
Mass of roots (g/plant) |
Above-ground mass (g/plant) |
Mass of roots (g/plant) |
Above-ground mass (g/plant) |
Mass of roots (g/plant) |
|
| Without inoculation (control) |
0,42 ± 0,02 | 0,12 ± 0,01 | 1,17 ± 0,06 | 1,12 ± 0,1 | 1,25 ± 0,02 | 2,25 ± 0,16 |
| N. PTV | 0,62 ± 0,02 | 0,18 ± 0,01 | 1,64 ± 0,09 | 1,79 ± 0,09 | 1,70 ± 0,07 | 2,11 ± 0,15 |
| S. meliloti Т17 | 0,65 ± 0,04 | 0,15 ± 0,01 | 1,59 ± 0,13 | 1,22 ± 0,11 | 1,83 ± 0,04 | 2,59 ± 0,24 |
| S. meliloti Т17 + N. PTV | 0,75 ± 0,08 | 0,22 ± 0,01 | 1,81 ± 0,18 | 1,82 ± 0,10 | 1,92 ± 0,03 | 2,95 ± 0,29 |
P ≤ 0,05.
Rhizogenesis was positively affected in plants, the seeds of which were treated with suspensions of microorganisms (Table 1). The largest increase of the plant root mass was detected after using the binary inoculation (S. meliloti T17 + N. PTV). Thus, in the phase of stem the mass of roots increased by 46.6% and 22.2%, in the budding stage by 49.2% and 13%, and in the early phase of flowering by 13.9% and 39.8%, compared with plants treated only by S. meliloti T17 or by only N. PTV, correspondingly.
Effective collaboration between all partners of symbiosis provides the activation of several metabolic processes and, above all, the fixation of atmospheric nitrogen. As a result of improved plant nutrition, their productivity increased and the quality of bioproducts improved.
In the phase of stem when the process of nitrogen fixation was still inactive, differences in NFA of nodules of alfalfa plants inoculated by mono- or binary bacterial complexes were not significant (Figure 2). However, in the budding stage and in the early flowering stage a nitrogen fixation in root nodules of plants infected with a mixture of S. meliloti T17 + N. PTV was more intensive. Positive regulatory role of cyanobacterium N. PTV is obvious according to the results presented in Table 2. Only in case of binary inoculation, plants demonstrate an increase in number and in weight of formed nodules (Table 2). Thus, the application of this microbial consortium provided increased NFA in nodules of alfalfa at the budding stage and maintained its relatively high level at the beginning of the early flowering stage (Figure 2). Therefore, this data proves that N. PTV has a stimulating effect on the functioning of root nodule bacteria S. meliloti T17.
Figure 2.
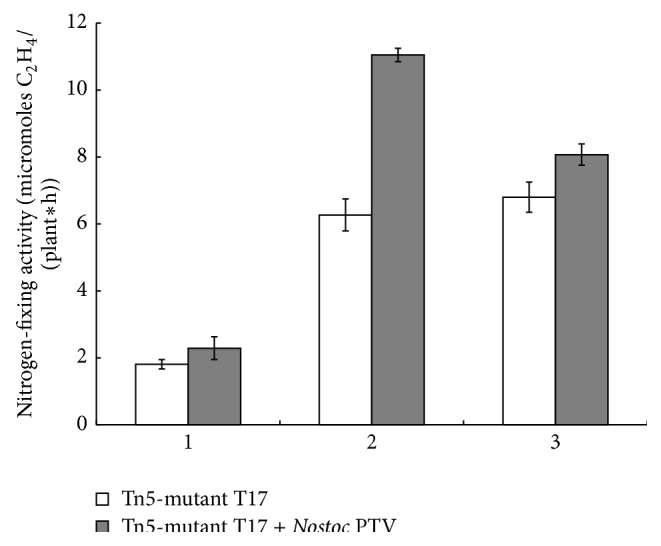
Dynamics of NFA of nodules of alfalfa plants inoculated by mono- and binary suspensions of microorganisms (micromoles of ethylene formed by nodules per plant per 1 h). 1: phase of stooling, 2: phase of budding, and 3: phase of flowering, P ≤ 0,05.
Table 2.
Number and mass of root nodules on alfalfa plants inoculated by mono- and binary suspensions of microorganisms.
| Inoculants | Phase of plant development | |||||
|---|---|---|---|---|---|---|
| Stooling | Budding | Flowering | ||||
| Number of root nodules (pcs/plant) |
Mass of root nodules (g/plant) |
Number of root nodules (pcs/plant) |
Mass of root nodules (g/plant) |
Number of root nodules (pcs/plant) |
Mass of root nodules (g/plant) |
|
| Without inoculation (control) | 0 | 0 | 0 | 0 | 0 | 0 |
| N. PTV | 0 | 0 | 0 | 0 | 0 | 0 |
| S. meliloti Т17 | 12,0 ± 1,0 | 0,010 ± 0,00 | 30,0 ± 8,5 | 0,115 ± 0,002 | 45,0 ± 0,5 | 0,135 ± 0,02 |
| S. meliloti Т17 + N. PTV | 14,0 ± 0,6 | 0,017 ± 0,04 | 57,0 ± 8,0 | 0,130 ± 0,001 | 70,0 ± 7,5 | 0,160 ± 0,02 |
Note. 15 plants of each variant of the experiment were analyzed for determination the average number of nodules on the roots of one plant.
P ≤ 0,05.
It is known that the use of active strains of root nodule bacteria and their associations with other microorganisms affect the formation and functioning of the photosynthetic complexes through the nitrogen status of a host plant. Presence of nitrogen available to plants determines the efficiency of symbiotic systems.
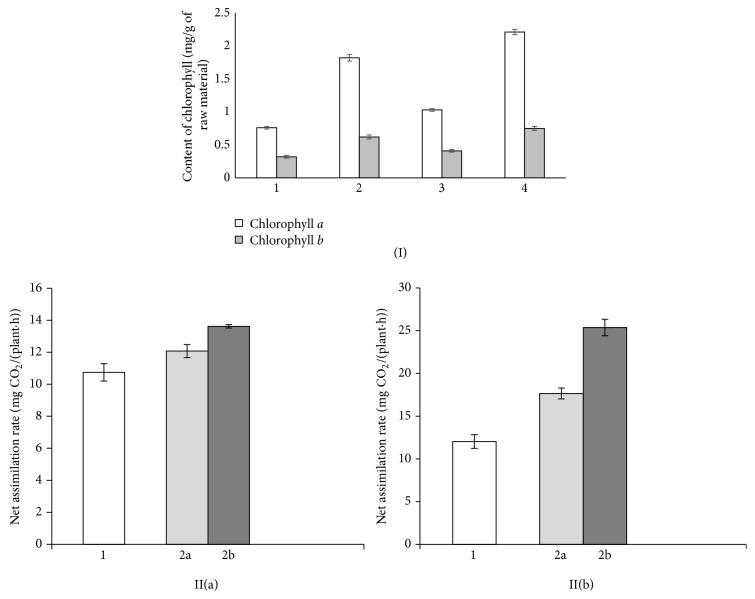
Mono- and binary suspensions inoculations of seeds showed positive dynamics of accumulation of photosynthetic pigments in the leaves of alfalfa compared with the absolute control (Figure 3). The most significant differences were observed in plants whose seeds had been inoculated with nitrogen-fixing consortium of microorganisms (chlorophyll a and chlorophyll b increased by 114.6 and 82.9%) compared with the corresponding option of treatment only by strain T17. It is known that the content of chlorophyll in the leaves is directly proportional to the intensity of nitrogen fixation and depends on symbiotic properties of root nodule bacteria [31–33]. Increasing the number of pigments in the leaves of alfalfa inoculated with binary bacterial suspension indicates the ability of N. PTV to enhance the functional activity of rhizobia, which are directly interfaced with the intensity of nitrogen fixation.
Figure 3.
(I): content of chlorophyll (mg/g of raw material) in leaves of alfalfa inoculated by mono- and binary suspensions of microorganisms S. meliloti Т17 and N. PTV. 1: control (without inoculation); 2: inoculation by N. PTV; 3: inoculation by S. meliloti Т17; 4: inoculation by consortium of S. meliloti Т17 + N. PTV. II: net assimilation rate (mg СО2/( plant·hour)) of alfalfa inoculated by mono- and binary suspensions of microorganisms S. meliloti Т17 and N. PTV. 1: inoculation by N. PTV; 2a: inoculation by S. meliloti Т17; 2b: inoculation by consortium of S. meliloti Т17 + N. PTV (II(a): phase of budding and II(b): phase of flowering).
Available forms of nitrogen, such as mineral and symbiotrophic, positively affect not only the formation of high grade, but also functional state of the plant photosynthetic apparatus. The net assimilation rate also demonstrates the effectiveness of the binary composition S. meliloti T17 + N. PTV. In particular, in the budding stage of these plants the net assimilation rate exceeded 12.7%, while in the phase of early flowering it increased by 43.7% of the corresponding rate during inoculation only by T17 (Figure 3).
The net assimilation rate of plant leaves typically is closely correlated with the content of nitrogen, and nitrogen is presented mainly in amino acids and proteins. Rubisco, the major cell photosynthetic enzyme of CO2 assimilation, represents more than half of the soluble cell proteins in leaf. Obviously, the intensification of NFA in the binary composition was the main reason for the increase of plant assimilation rate. However, it is possible that more active symbiotic apparatus, which is formed on the roots of plants through binary inoculation, enhanced “request” on assimilates by the root system, thereby stimulating the photosynthetic activity of plant leaves. There is a gradient of transport forms of carbon, particularly sucrose, between roots and leaves in the conduction system and it accelerates the outflow of carbon from the leaves. This, in turn, eliminates restrictions by photosynthesis products imposed on the feedback principle and further accelerates photosynthetic carbon assimilation. Thus, the efficient operation of the symbiotic apparatus in inoculated plants greatly stimulated the accumulation of photosynthetic pigments and increased the net assimilation rate. The accumulation of organic matter contributes to the formation of the plant biomass, because the basis of the biological productivity of the plant organism, including those capable of symbiotic nitrogen fixation, is photosynthetic carbon assimilation [31].
In consequence of artificial inoculation of alfalfa seeds by consortium of nitrogen-fixing microorganisms S. meliloti T17 + N. PTV the yield of green mass of plants increased by 17.9% and the protein content in the leaves increased by 12.0% compared to monoinoculation by strain T17 (Table 3). This is an evidence of the effective interaction of test organisms in the cyano-Rhizobium associations and their positive impact on the growth and physiological characteristics of alfalfa plants (Table 3).
Table 3.
Productivity and protein content in leaves of alfalfa, inoculated by mono- and binary suspensions of microorganisms.
| Inoculants | Harvest of green mass of plant, g/vessel |
Protein content in the leaves | ||||
|---|---|---|---|---|---|---|
| I mowing | II mowing | Total harvest |
% to monoinoculation by rhizobium |
% to monoinoculation by rhizobium | ||
| Control | 17,68 ± 0,51 | 19,70 ± 0,64 | 37,38 | 13,2 | ||
| N. PTV | 21,40 ± 0,52 | 24,22 ± 0,76 | 45,62 | 14,6 | ||
| S. meliloti Т17 | 21,81 ± 0,48 | 25,17 ± 0,30 | 46,98 | 18,32 | ||
| S. meliloti Т17 + N. PTV | 26,26 ± 0,37∗ | 29,15 ± 0,17∗ | 55,41 | 117,9 | 20,52 | 112,0 |
The amino acid composition is the main criterion of the biological value of proteins. An index of a total amino acid composition of vegetative mass of the experimental inoculated variants of alfalfa plants increased in comparison to the control (without bacterial inoculation). The maximum quantity of lysine, the most essential and deficient amino acid in humans and animals, was recorded in leaves of alfalfa (Table 4). As a result of using binary inoculation a total amino acid composition increased by 25.1%, compared with the case of inoculation only by T17. In particular, a quantity of essential amino acids increased by 33.9%, and a quantity of nonessential amino acids increased by 17.7% (Figure 4). At the same time, an increase of the content of methionine, histidine, arginine, and tyrosine was observed, which are present in small quantities in plant leaves, and this is one of the factors limiting the rate of biosynthesis of proteins, especially in generative organs. The results are a direct proof of the positive impact of cyanobacterial inoculation on the quality of agricultural products.
Table 4.
Amino acid content in leaves of alfalfa, inoculated by mono- and binary suspensions of microorganisms.
| Amino acid | Content of amino acids (mg/100 mg DW) | |||
|---|---|---|---|---|
| Control | N. punctiforme | Т17 | Т17 + N. punctiforme | |
| Gamma-aminobutyric acid | 0,065 | 0,088 | 0,085 | 0,123 |
| Lysine | 0,386 | 0,802 | 0,459 | 0,610 |
| Histidine | 0,094 | 0,301 | 0,171 | 0,232 |
| Arginine | 0,297 | 0,905 | 0,439 | 0,585 |
| Asparagine | 0,675 | 0,882 | 0,748 | 0,779 |
| Threonine | 0,278 | 0,623 | 0,383 | 0,496 |
| Serine | 0,321 | 0,698 | 0,413 | 0,533 |
| Glutamic acid | 0,876 | 1,931 | 1,083 | 1,395 |
| Proline | 0,376 | 0,610 | 0,419 | 0,548 |
| Glycine | 0,423 | 0,790 | 0,470 | 0,537 |
| Alanine | 0,479 | 0,895 | 0,581 | 0,644 |
| Cysteine | 0,054 | 0,262 | 0,057 | 0,079 |
| Valine | 0,245 | 0,651 | 0,301 | 0,423 |
| Methionine | 0,111 | 0,298 | 0,149 | 0,200 |
| Isoleucine | 0,175 | 0,439 | 0,233 | 0,273 |
| Leucine | 0,523 | 1,234 | 0,685 | 0,938 |
| Tyrosine | 0,201 | 0,534 | 0,285 | 0,253 |
| Phenylalanine | 0,398 | 0,835 | 0,453 | 0,626 |
| Total | 5,977 | 12,779 | 7,417 | 9,276 |
Figure 4.
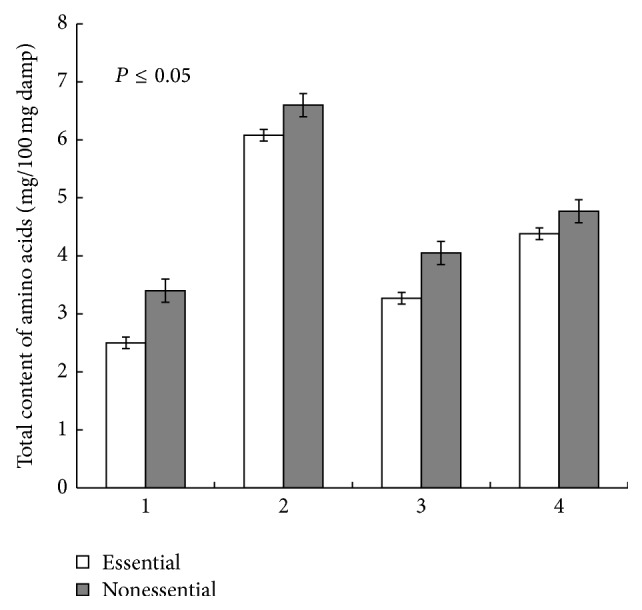
Total content of essential and nonessential amino acids in leaves of alfalfa grown after mono- and binary inoculation by cyanorhizobial compositions of microorganisms: 1: control (without inoculation), 2: monoinoculation of alfalfa seeds by cyanobacterium N. PTV, 3: inoculation of alfalfa seeds by Tn5-mutant strain of S. meliloti T17, and 4: binary inoculation of alfalfa seeds by Tn5-mutant strain of S. meliloti T17 + N. PTV.
3.2. Stress Response of Plants Inoculated with Microbial Consortium
It is known that the plant productivity rate and resistance index are inversely dependent values. A positive effect of the cyanobacterial consortium T17 + N. PTV on the productivity of alfalfa is shown in our study. It was logical to study the effect of the binary inoculation on plant resistance to the adverse effects of certain environmental factors. It is an extremely important issue. We have used herbicide diquat as a model stress factor. For a short duration (30 minutes) of diquat treatment the content of TBA-reactive products in photosynthetic tissues of plants that were inoculated with the strain T17 was reduced by 18% compared to plants without inoculation (control 2). In the case when plants were inoculated with the consortium S. meliloti T17 + N. PTV the content of TBA-reactive products remained at the level of control. In the experiments with more prolonged action of the stress factor (60 min) the content of TBA-reactive products in plants inoculated only by the strain T17 decreased by 16.9% and in the case of binary inoculation the content of TBA-reactive products decreased by 25%. It should be noted that after 24 h of plants exposure with diquat, regardless of the inoculation agent used, reducing the amount of TBA-active products in photosynthetic tissues was not observed compared with the control. At the same time, in the experiment with the use of the consortium of microorganisms a difference (15.4%) with the inoculation only by strain T17 was marked (Figure 5). At short-term action of diquat (30 min) a rate of SOD activity in photosynthetic tissues of plants inoculated with S. meliloti T17 + N. PTV was 2.5 times higher than in controls and by 42.5% in plants, inoculated by strain T17. After herbicide treatment during 60 minutes a significant altering of SOD activity in inoculated plants (irrespective of whether a mono- or binary inoculation) was not observed. However, in comparison to the control, this difference was significant; the enzyme activity was increased by 54%. Under long-term stress (24 h) in plants inoculated with strain T17, SOD activity remained at the same level as for short-term exposure. In plants, the seeds were treated with consortium S. meliloti T17 + N. PTV, for the same conditions; this index decreased by 23.4% compared with the control and 34.4%, in comparison with the plants inoculated by strain T17 (Figure 6). Thus, the plants inoculated with algae-rhizobial composition proved to be more resistant to oxidative stress. It is possible due to the increased level of NFA of their symbiotic system and thus the increase in the number of available forms of nitrogen for alfalfa plants and the possible participation of NO in the defense reactions. In the literature, there are two hypotheses about the mechanisms of NO action under conditions of stress. First, NO may act as an antioxidant, directly linking to ROS, thereby protecting cells from damaging their actions [34]. Secondly, NO can act as a signaling molecule that triggers a cascade of reactions that lead to the expression of specific genes [35]. In their chemical and physical properties small molecule, rapid metabolism, lack of charging, and high diffusion coefficient of NO are well suited for the role of intracellular signaling mediator of plant stress responses.
Figure 5.
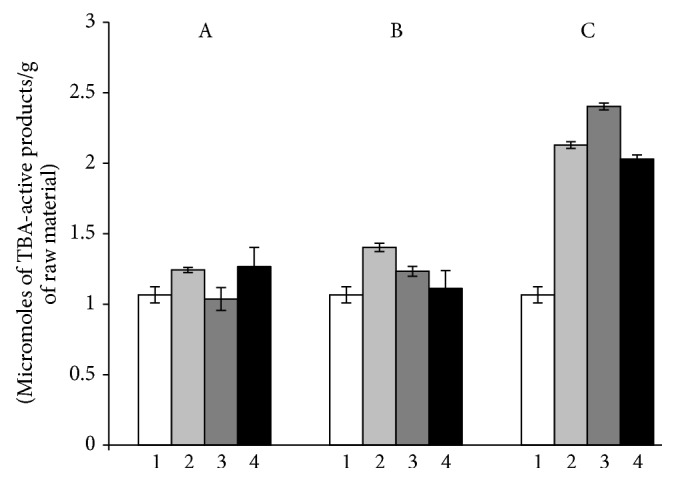
Content of TBA-active products in alfalfa leaves after herbicide diquat treatment: А: for 30 min, B: for 60 min, and C: for 24 h. 1: control (without inoculation and herbicide diquat treatment); 2: control (without inoculation, with herbicide diquat treatment); 3: inoculation by Tn5-mutant of S. meliloti Т17, with herbicide diquat treatment; 4: inoculation by Tn5-mutant of S. meliloti Т17+ N. PTV, with herbicide diquat treatment.
Figure 6.
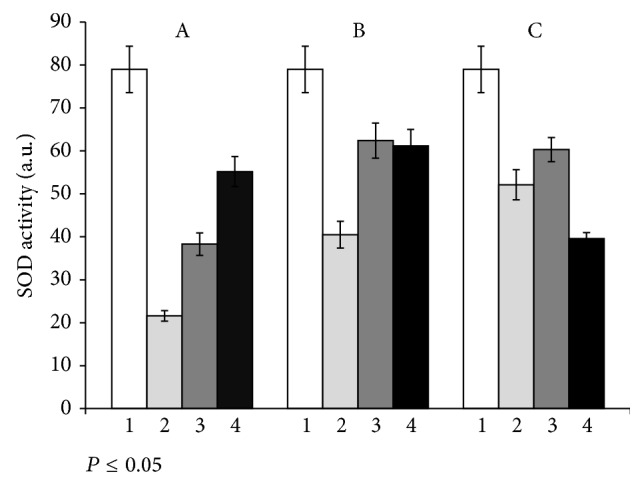
SOD activity in alfalfa leaves after herbicide diquat treatment: А: in 30 min, B: in 60 min, and C: in 24 h. 1: control (without inoculation and herbicide diquat treatment); 2: control (without inoculation, with herbicide diquat treatment); 3: inoculation by Tn5-mutant of S. meliloti Т17, with herbicide diquat treatment; 4: inoculation by Т17 + N. PTV, with herbicide diquat treatment.
Thus, the inoculation of alfalfa seeds by a consortium of nitrogen-fixing microorganisms S. meliloti T17 + N. PTV increased the nitrogenase activity of root nodules, increased the net assimilation rate, and increased productivity and product quality, and also the stability of alfalfa plants under the influence of oxidative stress induced by herbicides.
3.3. Molecular Typing and Phylogenetic Analysis of Cyanobacterium Nostoc PTV
One of the main goals of this study was the molecular typing and phylogenetic analysis of a new cyanobacterial isolate N. PTV originated from the Institute of Hydrobiology of Academy of Science of Ukraine. As it was shown above, this cyanobacterium is effective for soil algalization. As a component of algae-rhizobium compositions, this cyanobacterium stimulates germinative energy, growth, and productivity of legumes.
To identify and to determine the phylogenetic positions of the new cyanobacterial isolate N. PTV we used a partial sequence of the nifH gene (342 bp), encoding nitrogenase reductase, and 16S ribosomal RNA gene cluster (1765 bp) as molecular markers. Comparison of the nifH gene sequence and rRNA gene cluster sequence of cyanobacterium N. PTV with all the sequences present at the GenBank by using the program Blast (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome) shows that this strain has no full similarity with any of early investigated organisms.
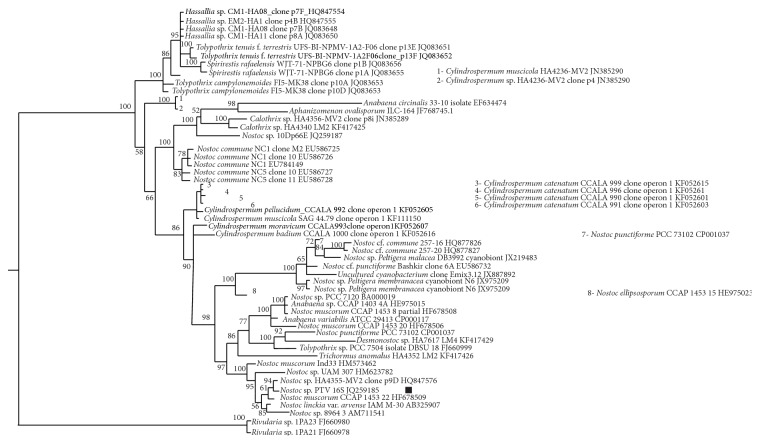
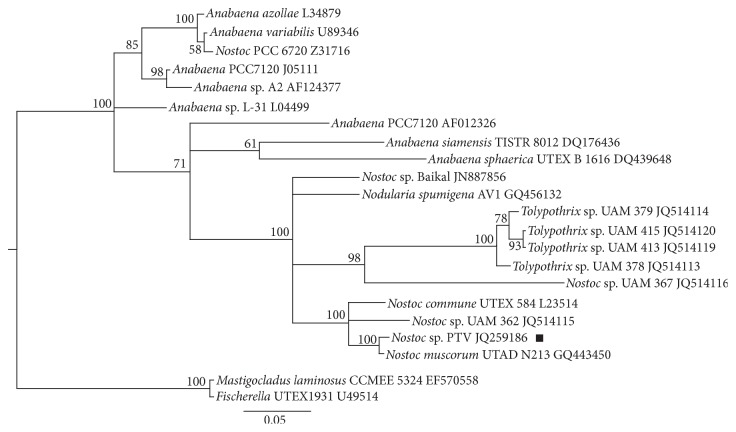
Comparison of rRNA gene cluster sequence (1765 bp) of the cyanobacterium Nostoc sp. PTV revealed that this cyanobacterium shows the highest similarity with several strains of N. muscorum and N. commune (Table 5). In general, support for branching in the tree, based on a fragment of 16S rRNA gene sequence (Figure 7), is worse in comparison with the reconstruction of the phylogeny of cyanobacteria based on the sequence of the gene nifH (Figure 8).
Table 5.
BLAST results obtained by querying the 16S–23S rRNA gene cluster of Nostoc sp. PTV with GenBank and geographical and ecological origins of the hits.
| Closest GenBank relative | GenBank access number | Query coverage, % | Score, % | Identity, % | E value | Origin of the strain and reference |
|---|---|---|---|---|---|---|
| Nostoc sp. HA4355-MV2 clone p9D | HQ847576 | 98 | 2872 | 97 | 0.0 | Maniniholo Cave wall, near Haena USA: Kauai, Hawaii |
|
| ||||||
| Nostoc muscorum CCAP 1453/22 | HF678509 | 98 | 2531 | 93 | 0.0 | Scottish Association for Marine Science, Molecular and Microbial Biology, Dunstaffnage Marine Laboratory, Oban, PA37 1QA, United Kingdom |
|
| ||||||
| Anabaena variabilis ATCC 29413 | CP000117 | 98 | 2457 | 92 | 0.0 | It has been studied extensively for over 40 years and is the strain of choice for many laboratories throughout the world |
|
| ||||||
| Nostoc sp. PCC 7120 | BA000019 | 98 | 2453 | 92 | 0.0 | Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. (Nostoc) strain PCC 7120 is available |
|
| ||||||
| Nostoc commune NC1 clone 10 | EU586726 | 98 | 2441 | 91 | 0.0 | John Carroll University, 20700, North Park Boulevard, University Heights, OH 44118, USA |
|
| ||||||
| Nostoc commune NC1 clone M2 | EU586725 | 98 | 2441 | 91 | 0.0 | John Carroll University, 20700, North Park Boulevard, University Heights, OH 44118, USA |
|
| ||||||
| Nostoc commune NC5 clone 10 | EU586727 | 98 | 2437 | 91 | 0.0 | Biology, John Carroll University, 20700, North Park Boulevard, University Heights, OH 44118, USA |
|
| ||||||
| Nostoc commune NC1 | EU784149 | 98 | 2428 | 91 | 0.0 | Nostoc commune NC1 was isolated from soil (subaerophyt) in Třeboň/Czech republic in 2006. |
|
| ||||||
| Nostoc commune NC5 clone 11 | EU586728 | 98 | 2423 | 91 | 0.0 | Biology, John Carroll University, 20700, North Park Boulevard, University Heights, OH 44118, USA |
|
| ||||||
| Nostoc ellipsosporum CCAP 1453/15 | HE975023 | 94 | 2399 | 92 | 0.0 | Scottish Association for Marine Science, Molecular and Microbial Biology, Dunstaffnage Marine Laboratory, Oban PA37 1QA, United Kingdom |
|
| ||||||
| Nostoc cf. punctiforme Bashkir clone 6A | EU586732 | 96 | 2378 | 92 | 0.0 | John Carroll University, 20700, North Park Boulevard, University Heights, OH 44118, USA |
|
| ||||||
| Calothrix sp. HA4356-MV2 clone p8i | JN385289 | 98 | 2361 | 90 | 0.0 | Cave wall scraping, Maniniholo Cave near Haena, USA: Kauai, Hawaii |
|
| ||||||
| Calothrix sp. HA4340 LM2 | KF417425 | 98 | 2361 | 90 | 0.0 | Cave, USA: Kauai, Hawaii, Maniniholo Cave |
|
| ||||||
| Anabaena sp. CCAP 1403/4A | HE975015 | 93 | 2360 | 92 | 0.0 | Scottish Association for Marine Science, Molecular and Microbial Biology, Dunstaffnage Marine Laboratory, Oban PA37 1QA, United Kingdom |
|
| ||||||
| Nostoc muscorum CCAP 1453/8 | HF678508 | 93 | 2358 | 92 | 0.0 | United Kingdom: Scotland |
|
| ||||||
| Calothrix sp. HA4356-MV2 clone p8i | JN385289 | 98 | 2356 | 90 | 0.0 | Cave wall scraping, Maniniholo Cave near Haena, USA: Kauai, Hawaii |
|
| ||||||
| Cylindrospermum moravicum CCALA 993 clone operon 1 | KF052607 | 97 | 2331 | 94 | 0.0 | Cave sediment, Czech Republic: Amaterska Cave, South Moravia |
|
| ||||||
| Nostoc punctiforme PCC 73102 | CP001037 | 97 | 2331 | 91 | 0.0 | A symbiont from a cycad |
|
| ||||||
| Nostoc sp. Peltigera malacea DB3992 cyanobiont | JX219483 | 97 | 2322 | 91 | 0.0 | Cyanobiont of lichenized fungi Peltigera malacea, Iceland |
|
| ||||||
| Nostoc muscorum CCAP 1453/20 | HF678506 | 95 | 2318 | 91 | 0.0 | Scottish Association for Marine Science, Molecular and Microbial Biology, Dunstaffnage Marine Laboratory, Oban PA37 1QA, United Kingdom |
|
| ||||||
| Nostoc sp. 10Dp66E | JQ259187 | 98 | 2318 | 90 | 0.0 | From association with Dynamena pumila L., White Sea, Russia |
|
| ||||||
| Cylindrospermum catenatum CCALA 999 clone operon 1 | KF052615 | 97 | 2309 | 94 | 0.0 | Soil, Slovakia: forest above Stara Brzotinska Cave, Slovak Karst |
|
| ||||||
| Trichormus anomalus HA4352 LM2 | KF417426 | 96 | 2309 | 92 | 0.0 | Cave, USA: Kauai, Hawaii, Maniniholo Cave |
|
| ||||||
| Cylindrospermum sp. HA4236-MV2 clone p4 | JN385290 | 98 | 2309 | 89 | 0.0 | Taro field, Makiki Nature Center, USA: Oahu, Hawaii |
|
| ||||||
| Cylindrospermum catenatum CCALA 996 clone operon 1 | KF052611 | 97 | 2307 | 94 | 0.0 | Soil, Czech Republic: Amaterska Cave, South Moravia |
|
| ||||||
| Tolypothrix campylonemoides FI5-MK38 clone p10D | JQ083654 | 98 | 2305 | 90 | 0.0 | Sand, USA: Fort Irwin NTC, San Bernardino Co., California |
|
| ||||||
| Cylindrospermum catenatum CCALA 990 clone operon 1 | KF052601 | 95 | 2302 | 94 | 0.0 | Soil, Czech Republic: Benešov nad Černou, South Bohemia |
|
| ||||||
| Spirirestis rafaelensis WJT-71-NPBG6 clone p1B | JQ083656 | 98 | 2300 | 90 | 0.0 | Joshua Tree National Park, USA: Joshua Tree Forest, San Bernardino Co., California |
|
| ||||||
| Cylindrospermum badium CCALA 1000 clone operon 1 | KF052616 | 97 | 2298 | 94 | 0.0 | Reclaimed coal mine soil, USA: Pyramid State Recreation Area, Illinois |
|
| ||||||
| Cylindrospermum catenatum CCALA 991 clone operon 1 | KF052603 | 97 | 2293 | 94 | 0.0 | Soil, Czech Republic: Most Region, North Bohemia |
|
| ||||||
| Spirirestis rafaelensis WJT-71-NPBG6 clone p1A | JQ083655 | 98 | 2291 | 89 | 0.0 | Joshua Tree National Park, USA: Joshua Tree Forest, San Bernardino Co., California |
|
| ||||||
| Nostoc sp. Peltigera membranacea cyanobiont N6 | JX975209 | 97 | 2289 | 91 | 0.0 | Symbiont of Peltigera membranacea lichen, Iceland |
|
| ||||||
| Cylindrospermum pellucidum CCALA 992 clone operon 1 | KF052605 | 97 | 2287 | 94 | 0.0 | Cave sediment, Slovakia: Dlha chodba in Domica Cave system, Slovak Karst |
|
| ||||||
| Hassallia sp. EM2-HA1 clone p4B | HQ847555 | 98 | 2282 | 89 | 0.0 | Soil, Mojave National Preserve, USA: San Bernardino Co., California |
|
| ||||||
| Tolypothrix tenuis f. terrestris UFS-BI-NPMV-1A2-F06 clone p13E | JQ083651 | 98 | 2277 | 89 | 0.0 | Arid soil after a burn, foothills of the Onaquee Mts. USA: Utah |
|
| ||||||
| Nostoc cf. commune 257-16 | HQ877826 | 96 | 2271 | 90 | 0.0 | Subaerial, on Bonampak's archeological building walls, Mexico: Chiapas |
|
| ||||||
| Hassallia sp. CNP3-B3-C04 clone p5D | HQ847556 | 98 | 2271 | 89 | 0.0 | Soil, Needles District, Virginia Park, Canyonlands National Park, USA: San Bernardino Co., California |
|
| ||||||
| Uncultured cyanobacterium clone Emix3.12 | JX887892 | 92 | 2269 | 91 | 0.0 | Freshwater microbial mat Konstanz, Germany |
|
| ||||||
| Cylindrospermum muscicola SAG 44.79 clone operon 1 | KF111150 | 96 | 2268 | 93 | 0.0 | Soil France: Gif-Sur-Yvette, Ile-de-France Region |
|
| ||||||
| Tolypothrix tenuis f. terrestris UFS-BI-NPMV-1A2-F06 clone p13F | JQ083652 | 98 | 2268 | 89 | 0.0 | Arid soil after a burn, foothills of the Onaquee Mts. USA: Utah |
|
| ||||||
| Hassallia sp. CM1-HA11 clone p8A | JQ083650 | 98 | 2268 | 89 | 0.0 | Sandy loam near gypsum mine, USA: Clark Mountains, San Bernardino Co., California |
|
| ||||||
| Hassallia sp. CM1-HA08 clone p7B | JQ083648 | 98 | 2268 | 89 | 0.0 | Sandy loam near gypsum mine, USA: Clark Mountains, San Bernardino Co., California |
|
| ||||||
| Nostoc cf. commune 257-20 | HQ877827 | 94 | 2266 | 91 | 0.0 | Biofilms of N. cf. commune were collected at Bonampak archeological area in 2008 from two sites on the building walls (Chiapas, Mexico). |
|
| ||||||
| Tolypothrix campylonemoides FI5-MK38 clone p10A | JQ083653 | 98 | 2266 | 89 | 0.0 | Sand USA: Fort Irwin NTC, San Bernardino Co., California |
|
| ||||||
| Hassallia sp. CM1-HA08 clone p7F | HQ847554 | 98 | 2266 | 89 | 0.0 | Soil, Clark Mountains, near gypsum mine USA: San Bernardino Co., California |
|
| ||||||
| Desmonostoc sp. HA7617 LM4 | KF417429 | 96 | 2241 | 90 | 0.0 | USA: Kauai, Hawaii, Waikapalae Cave |
|
| ||||||
| Camptylonemopsis sp. HA4241-MV5 clone B2-3 + p4 | JN385292 | 96 | 2223 | 93 | 0.0 | Moleka stream USA: Oahu, Hawaii |
|
| ||||||
| Anabaena circinalis 33-10 isolate | EF634474 | 98 | 2199 | 88 | 0.0 | Ohau Channel, New Zealand |
|
| ||||||
| Tolypothrix sp. PCC 7504 isolate DBSU 18 | FJ660999 | 90 | 2194 | 92 | 0.0 | Freshwater Aquarium, Sweden |
|
| ||||||
| Nostoc sp. UAM 307 | HM623782 | 70 | 2111 | 98 | 0.0 | Rock surface of calcareous river Spain: Matarranya River, Teruel, East Spain |
|
| ||||||
| Rivularia sp. 1PA23 | FJ660980 | 92 | 2021 | 88 | 0.0 | Pozas Azules I, Mexico Microbialite freshwater |
|
| ||||||
| Nostoc sp. 8964:3 | AM711541 | 66 | 2006 | 99 | 0.0 | Host is Gunneraprorepens (Angiospermae), New Zealand |
|
| ||||||
| Rivularia sp. 1PA21 | FJ660978 | 92 | 2004 | 88 | 0.0 | Institute of Ecology, UNAM (Mexico) |
|
| ||||||
| Nostoc linckia var. arvense IAM M-30 | AB325907 | 65 | 1999 | 99 | 0.0 | Cultivated samples from the Institute of Molecular Biosciences at the University of Tokyo |
|
| ||||||
| Aphanizomenon ovalisporum ILC-164 | JF768745 | 92 | 1988 | 93 | 0.0 | Lake Kinneret, Israel; Banker et al., 1997 [5] |
|
| ||||||
| Nostoc muscorum Ind33 | HM573462 | 65 | 1988 | 99 | 0.0 | Paddy field, India: Agricultural Farms, Banaras Hindu University, Varanasi, Uttar Pradesh |
Figure 7.
Phylogenetic relationships of Nostoc sp. PTV (designated by black square) inferred under the posterior probability criterion (MrBayes) from the gene for 16S rRNA, partial sequence information. Numbers at the nodes indicate the Bayesian statistical support values (posterior probabilities multiplied by 100); only values higher than 50% are given. The scale bar indicates the number of substitutions per nucleotide position.
Figure 8.
Phylogenetic relationships of Nostoc sp. PTV (designated by black square) inferred under the posterior probability criterion (MrBayes) from the gene for nifH, partial sequence information. Numbers at the nodes indicate the Bayesian statistical support values (posterior probabilities multiplied by 100); only values higher than 50% are given. The scale bar indicates the number of substitutions per nucleotide position.
The group of Nostoc strains and species, which includes Nostoc PTV, forms a cluster with the minimum of allowable support, 0.95. Hierarchy of strains (HA 4355-MV2, PTV, and 8964) and Nostoc species (N. muscorum and N. linckia) cannot be evaluated because of the low topological support of this site of the tree: credibility values range from 0.56 to 0.94. It is difficult to discuss a relation of Nostoc PTV strain to strain HA 4355-MV2, due to the very low values of the other branches in the cluster, and very scarce information about the HA-MV2 4355 strain clearly does not help to solve the problem of their possible relationship.
N. muscorum is discussed below. N. linckia is also a freshwater strain and could be isolated in some terrestrial niches. Interestingly, the Nostoc strain UAM 307 is quite clearly differentiated from the other representatives of Nostocaceae (0.95). Being sufficiently close to strain of Nostoc PTV, this cyanobacterium has some significant features that provided the separation of this strain into the other branch of the common with Nostoc PTV cluster.
Even more interesting detail is that the last significant branch (credibility value is equal to unity) of the cluster is formed by the strain of N. muscorum Ind33, which is significantly aside not only from the desired strain of PTV, but also from the different strains of the same species (CCAP 1453-22). Thus, the strain of Nostoc PTV has teamed up with members of their own genus.
Outgroup of this dendrogram is represented by two strains of Rivularia (this kind of cyanobacteria forms heteropolar threads; their trichomes are densely agglomerated, covered with a total mucus). The genus is represented only by the species often living on calcareous substrates, but there are rare epilithic and epiphytic species.
Comparison of nifH sequence of cyanobacterium PTV revealed that this strain shows the highest similarity with several strains of Nostoc (Table 6). The closest relative is Nostoc muscorum UTAD N213, purified from rice paddy in Mondego River Basin (Portugal). Phylogenetic analysis (Figure 8) revealed that the cyanobacterium PTV forms a minicluster with Nostoc muscorum UTAD N213. N. muscorum is a free-living filamentous cyanobacterium, which inhabits both terrestrial and freshwater aquatic environments. They are phototrophic organisms performing photosynthesis and also fixing atmospheric nitrogen [36].
Table 6.
BLAST results obtained by querying the nifH gene of Nostoc sp. PTV with GenBank and geographical and ecological origins of the hits.
| Closest GenBank relative |
GenBank number | Query coverage % | Score % | Identity % | E value | Origin of the strain and reference |
|---|---|---|---|---|---|---|
| Nostoc muscorum UTAD N213 | GQ443450.1 | 100 | 612 | 99 | 7e − 172 | Rice paddy in Mondego River Basin, Portugal |
|
| ||||||
| Nostoc muscorum clone CC1090A1 | AY221814.1 | 94 | 576 | 99 | 5e − 161 | Ocean Sciences Department, University of California, Santa Cruz, CA 95064, USA |
|
| ||||||
| Nostoc commune (UTEX 584) | L23514.1 | 99 | 565 | 97 | 9E − 158 | Scotland |
|
| ||||||
| Nostoc sp. UAM 362 | JQ514115.1 | 99 | 553 | 96 | 5e − 154 | Rock surface of calcareous river with brackish water, Spain: Muga, Girona (Northeast Spain) |
|
| ||||||
| Nostoc sp. Baikal | JN887856.1 | 99 | 517 | 94 | 4e − 143 | Nitrogen-fixing cyanobacteria from Lake Baikal |
|
| ||||||
| Nodularia spumigena AV1 | GQ456132.1 | 99 | 511 | 93 | 2e − 141 | The surface waters of the Baltic Sea, Stockholm, Sweden |
|
| ||||||
| Tolypothrix sp. UAM 379 | JQ514114.1 | 99 | 462 | 90 | 8E − 127 | Rock surface of calcareous river with brackish water, Spain: Muga, Girona (Northeast Spain) |
|
| ||||||
| Tolypothrix sp. UAM 378 | JQ514113.1 | 99 | 462 | 90 | 8E − 127 | Rock surface of calcareous river with brackish water, Spain: Muga, Girona (Northeast Spain) |
|
| ||||||
| Tolypothrix sp. UAM 415 | JQ514120.1 | 99 | 453 | 89 | 4E − 124 | Rock surface of calcareous river with brackish water, Spain: Muga, Girona (Northeast Spain) |
|
| ||||||
| Tolypothrix sp. UAM 413 | JQ514119.1 | 99 | 453 | 89 | 4E − 124 | Rock surface of calcareous river with brackish water, Spain: Muga, Girona (Northeast Spain) |
|
| ||||||
| Nostoc PCC 6720 | Z31716.1 | 99 | 430 | 88 | 4E − 117 |
Nostoc PCC 6720 was previously known as Anabaenopsis circularis. This is a freshwater species |
|
| ||||||
| Anabaena sp. L-31 | L04499.1 | 99 | 426 | 88 | 5E − 116 | The filamentous, heterocystous, nitrogen-fixing freshwater cyanobacterium |
|
| ||||||
| Anabaena PCC7120 | J05111.1 | 99 | 426 | 88 | 5E − 116 | http://wiki.annotation.jp/Kazusa:CyanoBase:Anabaena_sp._PCC_7120 |
|
| ||||||
| Nostoc sp. UAM 367 | JQ514116.1 | 100 | 423 | 87 | 7E − 115 | Rock surface of calcareous river with brackish water, Spain: Muga, Girona (Northeast Spain) |
|
| ||||||
| Anabaena siamensis TISTR 8012 | DQ176436.2 | 100 | 414 | 87 | 3E − 112 | Anabaena siamensis is a filamentous heterocystous nitrogen-fixing cyanobacterium which originally was isolated from a rice paddy field in Thailand |
|
| ||||||
| Mastigocladus laminosus CCMEE 5324 | EF570558.1 | 100 | 414 | 87 | 3E − 112 | The cosmopolitan thermophilic cyanobacterium Mastigocladus laminosus from the University of Oregon's Culture Collection of Microorganisms from Extreme Environments (CCMEE) |
|
| ||||||
| Fischerella UTEX1931 | U49514.1 | 100 | 414 | 87 | 3E − 112 | Thermophilic cyanobacterium (synonym: Mastigocladus laminosus) |
|
| ||||||
| Anabaena sphaerica UTEX “B 1616” | DQ439648.1 | 99 | 408 | 87 | 1E − 110 | Department of Chemistry and Chemical Engineering, University of Sheffield, Mappin Street, Sheffield, South Yorkshire S1 3JD, United Kingdom |
|
| ||||||
| Anabaena sp. A2 | AF124377.1 | 99 | 408 | 87 | 1.E − 110 | Molecular Evolution, BMC, Uppsala University, Husargatan 3, 751 24 Uppsala, Sweden |
|
| ||||||
| Anabaena azollae | L34879.1 | 99 | 435 | 88 | 1E − 118 | Anabaena azollae 1a, a putative symbiont of Azolla caroliniana |
|
| ||||||
| Anabaena variabilis | U89346.1 | 99 | 435 | 88 | 1E − 118 | Anabaena variabilis ATCC 29413 is a filamentous cyanobacterium that produces heterocysts and fixes nitrogen under a variety of environmental conditions |
N. muscorum is the most common type of Nostoc in terrestrial ecosystems and is widely spread, due to the adaptability to many adverse conditions. It forms a symbiotic relationship with many types of terrestrial plants and fungi.
It is known that N. muscorum has great effect on soil biology and productivity which makes it an attractive soil inoculant. This cyanobacterium is able to obtain carbon and nitrogen from the air and has an advantage over heterotrophic soil inoculants, which are usually limited by carbon [37].
It also benefits plants and other soil bacteria by increasing soil organic matter in the form of carbohydrates and provides biological organic nitrogen that can be assimilated by plants [38]. N. muscorum helps to create the environment conditions to further colonization and growth by plants and other microorganisms [39].
Inoculation of the N. muscorum isolates caused a significant effect on growth of wheat and maize plants. Cyanobacterial inoculation positively affected pigment content, increased plant shoot and root dry weight, and increased leaf area [40].
In general, the topology of the dendrogram (Figure 8) has a good support; the main branch nodes are characterized by high credibility values.
Nostoc PTV is in the same cluster with Nostoc sp. UAM-362 and Nostoc commune. Nostoc sp. UAM-362 was isolated from the rock surface of calcareous river with brackish water in Spain: Muga, Girona (Northeast Spain). N. commune is a colonial species of cyanobacterium. As well as N. punctiforme, N. commune is able to survive in extreme conditions such as polar regions and arid areas.
Three more clusters are presented as the parts of the large one: two single clusters, represented by Nostoc sp. Baikal (nitrogen-fixing cyanobacteria from Lake Baikal) and by Nodularia spumigena from family Nostocaceae. Nodularia occurs mainly in brackish or saline waters. Nodularia cells occasionally can form heavy algal blooms. Some strains produce a toxin (nodularin), which is harmful to human health [41].
The third cluster is formed by four strains of Tolypothrix and by one strain of Nostoc sp. UAM-367 (isolated from rock surface of calcareous river with brackish water in Spain: Muga, Girona). Cyanobacteria Tolypothrix grow in unpolluted waters; several species are found in swamps, known aerophilic species growing on the bark of trees, in the wet sands, on wet rocks, and so forth.
Two species, and one strain (PCC 7120) of Anabaena, representing a family of filamentous cyanobacteria Nostocaceae, belong to this large cluster with a poor resolution (a credibility value of branching is 0.71). These cyanobacteria exist in the form of plankton; some species are symbionts of plants. Anabaena is one of the four genera of cyanobacteria that produce neurotoxins. Anabaena is a model for the study of cell differentiation and differential gene expression during nitrogen fixation [42]. A. siamensis and A. sphaerica are freshwater species. This mega cluster consisting of described species of cyanobacteria is well differentiated from the other two clusters: a single one represented by strain Anabaena L-31 (freshwater cyanobacterium) and a poorly differentiated cluster, which consists of two strains of Anabaena (A2 and PCC 7120) and two well-differentiated species of Anabaena, A. azollae and A. variabilis. A. azollae forms symbiosis with water fern Azolla.
Outgroup for the described species of Nostoc and Anabaena is represented by Mastigocladus laminosus and by member of the genus Fischerella (strain UTEX 1931). The first organism is a typical representative of the genus Mastigocladus. Fischerella represents another squad, Stigonematales. Both types of reference are truly branching filamentous forms of thermophilic cyanobacteria.
3.4. Gene Transfer into Nostoc PTV Cells
Nostoc PTV cells were tested for their ability to conjugational DNA transfer. As a result of plasmid pRL692 transfer into cyanobacterial cells several hundred transconjugant colonies were selected on selective plates that contained solid BG-11 medium and antibiotics (Sp10 and Sm2). On control plates antibiotic-resistant colonies were absent (data not shown). In future experiments we plan to use this experimental approach for transposon mutagenesis of Nostoc PTV and selection of the new mutants with interesting characteristics.
4. Conclusions
The use of microbial consortium newly identified Nostoc PTV strain together with Sinorhizobium meliloti T17 was more efficient than the use of the single-rhizobium strain for alfalfa plant inoculation. This treatment provides an intensification of the processes of nitrogen fixation and photosynthesis and stimulates growth of above-ground plant mass and rhizogenesis and leads to increased productivity of Medicago sativa L. and improved amino acid composition of plant leaves. Phylogenetic analysis by using two different molecular markers showed that this new cyanobacterium belongs to a cluster of the genus Nostoc, with the closest relative of Nostoc muscorum. Gene transfer of transposon bearing plasmid DNA has been shown for cyanobacterium Nostoc PTV. It makes this strain very attractive model for future genetic and physiological experiments and biotechnological applications.
Acknowledgments
This work was partly supported by Grant no. 14-04-00656 of the Russian Foundation for Basic Research. Authors devote this study to memory of Professor Tatjana V. Parshikova, who initiated this research.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Rai A. N., Söderbäck E., Bergman B. Cyanobacterium—plant symbioses. New Phytologist. 2000;147(3):449–481. doi: 10.1046/j.1469-8137.2000.00720.x. [DOI] [PubMed] [Google Scholar]
- 2.Santi C., Bogusz D., Franche C. Biological nitrogen fixation in non-legume plants. Annals of Botany. 2013;111(5):743–767. doi: 10.1093/aob/mct048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subashchandrabose S. R., Ramakrishnan B., Megharaj M., Venkateswarlu K., Naidu R. Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnology Advances. 2011;29(6):896–907. doi: 10.1016/j.biotechadv.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Stal L. J. Physiological ecology of cyanobacteria in microbial mats and other communities. New Phytologist. 1995;131(1):1–32. doi: 10.1111/j.1469-8137.1995.tb03051.x. [DOI] [PubMed] [Google Scholar]
- 5.Banker R., Carmeli S., Hadas O., Teltsch B., Porat R., Sukenik A. Identification of cylindrospermopsin in Aphanizomenon ovalisporum (Cyanophyceae) isolated from lake kinneret, Israel. Journal of Phycology. 1997;33(4):613–616. doi: 10.1111/j.0022-3646.1997.00613.x. [DOI] [Google Scholar]
- 6.Kots S. Y., Vorobey N. A., Malichenko S. M., Butnitskiy I. M. Strain of bacteria Sinorhizobium meliloti Т17 (IMB B-7282) to produce of the bacterial fertilizers for alfalfa. Patent for utility of the model N 55432. Bulletin no. 23, 2010.
- 7.Novikova T. I., Sharypova L. A., Simarov B. V. Transposon mutagenesis of the strain СХМ1-105 Rhizobium meliloti . Molecular Genetics Microbiology and Virology. 1986;8:32–35. [Google Scholar]
- 8.Zehnder A., Gorham P. R. Factors influencing the growth of Microcystis aeruginosa Kutz Emend. Elenkin. Canadian Journal of Microbiology. 1960;6(6):645–660. doi: 10.1139/m60-077. [DOI] [PubMed] [Google Scholar]
- 9.Kiriziy D. A., Vorobey N. A., Kots S. Y. The relationship of nitrogen fixation and photosynthesis as the main components of the production process in alfalfa. Russian Journal of Plant Physiology. 2007;54:666–671. [Google Scholar]
- 10.Kobbia I. A., Battah M. G., Shabana E. F., Eladel H. M. Chlorophyll a fluorescence and photosynthetic activity as tools for the evaluation of simazine toxicity to Protosiphon botryoides and Anabaena variabilis . Ecotoxicology and Environmental Safety. 2001;49(2):101–105. doi: 10.1006/eesa.2000.1955. [DOI] [PubMed] [Google Scholar]
- 11.Papageorgiou G. C., Tsimilli-Michael M., Stamatakis K. The fast and slow kinetics of chlorophyll a fluorescence induction in plants, algae and cyanobacteria: a viewpoint. Photosynthesis Research. 2007;94(2-3):275–290. doi: 10.1007/s11120-007-9193-x. [DOI] [PubMed] [Google Scholar]
- 12.Grodzinskiy A. M., Grodzinskiy D. M. Quick Reference Guide for Plant Physiology. Naukova Dumka; 1964. [Google Scholar]
- 13.Hardy R. W. F., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiology. 1968;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wellburn A. R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology. 1994;144(3):307–313. doi: 10.1016/s0176-1617(11)81192-2. [DOI] [Google Scholar]
- 15.Coombs J., Hall D. O., Long S. P., Scurlock J. M. O., editors. Techniques in Bioproductivity and Photosynthesis. 2nd. Pergamon; 1985. [Google Scholar]
- 16.Lowry O. H., Rosenbrought N. Z., Farr A. L., Randall R. Z. Protein measurement with Folin fenol reagent. The Journal of Biological Chemistry. 1951;153:p. 265. [PubMed] [Google Scholar]
- 17.Zubay G. Biochemistry. New York, NY, USA: The McGraw-Hill Companies; 1998. [Google Scholar]
- 18.Andreeva L. I., Kozhemiakin L. A., Kishkun A. A. Modification of the method of determining lipid peroxidation in a test using thiobarbituric acid. Laboratory Work. 1988:41–43. [PubMed] [Google Scholar]
- 19.Heath R. L., Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics. 1968;125(1):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 20.Koksharova O. A., Schubert M., Shestakov S., Cerff R. Genetic and biochemical evidence for distinct key functions of two highly divergent GAPDH genes in catabolic and anabolic carbon flow of the cyanobacterium Synechocystis sp. PCC 6803. Plant Molecular Biology. 1998;36(1):183–194. doi: 10.1023/a:1005925732743. [DOI] [PubMed] [Google Scholar]
- 21.Koksharova O. A., Kravzova T. R., Lazebnaya I. V., et al. Molecular identification and ultrastructural and phylogenetic studies of cyanobacteria from association with the white sea hydroid Dynamena pumila (L., 1758) BioMed Research International. 2013;2013:11. doi: 10.1155/2013/760681.760681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nübel U., Garcia-Pichel F., Muyzer G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Applied and Environmental Microbiology. 1997;63(8):3327–3332. doi: 10.1128/aem.63.8.3327-3332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook J., Fritsch E. F., Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronquist F., Huelsenbeck J. P. MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 26.Elhai J., Wolk C. P. Conjugal transfer of DNA to cyanobacteria. Methods in Enzymology. 1988;167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- 27.Elhai J., Vepritskiy A., Muro-Pastor A. M., Flores E., Wolk C. P. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. Journal of Bacteriology. 1997;179(6):1998–2005. doi: 10.1128/jb.179.6.1998-2005.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koksharova O. A., Wolk C. P. A novel gene that bears a DnaJ motif influences cyanobacterial cell division. Journal of Bacteriology. 2002;184(19):5524–5528. doi: 10.1128/JB.184.19.5524-5528.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vorobey N. A., Patsko O. V., Kots S. Y., Parshikova T. V. Physiological traits of alfalfa plants development under the inoculation by mixed nitrogen fixing microorganisms cultures. Physiology and Biochemistry of the Cultural Plants. 2009;41(4):344–352. [Google Scholar]
- 30.Sergeeva E., Liaimer A., Bergman B. Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta. 2002;215(2):229–238. doi: 10.1007/s00425-002-0749-x. [DOI] [PubMed] [Google Scholar]
- 31.Voisin A. S., Salon C., Jeudy C., Warembourg F. R. Symbiotic N2 fixation activity in relation to C economy of Pisum sativum L. as a function of plant phenology. Journal of Experimental Botany. 2003;54(393):2733–2744. doi: 10.1093/jxb/erg290. [DOI] [PubMed] [Google Scholar]
- 32.Berman-Frank I., Lundgren P., Falkowski P. Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Research in Microbiology. 2003;154(3):157–164. doi: 10.1016/S0923-2508(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 33.Küpper H., Ferimazova N., Šetlík I., Berman-Frank I. Traffic lights in Trichodesmium. Regulation of photosynthesis for nitrogen fixation studied by chlorophyll fluorescence kinetic microscopy. Plant Physiology. 2004;135(4):2120–2133. doi: 10.1104/pp.104.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beligni M. V., Lamattina L. Nitric oxide interferes with plant photo-oxidative stress by detoxifying reactive oxygen species. Plant, Cell and Environment. 2002;25(6):737–748. doi: 10.1046/j.1365-3040.2002.00857.x. [DOI] [Google Scholar]
- 35.Wendehenne D., Pugin A., Klessig D. F., Durner J. Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends in Plant Science. 2001;6(4):177–183. doi: 10.1016/s1360-1385(01)01893-3. [DOI] [PubMed] [Google Scholar]
- 36.Blumwald E., Tel-Or E. Structural aspects of the adaptation of Nostoc muscorum to salt. Archives of Microbiology. 1982;132(2):163–167. doi: 10.1007/bf00508724. [DOI] [Google Scholar]
- 37.Rogers S. L., Burns R. G. Changes in aggregate stability, nutrient status, indigenous microbial populations, and seedling emergence, following inoculation of soil with Nostoc muscorum . Biology and Fertility of Soils. 1994;18(3):209–215. doi: 10.1007/bf00647668. [DOI] [Google Scholar]
- 38.de Caire G. Z., de Cano M. S., de Mulé M. C. Z., Palma R. M., Colombo K. Exopolysaccharide of Nostoc muscorum (Cyanobacteria) in the aggregation of soil particles. Journal of Applied Phycology. 1997;9(3):249–253. doi: 10.1023/a:1007994425799. [DOI] [Google Scholar]
- 39.Dodds W. K., Gudder D. A., Mollenhauer D. The ecology of Nostoc . Journal of Phycology. 1995;31(1):2–18. doi: 10.1111/j.0022-3646.1995.00002.x. [DOI] [Google Scholar]
- 40.Sholkamy E. N., El-Komy H., Al-Arfaj A. A., Abdel-Megeed A., Mostafa A. A. Potential role of Nostoc muscorum and Nostoc rivulare as biofertilizers for the enhancement of maize growth under different doses of n-fertilizer. African Journal of Microbiology Research. 2012;6(48):7435–7448. [Google Scholar]
- 41.Dittmann E., Fewer D. P., Neilan B. A. Cyanobacterial toxins: biosynthetic routes and evolutionary roots. FEMS Microbiology Reviews. 2013;37(1):23–43. doi: 10.1111/j.1574-6976.2012.12000.x. [DOI] [PubMed] [Google Scholar]
- 42.Cai Y., Wolk C. P. Anabaena sp. strain PCC 7120 responds to nitrogen deprivation with a cascade-like sequence of transcriptional activations. Journal of Bacteriology. 1997;179(1):267–271. doi: 10.1128/jb.179.1.267-271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]