Abstract
Background/Aim
Basal plasma leptin levels are higher in women than in men and also higher in obese than in lean subjects, but the dynamic leptin secretion has not been well studied. We tested whether the leptin secretory response to glucocorticoid or insulin differs by gender and adiposity status.
Methods
Seventy-nine nondiabetic adults, comprising lean [body mass index (BMI; kg/m2) ≤25; n = 27], obese (BMI 30-40; n = 28), and massively obese (BMI >40; n = 24) subjects, participated in two separate studies. In study 1, the subjects received oral dexamethasone (4 mg), with blood sampling before and 8 and 16 h after ingestion. In study 2, the subjects underwent a two-step hyperinsulinemic (1.0 mU·kg−1/min for 3 h, then 2.0 mU·kg−1/min for 3 h), euglycemic (∼100 mg/dl) clamp. Blood samples were obtained at baseline and every 20 min during the clamp.
Results
Basal and stimulated leptin levels were higher in women than in men, and higher in the obese groups than in lean subjects. The percentage increase above baseline leptin was similar among men and women within each group, but was ∼30% lower in massively obese compared to lean subjects.
Conclusion
Leptin secretory responses to glucocorticoid or insulin stimulation are preserved across a broad adiposity range, with higher absolute responses in women than in men.
Keywords: Leptin secretagogues, Gender dimorphism, Glucocorticoids, Insulin, Obesity
Introduction
Basal (fasting) plasma leptin levels are two- to threefold higher in women than in men, and higher in obese than in lean subjects [1–4]. In contrast, the effects of gender and adiposity on leptin secretory responses to stimuli have not been fully investigated. The basal hyperleptinemia of obesity is the result of leptin synthesis and secretion by the abundant fat cell mass [4–6]. Because leptin exerts anorectic and weight-reducing effects, the near-universal hyperleptinemia observed in obese persons constitutes a physiological paradox that has generally been attributed to ‘leptin resistance’ [7]. The mechanism(s) of leptin resistance are not fully understood, but factors such as limited leptin delivery across the blood-brain barrier [8] or mutations in postreceptor signaling [9, 10] have been proposed.
States of hormone resistance often are accompanied by increased secretion of the index hormone. In the case of glucoregulation, the majority of obese subjects with insulin resistance escape diabetes by augmenting their insulin secretion [11, 12]. At diagnosis, patients with type 2 diabetes typically have normal or even elevated fasting plasma insulin levels, but the dynamic insulin secretory response to glucose is markedly impaired [11–13]. In fact, loss of first-phase insulin secretion in response to intravenous glucose precedes and ultimately permits the development of diabetes [11–15].
Many overweight or obese individuals maintain their body weight for long periods without progressing to massive obesity, whereas others break through into extreme obesity. The factors regulating progression from normal weight to obesity, and thence to massive or extreme obesity, are not fully understood. Extrapolating from the well-known impairment of glucose-stimulated insulin secretion during evolution of type 2 diabetes, we investigated whether an analogous blunting of stimulated leptin secretory responses could be demonstrated across the spectrum from normal weight to massive obesity. To explore this concept, we have utilized two different leptin secretagogues –dexamethasone [16–18] and insulin [19, 20] – in two separate studies to compare leptin secretory responses in three groups of nondiabetic subjects who are lean, obese, or massively obese.
Subjects and Methods
We studied a total of 79 nondiabetic subjects (30 male, 49 female; mean age 36.7 ± 1.64 years) comprising lean [body mass index (BMI; kg/m2) <25, n = 27], obese (BMI 30–40, n = 28), and massively obese (BMI >40, n = 24) participants. All subjects gave written informed consent for participation in this study protocol, which was approved by the Washington University Human Studies Committee. These human studies were conducted according to the principles expressed in the Declaration of Helsinki. The study participants were instructed to follow their usual diet and lifestyle practices, and to avoid fasting [21], overfeeding [22], or strenuous exercise [23] during the period of study. The subjects had no history of diabetes or evidence of diabetes, as determined by fasting glucose and 75-gram oral glucose tolerance tests [24]. No subject had a history of current or previous use of glucocorticoids or other medications that alter appetite, body weight, or glucoregulatory physiology. None of the subjects was enrolled in any active weight loss program.
Study 1: Single-Dose Dexamethasone Challenge
For study 1, we enrolled 30 nondiabetic subjects (11 male, 19 female; mean age 37.1 ± 1.5 years). Ten of the subjects were lean (BMI ≤25), 10 were obese (BMI 30–40), and 10 were massively obese (BMI >40). The study participants were seen in the outpatients department of the Washington University General Clinical Research Center on three occasions. During the first visit, between 07.00 and 08.00 h on day 1, a fasting blood specimen was obtained, and each subject was given a single tablet of dexamethasone (4 mg) to be ingested between 23.00 h and midnight [25]. The subjects then returned to the General Clinical Research Center for repeat blood sampling at 08.00 h (8-hour specimen) and 16.00 h on day 2 (16-hour specimen). Table 1 shows the baseline characteristics of the study participants.
Table 1.
Characteristics of the study subjects
| Lean | Obese | Massive | |
|---|---|---|---|
| Study 1 (dexamethasone) | |||
| Subjects (F/M), n | 10 (6/4) | 10 (6/4) | 10 (7/3) |
| Age, years | 32.5 ± 2.6 | 36.2 ± 1.8 | 43.6 ± 2.7b |
| BMI | 21.0 ± 0.9 | 38.0 ± 1.1 | 53 ± 4.3 |
| Fasting plasma glucose, mg/dl | 91.3 ± 3.8 | 101 ± 3.8a | 92.7 ± 6.8 |
| Cortisol nadir, μg/dl | 1.7 ± 0.3 | 1.5 ± 0.2 | 1.2 ± 0.2 |
|
| |||
| Study 2 (insulin) | |||
| Subjects (F/M), n | 17 (9/8) | 18 (11/7) | 14 (9/5) |
| Age, years | 36.3 ± 1.4 | 39.3 ± 2.5 | 39.4 ± 1.5 |
| BMI | 21.7 ± 0.4 | 31.8 ± 0.8 | 49.1 ± 3.1 |
| Fasting plasma glucose, mg/dl | 86.2 ± 2.1 | 92.1 ± 2.9 | 99.4 ± 2.3c, d |
The subjects in study 1 and study 2 were not the same individuals; the two studies were conducted on two different populations of lean, obese, and massively obese subjects.
p = 0.02 vs. lean subjects;
p < 0.01 vs. lean or obese subjects;
p < 0.001 vs. lean subjects;
p = 0.02 vs. obese subjects.
Study 2: Two-Step Hyperinsulinemic-Euglycemic Clamp
For study 2, we enrolled 49 subjects (20 male, 29 female; mean age 36.3 ± 1.78 years). Using the BMI criteria described under study 1, 17 of the subjects were lean, 18 were obese, and 14 were massively obese (table 1). The study participants were admitted to the General Clinical Research Center at 07.00 h after a 12-hour overnight fast and discharged at approximately 17.00 h the same day. On arrival, intravenous lines were placed in an antecubital vein for infusion of insulin and dextrose and in a contralateral hand vein for arterialized blood sampling. Beginning at 08.00 h, regular human insulin (Eli Lilly Co., Indianapolis, Ind., USA) was infused continuously at a rate of 1.0 mU·kg−1/min for 180 min, followed by 2.0 mU·kg−1/min for another 180 min; dextrose (20%) was infused at a variable rate to maintain euglycemia (∼100 mg/dl). Bedside plasma glucose levels were assessed every 10 min during the clamp procedure, and samples for measurement of insulin and leptin levels were obtained at 60, 40, 20, and 0 min before and every 20 min during the insulin infusion.
Biochemical Analyses
Plasma leptin was measured with an in-house RIA using a commercial kit (Linco Research, Inc., St. Charles, Mo., USA). Samples were assayed in duplicate, and the limits of detection and linearity for the leptin RIA were 0.5 and 100 ng/ml, respectively; the intra- and interassay coefficients of variation were <7% [26]. Insulin [27], C-peptide [27], and cortisol [28] levels were measured by RIA. Plasma glucose was measured with a glucose oxidase method (Beckman Instruments, Inc., Fullerton, Calif., USA).
Statistical Analyses
Data are expressed as mean ± SEM. To account for individual and gender differences in baseline leptin levels, pre- and post-intervention leptin data are expressed as a percentage of baseline values, following dexamethasone or insulin stimulation. We used repeated-measures ANOVA to verify treatment effect (i.e., hormonal changes following dexamethasone or insulin stimulation) and Dunnett's post hoc test to confirm specific differences. The differences in mean baseline values of continuous variables in the different groups of subjects were compared using Student's t tests. Statistical analyses were run on an IBM StatView program (SAS Institute, Inc., Cary, N.C., USA). p <0.05 was accepted as significant.
Results
The main outcome measure was the change in plasma leptin levels following dexamethasone ingestion or insulin infusion, compared across the lean, obese, and massively obese groups.
Study 1: Single-Dose Dexamethasone Challenge
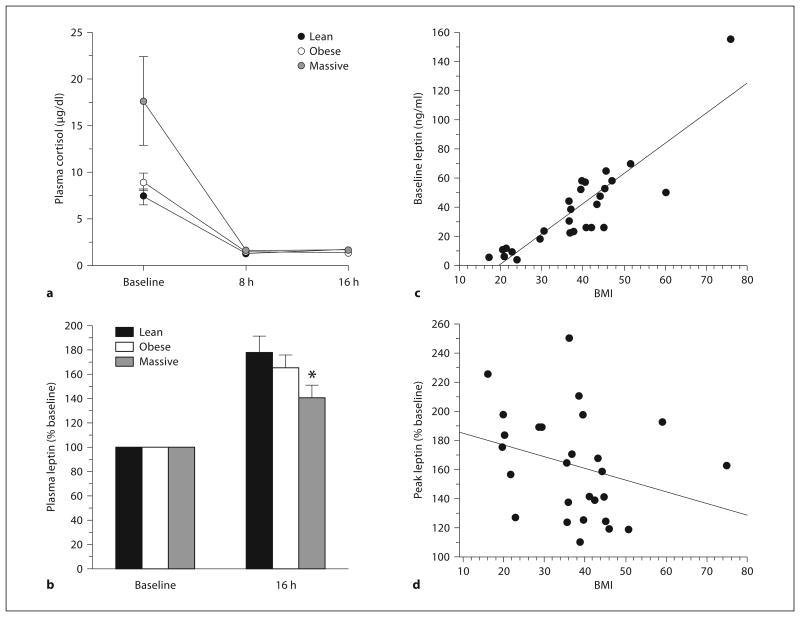
Figure 1a shows the fidelity of intervention for the dexamethasone study, as indicated by feedback suppression of endogenous cortisol production. The plasma cortisol levels were adequately and similarly suppressed in all study groups. The plasma leptin response to oral dexamethasone peaks at 16 h following ingestion [16, 17, 25]. Because of the known gender dimorphism in plasma leptin levels, we initially analyzed the data separately for each gender. Table 2 shows baseline and peak (16 h) plasma leptin levels after dexamethasone in lean, obese, and massively obese men and women. The baseline and post-stimulation leptin levels were higher (p < 0.001) in women than in men across the three groups.
Fig. 1.
Changes in plasma cortisol (a) and plasma leptin (b) following dexamethasone ingestion in lean, obese, and massively obese subjects, and the relationship between BMI and baseline (fasting) plasma leptin (c) or leptin levels af ter dexa met hasone (d). Baseline (fasting) leptin levels were positively correlated with BMI (R2 = 0.76) but peak dexamethasone-stimulated leptin levels showed a trend toward an inverse correlation. (To convert cortisol from μg/dl to nmol/l, multiply by 27.59.) * p = 0.02 versus lean and p = 0.05 versus obese.
Table 2.
Basal and peak stimulated leptin levels
| Lean | Obese | Massive | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| male | female | male | female | male | female | |
| Study 1 (dexamethasone) | ||||||
| Basal leptin, ng/mla | 5.7 ± 0.7 | 10.8 ± 0.6 | 23.4 ± 1.9 | 41.9 ± 4.1 | 26.0 ± 2.5 | 59.1 ± 3.8 |
| Peak leptin, ng/mla | 10.5 ± 1.6 | 18.7 ± 1.8 | 36.7 ± 1.7 | 71.2 ± 9.2 | 41.4 ± 4.7 | 84.5 ± 6.0 |
| Peak/basal, % b | 184 ± 11 | 173 ± 8.1 | 157 ± 14 | 170 ± 14 | 159 ± 11 | 143 ± 12 |
| Study 2 (insulin) | ||||||
| Basal leptin, ng/mla | 5.9 ± 2.6 | 14.8 ± 2.2 | 13.2 ± 3.9 | 23.9 ± 4.2 | 14.0 ± 1.8 | 39.2 ± 7.9 |
| Peak leptin, ng/mla | 8.4 ± 3.1 | 18.4 ± 2.2 | 16.9 ± 4.9 | 34.7 ± 5.8 | 19.5 ± 3.8 | 47.3 ± 7.9 |
| Peak/basal, %b | 150 ± 8.4 | 153 ± 11 | 137 ± 6.7 | 148 ± 8.2 | 130 ± 10 | 126 ± 3.8 |
The subjects in study 1 and study 2 were not the same individuals; the two studies were conducted on two different populations of lean, obese, and massively obese subjects.
The basal and peak leptin levels were significantly higher (p < 0.01) in women than in men in each adiposity group.
The mean percentage increases in leptin above baseline values were similar among men and women within each adiposity group, but significantly lower in the massively obese than in lean or obese subjects. The data on basal and poststimulation leptin patterns across gender and adiposity groups, obtained using dexamethasone or insulin as leptin secretagogue, were concordant, although insulin was a weaker leptin stimulant than glucocorticoid.
Following dexamethasone ingestion, plasma leptin levels increased significantly in lean men (p = 0.05) and women (p = 0.005), obese men (p = 0.003) and women (p = 0.01), and massively obese men (p = 0.02) and women (p = 0.006). The absolute increases above baseline leptin values in response to dexamethasone were approximately 5 ng/ml in lean men and 8 ng/ml in lean women; 13 ng/ml in obese men and 30 ng/ml in obese women, and 15 ng/ml (men) and 25 ng/ml (women) among massively obese subjects (table 2). Because of the marked gender-related and interindividual differences in baseline plasma leptin levels, we expressed poststimulation leptin levels as a percentage of baseline values (peak/basal), to enable comparison across gender and study groups. As shown in table 2, the percent peak/basal incremental plasma leptin responses to glucocorticoid stimulation did not display any significant gender differences. Thus, women have higher basal leptin levels and maintained the higher secretion rate during dexamethasone stimulation compared to men, but the percentage increases above baseline leptin values were similar in both genders.
To assess the effect of adiposity, we combined the percent peak/basal incremental leptin responses to dexamethasone for all subjects in each group. As shown in figure 1b, the peak leptin levels (% baseline) observed 16 h following dexamethasone ingestion were 178 ± 13.9% in lean, 166 ± 10.5% in obese, and 145 ± 10.4% in massively obese (p = 0.02 vs. lean and 0.05 vs. obese) subjects. These data indicate that the percentage incremental leptin responses were inversely related to basal leptin levels.
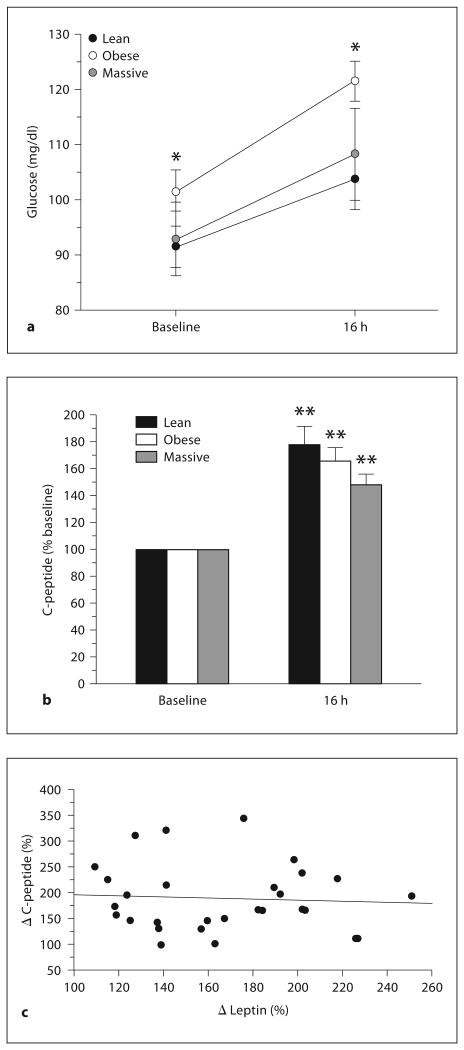
Baseline (fasting) leptin levels displayed the well-known positive correlation with BMI (R2 = 0.76, fig. 1c), but peak dexamethasone-stimulated leptin levels showed an insignificant trend toward an inverse correlation (fig. 1d). As already noted, none of the study subjects had diabetes. Plasma glucose levels increased following dexamethasone ingestion in all subjects, the peak level being significantly higher in obese subjects (p < 0.02) than in lean or massively obese subjects (fig. 2a). Insulin secretion (indicated by C-peptide levels) also increased following dexamethasone ingestion; the peak C-peptide level following dexamethasone challenge tended to be lower in massively obese subjects than in lean or obese groups, but the differences were not significant (fig. 2b). Furthermore, the change in C-peptide did not predict the change in leptin, following dexamethasone ingestion (fig. 2C).
Fig. 2.
Plasma glucose (a) and C-peptide (b) levels following dexamethasone ingestion in lean, obese, and massively obese subjects. c Relationship between changes in leptin and C-peptide levels after dexamethasone for all subjects. * p < 0.02 versus lean or massively obese subjects; ** p < 0.01 versus baseline values.
Study 2: Two-Step Hyperinsulinemic-Euglycemic Clamp
Plasma glucose and insulin levels were maintained within the desired targets during the two-step hyperin-sulinemic-euglycemic clamps. The target plasma glucose concentration was ∼100 mg/dl and the targets for plasma insulin were ∼50 μU/ml (step 1) and ∼100 μU/ml (step 2). Thus, the ambient insulinemia of 50–100 μU/ml reached during the two-step euglycemic clamp was well within the high-physiological range for postprandial insulin levels among human beings [14]. Slight increases in plasma leptin levels (6–10% above baseline) were noted during the initial 3 h of infusion of the lower dose (1.0 mU·kg−1/min) of insulin, and maximal responses were observed at the end of the second step, when insulin was infused at 2.0 mU·kg−1/min for an additional period of 3 h. Thus, the plasma leptin level at 6 h following the start of insulin infusion was recorded as the peak leptin response during study 2.
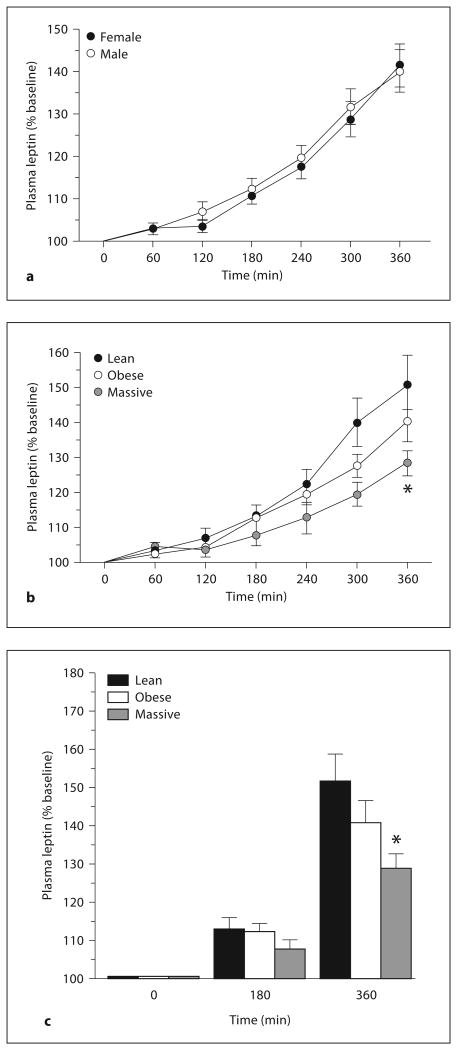
Table 2 summarizes the baseline and peak leptin levels attained during insulin infusion in lean, obese, and massively obese men and women. Both the baseline and peak stimulated leptin levels were higher (p < 0.01) in women than in men in each of the study groups. However, the peak incremental leptin response, expressed as a percentage of baseline values, was similar among men and women within each study group (table 2; fig. 3a). The basal and poststimulation leptin patterns observed across gender and all study groups were qualitatively similar using insulin as leptin secretagogue as compared with dexamethasone. However, the leptin secretory responses to insulin were less robust and displayed greater individual variability than the responses to dexamethasone (table 2).
Fig. 3.
Changes in plasma leptin in all male and female subjects (a) and in lean, obese, and massively obese subjects (b, c) during two-step hyperinsulinemic-euglycemic clamp. * p = 0.007 versus lean and p = 0.035 versus obese subjects.
Because the incremental plasma leptin response to insulin stimulus, expressed as a percentage of baseline values, was similar in male and female subjects (fig. 3a), we combined the data for men and women within each group and compared the leptin responses across the groups. The combined data (fig. 3b, c) show that the peak incremental leptin responses to insulin were 152 ± 7.6% in lean, 141 ± 5.9% in obese, and 128 ± 3.7% in massively obese (p = 0.007 vs. lean and p = 0.035 vs. obese) subjects.
Discussion
Given that gender and fat mass are major regulators of basal leptin secretion, the present study determined whether dynamic leptin secretion also was under the control of gender and adiposity. We compared plasma leptin responses to dexamethasone or insulin in lean, obese, and massively obese men and women. Our data show that both basal and stimulated leptin levels were higher in women than in men, but the percentage increase in leptin above baseline values was similar among men and women of approximately similar body mass. After normalization for individual differences in baseline leptin values, men and women in each of our prespecified study groups (lean, obese, massively obese) mounted similar proportionate responses to dexamethasone or insulin.
Remarkably, the data obtained using insulin as leptin secretagogue were in accord with those generated by dexamethasone. To our knowledge, this is the first report that has evaluated the effects of gender and adiposity on insulin-stimulated leptin secretion in nondiabetic subjects.
We observed that responses to leptin secretagogues (both insulin and dexamethasone) were remarkably preserved across a broad range of adiposity (BMI <25 to >40). Indeed, obese and massively obese subjects, all of whom had markedly elevated basal leptin levels, were able to secrete substantial amounts of additional leptin in response to either dexamethasone or insulin administration. Notably, the leptin response to a single oral dose of dexamethasone (4 mg) was more robust than the response to 6 h of hyperinsulinemic (euglycemic) infusion, which makes oral glucocorticoid the leptin secretagogue of choice.
Our data indicate preservation of adipocyte sensitivity to leptin secretagogues across the range from leanness to massive obesity, although the proportionate increase above basal leptin levels appears to be diminished in massively obese subjects. Overall, compared with lean subjects, the proportional incremental leptin responses (% baseline) to dexamethasone or insulin were decreased by ∼12% in obese subjects and by ∼30% in massively obese subjects. Thus, massive obesity (BMI >40) appears to be associated with a significant attenuation of the fold increase in leptin levels in response to glucocorticoid or insulin stimulation. The fold increases in leptin responses among subjects with submassive obesity (BMI 30–40) were somewhat lower than in lean subjects but significantly greater than those of massively obese subjects.
The significance of the modest decline in incremental leptin responses to secretagogues with increasing adiposity is unclear due to the cross-sectional design of the present study. A possible explanation for the apparent decrease in dynamic leptin responses (expressed as % baseline) among the massively obese subjects is that these subjects started with markedly elevated basal leptin levels (compared to the lean subjects). Thus, an absolute increase of 5 ng/ml (i.e., from 5 to 10 ng/ml) in a lean subject would yield an increase of 100% above baseline, whereas the same absolute increase would represent 25% above baseline for an obese subject with a baseline leptin level of 20 ng/ml. Nonetheless, the fact that further increases in absolute leptin levels were noted, following dexamethasone or insulin stimulation, among hyperleptinemic obese and massively obese subjects, argues against leptin deficiency as a major mechanism for the development of massive obesity.
However, because exogenous leptin treatment induces weight loss [29] and prevents weight regain [30] in hyperleptinemic obese subjects, it can be argued that obese subjects with leptin resistance need to produce even greater amounts of leptin in order to prevent progression to massive obesity. Viewed in that light, it is tempting to speculate that the ∼30% decrease in incremental leptin response to secretagogues observed among the massively obese subjects could have some significance.
Theoretically, failure to augment circulating leptin levels in response to endogenous stimuli could permit progression to massive obesity [29, 30]. Many individuals remain overweight or obese for long periods without progressing to massive obesity, and are able to defend against upward or downward pressures on their stable body weight through metabolic adjustments [31, 32]. The regulation of progression from submassive obesity to massive or extreme obesity is poorly understood. Under physiological conditions, the circulating levels of insulin, cortisol [33], and leptin [22] all increase postprandially. The postprandial increases in insulin and cortisol precede those of leptin by several hours [34], and fasting abolishes the glucocorticoid-induced hyperleptinemia [35, 36]. Furthermore, suppression of cortisol biosynthesis with metyrapone blunts the meal-stimulated rise in circulating leptin levels, indicating that cortisol is permissive of the postprandial insulin-stimulated rise in leptin [34, 37]. Moreover, augmentation of endogenous leptin secretion decreases voluntary food intake [38].
Thus, the physiological hyperleptinemia induced by postprandial increases in the circulating levels of insulin or cortisol (or both) could subserve a metabolic role of limiting hunger and energy intake, particularly during the several hours between meals. An impaired response to endogenous leptin secretagogues, thus, could permit hunger, frequent snacking, excessive energy intake, and progression of obesity.
In conclusion, leptin responses to glucocorticoid and insulin are demonstrable across the spectrum of human adiposity. Obesity, even in its massive form, is associated with the ability to augment the already high circulating leptin levels in response to stimulation by glucocorticoid or insulin. However, the fold increase in stimulated leptin levels over baseline values is diminished among massively obese subjects compared with lean or obese subjects.
Acknowledgments
This study was supported by an American Diabetes Association Clinical Research grant and National Institutes of Health grants MO1 RR00036 and P60 DK20579. Dr. Dagogo-Jack is supported in part by NIH grants R01 DK067269 and MO1 RR00211.
References
- 1.Considine RV, Sinha MK, Heiman ML, Kriaciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 2.Dagogo-Jack S, Fanelli C, Paramore D, Brothers J, Landt M. Plasma leptin and insulin relationships in obese and nonobese humans. Diabetes. 1996;45:695–698. doi: 10.2337/diab.45.5.695. [DOI] [PubMed] [Google Scholar]
- 3.Ostlund RE, Jr, Yang JW, Klein S, Gingerich R. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab. 1996;81:3909–3913. doi: 10.1210/jcem.81.11.8923837. [DOI] [PubMed] [Google Scholar]
- 4.Lonnqvist F, Aener P, Nordfors L, Schalling M. Overexpression of the obese (ob) gene in adipose tissue of obese subjects. Nat Med. 1995;1:950–953. doi: 10.1038/nm0995-950. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton BS, Paglia D, Kwan AYM, Deitel M. Increased obese mRNA expression in omental fat cells from massively obese humans. Nat Med. 1995;1:953–956. doi: 10.1038/nm0995-953. [DOI] [PubMed] [Google Scholar]
- 6.Levy JR, Gyarmati J, Lesko JM, Adler RA, Stevens W. Dual regulation of leptin secretion: intracellular energy and calcium dependence of regulated pathway. Am J Physiol Endocrinol Metab. 2000;278:E892–E901. doi: 10.1152/ajpendo.2000.278.5.E892. [DOI] [PubMed] [Google Scholar]
- 7.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz MW, Peskind E, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2:589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 9.Blorbaek C, El-Haschimi K, Fratz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1996;274:30059–30065. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg GR, McAinch AJ, Chen MB, O'Brien PE, Dixon JB, Cameron-Smith D, Kemp BE. The suppressor of cytokine signalling 3 (SOCS3) inhibits leptin activation of AMP-kinase in cultured skeletal muscle of obese humans. J Clin Endocrinol Metab. 2006;91:3592–3597. doi: 10.1210/jc.2006-0638. [DOI] [PubMed] [Google Scholar]
- 11.Dagogo-Jack S, Santiago JV. Pathophysiology of type 2 diabetes and modes of action of therapeutic interventions. Arch Intern Med. 1997;157:1802–1817. [PubMed] [Google Scholar]
- 12.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pimenta W, Korytkowski M, Mitrakou A, Jenssen T, Yki-Jarvinen H, Evron W, Daily G, Gerich J. Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM. JAMA. 1995;273:1855–1861. [PubMed] [Google Scholar]
- 14.Pfeifer MA, Halter JB, Porte D., Jr Insulin secretion in diabetes mellitus. Am J Med. 1981;70:578–588. doi: 10.1016/0002-9343(81)90579-9. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Castaner M, Biarnes J, Camps I, Ripolles J, Gomez N, Soler J. Beta-cell dysfunction in first degree relatives of patients with non-insulin-dependent diabetes mellitus. Diabet Med. 1996;13:953–959. doi: 10.1002/(SICI)1096-9136(199611)13:11<953::AID-DIA257>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 16.Larsson H, Ahren B. Short-term dexamethasone treatment increases plasma leptin independently of changes in insulin sensitivity in healthy women. J Clin Endocrinol Metab. 1996;81:4428–4432. doi: 10.1210/jcem.81.12.8954054. [DOI] [PubMed] [Google Scholar]
- 17.Dagogo-Jack S, Selke G, Melson AK, Newcomer JW. Robust leptin secretory responses to dexamethasone in obese subjects. J Clin Endocrinol Metab. 1997;82:3230–3233. doi: 10.1210/jcem.82.10.4154. [DOI] [PubMed] [Google Scholar]
- 18.Papaspyrou-Rao S, Schneider SH, Petersen RN, Fried SK. Dexamethasone increases leptin expression in humans in vivo. J Clin Endocrinol Metab. 1997;82:1635–1637. doi: 10.1210/jcem.82.5.3928. [DOI] [PubMed] [Google Scholar]
- 19.Saad MF, Khan A, Sharma A, Michael R, Riad-Gabriel MG, Boyadjian R, Jinagouda SD, Steil GM, Kamdar V. Physiological insulinemia acutely modulates plasma leptin. Diabetes. 1998;47:544–549. doi: 10.2337/diabetes.47.4.544. [DOI] [PubMed] [Google Scholar]
- 20.Askari H, Liu J, Dagogo-Jack S. Hormonal regulation of human leptin in vivo: effects of hydrocortisone and insulin. Int J Obes Relat Metab Disord. 2000;24:1254–1259. doi: 10.1038/sj.ijo.0801379. [DOI] [PubMed] [Google Scholar]
- 21.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in human subjects. J Clin Endocrinol Metab. 1996;81:3419–3423. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 22.Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF. Response of leptin to short-term and prolonged overfeeding in humans. J Clin Endocrinol Metab. 1996;81:4162–4165. doi: 10.1210/jcem.81.11.8923877. [DOI] [PubMed] [Google Scholar]
- 23.Perusse L, Collier G, Gagnon J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Nadeau A, Zimmet PZ, Bouchard C. Acute and chronic effects of exercise on leptin levels in humans. J Appl Physiol. 1997;83:5–10. doi: 10.1152/jappl.1997.83.1.5. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Askari H, Dagogo-Jack S. Basal and stimulated plasma leptin in diabetic subjects. Obes Res. 1999;7:537–544. doi: 10.1002/j.1550-8528.1999.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 26.Ma Z, Gingerich RL, Santiago JV, Klein S, Smith CH, Landt M. Radioimmunoassay of leptin in human plasma. Clin Chem. 1996;42:942–946. [PubMed] [Google Scholar]
- 27.Kuzuya H, Blix P, Horwitz D, Steiner D, Rubenstein A. Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes. 1977;26:22–29. doi: 10.2337/diab.26.1.22. [DOI] [PubMed] [Google Scholar]
- 28.Farmer R, Pierce C. Plasma cortisol determination. Radioimmunoassay and competitive binding compared. Clin Chem. 1974;20:411–414. [PubMed] [Google Scholar]
- 29.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 30.Fogteloo AJ, Pijl H, Frölich M, McCamish M, Meinders AE. Effects of recombinant human leptin treatment as an adjunct of moderate energy restriction on body weight, resting energy expenditure and energy intake in obese humans. Diabetes Nutr Metab. 2003;16:109–114. [PubMed] [Google Scholar]
- 31.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 32.Dagogo-Jack S. Neuroendocrine regulation of food intake. In: Opara E, editor. Diabetes and Nutrition. Boca Raton: CRC Press; 2006. pp. 5–25. [Google Scholar]
- 33.Kirschbaum C, Bono EG, Rohleder N, Gessner C, Pirke KM, Salvador A, Hellhammer DH. Effects of fasting and glucose load on free cortisol responses to stress and nicotine. J Clin Endocrinol Metab. 1997;82:1101–1105. doi: 10.1210/jcem.82.4.3882. [DOI] [PubMed] [Google Scholar]
- 34.Laferrère B, Abraham C, Awad M, Jean-Baptiste S, Hart AB, Garcia-Lorda P, Kokkoris P, Russell CD. Inhibiting endogenous cortisol blunts the meal-entrained rise in serum leptin. J Clin Endocrinol Metab. 2006;91:2232–2238. doi: 10.1210/jc.2005-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laferrère B, Fried SK, Hough K, Campbell SA, Thornton J, Pi-Sunyer FX. Synergistic effects of feeding and dexamethasone on serum leptin levels. J Clin Endocrinol Metab. 1998;83:3742–3745. doi: 10.1210/jcem.83.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umamaheswaran I, Askari H, Tykodi G, Dagogo-Jack S. Plasma leptin response to glucocorticoid occurs at physiological doses, and is abolished by fasting. Obes Res. 2003;11:232–237. doi: 10.1038/oby.2003.36. [DOI] [PubMed] [Google Scholar]
- 37.Dagogo-Jack S, Tykodi G, Umamaheswaran I. Inhibition of cortisol biosynthesis decreases circulating leptin levels in obese humans. J Clin Endocrinol Metab. 2005;90:5333–5335. doi: 10.1210/jc.2005-0803. [DOI] [PubMed] [Google Scholar]
- 38.Askari H, Liu J, Dagogo-Jack S. Energy adaptation to glucocorticoid-induced hyperleptinemia in human beings. Metabolism. 2005;54:876–880. doi: 10.1016/j.metabol.2005.01.035. [DOI] [PubMed] [Google Scholar]