Abstract
Chronic graft-versus-host disease (cGVHD) induces significant morbidity and mortality after allogeneic hematopoietic stem cell transplantation. Corticosteroids are standard initial therapy despite limited efficacy and long-term toxicity. Based on our experience using bortezomib as effective acute GVHD prophylaxis, we hypothesized that proteasome-inhibition would complement the immunomodulatory effects of corticosteroids to improve outcomes in cGVHD. We undertook a single-arm phase II trial of bortezomib plus prednisone for initial therapy of cGVHD. Bortezomib was administered at 1.3 mg/m2 IV on days 1, 8, 15, 22 of each 35-day cycle for 3 cycles (15 weeks). Prednisone was dosed at 0.5-1 mg/kg/day with a suggested taper after cycle 1. All 22 enrolled participants were evaluable for toxicity, 20 were evaluable for response. Bortezomib plus prednisone therapy was well-tolerated, with one occurrence of grade 3 sensory peripheral neuropathy possibly related to bortezomib. The overall response rate at week 15 in evaluable participants was 80%, including 2 (10%) complete and 14 (70%) partial responses. The organ-specific complete response rate was 73% for skin, 53% for liver, 75% for gastrointestinal tract, and 33% for joint, muscle, or fascia involvement. The median prednisone dose decreased from 50 mg/day to 20 mg/day at week 15 (p < 0.001). The combination of bortezomib and prednisone for initial treatment of cGVHD is feasible and well-tolerated. We observed a high response rate to combined bortezomib and prednisone therapy, but, in this single-arm study, we could not directly measure the impact of bortezomib. Proteasome inhibition may offer benefit in the treatment of cGVHD and should be further evaluated
Introduction
Chronic graft-versus-host disease (cGVHD) is associated with significant morbidity, decreased quality-of-life, and late mortality after allogeneic hematopoietic stem cell transplantation (HSCT).[1-3] Corticosteroids are standard-of-care for initial cGVHD therapy despite limited efficacy and significant long-term toxicity.[4] Novel cGVHD therapies are a major unmet need.
Bortezomib is a proteasome-inhibitor that exerts immunomodulatory effects, in part, through inhibition of nuclear factor-kappa B (NFκB), a regulator of cytokine signaling, and T- and B-cell development, activation, differentiation, and survival.[5-11] Bortezomib depletes proliferating allo-reactive T-cells, decreases Type 1 helper T-cell cytokine production, and inhibits antigen presenting cell (APC) function, with limited impact on the number and function of regulatory T-cells (Tregs) that are important mediators of GVHD control.[12-17] Bortezomib is effective in preventing acute GVHD in patients undergoing mismatched unrelated donor reduced-intensity allogeneic HSCT.[18, 19] Case reports of bortezomib treatment in patients with relapsed multiple myeloma after allogeneic HSCT describe cGVHD responses.[20-22] Prospective trials evaluating bortezomib for cGVHD treatment have not been reported. We undertook a phase II trial to assess the feasibility and efficacy of adding bortezomib to corticosteroids for the initial treatment of cGVHD.
Methods
This was a single-arm, single-center, prospective phase II trial to evaluate the safety and efficacy of combined bortezomib and prednisone therapy for initial treatment of cGVHD. It was approved by the Institutional Review Board of the Dana-Farber Cancer Institute/Harvard Cancer Center. Informed consent was obtained at enrollment. The trial was registered at ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT00815919).
Cohort Selection
Participants had cGVHD necessitating initiation of systemic therapy. Recipients of matched or mismatched, related or unrelated allogeneic HSCT with myeloablative or reduced-intensity conditioning who were non-pregnant, ≥18 years, and ≥100 days post-transplantation were eligible. Major exclusion criteria included peripheral neuropathy grade ≥1 with pain in the 4 weeks prior, active cancer or infection, ECOG PS > 2, active heart failure, cardiac ischemia, or ventricular arrhythmias, abnormal renal function (serum creatinine >3 mg/dL or hemodialysis), or severe cytopenias (absolute neutrophil count <1500/mm3 or platelet count <50,000/mm3). Patients with abnormal liver function tests (serum total bilirubin >2 mg/dL, serum alanine or aspartate aminotransferase > 2× ULN) were included if hepatic dysfunction was considered a cGVHD manifestation. Recipients of systemic corticosteroid therapy within 4 weeks prior to enrollment were not eligible.
Study Treatment
Enrolled subjects received 1.3 mg/m2 bortezomib (Millenium Pharmaceuticals, Inc) intravenously on days 1, 8, 15 and 22 of each 35 day cycle for 3 cycles (15 weeks). The dosing schedule was chosen for its minimal toxicity and effective proteasome-inhibition in phase I studies, and to limit the number of treatment visits.[23] Concurrently, participants initiated 0.5-1 mg/kg of prednisone daily. The initial study dose was 0.5 mg/kg daily, but the study protocol was later amended to allow a starting dose of up to 1 mg/kg daily per the discretion of the treating physician. The suggested prednisone taper was 10-25% every 1-2 weeks after cycle 1. Organ-specific topical therapies were allowed. No addition or subtraction of other systemic immunosuppressive medications was permitted during the 15-week study. In participants receiving other immunosuppressive medications at enrollment, doses were adjusted based on the therapeutic range of that medication. Participants received standard antimicrobial prophylaxis per institutional guidelines.
Study Assessments
Participants were evaluated at enrollment and on days of bortezomib infusion. Global and organ-specific cGVHD assessments at involved sites were performed at baseline and week 15 per NIH consensus criteria (see Supplementary Appendix).[24] Participants completed a validated neurotoxicity assessment at baseline and week 15.[25]
Laboratory Analysis
Routine laboratory examination and serum cytomegalovirus viral load assessment were performed weekly during study treatment. Quantitative immunoglobulin measurement was performed at baseline and week 15, and immunophenotypic analysis of peripheral blood mononuclear cells were performed at baseline, week 6, week 11, and at the completion of study therapy. Treg cells were defined as CD3+CD4+CD25med-highCD127neg-low, Tcon cells as CD3+CD4+CD25neg-lowCD127med-high, naïve Tregs as CD4+CD25med-highCD127neg-lowCD45RO-, memory Tregs as CD4+CD25med-highCD127neg-lowCD45RO+, dendritic cells (DC) as HLA-DR+ lineage marker negative, myeloid DC as CD11c+, plasmacytoid DC as CD123+, natural killer (NK) cells as CD3-CD56+, T-cells as CD3+, and B-cells as CD19+.
Fifty μL of whole blood (15% EDTA) was incubated with the following fluorophore-conjugated monoclonal antibodies: anti-CD3 V450 (clone UCHT1, BD Biosciences), anti-CD4 APC-H7 (clone RPA-T4, BD Biosciences), anti-CD8 Pacific Orange (clone 3B5, Invitrogen), anti-CD25 PE-Cy7 (clone M-A251, BD Biosciences), anti-CD127 PE-Cy5 (clone eBioRDR5, eBioscience) anti-CD56 PE (clone B159, BD Biosciences), anti-CD19 APC (clone HIB19, BD Biosciences), anti-CD11c+ PE-Cy5 (clone B-ly6, BD Biosciences), and anti-CD123 FITC (clone AC145, Miltenyi). Red-cell lysis with 500 μl of BD PharmLyse lysing solution followed. Cell analysis was performed with the use of the FACSCanto II system (BD Biosciences) and FACSDiva software (BD Biosciences).
Study Endpoints
The primary endpoint was the overall response rate (ORR) at week 15 - the completion of study treatment. Secondary endpoints included: toxicity assessment, the proportion of patients tolerating ≥50% steroid dose reduction at week 15, overall and relapse-free survival (OS, RFS), and the proportion of patients requiring prednisone at 1 year after completion of bortezomib treatment. A complete response (CR) was defined as resolution of all reversible manifestations o cGVHD. Partial response (PR) was defined per NIH response consensus criteria guidelines[26] as improvement of manifestations of cGVHD with clinical evidence of residual cGVHD - a decrease ≥1 point on a 0 to 3-point organ-specific categorical scale or ≥2 points on a physician-reported 0 to 10-point global scale (see the Supplementary Appendix) - without progression in any organs or sites. Progressive cGVHD was defined as an increase of ≥1 point on an organ-specific 0 to 3-point categorical scale, or the institution of a new immunosuppressive agent or any increase in the corticosteroid dose above a participant';s baseline dose prior to the completion of study therapy. Stable disease (SD) was defined as no evidence of cGVHD response without evidence of progressive cGVHD. Mixed response was defined as a response in primary sites of cGVHD involvement but interval progressive cGVHD in other organs or sites. Oral or ocular cGVHD involvement were not evaluated for organ-specific response and were not included in global response assessment, since topical therapies were permitted during the study.
Statistical Analysis
Baseline characteristics were reported descriptively. Pre-to-post treatment change in cGVHD score and immunophenotype data were compared using the Wilcoxon-signed-rank test. The initial prednisone dose between responders and non-responders was compared using the Wilcoxon rank-sum test. OS and RFS were calculated using the Kaplan-Meier method. OS was the start of treatment to death from any cause. Participants alive or lost to follow-up were censored at the time last seen alive. RFS was the start of treatment to malignant disease relapse/progression or death from any cause, whichever occurred first. Participants alive without disease relapse/progression were censored at the time last seen alive and relapse-free. Cumulative incidence of non-relapse mortality (NRM) and relapse with or without death were constructed reflecting time to relapse and time to non-relapse death, respectively, as competing risks. Failure-free survival was the start of treatment to cGVHD progression necessitating additional systemic therapy, malignant disease relapse/progression or death from any cause, whichever occurred first. All calculations were done using SAS 9.3 (SAS Institute Inc, Cary, NC), and R version 2.13.2 (the CRAN project).
It was prospectively determined that the study treatment would be considered promising if ORR (CR+PR) was ≥60% and unacceptable if ≤30%. With this design, the probability of concluding the treatment as promising was 0.94 if the true but unknown response rate was 60%, but 0.09 if the true rate was 30%. This decision rule was calculated using an exact binomial distribution.
Results
Patients
Between March 2009 and November 2011, 22 participants with cGVHD were enrolled. Their baseline demographic, transplantation, and GVHD characteristics are reported in Table 1. Nineteen participants had de novo cGVHD, while 3 had prior grade II-IV acute GVHD. Participants had a median of 3.5 (range, 1-7) sites of baseline cGVHD involvement. The degree of baseline cGVHD involvement in each participant by organ is depicted in Figure 1. Three participants had only liver involvement, and GVHD was confirmed by liver biopsy in each case. Seven participants with diagnsotic features of cGVHD in an organ other than skin, had erythematous or maculopapular skin GVHD lesions without other diagnostic or distinctive findings of skin cGVHD. Eight participants were on systemic immunosuppressive agents at onset of cGVHD and continued on these medications. Two participants developed cGVHD despite having previously received prophylactic rituximab (Genentech, South San Francisco, CA) as part of a clinical trial evaluating its safety and efficacy in the prevention of cGVHD (details in Supplementary Tables A and B). The median duration of follow-up in survivors was 25 months (range, 19.3-48.5).
Table 1. Baseline Characteristics of the Participants.
| Baseline Characteristics | N | % | |
|---|---|---|---|
| Participants Enrolled | 22 | (100) | |
| Participants Evaluable for Response | 20 | (90.9) | |
| Age, median (range) | 51 | 25-69 | |
| Male | 10 | (45.5) | |
| Primary Diagnosis | AML | 9 | (40.9) |
| CLL, SLL, PLL | 1 | (4.5) | |
| Hodgkin's Lymphoma | 1 | (4.5) | |
| ALL | 3 | (13.6) | |
| MDS | 1 | (4.5) | |
| MPD | 2 | (9) | |
| Mixed MDS/MPD | 1 | (4.5) | |
| NHL | 4 | (18.2) | |
| Stem Cell Source | PBSC | 22 | (100) |
| Donor Source | 8/8 MUD | 13 | (59.1) |
| 8/8 MRD | 5 | (22.7) | |
| 7/8 MMUD | 4 | (18.2) | |
| Patient-Donor Gender | F-F | 5 | (22.7) |
| F-M | 7 | (31.8) | |
| M-F | 1 | (4.5) | |
| M-M | 9 | (40.9) | |
| Prior Acute GVHD | Grade II-IV | 3 | (13.6) |
| Grade 0-I | 19 | (86.4) | |
| Platelets × 109/L, median (range) | 197 | 90-378 | |
| Days after HSCT to cGVHD onset, median (range) | 215 | 133-666 | |
| Days after HSCT to initiation of cGVHD therapy, median (range) | 257 | 186-2070 | |
| Concurrent Agents | Tacrolimus | 6 | (27.3) |
| Sirolimus | 3 | (13.6) | |
| Mycophenolate mofetil | 1 | (4.5) |
Figure 1. Heat Map Baseline Depicting Baseline Chronic GVHD Involvement by Organ Site and Week 15 Response at All Evaluable Sites.
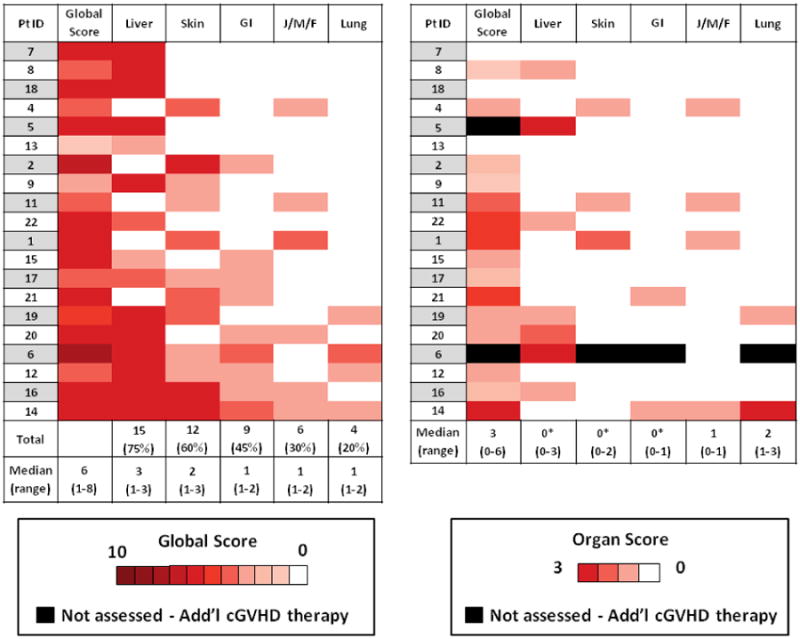
Global and organ site chronic GVHD responses at week 15 (right panel) in each evaluable participants at each site of cGVHD involvement compared to baseline involvement (left panel) scored according to NIH consensus criteria. 2 participants had early progression of liver cGVHD and came off study prior to week 15, liver organ scores are included. Median provider-reported global cGVHD scores and NIH organ-site cGVHD scores at baseline and week 15 are included. Asterisk denotes statistically significant difference between baseline and week 15 (all p ≤ 0.02)
Feasibility and Safety
Twenty-two participants were evaluable for toxicity. Eleven (50%) received an initial prednisone dose of ∼0.5mg/kg/day, 3 (14%) received an initial prednisone dose of ∼0.75mg/kg/day, and 8 participants (36%) received an initial prednisone dose of ∼1mg/kg/day. One participant was withdrawn from the study with primary coccidiomycosis from an environmental exposure during cycle 1 of treatment, and another died of relapsed acute myelogenous leukemia during cycle 2 -both deemed unrelated to bortezomib. One participant developed grade 3 CTCAE v3.0 sensory neuropathy possibly related to bortezomib. There were no other grade ≥3 AEs probably or possibly related to bortezomib. Eighteen participants (82%) completed all 3 cycles of combination therapy.
Clinical Response
Twenty participants were evaluable for response, and 16 (80%) exhibited an objective response after 15 weeks per NIH consensus response criteria. Two (10%) had a CR and 14 participants (70%) had a PR to study treatment. One participant (5%) had SD. One participant (5%) had a mixed response, with complete resolution of cGVHD in sites of severe involvement (liver and skin), but interval progression of bronchiolitis obliterans. Two participants (10%), both with liver involvement, had progressive cGVHD with rising transaminases on days 11 and 15 after treatment initiation, respectively, for which an increased prednisone dose above the initial 0.5 mg/kg daily was required. There was no association between daily prednisone dose and response, with the median initial prednisone dose in responders (CR/PR) at 0.73 mg/kg/day compared to 0.54 mg/kg/day in non-responders (mixed response/SD/PD) (p = 0.28). The single participant (100%) with mild cGVHD, 5 of 6 participants (83%) with moderate cGVHD, and 10 of 13 participants (77%) with severe cGVHD per NIH global score criteria had objective evidence of response. Both participants achieving CR had severe cGVHD at baseline without fixed, “irreversible” manifestations of cGVHD (see Supplementary Table A and B for details).
Responses by evaluable organ site included, skin (n = 11): 8 (73%) CR, 1 (9%) PR, and 2 (18%) SD; liver (n = 15): 8 (53%) CR, 5 (33%) PR, and 2 (13%) participants with progressive liver cGVHD; gastrointestinal (GI) tract (n = 8): 6 (75%) CR, 1 (12.5%) PR, and 1 (12.5%) SD; joint, muscle, or fascia involvement (n = 6): 2 (33%) CR, 1 (17%) PR, and 3 (50%) SD; lung (n = 3): 1 (33%) CR, 1 (33%) SD, and 1 (33%) participant with progressive bronchiolitis obliterans (BO). Clinically, the participant's course was consistent with steady progression of BO/cGVHD despite study treatment, but the potential of pulmonary toxicity associated with bortezomib cannot be excluded. The 3 participants (100%) with severe skin cGVHD had complete resolution of skin cGVHD. All participants (n = 7) with erythema, pruritis, and maculopapular rash (range, 7-90% body surface area involved) as predominant skin GVHD manifestations had complete resolution of their skin GVHD. Four evaluable participants had lichen-planus or sclerotic skin cGVHD, with 2 exhibiting stability of these skin cGVHD manifestations, one participant with a PR, and one participant had resolution of moderate severity sclerotic skin cGVHD. Five of 11 participants (45%) with severe liver cGVHD had complete resolution of liver cGVHD. Responses included improvement in all liver function tests, including transaminases, alkaline phosphatase, and bilirubin. Participants with GI cGVHD primarily had upper GI manifestations, and responses included resolution or improvement of dysphagia, esophagitis, and nausea. Participants with musculoskeletal involvement all had fasciitis without joint contractures, and responses included softening of fasciitis with improvement in range of motion. Responses in each participant by specific organ-site, including organ-site severity, and the median cGVHD global and NIH cGVHD organ-site scores in the cohort at baseline and week 15 are detailed in Figure 1.
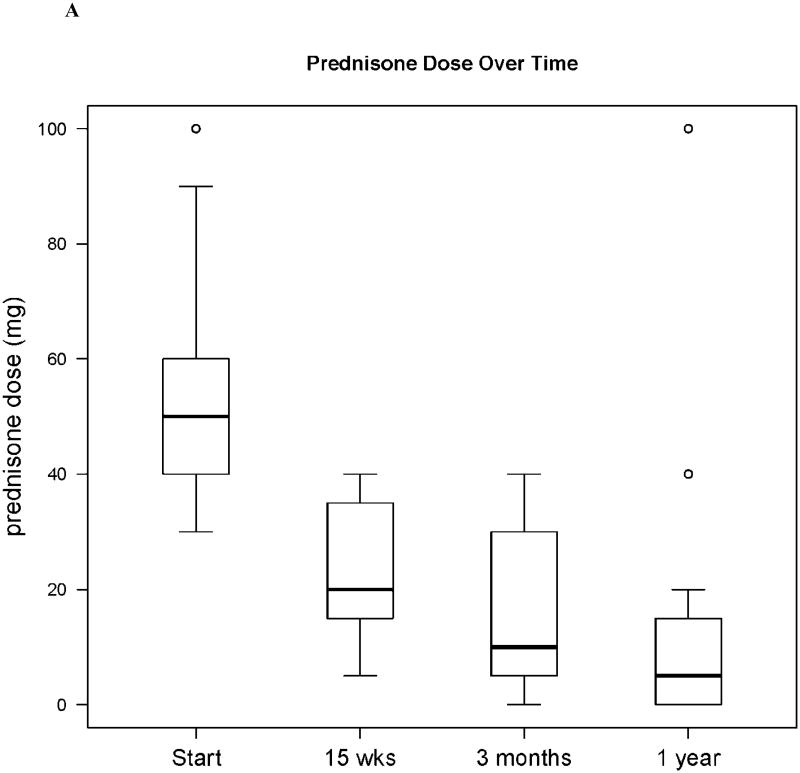
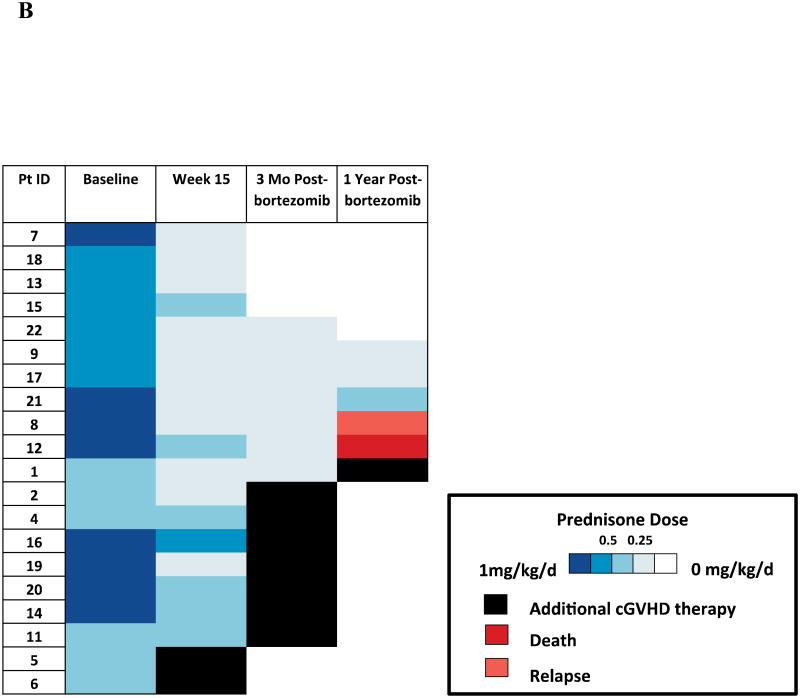
Fourteen participants (70%) tolerated a ≥ 50% steroid dose reduction by week 15. The median prednisone dose of the cohort decreased from 50 mg (range, 30-100) at baseline to 20 mg (range, 5-40) at week 15 (p < 0.001). The end of study failure-free survival at 15 weeks in the entire cohort (n = 22) was 82%. One year after completing study treatment, 5 (25%) evaluable participants were off prednisone completely, 2 (10%) participants were receiving less than 0.25 mg/kg daily of prednisone, 10 (50%) participants required additional cGVHD therapy, 1 participant died of relapsed acute lymphoblastic leukemia, and 1 participant died of autopsy-proven pulmonary veno-occlusive disease. [Figure 2].
Figure 2. Prednisone Dose Over Time.
Prednisone dose at baseline, study week 15 (the completion of bortezomib therapy), 3 months after the completion of bortezomib therapy, and 1 year after the completion of bortezomib therapy. Panel (A) shows median prednisone dose in the cohort over time, Note: N=22, N=22, N=18, N=17, respectively; Panel (B) shows the prednisone dose in each participant over time.
The 2-year OS and RFS were both 73% in the cohort (n = 22) and the cumulative incidence of NRM and relapse were 14% each for the entire cohort [Figure 3]. For responders (n = 16), the 2-year OS and RFS were 88% and the cumulative incidence of NRM and relapse were 6.3% each.
Figure 3. Overall and Relapse-Free Survival.
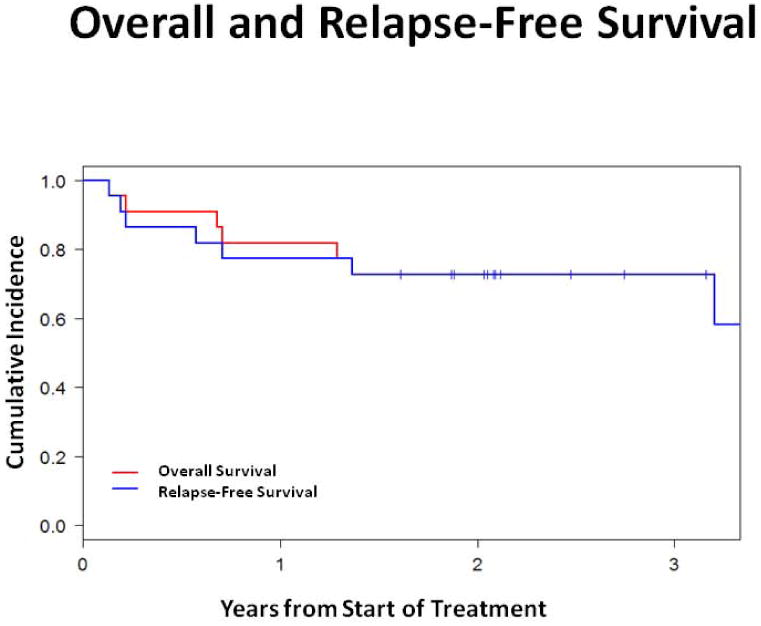
Kaplan-Meier analysis of overall survival and relapse-free survival in the cohort over time.
Laboratory and Correlative Analyses
Fifteen weeks of bortezomib and prednisone therapy did not induce clinically significant leukopenia, anemia, or thrombocytopenia. The median peripheral blood total lymphocyte, total T-cell, and CD8+ T-cell counts in the cohort declined after 15 weeks of study therapy (all p = 0.04). Median peripheral blood B-cell counts were unaffected by bortezomib and prednisone therapy, and NK-cell counts initially declined but were not at 15 weeks. [Figure 4A]. The median total Treg count and Treg:Tcon ratio at baseline were 39.5 cells/mm3 and 0.15, respectively, and declined after 15 weeks of bortezomib and prednisone to 20.8 cells/mm3 (p < 0.0001) and 0.08 (p = 0.0002), respectively [Figure 4B]. The median memory Treg count was decreased at 15 weeks compared to baseline (p < 0.0001). However, the median naïve Treg count was not significantly affected at 15 weeks (p = 0.12) [Figure 4C]. Median total DC, myeloid DC, and plasmacytoid DC counts were decreased at 15 weeks compared to baseline (p = 0.039, p = 0.011, p = 0.005, respectively) [Figure 4D]. Total lymphocytes, total and CD8+ T-cells, total and memory Tregs, and total and subset DC counts recovered to baseline by 3 months after the last bortezomib dose.
Figure 4. Immune Correlates.
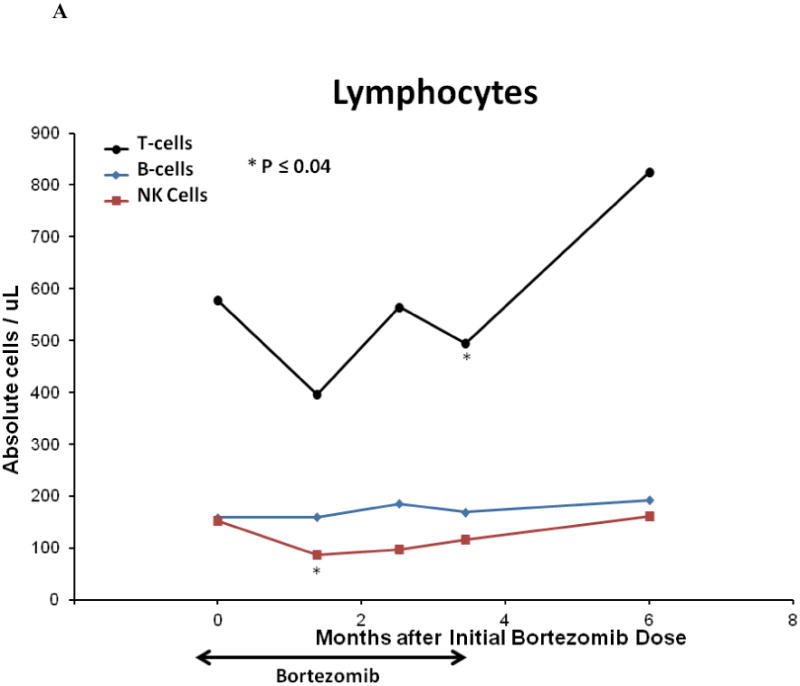
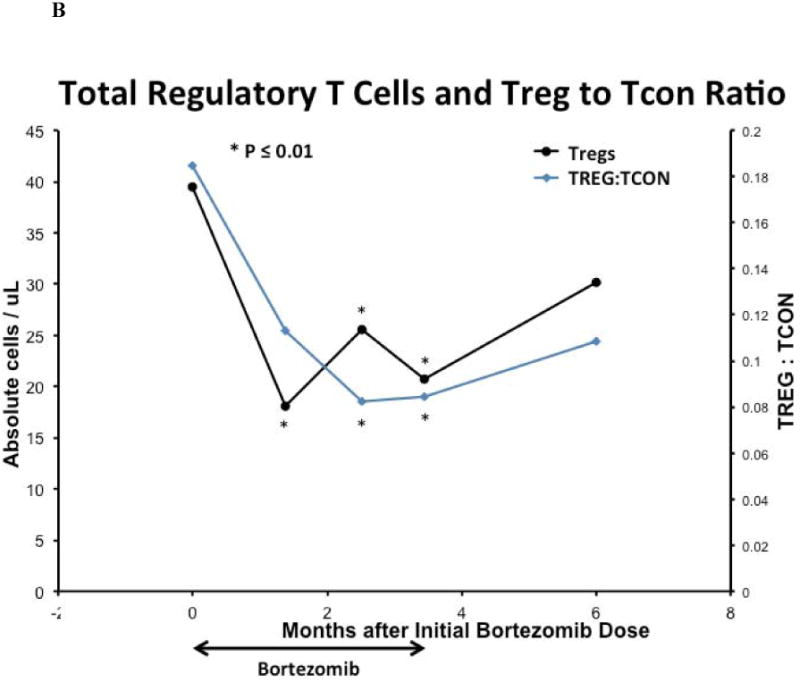
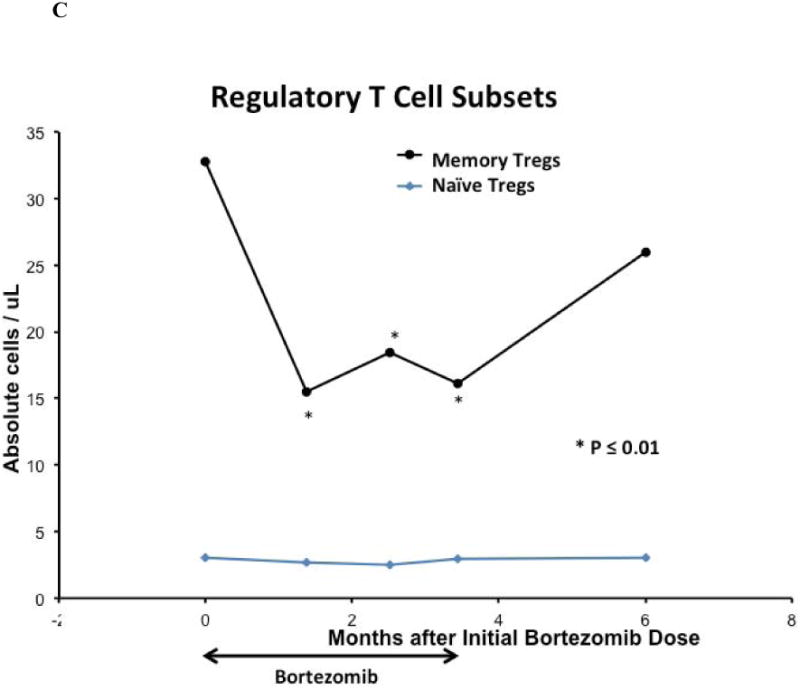
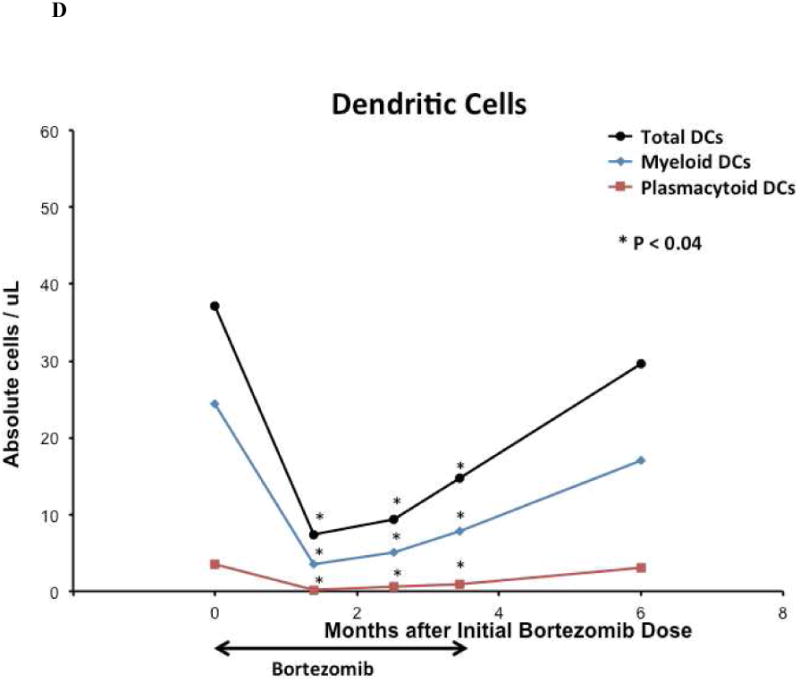
(A) Median peripheral blood T, B, NK lymphocyte lineage counts, (B) median peripheral blood total regulatory T-cell count and Treg:Tcon ratio, (C) median peripheral blood regulatory T-cell subset counts, (D) and median peripheral blood total and dendritic cell subset cell counts on bortezomib and prednisone therapy. For all analyses, n = 21 at baseline; n = 18 at week 6, n = 19 at week 11, n = 19 at week 15, and n = 10 at 6 months after initial bortezomib dose. Cell counts were compared between time points using the Wilcoxon-signed-rank test. Asterisk denotes statistically significant (p ≤ 0.05) change at a time point compared to baseline.
Discussion
Chronic GVHD is an important source of morbidity and mortality after allogeneic HSCT with limited treatment options. Corticosteroids are the mainstay of upfront therapy, and efforts to improve cGVHD outcomes by adding other agents such as thalidomide or mycophenolate mofetil (MMF) have been unsuccessful.[27-29] Bortezomib is a proteasome inhibitor with immunomodulatory effects that we hypothesized may be useful in treating cGVHD.
Because of the protocol-specified exclusion of patients receiving recent systemic glucocorticoid therapy, the study cohort was primarily composed of participants with de novo cGVHD, with some participants exhibiting quiescent cGVHD. There were no patients with progressive cGVHD. This proportion of de novo cGVHD is in line with practice patterns at our center. In our study, the addition of 15 weeks of bortezomib to prednisone for the initial treatment of cGVHD was feasible and well-tolerated. Toxicity was minimal, with only one occurrence of grade 3 sensory peripheral neuropathy possibly related to bortezomib. There were no grade ≥ 3 infectious or other adverse events related to study treatment (see Supplementary Table B for list of grade ≥3 AEs).
The response rate was high at 15 weeks - the completion of study treatment. In particular, liver cGVHD and erythematous or maculopapular skin GVHD appeared sensitive to combined bortezomib and prednisone therapy, despite severe baseline organ involvement. As a study of initial therapy for incident cGVHD, we had few participants with lichen planus-like or sclerotic skin cGVHD. The median corticosteroid dose at 15 weeks in the cohort also decreased substantially, suggesting that bortezomib may offer steroid-sparing efficacy in cGVHD therapy.
The required weekly bortezomib infusions were a barrier to accrual and selected for a study population with severe cGVHD. For instance, 50% of our participants had severe liver cGVHD and 64% had severe global cGVHD per the NIH scale, as compared to 1% severe liver cGVHD and 4% severe global cGVHD in the prednisone-only control arm of a randomized upfront cGVHD treatment study.[28] Therefore, while response rates in our cohort appear comparable with published corticosteroid-alone control cohorts in other trials,[28, 30] we could not identify an appropriate clinical comparator cohort in the literature.
In our study, immunophenotypic analyses indicated that the bortezomib and prednisone combination reduced in vivo peripheral blood DC counts throughout the study period. While prior reports suggested Treg survival was unaffected by bortezomib, the median total Treg count and the Treg to Tcon ratio declined in our study during the 15 weeks of bortezomib and prednisone therapy.[12, 17] Naïve Tregs remained stable. This may be of interest, as there is evidence suggesting that naïve Tregs may help control GVHD and that severe cGVHD is associated with a naive Treg deficit.[31, 32] B-cells, also implicated in the pathogenesis of cGVHD, were unaffected.[33-40] As there was only 1 male participant with a female donor, we were unable to evaluate treatment impact on allo-reactive H-Y antibodies. While potentially interesting as biomarkers of response, these findings may reflect the impaired immune reconstitution and Treg deficit observed in severe cGVHD.[31, 41, 42] We further invoke caution, as it is not possible to isolate the impact of bortezomib in this study of combination therapy, and our findings may represent the effect of corticosteroids.
In this study, bortezomib therapy was discontinued after 15 weeks. Hence, we reported failure-free survival at 15 weeks. Classifying cGVHD progression after the discontinuation of bortezomib therapy as a treatment failure would imply that bortezomib has sustained, long-term immunomodulatory effects in cGVHD, which has not been demonstrated. Within 3 months of bortezomib discontinuation, corticosteroid taper continued in the majority of participants, but many developed progressive cGVHD that required additional therapy. This may be due to the severity of baseline cGVHD involvement in the cohort. It also suggests that any benefit conferred by the addition of bortezomib to corticosteroids may require continued bortezomib exposure. Indeed, other immunomodulatory therapies for cGVHD, such as low-dose interleukin-2, have resulted in durable responses with continued administration.[43] However, long-term bortezomib therapy early after allogeneic HSCT has been associated with neurologic, gastrointestinal, and hematologic toxicities.[22, 44, 45] Consideration of maintenance bortezomib for cGVHD therapy would require weighing the adverse effects against any potential benefits in a clinical trial. This issue and accrual challenges may be circumvented in future studies by using oral proteasome inhibitors. These newer agents are associated with less neurotoxicity, which may evade another obstacle to long-term proteasome inhibitor use.
Although the cGVHD responses in this study are encouraging, the lack of a comparator arm precludes a definitive determination of the impact of adding bortezomib to prednisone for initial therapy of cGVHD. Previous studies of initial cGVHD therapy have demonstrated high response rates in corticosteroid-alone control arms, and it remains possible we may have observed similar responses with single-agent corticosteroid therapy if our trial had a control arm. A randomized study would be necessary to determine whether proteasome inhibition confers a benefit in this setting. We caution against the use of bortezomib or other proteasome inhibitors for the treatment of cGVHD outside of the setting of a clinical trial. However, our phase II results indicate that study of proteasome inhibitors for treatment of cGVHD is of interest, and further exploration of proteasome inhibition (e.g. different agents, route of administration, or dosing schedule) alone or in combination with other cGVHD treatments may be worthwhile.
Supplementary Material
Supplementary Appendix
Supplemental Table A: Additional Baseline Characteristics in Each Patient
Supplemental Table B: Response and Adverse Event Information in Each Patient
Acknowledgments
We thank the study's registered nurses, Susan Stephenson and Mildred Pasek; the stem-cell transplant program nurse practitioners, Melissa Cochran, Amy Joyce, Bonnie Dirr, and Katherine Stephans; and all the study participants. This study was supported in part by Millennium Pharmaceuticals, Inc and by the National Institutes of Health/National Cancer Institute (P01 CA142106, R01 CA183559). J.K. is a Clinical Research Scholar of the Leukemia and Lymphoma Society.
Footnotes
A preliminary version of this work was presented in abstract form at the 55th American Society of Hematology Annual Meeting and Exposition in New Orleans, Louisiana, in December 2013
Contribution: J.K., H.T.K., E.P.A, P.A., C.S.C, V.T.H, J.R., B.R.B, J.H.A., and R.J.S contributed to the conception and design of the study; A.F.H., H.T.K., B.B., K.T.J., E.P.A, P.A., C.S.C, V.T.H, S.N., J.R., J.H.A., R.J.S, and J.K. collected and assembled the data; A.F.H., H.T.K., and J.K. analyzed the data; A.F.H., H.T.K., E.P.A, P.A., C.S.C, V.T.H, S.N., J.R., B.R.B, J.H.A., R.J.S, and J.K. contributed to the interpretation of data; A.F.H., H.T.K., and J.K. prepared the manuscript; all authors assisted in the critical review of the manuscript and approved the final version of the manuscript for submission.
Conflict of Interest Disclosure: This manuscript describes the off-label use of bortezomib. This study was sponsored, in part, by Millenium Pharmaceuticals, Inc. J.K. has received honoraria from OptumHealth, has served as an advisor/consultant for Takeda Pharmaceuticals, Spectrum Pharmaceuticals, and Eleven Biotherapeutics and has received research funding from Millennium Pharmaceuticals, Otsuka Pharmaceuticals, and Prometheus Laboratories. C.S.C has received consulting honoraria and research funding from Millenium Pharmaceuticals, Inc. J.H.A. and R.J.S have served as consultants for Millenium Pharmaceuticals, Inc.
Supplemental Information: Provider Case Report Form
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perez-Simon JA, Encinas C, Silva F, Arcos MJ, Diez-Campelo M, Sanchez-Guijo FM, et al. Prognostic factors of chronic graft-versus-host disease following allogeneic peripheral blood stem cell transplantation: the national institutes health scale plus the type of onset can predict survival rates and the duration of immunosuppressive therapy. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14:1163–71. doi: 10.1016/j.bbmt.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Pidala J, Kurland B, Chai X, Majhail N, Weisdorf DJ, Pavletic S, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117:4651–7. doi: 10.1182/blood-2010-11-319509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–92. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100:48–51. doi: 10.1182/blood.v100.1.48. [DOI] [PubMed] [Google Scholar]
- 5.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer research. 2001;61:3071–6. [PubMed] [Google Scholar]
- 6.Palombella VJ, Conner EM, Fuseler JW, Destree A, Davis JM, Laroux FS, et al. Role of the proteasome and NF-kappaB in streptococcal cell wall-induced polyarthritis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15671–6. doi: 10.1073/pnas.95.26.15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Luo H, Chen H, Duguid W, Wu J. Role of proteasomes in T cell activation and proliferation. Journal of immunology. 1998;160:788–801. [PubMed] [Google Scholar]
- 8.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annual review of immunology. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 9.Finn PW, Stone JR, Boothby MR, Perkins DL. Inhibition of NF-kappaB-dependent T cell activation abrogates acute allograft rejection. Journal of immunology. 2001;167:5994–6001. doi: 10.4049/jimmunol.167.10.5994. [DOI] [PubMed] [Google Scholar]
- 10.Sunwoo JB, Chen Z, Dong G, Yeh N, Crowl Bancroft C, Sausville E, et al. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:1419–28. [PubMed] [Google Scholar]
- 11.Kaileh M, Sen R. NF-kappaB function in B lymphocytes. Immunological reviews. 2012;246:254–71. doi: 10.1111/j.1600-065X.2012.01106.x. [DOI] [PubMed] [Google Scholar]
- 12.Blanco B, Perez-Simon JA, Sanchez-Abarca LI, Caballero-Velazquez T, Gutierrez-Cossio S, Hernandez-Campo P, et al. Treatment with bortezomib of human CD4+ T cells preserves natural regulatory T cells and allows the emergence of a distinct suppressor T-cell population. Haematologica. 2009;94:975–83. doi: 10.3324/haematol.2008.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco B, Perez-Simon JA, Sanchez-Abarca LI, Carvajal-Vergara X, Mateos J, Vidriales B, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006;107:3575–83. doi: 10.1182/blood-2005-05-2118. [DOI] [PubMed] [Google Scholar]
- 14.Nencioni A, Schwarzenberg K, Brauer KM, Schmidt SM, Ballestrero A, Grunebach F, et al. Proteasome inhibitor bortezomib modulates TLR4-induced dendritic cell activation. Blood. 2006;108:551–8. doi: 10.1182/blood-2005-08-3494. [DOI] [PubMed] [Google Scholar]
- 15.Sun K, Welniak LA, Panoskaltsis-Mortari A, O'Shaughnessy MJ, Liu H, Barao I, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8120–5. doi: 10.1073/pnas.0401563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanco B, Sanchez-Abarca LI, Caballero-Velazquez T, Santamaria C, Inoges S, Perez-Simon JA. Depletion of alloreactive T-cells in vitro using the proteasome inhibitor bortezomib preserves the immune response against pathogens. Leukemia research. 2011;35:1412–5. doi: 10.1016/j.leukres.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Kim JS, Lee JI, Shin JY, Kim SY, Shin JS, Lim JH, et al. Bortezomib can suppress activation of rapamycin-resistant memory T cells without affecting regulatory T-cell viability in non-human primates. Transplantation. 2009;88:1349–59. doi: 10.1097/TP.0b013e3181bd7b3a. [DOI] [PubMed] [Google Scholar]
- 18.Koreth J, Stevenson KE, Kim HT, Garcia M, Ho VT, Armand P, et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood. 2009;114:3956–9. doi: 10.1182/blood-2009-07-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koreth J, Stevenson KE, Kim HT, McDonough SM, Bindra B, Armand P, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3202–8. doi: 10.1200/JCO.2012.42.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mateos-Mazon J, Perez-Simon JA, Lopez O, Hernandez E, Etxebarria J, San Miguel JF. Use of bortezomib in the management of chronic graft-versus-host disease among multiple myeloma patients relapsing after allogeneic transplantation. Haematologica. 2007;92:1295–6. doi: 10.3324/haematol.10820. [DOI] [PubMed] [Google Scholar]
- 21.Todisco E, Sarina B, Castagna L, Mazza R, Rahal D, Nozza A, et al. Inhibition of chronic graft-vs-host disease with retention of anti-myeloma effects by the proteasome inhibitor bortezomib. Leukemia & lymphoma. 2007;48:1015–8. doi: 10.1080/10428190701253843. [DOI] [PubMed] [Google Scholar]
- 22.El-Cheikh J, Michallet M, Nagler A, de Lavallade H, Nicolini FE, Shimoni A, et al. High response rate and improved graft-versus-host disease following bortezomib as salvage therapy after reduced intensity conditioning allogeneic stem cell transplantation for multiple myeloma. Haematologica. 2008;93:455–8. doi: 10.3324/haematol.12184. [DOI] [PubMed] [Google Scholar]
- 23.Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X, et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:2108–21. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 24.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2005;11:945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Calhoun EA, Welshman EE, Chang CH, Lurain JR, Fishman DA, Hunt TL, et al. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2003;13:741–8. doi: 10.1111/j.1525-1438.2003.13603.x. [DOI] [PubMed] [Google Scholar]
- 26.Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006;12:252–66. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood. 2000;96:3995–6. [PubMed] [Google Scholar]
- 28.Martin PJ, Storer BE, Rowley SD, Flowers ME, Lee SJ, Carpenter PA, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009;113:5074–82. doi: 10.1182/blood-2009-02-202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arora M, Wagner JE, Davies SM, Blazar BR, Defor T, Enright H, et al. Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2001;7:265–73. doi: 10.1053/bbmt.2001.v7.pm11400948. [DOI] [PubMed] [Google Scholar]
- 30.Martin PJ, Storer BE, Carpenter PA, Couriel DR, Flowers ME, Gupta V, et al. Comparison of short-term response and long-term outcomes after initial systemic treatment of chronic graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:124–32. doi: 10.1016/j.bbmt.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Science translational medicine. 2013;5:179ra43. doi: 10.1126/scitranslmed.3005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imanguli MM, Cowen EW, Rose J, Dhamala S, Swaim W, Lafond S, et al. Comparative analysis of FoxP3 regulatory T Cells in the target tissues and blood in chronic graft versus host disease. Leukemia. 2014 doi: 10.1038/leu.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–74. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–8. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arai S, Sahaf B, Narasimhan B, Chen GL, Jones CD, Lowsky R, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119:6145–54. doi: 10.1182/blood-2011-12-395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cutler C, Kim HT, Bindra B, Sarantopoulos S, Ho VT, Chen YB, et al. Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122:1510–7. doi: 10.1182/blood-2013-04-495895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–62. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miklos DB, Kim HT, Zorn E, Hochberg EP, Guo L, Mattes-Ritz A, et al. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103:353–9. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarantopoulos S, Stevenson KE, Kim HT, Bhuiya NS, Cutler CS, Soiffer RJ, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:6107–14. doi: 10.1158/1078-0432.CCR-07-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zorn E, Miklos DB, Floyd BH, Mattes-Ritz A, Guo L, Soiffer RJ, et al. Minor Histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. The Journal of experimental medicine. 2004;199:1133–42. doi: 10.1084/jem.20031560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106:2903–11. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuoka K, Kim HT, McDonough S, Bascug G, Warshauer B, Koreth J, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. The Journal of clinical investigation. 2010;120:1479–93. doi: 10.1172/JCI41072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, 3rd, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. The New England journal of medicine. 2011;365:2055–66. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroger N, Zabelina T, Ayuk F, Atanackovic D, Schieder H, Renges H, et al. Bortezomib after dose-reduced allogeneic stem cell transplantation for multiple myeloma to enhance or maintain remission status. Experimental hematology. 2006;34:770–5. doi: 10.1016/j.exphem.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 45.van de Donk NW, Kroger N, Hegenbart U, Corradini P, San Miguel JF, Goldschmidt H, et al. Remarkable activity of novel agents bortezomib and thalidomide in patients not responding to donor lymphocyte infusions following nonmyeloablative allogeneic stem cell transplantation in multiple myeloma. Blood. 2006;107:3415–6. doi: 10.1182/blood-2005-11-4449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix
Supplemental Table A: Additional Baseline Characteristics in Each Patient
Supplemental Table B: Response and Adverse Event Information in Each Patient