Abstract
Introduction
Excessive pain during medical procedures is a widespread problem but is especially problematic during daily wound care of patients with severe burn injuries.
Methods
Burn patients report 35–50% reductions in procedural pain while in a distracting immersive virtual reality, and fMRI brain scans show associated reductions in pain-related brain activity during VR. VR distraction appears to be most effective for patients with the highest pain intensity levels. VR is thought to reduce pain by directing patients’ attention into the virtual world, leaving less attention available to process incoming neural signals from pain receptors.
Conclusions
We review evidence from clinical and laboratory research studies exploring Virtual Reality analgesia, concentrating primarily on the work ongoing within our group. We briefly describe how VR pain distraction systems have been tailored to the unique needs of burn patients to date, and speculate about how VR systems could be tailored to the needs of other patient populations in the future.
Keywords: Virtual reality, Pain distraction, Analgesia
Introduction
The Problem: Uncontrolled Pain
The treatment of severely burn-injured patients is one of the most painful processes in medicine. Few injuries involve more painful and numerous procedures than severe burns. In the USA, each year, an estimated 700,000 people visit the emergency room for treatment of burns. Of these, 45,000 have burns significant enough to require inpatient hospitalization [1]. In order to prevent infection and promote healing, patients with severe burns typically must have their bandages removed and have their wounds cleaned daily for weeks or even months. During cleaning/debridement, foreign materials and dead tissue are removed from the open wound, antiseptic ointments are applied, and the wound is re-dressed/re-bandaged. These wound care sessions allow caregivers to look at the wound and monitor healing progress. Surgeons may need to surgically remove damaged skin and transplant fresh skin from another part of the body, e.g., the patient’s own unburned thigh to their burned hands, or in some cases, with donated skin from a cadaver. Once the graft takes hold on the burn site, staples or other adhesive devices that have been temporarily holding the transplanted skin in place must be removed. Wound care sessions involving staple removal from healing skin grafts are often especially painful. Furthermore, the site where the healthy skin was “harvested” from a non-joint area is now an additional painful raw wound that must also be kept clean. While most patients report only mild pain when lying still (termed “resting pain”), most patients with burn injuries report severe pain during burn wound care [2–4].
Under-medication contributes to severe pain [5]. Repeated administration of opioids often results in gradually reduced analgesic effects, a phenomenon known as tolerance. With frequent medications over days, weeks or months, escalating doses of opioid analgesics are needed to achieve the same analgesic effect. Over time, daily use of opioids is frequently accompanied by physical dependence, the need for continued drug use to prevent physical and emotional withdrawal symptoms [6]. Even maximal opioid doses often fail to control all pain [7, 8]. Opioid side effects can include nausea, excessive sedation, cognitive dysfunction, constipation, and other concerns, and become increasingly problematic with higher dose levels [7], limiting what dose is considered appropriate.
In addition to numerous wound-cleaning procedures, burn patients must also endure weeks or months of daily physical therapy exercises both as inpatients and after discharge as outpatients. Hand burns are very common. After healing, patients who sustain burns in vulnerable joints such as fingers may find it challenging to move their fingers enough to grasp objects or type on a computer. To counteract the tendency of healing burned skin to harden, contract, and lose its elasticity, frequent physical therapy is conducted to help retain full use of their injured limbs. This is especially important for burn wounds that cross joints such as fingers, elbows, shoulders, and knees. Physical therapy is essential for maximizing functionality and can also help minimize the number of skin grafts needed to surgically release skin that has contracted during healing. But pain can interfere with compliance [9]. Adjunctive non-pharmacologic techniques, including use of hypnosis [10–13] and related cognitive behavioral approaches may be used in addition to traditional pain medications to help reduce severe procedural pain. There are numerous studies reporting evidence that conventional distraction such as music can help reduce pain [14, 15]. However, according to a recent systematic Cochrane review meta-analysis, listening to music only reduced pain intensity levels by one half of one point on a ten-point rating scale and only slightly reduced opioid analgesic use. According to Cepeda et al. [16], “the magnitude of these benefits is small, and, therefore, its clinical importance unclear” (p. 1). A much stronger, more robust adjunctive non-pharmacologic analgesic is needed.
Immersive Virtual Reality Pain Distraction
Interdisciplinary research teams are exploring ways to use emerging computer technologies to help address this important medical problem of how to better control acute procedural pain. Immersive virtual reality (VR) visually isolates patients from the “real world.” The helmet typically used to deliver VR, blocks the patients’ view of the hospital room and substitutes computer-generated images via small computer screens and lenses positioned near the patient’s eyes. Noise canceling earphones block/replace hospital noises with sound effects and relaxing background music from the virtual world. The goal of immersive VR is to give patients the illusion they are inside the 3D computer-generated world, as if the virtual world is a place they are visiting. In theory, while health care professionals are conducting invasive procedures, instead of cognitively remaining in the painful real world, the patient is allowed to perceptually escape into a pleasant alternative 3D virtual world.
The logic for how VR works is as follows. Pain requires attention [17, 18]. Humans have limited attentional capacity [19]. Interacting with virtual reality uses a substantial amount of the patient’s limited controlled attentional resources. For example, VR has been found to reduce performance on a divided attention task [20]. Consequently, when in VR, the patient has less attention available to process incoming signals from pain receptors. As a result, patients report less pain while in VR, they spend less time thinking about their pain during VR, and often report having more fun during wound care while in VR compared with wound care with no VR [2, 21, 22].
The first immersive VR software designed for pain control was named SnowWorld (www.vrpain.com)1. In SnowWorld, patients interact with snowmen, igloos, penguins, woolly mammoths, and flying fish by throwing snowballs. Patients aim with a computer mouse (or sometimes via head tracking) and left click the mouse to throw snowballs. The virtual objects respond in various ways when hit by snowballs (e.g., snowmen shatter in 3D with sound effects and mammoths trumpet angrily, with Paul Simon songs from the album Graceland playing in the background).
In the series of preliminary studies with patients undergoing painful medical procedures, patients report feeling 35–50% less pain while in VR with immersive VR (standard medications+VR) compared with treatment as usual (standard medications alone+no VR). VR analgesia has been demonstrated in burn patients both during wound care [2, 22–24] and during physical therapy [25–29].
Is VR Analgesia Effective for Patients Experiencing Severely Intense Pain?
Previous pain researchers have theorized that distraction will be less effective at reducing severe pain intensity levels compared with reducing mild to moderate pain intensities. For example, McCaul and Malott [30] proposed that “stimulus intensity is an important determinant of whether and when a distraction will occur. In other words, as a painful stimulus reaches some intense level, it will begin to attract attention and impede the effectiveness of the distraction” (p. 518). Other researchers have argued that distraction will be less effective if the pain is perceived as very threatening (affective factors), for instance in high pain catastrophizers who have trouble disengaging their attention from pain [31]. According to these researchers, distraction should become less effective during severe and higher pain intensity. In other words, McCaul and Mallot, and others predict that distraction will fail exactly when an effective treatment is needed the most.
To explore whether VR can reduce severe and higher pain, patients received VR during burn wound debridement in a hydrotherapy tank, where some of the most painful burn wound care is conducted [2]. Eleven patients were studied using a custom fiberoptic water-friendly VR system that can safely be used in water (see Fig. 1). Each patient spent a portion of their wound debridement with no distraction and spent an equivalent portion of wound care in VR during the same wound care session (within-subject condition order randomized). After each condition, patients completed three subjective pain ratings using 0 to 10 labeled Graphic Rating Scales (GRS) with respect to the preceding portion of wound care. Such pain rating scales have been shown to be valid through their strong associations with other measures of pain intensity, and through their ability to detect treatment effects [32, 33]. These queries were designed to assess the cognitive component of pain (amount of time spent thinking about pain), the affective component of pain (unpleasantness), and the sensory/intensity component of pain (worst pain). Affective and sensory pains are two separately measurable and sometimes differentially influenced components of the pain experience [34]. Gracely et al. [34] have shown ratio scale measures such as the labeled GRS to be highly reliable. In addition, a single GRS rating of “fun” during wound care was measured.
Fig. 1.
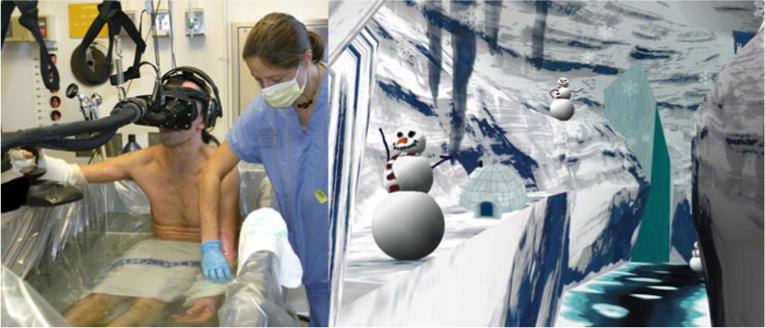
Left, a burn patient getting wound cleaning/debridement in a hydro(scrub) tank “goes into” the immersive virtual world (shown on the right) to distract him from his excessive pain. The custom water-friendly system uses fiberoptic image guides to safely transmit computer-generated images from the immersive virtual reality to the patient via photons (light). (Photo credits—left photo by Hunter Hoffman, UW; image on the right by Stephen Dagadakis, UW, shows the 2003 version of SnowWorld, (designed at the University of Washington, www.vrpain.com, created by Jeff Bellinghausen and Chuck Walter from Multigen, and upgraded by Brian Stewart from SimWright Inc., Howard Abrams (freelance worldbuilder), and Duff Hendrickson, UW))
Overall, patients (n=11) reported a large, statistically significant and clinically meaningful reduction in pain during VR [2]. The six patients who reported the highest pain intensity during “no VR” (worst pain, >7.6; n=6) reported a 41% reduction in pain intensity (worst pain) during VR. Although other VR analgesia studies have commonly included burn patients experiencing severely intense pain, this was the first study to analyze VR analgesia in a subgroup of burn patients who were all experiencing severe pain intensity. Although preliminary, these results suggest that immersive VR can be an effective adjunctive non-pharmacologic pain reduction technique, even for burn patients experiencing severe pain during wound care that is taking place in a hydrotherapy tank.
The Relationship Between the Immersiveness of the VR System and Analgesic Effectiveness
Using the concept of immersion as a theoretical framework, researchers have begun to analyze what makes VR effective for reducing pain. Slater and Wilbur define immersion as an objective, quantifiable description of what a particular VR system can provide to a participant. Immersion is different from the subjective psychological illusion of going into the virtual world, known as presence. According to Slater and Wilbur [35], presence is a psychological state of consciousness and is reliant on subjective measures (asking users to rate on a scale from 1 to 10 how much they felt like they went into the computer-generated world as if it is a place they visited). In contrast, immersiveness is objectively measurable (e.g., using trigonometry to calculate the “field of view” or amount of peripheral vision stimulated by a VR helmet’s displays).
In several laboratory studies exploring the relationship between immersion and analgesic effectiveness, healthy volunteers received brief thermal pain stimuli at carefully controlled temperatures and rated how painful they found the stimuli. These studies found that highly immersive VR systems are more effective at reducing pain than less-immersive VR systems [36–38] and as described next, the difference in amount of analgesia achieved with a highly immersive VR system can be considerable.
In one laboratory study, high-technology VR goggles increased the patient’s peripheral vision in the virtual world, increasing how much VR reduced pain [36]. Researchers [36] randomly assigned participants (healthy volunteers) to either a low-technology VR helmet group (n=28), a high-technology VR helmet group (n=26) or to a no VR group (n=23). To help minimize demand characteristics, both the subjects and the research assistant collecting the experimental pain ratings remained unaware that helmet quality was being manipulated. Compared with the group that received the low-technology VR helmet (35° field of view diagonal), the high-technology VR goggles group (60° field of view diagonal) reported 34% more reduction in worst pain, 46% more reduction in pain unpleasantness, 29% more reduction in time spent thinking about pain, and 32% more fun during the pain stimulus during VR. Sixty-five percent of participants in the high-technology VR goggles group showed a clinically meaningful reduction in pain intensity during virtual reality, compared with only 29% for the low-technology VR helmet group. These results suggest that helmet quality (i.e., goggle size/field of view/amount of peripheral vision looking into VR) is an especially important factor for achieving clinically meaningful reductions in pain intensity, and the study design helps reduce the likelihood that VR analgesia is due to an artifact such as demand characteristics.
In a related study [38], instead of manipulating helmet quality, the objective immersiveness of the VR system was manipulated via interactivity, i.e., whether participants interacted with the virtual world or not. Twenty-one participants (healthy volunteers) were randomly assigned to one of two treatment groups. All participants individually glided through the virtual world SnowWorld, but one group could look around and interact with the virtual world via a trackball, and the other group could not interact with the virtual world (no trackball). Afterwards, each participant provided subjective 0–10 pain ratings.
The more-immersive VR group who interacted with the virtual world via a trackball showed significantly more pain reduction than the less-immersive VR group who received non-interactive VR with no track ball [38, see also 39]. Compared with the non-interactive VR group, participants in the interactive VR group showed 75% more reduction in pain unpleasantness (p<.005) and 74% more reduction in worst pain (p<.005) and 32% more reduction in time spent thinking about pain (p=.01). Interactivity increased the objective immersiveness of the VR system, and as predicted, increased the analgesic effectiveness. In summary, so far, high-technology VR helmet quality (wide field of view goggles), and interactivity (playing with a mouse-like trackball or other input device) have been isolated as especially important factors contributing to VR analgesia.
Using fMRI Brain Scans to Measure Pain-Related Brain Activity
What is going on in people’s brains when they feel pain, and how are those patterns of brain activity altered (if at all) when participants go into immersive virtual reality and experience large reductions in how much pain they feel? To explore these topics, Hoffman and colleagues [40, 41] measured the objective physiological neural correlates of VR analgesia. Custom magnet-friendly VR goggles [42] were designed and built that allowed participants to experience the illusion of going inside the computer-generated world while simultaneously assessing neural activity using fMRI brain scans. A thermal pain stimulator was attached to the healthy volunteer’s foot. Participants received 30 s of thermal stimulation at a painful but tolerable temperature pre-approved by each participant, then 30 s with lukewarm temperature, and this cycle of “pain on/pain off” was repeated three times over a 6-min fMRI brain scan.
During half of the brain scan, the control condition, participants looked at a fixation cross and saw no VR, and heard no music and no VR sound effects. During the other half of their fMRI brain scan they went into the 3D computer-generated world, and interacted with the virtual world by throwing snowballs at snowmen, igloos, robots and penguins, which responded with 3D visual and 3D sound effects when hit. The treatment order was randomized such that approximately half of the participants received immersive virtual reality for 3 min followed immediately by “no VR” for 3 min and vice versa (see Fig. 2). Immediately after the 6-min fMRI brain scan, subjects rated how much pain they had experienced during VR and during no VR, on 10 point rating scales. The subjective pain ratings replicated previous results, i.e., participants reported feeling moderate to severe pain during the pain stimuli with no VR, and subjects reported much less pain when in VR. In addition to reporting less subjective pain, objective measures of the neural correlates of pain showed large (50% or greater) statistically significant reductions in pain-related brain activity in all five regions of the brain studied (the anterior cingulate cortex, insula, thalamus, the primary and the secondary somatosensory cortex, see the figure provided in the Electronic Supplementary Material).
Fig. 2.
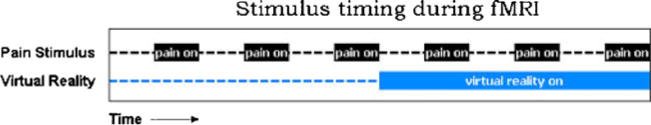
A schematic showing the laboratory pain stimulation paradigm used in an fMRI brain scan study exploring whether virtual reality changes the amount of pain-related brain activity
A second recent laboratory fMRI brain scan study involving nine healthy volunteer participants (also using a within-subjects design) compared and contrasted VR analgesia vs. systemic opioid analgesia, both via subjective pain ratings as well as objective measures of brain activity patterns. Thermal pain stimuli were applied to the patient’s foot during fMRI [43]. Results showed that when used alone, VR and opioid analgesia each reduced pain ratings and pain-related brain activity. Furthermore, adding immersive VR to opioids resulted in significantly more reduction in pain ratings than opioids alone, and patterns of pain-related brain activity were consistent with subjective analgesic reports.
Another laboratory study compared, contrasted, and combined VR analgesia with conventional post-hypnotic analgesia. Researchers [44] experimentally induced thermal pain to test healthy normal volunteer participants. Posthypnotic suggestions were administered via an audiotape of a hypnotist. Using a 2×2, between-groups design, participants were randomly assigned to one of the following four conditions: (1) no hypnosis+no VR, (2) no hypnosis+yes VR, (3) yes hypnosis+no VR, or (4) yes hypnosis+yes VR. The impact of post-hypnotic suggestions for analgesia was specific to high hypnotizables. Only highly hypnotizable participants (i.e., who scored high on the Stanford Hypnotizability Scale, which turned out to be approximately 25% of the participants) reported post-hypnotic analgesia after listening to an audiotape containing post-hypnotic suggestions for analgesia (see also Patterson and Jensen [13]). In contrast, VR analgesia was effective regardless of hypnotizability. These results suggest that hypnosis and virtual reality work via different mechanisms. Results of high hypnotizables showed a non-significant but predicted pattern for high hypnotizables: audio hypnosis combined with immersive VR distraction reduced pain unpleasantness 25% more and reduced worst pain 22% more than did VR distraction alone. Although not statistically significant, results showed the predicted pattern for subjects who were highly hypnotizable. Further research is needed to explore whether hypnotic suggestions could be customized to potentiate or amplify the amount of pain reduction from virtual reality distraction and pharmacologic analgesia.
Encouraged by the small but growing literature on VR analgesia in civilian burn patients, military researchers are beginning to explore the use of VR analgesia in patients with combat-related burn injuries, such as U.S. troops severely burned in Iraq and Afghanistan during terrorist roadside bomb attacks on humvee convoys [45]. A custom “robot-like” arm (see Fig. 3) allows the soldiers to use the immersive VR world without the discomfort of wearing a 1.75 lb VR helmet on their head. In addition, the robot-like arm reduces or eliminates contact between the patient and the equipment (making the goggles easier to clean/sterilize) and makes VR available to patients with bandaged face and head burns.
Fig. 3.
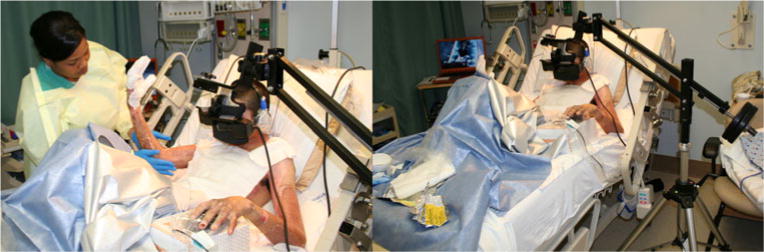
Patient with combat-related burn injuries receiving wound care in immersive virtual reality via robot-like arm mounted VR goggles which do not require wearing a head mounted VR helmet. (Photo credits by Hunter Hoffman, www.vrpain.com)
Using graphic pain rating scales, each of the two soldiers rated their pain during VR vs. no VR (order randomized). Both patients were severely burned in separate incidents when their humvees were attacked by terrorists using improvised explosive devices in Iraq (a roadside bomb for patient 1 and a rocket propelled grenade for patient 2). Both patients were evacuated from Iraq to a military burn trauma center in the USA. Averaged across the two patients, worst pain dropped from severe pain intensity (mean=7.5/10) to moderate pain intensity (4.5/10). Pain ratings of “time spent thinking about pain” dropped from 100% of the time during no VR to 08% of the time during VR, and “pain unpleasantness” ratings dropped from “moderate” (mean= 6.5/10) to “mild” (mean=2.0/10). The patients rated wound care as “no fun at all” (0/10) during no VR but “pretty fun” (9/10) during VR. These preliminary results suggest that immersive VR has feasibility as a potential adjunctive non-pharmacologic analgesic for military patients with combat-related burn injuries. Larger controlled military studies are warranted and needed.
Studies Exploring the Use of VR Analgesia for Blunt Force Trauma, Dental Fears, Claustrophobia, Cerebral Palsy, Cancer, and Urological Endoscopy Patients
Because burn wounds are unusually painful injuries, techniques that are effective for reducing pain in burn patients are also likely to be effective in treating procedural pain in other patient populations besides burns. Consistent with this notion, preliminary case studies have found that VR reduced pain during physical therapy in a non-burn blunt force trauma injury (a pedestrian who was hit by a semi truck, undergoing physical therapy in the trauma unit) [46]. VR reduced pain and fear in two patients during dental/periodontal procedures in patients with dental fears [47, 48], and VR reduced fear/anxiety in a claustrophobic patient during a brain scan [49]. VR has reduced pain during a urological procedure in an older man receiving endoscopic transurethral microwave thermotherapy ablation of the prostate [50], and in pediatric cerebral palsy patients during painful physical therapy rehabilitation after single event multilevel surgery to increase ambulation [51]. VR has reduced discomfort during subcutaneous vascular port access and venipuncture in children and adolescents with cancer [52, 53], and VR reduced pain in children getting venipuncture in general [54]. A growing number of researchers using a variety of distraction software have also found evidence that VR reduces clinical and laboratory pain [24, 39, 51, 55–59] or itching [60]. VR systems may be tailored to the specific needs of different patient populations in the future. For example, although highly immersive VR systems are typically needed for severe burn patients, less-immersive VR systems may be adequate for some other medical procedures such as blood draws, cannula implants, and dental procedures.
Future Directions: Repeated Use of Virtual Reality Pain Distraction
Researchers conducted a preliminary study exploring whether VR continues to be effective when used for longer, clinically relevant treatment durations, for several days in a row. Four children with large severe burns ranging in size from 45% to 82% total body surface area (TBSA), with average 64.5% TBSA, were studied for 10 days each. Occupational and physical therapists orchestrated passive range of motion exercises with each patient for 5 days during VR compared with similar treatment for 5 days without VR. Treatment order was randomized. Some patients received 5 days of physical therapy with VR vs. 5 days with no VR, and others received 5 days of no VR followed by 5 days of VR. Results showed large reductions in worst pain intensity (approximately 45% reduction in worst pain), pain unpleasantness, and time spent thinking about pain, and more fun during VR compared with no VR during the 25-min VR treatments per day, for 5 days in a row per patient [61]. There was no diminishment in analgesic effectiveness over the 5 day period (see Fig. 4), and equivalent range of motion was achieved with VR as compared with standard care without VR.
Fig. 4.
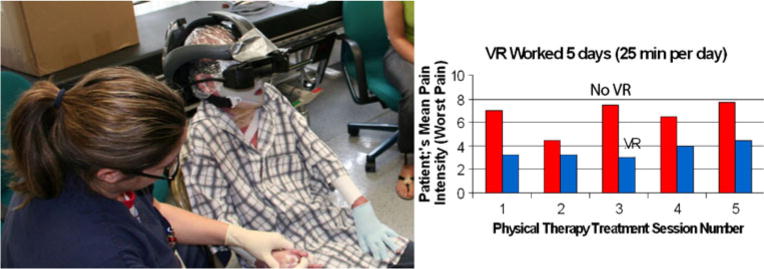
Pediatric burn patient in virtual reality during passive range of motion exercises. The VR helmet shown has high-technology 80° diagonal field of view goggle views of the virtual world, as seen by the patient. (Photo credit—left photo by Hunter Hoffman, www.vrpain.com)
Larger, multisite studies using VR for longer treatment durations on multiple days are needed to determine the clinical value of VR for everyday burn care, and to explore whether there are any long term benefits to repeatedly using virtual reality pain distraction. Better control of repeated procedural pain could potentially improve long term physiological and/or psychological outcome [62, 63]. In addition to reduced procedural pain during VR, we predict that frequent use of adjunctive VR analgesia can potentially have impact on use of opioid analgesics, can reduce PTSD symptoms and/or depression, improve functionality (range of motion), and may improve sleep. In addition, there is speculation that development of chronic pain may in some cases be prevented by reducing the amount of repeated severe procedural pain experienced by patients during their hospital stay, i.e., via preventative analgesia [63].
Future Directions: VR Hardware and Software Tailored to the Needs of Burn Patients
To date, researchers have been able to design and build several unique pieces of equipment specifically tailored to the custom needs of burn patients. For example, a custom fiberoptic VR helmet was developed that could be safely worn by burn patients sitting in a tub of water known as a hydrotank/scrubtank [2]. Similarly, the first two fMRI neuroimaging studies on VR analgesia [40, 43] required researchers to design and build the first pair of custom wide field of view magnet-friendly fiberoptic “photonic” VR goggles (only light, no electricity, reaches the participants) [42]. Laboratory studies suggest that participants who show only modest VR analgesia are likely to show larger reductions in pain if a more-immersive VR system is used [36]. In addition, patients who find VR helmets uncomfortable or who have head or face burns that preclude the use of conventionally helmets may be able to use the new robot-like arm mounted VR goggles. Currently VR systems using robot-like arm [45] mounted nvisinc.com MX90 VR goggles are the state of the art in high-technology VR hardware for acute procedural pain distraction. For severely burn-injured patients, more research and development is needed to increase the immersiveness of the VR system, to increase the amount of pain reduction experienced by burn patients during medical procedures, for those needing a stronger “dose” of virtual reality distraction. More rugged, less expensive, more portable plug and play VR systems are also needed. Future VR analgesia systems will capitalize on new display technologies, more sophisticated virtual worlds, and a growing understanding of how to make VR even more distracting. Laboratory and clinical research is accelerating how quickly and successfully VR analgesia gets translated into clinical practice.
Because of the pervasive prevalence of excessive pain during medical procedures, and especially in light of the large numbers of children severely burned each year [64], more research exploring the use of virtual reality analgesia is justified, and further improvement/development of VR equipment hardware and software tailored to the needs of patients receiving VR during medical procedures is warranted.
Supplementary Material
Acknowledgments
This manuscript was funded by the following NIH grants to Drs. Patterson and Sharar at the UW: NIH HD40954-01, 1R01AR054115-01A1, R01GM042725-17A1, the Scan Design Foundation by Inger and Jens Bruun, and NIH grant RO1 HD049471 to Dr. Oscar E. Suman (UTMB and Shriners Galveston).
Footnotes
Snow World is made available free of charge to eligible medical centers by the Hoffman and Patterson via Hunthoff@uw.edu. The most recent build of SnowWorld 2006 was designed by our UW team with creative input and worldbuilding by Firsthand Inc, Seattle.
Electronic supplementary material The online version of this article (doi:10.1007/s12160-010-9248-7) contains supplementary material, which is available to authorized users.
Conflicts of Interest Statement The authors have no conflict of interest to disclose.
Contributor Information
Hunter G. Hoffman, Email: hunter@hitL.washington.edu, hunthoff@uw.edu, University of Washington Seattle, WA, USA; Human Interface Technology Laboratory, Human Photonics Lab, and Department of Mechanical Engineering, University of Washington, Seattle, WA, USA.
Gloria T. Chambers, University of Washington Seattle, WA, USA.
Walter J. Meyer, III, University of Texas Medical Branch and Shriners Children’s Hospital Galveston TX, Galveston, TX, USA.
Lisa L. Arceneaux, University of Texas Medical Branch and Shriners Children’s Hospital Galveston TX, Galveston, TX, USA.
William J. Russell, University of Texas Medical Branch and Shriners Children’s Hospital Galveston TX, Galveston, TX, USA.
Eric J. Seibel, University of Washington Seattle, WA, USA.
Todd L. Richards, University of Washington Seattle, WA, USA.
Sam R. Sharar, University of Washington Seattle, WA, USA.
David R. Patterson, University of Washington Seattle, WA, USA.
References
- 1.American Burn Association. Burn Incidence and Treatment in the US: 2007 Fact Sheet. Available from: http://www.ameriburn.org/resources_factsheet.php.
- 2.Hoffman HG, Patterson DR, Seibel E, et al. Virtual reality pain control during burn wound debridement in the hydrotank. Clin J Pain. 2008;24:299–304. doi: 10.1097/AJP.0b013e318164d2cc. [DOI] [PubMed] [Google Scholar]
- 3.Perry S, Heidrich G, Ramos E. Assessment of pain by burn patients. Journal of Burn Care and Rehabilitation. 1981;2:322–326. [Google Scholar]
- 4.Ptacek J, Patterson D, Doctor J. Describing and predicting the nature of procedural pain after thermal injuries: Implications for research. Journal of Burn Care and Rehabilitation. 2000;21:318–326. doi: 10.1067/mbc.2000.108146. [DOI] [PubMed] [Google Scholar]
- 5.Melzack R. The tragedy of needless pain. Scientific American. 1990;262:27–33. doi: 10.1038/scientificamerican0290-27. [DOI] [PubMed] [Google Scholar]
- 6.Berger AC, Whistler JL. How to design an opioid drug that causes reduced tolerance and dependence. Ann Neurol. 2010;67:559–569. doi: 10.1002/ana.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherny N, Ripamonti C, Pereira J, et al. Strategies to manage the adverse effects of oral morphine: An evidence-based report. Journal of Clinical Oncology. 2001;19:2542–2554. doi: 10.1200/JCO.2001.19.9.2542. [DOI] [PubMed] [Google Scholar]
- 8.Shang AB, Gan TJ. Optimising postoperative pain management in the ambulatory patient. Drugs. 2003;63:855–867. doi: 10.2165/00003495-200363090-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ward R. Physical Rehabilitation. In: Carrougher G, editor. Burn Care and Therapy. New York: Mosby; 1998. pp. 293–327. [Google Scholar]
- 10.Montgomery GH, DuHamel KN, Redd WH. A meta-analysis of hypnotically induced analgesia: How effective is hypnosis? Int J Clin Exp Hypn. 2000;48:138–153. doi: 10.1080/00207140008410045. [DOI] [PubMed] [Google Scholar]
- 11.Patterson DR. Is hypnotic pain control effortless or effortful? Hypnos. 2001;28:132–134. [Google Scholar]
- 12.Patterson DR. Clinical Hypnosis for Pain Control. Washington, DC: American Psychological Association; 2010. [Google Scholar]
- 13.Patterson DR, Jensen MP. Hypnosis and Clinical Pain. Psychological Bulletin. 2003;129:495–521. doi: 10.1037/0033-2909.129.4.495. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez E, Turk DC. The utility of cognitive coping strategies for altering pain perception: A meta-analysis. Pain. 1989;38:123–135. doi: 10.1016/0304-3959(89)90230-3. [DOI] [PubMed] [Google Scholar]
- 15.Klassen JA, Liang Y, Tjosvold L, Klassen TP, Hartling L. Music for pain and anxiety in children undergoing medical procedures: A systematic review of randomized controlled trials. Ambul Pediatr. 2008;8:117–128. doi: 10.1016/j.ambp.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Cepeda MS, Carr DB, Lau J, Alvarez H. Music for pain relief. Cochrane Database of Systematic Reviews. 2006;(2) doi: 10.1002/14651858.CD004843.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Eccleston C. Role of psychology in pain management. Br J Anaesth. 2001;87:144–152. doi: 10.1093/bja/87.1.144. [DOI] [PubMed] [Google Scholar]
- 18.Eccleston C, Crombez G. Pain demands attention: A cognitive-affective model of the interruptive function of pain. Psychological Bulletin. 1999;125:356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- 19.Kahneman D. Attention and effort. Englewood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- 20.Hoffman HG, Garcia-Palacios A, Kapa VA, Beecher J, Sharar SR. Immersive virtual reality for reducing experimental ischemic pain. International Journal of Human-Computer Interaction. 2003;15:469–486. [Google Scholar]
- 21.Hoffman HG. Virtual Reality Therapy. Scientific American. 2004;291:58–65. doi: 10.1038/scientificamerican0804-58. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman HG, Doctor JN, Patterson DR, Carrougher GJ, Furness TA., 3rd Use of virtual reality as an adjunctive treatment of adolescent burn pain during wound care: A case report. Pain. 2000;85:305–309. doi: 10.1016/s0304-3959(99)00275-4. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman HG, Patterson DR, Magula J, et al. Water-friendly virtual reality pain control during wound care. Journal of Clinical Psychology. 2004;60:189–195. doi: 10.1002/jclp.10244. [DOI] [PubMed] [Google Scholar]
- 24.van Twillert B, Bremer M, Faber AW. Computer-generated virtual reality to control pain and anxiety in pediatric and adult burn patients during wound dressing changes. J Burn Care Res. 2007;28:694–702. doi: 10.1097/BCR.0B013E318148C96F. [DOI] [PubMed] [Google Scholar]
- 25.Carrougher GJ, Hoffman HG, Nakamura D, et al. The effect of virtual reality on pain and range of motion in adults with burn injuries. J Burn Care Res. 2009;30:785–791. doi: 10.1097/BCR.0b013e3181b485d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman HG, Patterson DR, Carrougher GJ. Use of virtual reality for adjunctive treatment of adult burn pain during physical therapy: A controlled study. Clin J Pain. 2000;16:244–250. doi: 10.1097/00002508-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman HG, Patterson DR, Carrougher GJ, Sharar SR. Effectiveness of virtual reality-based pain control with multiple treatments. Clin J Pain. 2001;17:229–235. doi: 10.1097/00002508-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt YS, Hoffman HG, Blough DK, et al. A randomized, controlled trial of immersive virtual reality analgesia, during physical therapy for pediatric burns. Burns. 2010 doi: 10.1016/j.burns.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharar SR, Carrougher GJ, Nakamura D, et al. Factors influencing the efficacy of virtual reality distraction analgesia during postburn physical therapy: Preliminary results from 3 ongoing studies. Arch Phys Med Rehabil. 2007;88:S43–49. doi: 10.1016/j.apmr.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 30.McCaul KD, Malott JM. Distraction and coping with pain. Psychological Bulletin. 1984;95:516–533. [PubMed] [Google Scholar]
- 31.Crombez G, Eccleston C, Baeyens F, Eelen P. When somatic information threatens, catastrophic thinking enhances attentional interference. Pain. 1998;75:187–198. doi: 10.1016/s0304-3959(97)00219-4. [DOI] [PubMed] [Google Scholar]
- 32.Jensen MP. The validity and reliability of pain measures in adults with cancer. Journal of Pain. 2003;4:2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 33.Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of pain assessment. New York: Guilford Publications; 2001. pp. 15–34. [Google Scholar]
- 34.Gracely RH, McGrath P, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5:5–18. doi: 10.1016/0304-3959(78)90020-9. [DOI] [PubMed] [Google Scholar]
- 35.Slater M, Wilbur S. A framework for immersive virtual environments (FIVE): speculations on the role of presence in virtual environments. Presence Teleoper Virtual Environ. 1997;6:603–616. [Google Scholar]
- 36.Hoffman HG, Seibel EJ, Richards TL, et al. Virtual reality helmet display quality influences the magnitude of virtual reality analgesia. J Pain. 2006;7:843–850. doi: 10.1016/j.jpain.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman HG, Sharar SR, Coda B, et al. Manipulating presence influences the magnitude of virtual reality analgesia. Pain. 2004;111:162–168. doi: 10.1016/j.pain.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Wender R, Hoffman HG, Hunner HH, et al. Interactivity Influences the Magnitude of Virtual Reality Analgesia. J Cyber Ther Rehabil. 2009;2:27–33. [PMC free article] [PubMed] [Google Scholar]
- 39.Dahlquist LM, McKenna KD, Jones KK, et al. Active and passive distraction using a head-mounted display helmet: Effects on cold pressor pain in children. Health Psychol. 2007;26:794–801. doi: 10.1037/0278-6133.26.6.794. [DOI] [PubMed] [Google Scholar]
- 40.Hoffman HG, Richards TL, Coda B, et al. Modulation of thermal pain-related brain activity with virtual reality: Evidence from fMRI. Neuroreport. 2004;15:1245–1248. doi: 10.1097/01.wnr.0000127826.73576.91. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman HG, Richards TL, Bills AR, et al. Using fMRI to study the neural correlates of virtual reality analgesia. CNS Spectrums. 2006;11:45–51. doi: 10.1017/s1092852900024202. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman HG, Richards TL, Magula J, et al. A magnet-friendly virtual reality fiberoptic image delivery system. Cyberpsychol Behav. 2003;6:645–648. doi: 10.1089/109493103322725423. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman HG, Richards TL, Van Oostrom T, et al. The analgesic effects of opioids and immersive virtual reality distraction: Evidence from subjective and functional brain imaging assessments. Anesth Analg. 2007;105:1776–1783. doi: 10.1213/01.ane.0000270205.45146.db. table of contents. [DOI] [PubMed] [Google Scholar]
- 44.Patterson DR, Hoffman HG, Palacios AG, Jensen MP. Analgesic effects of posthypnotic suggestions and virtual reality distraction on thermal pain. J Abnorm Psychol. 2006;115:834–841. doi: 10.1037/0021-843X.115.4.834. [DOI] [PubMed] [Google Scholar]
- 45.Maani C, Hoffman HG, DeSocio PA, et al. Pain control during wound care for combat-related burn injuries using custom articulated arm mounted virtual reality goggles. Journal of CyberTherapy and Rehabilitation. 2008;1:193–198. [Google Scholar]
- 46.Hoffman HG, Patterson DR, Soltani M, et al. Virtual reality pain control during physical therapy range of motion exercises for a patient with multiple blunt force trauma injuries. Cyberpsychol Behav. 2008;19:47–49. doi: 10.1089/cpb.2008.0056. [DOI] [PubMed] [Google Scholar]
- 47.Furman E, Jasinevicius TR, Bissada NF, et al. Virtual reality distraction for pain control during periodontal scaling and root planing procedures. J Am Dent Assoc. 2009;140:1508–1516. doi: 10.14219/jada.archive.2009.0102. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman HG, Garcia-Palacios A, Patterson DR, Jensen MP, Furness TA., III The effectiveness of virtual reality for dental pain control: A case study. Cyberpsychol Behav. 2001;4:527–535. doi: 10.1089/109493101750527088. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Palacios A, Hoffman HG, Richards TR, Seibel EJ, Sharar SR. Use of virtual reality distraction to reduce claustrophobia symptoms during a mock magnetic resonance imaging brain scan: a case report. Cyberpsychol Behav. 2007;10:485–488. doi: 10.1089/cpb.2006.9926. [DOI] [PubMed] [Google Scholar]
- 50.Wright JL, Hoffman HG, Sweet RM. Virtual reality as an adjunctive pain control during transurethral microwave thermo-therapy. Urology. 2005;66:1320. doi: 10.1016/j.urology.2005.06.123. [DOI] [PubMed] [Google Scholar]
- 51.Steele E, Grimmer K, Thomas B, et al. Virtual reality as a pediatric pain modulation technique: A case study. Cyberpsychol Behav. 2003;6:633–638. doi: 10.1089/109493103322725405. [DOI] [PubMed] [Google Scholar]
- 52.Gershon J, Zimand E, Pickering M, Rothbaum BO, Hodges L. A pilot and feasibility study of virtual reality as a distraction for children with cancer. J Am Acad Child Adolesc Psychiatry. 2004;43:1243–1249. doi: 10.1097/01.chi.0000135621.23145.05. [DOI] [PubMed] [Google Scholar]
- 53.Windich-Biermeier A, Sjoberg I, Dale JC, Eshelman D, Guzzetta CE. Effects of distraction on pain, fear, and distress during venous port access and venipuncture in children and adolescents with cancer. J Pediatr Oncol Nurs. 2007;24:8–19. doi: 10.1177/1043454206296018. [DOI] [PubMed] [Google Scholar]
- 54.Gold JI, Kim SH, Kant AJ, Joseph MH, Rizzo AS. Effectiveness of virtual reality for pediatric pain distraction during i.v. placement. Cyberpsychol Behav. 2006;9:207–212. doi: 10.1089/cpb.2006.9.207. [DOI] [PubMed] [Google Scholar]
- 55.Chan EA, Chung JW, Wong TK, Lien AS, Yang JY. Application of a virtual reality prototype for pain relief of pediatric burn in Taiwan. J Clin Nurs. 2007;16:786–793. doi: 10.1111/j.1365-2702.2006.01719.x. [DOI] [PubMed] [Google Scholar]
- 56.Das D, Grimmer K, Sparnon A, McRae S, Thomas B. The efficacy of playing a virtual reality game in modulating pain for children with acute burn injuries: A randomized controlled trial. BMC Pediatric. 2005;5:1. doi: 10.1186/1471-2431-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malloy KM, Milling LS. The effectiveness of virtual reality distraction for pain reduction: A systematic review. Clin Psychol Rev. 2010;30:1011–1018. doi: 10.1016/j.cpr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Morris LD, Louw QA, Grimmer-Somers K. The effectiveness of virtual reality on reducing pain and anxiety in burn injury patients: A systematic review. Clin J Pain. 2009;25:815–826. doi: 10.1097/AJP.0b013e3181aaa909. [DOI] [PubMed] [Google Scholar]
- 59.Schneider SM, Prince-Paul M, Allen MJ, Silverman P, Talaba D. Virtual reality as a distraction intervention for women receiving chemotherapy. Oncol Nurs Forum. 2004;31:81–88. doi: 10.1188/04.ONF.81-88. [DOI] [PubMed] [Google Scholar]
- 60.Liebovici V, Magora F, Cohen S, Ingber A. Effects of virtual reality immersion and audiovisual distraction techniques for patients with pruritus. Pain Res Manag. 2009;14:283–286. doi: 10.1155/2009/178751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flores A, Hoffman HG, Russell W, et al. CyberTherapy Conference. San Diego, CA: 2008. Longer, multiple virtual reality pain distraction treatments of Hispanic and Caucasian children with large severe burns. [Google Scholar]
- 62.Patterson DR, Sharar SR. Burn pain. In: Loeser JD, editor. Bonica’s Management of Pain. Philadelphia, PA: Lippincott, Williams & Wilkins; 2001. pp. 780–787. [Google Scholar]
- 63.Malchow RJ, Black IH. The evolution of pain management in the critically ill trauma patient: Emerging concepts from the global war on terrorism. Crit Care Med. 2008;36:S346–357. doi: 10.1097/CCM.0b013e31817e2fc9. [DOI] [PubMed] [Google Scholar]
- 64.D’Souza AL, Nelson NG, McKenzie LB. Pediatric burn injuries treated in US emergency departments between 1990 and 2006. Pediatrics. 2009;124:1424–1430. doi: 10.1542/peds.2008-2802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


