SUMMARY
The prevalence of Alzheimer’s disease is increasing rapidly in the absence of truly effective therapies. A promising strategy for developing such therapies is the treatment of brain insulin resistance, a common and early feature of Alzheimer’s disease, closely tied to cognitive decline and capable of promoting many biological abnormalities in the disorder. The proximal cause of brain insulin resistance appears to be neuronal elevation in the serine phosphorylation of IRS-1, most likely due to amyloid-β-triggered microglial release of proinflammatory cytokines. Preclinically, the first line of defense is behavior-lowering peripheral insulin resistance (e.g., physical exercise and a Mediterranean diet supplemented with foods rich in flavonoids, curcumin and ω-3 fatty acids). More potent remediation is required, however, at clinical stages. Fortunately, the US FDA-approved antidiabetics exenatide (Byetta®; Amylin Pharmaceuticals, Inc., CA, USA) and liraglutide (Victoza®; Novo Nordisk A/S, Bagsvaerd, Denmark) are showing much promise in reducing Alzheimer’s disease pathology and in restoring normal brain insulin responsiveness and cognitive function.
Until recently, Alzheimer’s disease (AD) was defined as a type of neurodegenerative dementia associated with abnormally high densities of amyloid-β (Aβ) plaques and neurofibrillary tangles in the forebrain. The disorder was thus synonymous with a form of dementia. Today, however, AD is more broadly defined to include the underlying pathophysiological processes that gradually lead to a dementia [1,2]. Over several decades, AD pathology develops in three stages [3,4]: preclinical periods with no more than subtle behavioral symptoms [2,5]; a prodromal period known as mild cognitive impairment (MCI) due to AD with the first clear, but not incapacitating, symptoms [6,7]; and dementia due to AD [4,8]. This last stage is among the most devastating of human disorders, ultimately robbing its individuals of their identity, their capacity to care for themselves and their ability to recognize or communicate with others. Such dementia most often manifests at the age of 65 years or older, but it can manifest as early as 30 years of age in relatively rare familial cases [3].
AD dementia, the most common of all neurodegenerative dementias, is of special concern because it poses a worldwide public health risk of epidemic proportions [9,10] and there is, as yet, a lack of effective treatment. While over 100 pharmacological treatments for AD have been proposed and tested, most seeking to reduce Aβ levels in the brain, none have proven more than minimally effective [11] for more than approximately 1 year after diagnosis [12]. If this situation persists, it is expected that at least 13.8 million Americans will be afflicted with AD dementia by the year 2050, with healthcare costs for them costing US$1.2 trillion [3].
There is consequently an urgent need to develop novel treatments of AD within the next decade [13]. Among the most promising of those now in development are treatments that target brain insulin resistance (i.e., reduced neuronal responsiveness to extracellular insulin), which is an early, common and major feature in patients with AD, with and without diabetes [14,15]. This review describes the significance, nature and potential treatment of brain insulin resistance with a relatively new class of antidiabetics.
Significance of brain insulin resistance in AD
Insulin is best known as a pancreatic β-cell hormone secreted in response to elevated plasma glucose after meals. Its classic functions are stimulation of glucose uptake by adipose and muscle tissue, and inhibition of no longer needed free fatty acid released by adipose tissue and glucose production by the liver. However, insulin is also synthesized in brain neurons [16], including many pyramidal and granule cells in the adult cerebral cortex and hippocampus [17], where the density of insulin receptors is appreciable [18]. While pancreatic insulin is transported in small amounts across the blood–brain barrier in many brain regions and exerts effects on brain function, especially in the hypothalamus [19], most insulin in the brain outside the hypothalamus seems locally derived since vascular hypo- and hyper-insulinemia has little, if any, effect on total brain insulin [20]. Therefore, it seems likely that outside the hypothalamus insulin resistance in the brain largely reflects reduced responsiveness to endogenous, not pancreatic, insulin.
Unlike the case in peripheral tissues, insulin in the brain does not control cellular uptake of glucose [14]. However, insulin has many other functions. In the brain, it promotes most functions disrupted in AD, including regulation of cerebral blood flow, inflammatory responses, oxidative stress, Aβ clearance, tau phosphorylation, apoptosis, lipid metabolism, transmitter receptor trafficking, synaptic plasticity and memory formation [21,22]. Brain insulin resistance may thus cause or contribute to the full spectrum of AD pathology and symptoms. For that reason, the rate at which insulin resistance develops in the brain may play a large role in determining the rate at which AD progresses.
Nature of brain insulin resistance in AD
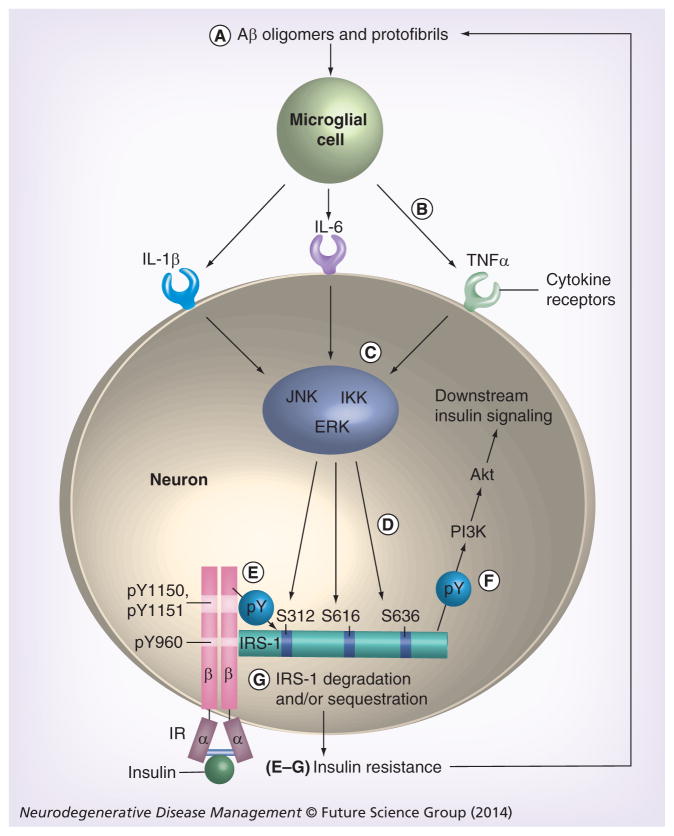
While not affecting neuronal glucose uptake, brain insulin resistance in AD is similar to muscle insulin resistance in Type 2 diabetes (T2D) [14]. In both circumstances, insulin is much less able to activate a specific signaling pathway than is normally the case. In that pathway (Figure 1), insulin binding of the insulin receptor (IR) at the cell surface activates IRS-1 intracellularly, which in turn activates PI3K, then Akt and, finally, the many downstream targets of Akt, including mTOR [14,23]. Since IRS-1 in the brain is neuronal not glial [14,24], brain insulin resistance in the signaling pathway just described is a neuronal phenomenon. Another IRS isoform (IRS-2) is abundant in the brain, but it does not mediate insulin signaling at or near physiological doses of insulin [14]. IRS-2 instead mediates IGF-1 signaling at such doses [14].
Figure 1. Neuronal insulin signaling pathway found resistant to insulin in Alzheimer’s disease and the most likely mechanism for that abnormality.
Insulin binding to the α-chains of the IR triggers pY of the β-chains of the receptor inside neurons. Thus activated, the IR binds and tyrosine phosphorylates IRS-1. This leads to activation of PI3K, then Akt and thereafter diverse downstream signaling molecules. (A–G) The sequence of steps likely to inhibit this pathway in AD. (A) Aβ oligomers and protofibrils activate microglial cells, (B) which consequently secrete a number of proinflammatory cytokines, including IL-1β, IL-6 and TNF-α. (C) Via their receptors on neurons, each of these cytokines activate one or more of three major IRS-1 serine kinases, JNK, IKK and ERK. (D) Each of these kinases phosphorylate one or more IRS-1 sites, including S312, S616 and/or S636/639 (S307, S612 and S632/635 in rodents, respectively). For simplicity, S636/639 in this review is simply called S636. Such phosphorylation, which is abnormally high in cerebral pyramidal cells of Alzheimer’s disease cases, inhibits IRS-1 interactions with the (E) IR upstream and (F) with PI3K downstream, thereby inhibiting transmission of insulin signals to downstream targets such as GSK-3 and mTOR complex 1. (G) Serine phosphorylation of IRS-1 can also promote its sequestration and/or degradation. (E–G) These last three events lead to insulin resistance, which is known to impair clearance of extracellular Aβ [90], presumably leading to further insulin resistance through the steps shown here. Aβ: Amyloid-β; IR: Insulin receptor; pY: Tyrosine phosphorylation.
The first clear indications that the brain may be insulin resistant in AD came from postmortem studies on baseline properties of the cerebral cortex and hippocampal formation, the latter referring collectively to the hippocampus, dentate gyrus and subiculum. These studies found, for example, that such tissue in AD cases exhibits decreased binding of insulin [25], reduced levels of activated IR [26] and increased serine phosphorylation of IRS-1 at sites known to inhibit insulin signaling, as discussed below [14,27].
Only recently, however, has it been demonstrated that the brain in AD actually is insulin resistant. Our group showed this using ex vivo stimulation [14]. We measured brain responses to physiological dose of insulin (1 nM) applied to brain tissue from AD dementia patients and healthy controls of the same sex and similar age who had died within approximately 6 h of autopsy. To reveal whether any abnormality in brain responses to insulin was a general factor in AD, we excluded cases with a history of diabetes.
In all the brain areas our group has studied (hippocampal formation, prefrontal cortex and cerebellar cortex [14,28]), insulin applied to AD tissue induced significantly less activation of the signaling pathway tested than in healthy tissue, as assessed by insulin-induced levels of tyrosine or serine phosphorylated forms (pY or pS, respectively) of insulin signaling molecules or binding among those molecules. Compared with control cases, insulin in AD cases induced 29–34% less activation of the IR (pY), 90% less activation of IRS-1 (pY), 96% less binding of PI3K to IRS-1, 89% less activation of Akt (pS) and 74% less activation of mTOR (pS) in the hippocampal formation (Figure 2) [14]. The first molecule in this signaling pathway to show severe dysfunction was IRS-1, which thus seems to be a central factor in brain insulin resistance in AD. Increasing the dose of insulin tested to 10 nM, which may be higher than can be achieved safely with intranasal insulin, was unable to significantly increase tissue responsiveness.
Figure 2. Physiologically demonstrated insulin resistance in the hippocampal formation of Alzheimer’s disease cases compared with age- and sex-matched controls.
Tissue with low postmortem intervals (~6 h) was used to test responses to 1–10 nM insulin in the IR–IRS-1–PI3K–Akt–mTOR pathway. Each bar in the graphs shows the mean percentage increase (± standard error of the mean) in activation or recruitment of a signaling molecule above the level seen without insulin exposure in the same diagnostic group. Reduced responsiveness was seen in (A) activation of the IR and (B) recruitment of IRS-1 to the IR, but the magnitude of the reduction was much less than in (C) total IRS-1 activation, (D) recruitment of the regulatory subunit of PI3K to IRS-1, (E) Akt activation via its S473 site or (F) mTOR activation via its S2448 site.
*p < 0.05; **p < 0.01; ***p < 0.0001.
AD: Alzheimer’s disease; N: Normal control.
Data taken from [14].
Our subsequent ex vivo stimulation studies have shown lesser, but significant, brain insulin resistance in the hippocampal formation from nondiabetic individuals with MCI [Wang H-Y et al., Liraglutide markedly reduces hippocampal insulin resistance in APP/PS1 mice and MCI cases (2014), Manuscript in preparation], which often progresses to AD dementia [29]. For this reason, brain insulin resistance appears to be an early feature of AD pathogenesis. Our additional finding that a very high percentage of the nondiabetic MCI and AD dementia cases studied show brain insulin resistance suggests that this is a common feature of AD even in the absence of diabetes [14].
According to our ex vivo stimulation studies on the hippocampal formation, brain insulin resistance in AD is accompanied by brain IGF-1 resistance (i.e., impaired IGF-1 signaling via IRS-2) [14]. Unlike the case with insulin resistance, IGF-1 resistance was severe even at the level of the hormone receptor. The significance of this phenomenon remains to be determined.
Search for the causes of brain insulin resistance
Many causes have been proposed to explain evidence of decreased insulin signaling in AD brains. Among the most often cited are reduced extracellular insulin estimated from cerebrospinal fluid assays [30], reduced total [26] or cell surface [27,31] IR expression, and reduced IR affinity for insulin [25]. There are, however, reasons to doubt that these are major factors in the reduced brain insulin signaling seen in AD. Deficient extracellular insulin in the AD brain is not clear given contrary cerebrospinal fluid findings [32,33]. Similarly, deficiencies in the total IR content of AD brain tissues have not been found in studies explicitly using age-matched controls [14,15,27,34,35], and cell fractionation fails to reveal deficiencies in cell surface IR levels in such tissues [14]. While insulin binding of the IR may be reduced in AD brain tissue [25], insulin still manages to activate the catalytic domain of IR at 71–74% of normal levels even in the hippocampal formation of AD dementia cases [13]. As noted above, far greater reductions in insulin responsiveness are seen below the IR in the AD brain beginning with IRS-1, which is activated by insulin at only 10% of normal levels in the hippocampal formation [14].
The most likely proximal cause of reduced brain insulin signaling in AD is thus brain insulin resistance due to dysfunctional IRS-1. This probably reflects Aβ-induced glial secretion of proinflammatory cytokines (Figure 1). Among the earliest abnormalities in AD is elevated soluble Aβ [36], monomers of which aggregate into oligomers that can assemble into fibrils later, which form amyloid plaques or into amylospheroids [37,38]. Also early in AD [39], Aβ oligomers and nascent fibrils (i.e., protofibrils) activate microglia, resulting in their secretion of proinflammatory cytokines, such as IL-1, IL-6 and TNF-α [40]. Such microglial activation may be a critical event in AD pathogenesis given the recent finding that knocking out a gene in an animal model of AD that encodes a microglial receptor (i.e., NOD-like receptor 3), which can sense inflammatory pathogens, including Aβ, prevents development of AD pathology and cognitive deficits that normally occur in that animal model [41]. Via neuronal receptors, microglial IL-1, IL-6 and TNF-α activate IRS-1 serine kinases known by the acronyms IKK, JNK and Erk2 [13]. In this way, Aβ oligomers administered to neuronal cultures or cerebral ventricles markedly elevate IRS-1 serine phosphorylation (IRS-1 pS) at multiple sites, namely S312, S616 and/or S636 (S307, S612 and S632 in rodents) (Figure 1) [42,43].
Elevated neuronal IRS-1 pS is prominent in the cerebral cortex and hippocampal formation of AD cases and appears to be the major cause of IRS-1 dysfunction in AD [14,27]. Since such phosphorylation inhibits the transmission of insulin-induced receptor activation to more downstream molecules, it is understandably an established cause of insulin resistance in peripheral tissues, especially muscle tissue [44]. The same appears to be true in AD brains, where insulin-induced IRS-1 activation is consistently reduced in tissues with significantly elevated levels of IRS-1 pS616 and IRS-1 pS636, which are thus potential biomarkers of brain insulin resistance [14]. As expected, levels of these candidate biomarkers are significantly correlated with oligomeric Aβ plaque loads and are negatively associated to a high degree with measures of cognitive ability, including episodic and working memory [14].
Chronically elevated neuronal IRS-1 pS resulting from Aβ-induced proinflammatory processes is thus the most likely explanation for brain insulin resistance in AD and a major factor in its cognitive deficits. This may explain why peripheral insulin resistance due to obesity and/or T2D exacerbates brain insulin resistance in AD [15] and in animal models of AD [45]. Obesity and T2D are, in fact, risk factors for AD [46] and are both associated with elevated vascular proinflammatory cytokines [47,48]. Especially in AD, where cerebral vasculature is damaged, cytokines can cross the blood–brain barrier [49] and activate IRS-1 serine kinases in the same way that microglial-derived cytokines can. Peripheral insulin resistance can also raise brain IRS-1 pS by decreasing clearance of brain Aβ because insulin facilitates hepatic clearance of plasma Aβ [50], interference with which impairs brain clearance of that peptide [51].
Slowing age-related increases in brain insulin resistance
Between the ages of 45 and 64 years, the prevalence of prediabetes [52] and T2D [53] in the USA rises steeply, indicating a steep rise in peripheral insulin resistance starting in midlife. For the reasons given above, this phenomenon could promote brain insulin resistance, a view supported by our finding that brain levels of IRS-1 pS616 increase significantly from middle to old age, even in those without T2D or cognitive decline. It is thus important, especially from middle-age onward, to continue (or to adopt) lifestyles known to lower peripheral insulin resistance and reduce the risk of progressing to MCI, which elevates risk of AD dementia [29] as noted earlier. The most effective lifestyle changes for these goals are loss of excess weight, regular physical exercise and adherence to a Mediterranean diet specified by Estruch et al. [54] supplemented with nutrients in other diets lowering peripheral insulin resistance, reducing Aβ pathology in the brain, and improving cognition and IRS-1 pS levels [55]. These additional nutrients are flavonoids in blueberries and green tea, curcumin in the spice turmeric and the ω-3 fatty acid docosahexaenoic acid enriched in fatty fishes, such as salmon [55].
Treating brain insulin resistance in AD
While weight loss, exercise and better diets may slow progression to clinical stages of AD and even mitigate symptom severity in MCI [56–59], randomized clinical trials have not provided consistent evidence that such lifestyle changes initiated after diagnosis of MCI or AD dementia markedly slow cognitive decline [52,53,60]. At those stages of AD, simply reducing peripheral insulin resistance is ineffective, as shown by clinical studies documenting the failure of many T2D treatments to reduce AD risk or improve cognition in AD dementia, namely treatments with peripherally administered insulin, metformin, sulfonylureas and thiazolidinediones, such as rosiglitazone and pioglitazone [61,62]. The thiazolidinediones are also clinically compromised by their elevation of risk for heart failure in those with prediabetes or T2D [63].
Despite the failure of many antidiabetics to reduce AD risk or treat AD cognitive deficits the demonstrated ability of intranasal insulin to improve cognition in MCI and early AD dementia cases [64,65] shows that enhancing brain insulin signaling remains a viable means of treating AD. Intranasal insulin administration by itself, however, is unlikely to overcome the levels of brain insulin resistance seen in AD. The T2D drugs specified above may have failed as AD treatments for a number of reasons, such as rapid degradation, poor penetrance of the blood–brain barrier and/or ineffectiveness in reducing neuronal insulin resistance in vivo.
Fortunately, the antidiabetic GLP-1 analogs/mimetics do not have these limitations and are priority candidates among marketed drugs for development as AD therapeutic agents [12]. GLP-1 itself is one of two well-known incretin peptides, which are so named because their secretion by the intestines in response to food increases glucose-stimulated insulin release by the pancreas [66]. Like insulin, GLP-1 is produced in the brain [67] and has many functions outside the pancreas, including neuroprotection [68,69], promotion of neurogenesis [69,70] and potentiation of insulin signaling [71,72].
Since GLP-1 is quickly metabolized, degradation-resistant analogs have been developed for use in treating T2D. Two of those approved by the US FDA are exenatide (synthetic form of exendin-4 marketed as Byetta®; Amylin Pharmaceuticals, Inc., CA, USA) and liraglutide (Victoza®; Novo Nordisk A/S, Bagsvaerd, Denmark). Both effectively reduce peripheral insulin resistance [72,73] and have excellent safety profiles with a low incidence of hypoglycemia [74,75], which is expected given that GLP-1 increases glucose-stimulated, not basal, pancreatic insulin secretion. Pancreatitis has occurred in a very small number of those taking GLP-1 analogs, which may reflect the fact that the drug is prescribed for diabetes, which is a risk factor for pancreatitis [74,75]. A recent meta-analysis, however, found no evidence that GLP-1 analogs increase risk of pancreatitis [76]. Clinical trials are needed to determine if the weight loss induced by GLP-1 analogs in normal and T2D cases poses a problem in AD cases [77].
Peripherally administered GLP-1 analogs, including exendin-4 and liraglutide, cross the blood–brain barrier [70,78] and are thus able to bind GLP-1 receptors widely found in the brain, including pyramidal cells of the cerebral cortex and hippocampal formation [79]. The GLP-1 analogs have a remarkable number of beneficial effects on neurons, many of which may derive from their ability to block Aβ-induced neuronal insulin resistance [43]. In mouse models of AD, including aged animals, these drugs reduce Aβ plaque loads, block Aβ-stimulated inflammatory responses, and promote neurogenesis, neuronal survival and synaptic integrity, restore long-term potentiation and reduce cognitive deficits [43,68–70,80,81]. Given that elevated IRS-1 pS in the brain may be the primary cause of brain insulin resistance, it is notable that exendin-4 and liraglutide reduce levels of IRS-1 pS616 and IRS-1 pS636 in the APP/PS1 mouse model of AD [43,82].
Our group has recently demonstrated that liraglutide essentially restores brain insulin sensitivity in APP/PS1 mice [83]. Using ex vivo stimulation, we showed that the hippocampal formation in such mice is as insulin resistant at 7.5 months as the same brain area in elderly AD cases, and that 2 months of daily liraglutide administration (25 nmol/kg intraperitoneally) beginning at 5 months virtually restored normal hippocampal formation responses to insulin in the IR–IRS-1–PI3K–Akt pathway. The same drug treatment was previously found to restore long-term potentiation in the HF and greatly improve cognition in this animal model of AD at 7 [80] and 14 months [81] of age.
Our most recent work suggests that liraglutide may be quite potent in reducing brain insulin resistance in MCI cases [Wang H-Y et al., Liraglutide markedly reduces hippocampal insulin resistance in APP/PS1 mice and MCI cases (2014), Manuscript in preparation]. As noted above, such tissue in MCI cases is insulin resistant to a lesser degree than the same brain area from AD cases. After exposure to 100 nM of liraglutide for 1 h, the hippocampal formation of MCI cases was found to be much more responsive to 1 nM insulin. Indeed, this treatment resulted in virtually normal insulin responsiveness in tissue from nonamnestic MCI cases and substantially improved insulin responsiveness in tissue from amnestic MCI cases. The same treatment also significantly improved insulin responsiveness in the hippocampal formation of AD cases, but the improvement in responsiveness remained far from normal.
GLP-1 analogs thus emerge as very promising therapeutic agents in AD at an early clinical stage before extensive, irreversible neurodegeneration occurs. This puts a premium on early diagnosis of MCI due to AD, which is becoming possible with current methods to image Aβ plaque levels with PET scans [84]. The results of the first clinical trials of GLP-1 analogs on MCI cases that were started in the last 2 years in the USA and the UK [85] are thus eagerly anticipated. Hopes are raised by the significant improvement in cognition reported recently in the first clinical trial of a GLP-1 analog (exenatide) on a neurodegenerative disorder, namely Parkinson’s disease [86]. As in AD, dementia in Parkinson’s disease is associated with peripheral insulin resistance [87].
Conclusion & future perspective
Since insulin normally regulates many brain functions disrupted in AD, correcting brain insulin resistance in AD may be one of the most efficient means of treating it. Given the research summarized above, there is reason to believe we may soon be able to markedly reduce brain insulin resistance in AD at the MCI stage using FDA-approved GLP-1 analogs. This treatment strategy is especially promising, because it may soon be improved by the development of dual agonists of GLP-1 and the other incretin, GIP. Acting on its own receptors in the brain, GIP also reduces brain pathology and cognitive deficits in a mouse model of AD [88]. Newly developed dual agonists of this type have proven more effective than either GLP-1 or GIP agonists alone in reducing peripheral insulin resistance [89]. Within the next 5–10 years, we may have a second generation of especially potent incretin treatments for AD ready for clinical use.
Practice Points.
Brain insulin resistance is a significant feature of Alzheimer’s disease (AD). This phenomenon by itself can promote many of the neural and cognitive abnormalities of AD. In individuals with or without a history of diabetes, brain insulin resistance is an early and common feature of AD, closely associated with cognitive decline.
Brain insulin resistance in AD is a neuronal phenomenon that reflects a decreased responsiveness to insulin at all levels of the insulin receptor–IRS-1–PI3K–Akt signaling pathway. The first major decrease in insulin responsiveness occurs, however, below the receptor, beginning with IRS-1.
The most immediate cause of brain insulin resistance in AD appears to be amyloid-β-triggered microglial release of proinflammatory cytokines, which inhibit insulin signaling by promoting serine phosphorylation of IRS-1.
It is likely that the rate of age-related increase in brain insulin resistance can be reduced. While there are no validated methods for detecting it in vivo, brain insulin resistance is more likely in those with peripheral insulin resistance (i.e., those with prediabetes or Type 2 diabetes [T2D]) since such resistance can promote brain insulin resistance. Individuals with prediabetics should, therefore, make lifestyle changes to lower peripheral insulin resistance (e.g., losing excess weight, getting regular physical exercise and adopting a Mediterranean diet) to avoid progression not only to T2D, but also to clinical stages of AD.
Once clinical stages of this disorder manifest, lifestyle changes are unlikely to normalize brain insulin responsiveness. This might be accomplished, however, by two GLP-1 analogs approved by the US FDA for T2D, namely exenatide (Byetta®; Amylin Pharmaceuticals, Inc., CA, USA) and liraglutide (Victoza®; Novo Nordisk A/S, Bagsvaerd, Denmark). These drugs show promise in restoring normal brain insulin responsiveness at the mild cognitive impairment stage of AD, but not in AD dementia. Clinical trials are now being conducted on mild cognitive impairment cases with these promising agents.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial competing interests disclosure
This work was supported by grants from the National Institute on Aging (R01 AG15819, P30 AG10124 and P30 AG10161) and from the Alzheimer’s Association. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2▪▪.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stage of Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s Association Workgroup. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. Provides a clear explanation of the new definition of Alzheimer’s disease (AD) and its rationale. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR., Jr Alzheimer’s disease: new concepts on its neurobiology and the clinical role imaging will play. Radiology. 2012;263(2):344–361. doi: 10.1148/radiol.12110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langbaum JB, Fleisher AS, Chen K, et al. Ushering in the study and treatment of preclinical Alzheimer disease. Nat Rev Neurol. 2013;9(7):371–381. doi: 10.1038/nrneurol.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging and Alzheimer’s Association Workgroup. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephan BC, Hunter S, Harris D, et al. The neuropathological profile of mild cognitive impairment (MCI): a systematic review. Mol Psychiatry. 2012;17(11):1056–1076. doi: 10.1038/mp.2011.147. [DOI] [PubMed] [Google Scholar]
- 8.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging and Alzheimer’s Association Workgroup. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sosa-Ortiz A, Acosta-Catillo I, Prince MJ. Epidemiology of dementias and Alzheimer’s disease. Arch Med Res. 2012;43(8):600–608. doi: 10.1016/j.arcmed.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 11▪.Mullane K, Williams M. Alzheimer’s therapeutics: continued clinical failures question the amyloid hypothesis – but what lies beyond? Biochem Pharmacol. 2013;85(3):289–305. doi: 10.1016/j.bcp.2012.11.014. Comprehensive review of the failures in the search for effective treatments of AD, the reasons for that outcome and potential ways to overcome the problem. [DOI] [PubMed] [Google Scholar]
- 12▪.Corbett A, Pickett J, Burns A. Drug repositioning for Alzheimer’s disease. Nat Rev Drug Discov. 2012;11(11):833–846. doi: 10.1038/nrd3869. Reports the systematic search of marketed drugs for novel use as AD therapeutic agents, concluding that a high priority should be given to the the antidiabetic GLP-1 analogs. [DOI] [PubMed] [Google Scholar]
- 13.Naylor MD, Karlawish JH, Arnold SE. Advancing Alzheimer’s disease diagnosis, treatment, and care: recommendations from the Ware Invitational Summit. Alzheimers Dement. 2012;8(5):445–452. doi: 10.1016/j.jalz.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪▪.Talbot K, Wang H-Y, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–1338. doi: 10.1172/JCI59903. Supplies the first physiological evidence of brain insulin resistance in AD, identifies its likely proximal causes and biomarkers, and establishes that the likely biomarkers are associated with cognitive decline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Liu F, Grundke-Iqbal I, et al. Deficient brain insulin signalling in Alzheimer’s disease and diabetes. J Pathol. 2011;225(1):54–62. doi: 10.1002/path.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devaskar SU, Giddings SJ, Rajakumar PA, et al. Insulin gene expression and insulin synthesis in mammalian neuronal cells. J Biol Chem. 1994;269(11):8445–8454. [PubMed] [Google Scholar]
- 17▪▪.Kuwabara T, Kagalwala MN, Onuma Y, et al. Insulin biosynthesis in neuronal progenitors derived from adult hippocampus and the olfactory bulb. EMBO Mol Med. 2011;3(12):742–754. doi: 10.1002/emmm.201100177. Gives a definitive demonstration that insulin is synthesized in the adult mammalian brain, specifically granule and/or pyramidal neurons in the neocortex and hippocampal formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unger J, McNeill TH, Moxley RT, et al. Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience. 1989;31(1):143–157. doi: 10.1016/0306-4522(89)90036-5. [DOI] [PubMed] [Google Scholar]
- 19.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136(1):82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Roth D, Hendricks SA, Lesniak MA, et al. Insulin in brain and other extrapancreatic tissues of vertebrates and non-vertebrates. Adv Metab Disord. 1983;10:303–340. doi: 10.1016/b978-0-12-027310-2.50017-7. [DOI] [PubMed] [Google Scholar]
- 21▪▪.Craft S, Cholerton B, Baker LD. Insulin and Alzheimer’s disease: untangling the web. J Alzheimers Dis. 2013;33(Suppl 1):S263–S275. doi: 10.3233/JAD-2012-129042. Provides the most comprehensive and current review of the role dysfunctional insulin signaling in the brain may play in producing many of the major abnormalities in AD. [DOI] [PubMed] [Google Scholar]
- 22.Ghasemi R, Haeri A, Dargahi L, et al. Insulin in the brain: sources, localization and functions. Mol Neurobiol. 2013;47(1):145–171. doi: 10.1007/s12035-012-8339-9. [DOI] [PubMed] [Google Scholar]
- 23.Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:476279. doi: 10.1155/2010/476279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folli F, Bonfanti L, Renard E, et al. Insulin receptor substrate-1 (IRS-1) distribution in the rat central nervous system. J Neurosci. 1994;14(11):6412–6422. doi: 10.1523/JNEUROSCI.14-11-06412.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivera EJ, Goldin A, Fulmer N, et al. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8(3):247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 26.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease – is this Type 3 diabetes? J Alzheimers Dis. 2005;7(1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 27▪.Moloney AM, Griffin RJ, Timmons S, et al. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurbiol Aging. 2010;31(2):224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. Reports some of the first clear evidence for brain insulin resistance in AD and for a role of IRS-1 serine phosphorylated insulin (pS) in that phenomenon. [DOI] [PubMed] [Google Scholar]
- 28.Wang H-Y, Bakshi K, Frankfurt M, et al. Reducing amyloid-related Alzheimer’s disease pathogenesis by a small molecule targeting filamin A. J Neurosci. 2012;32(29):9773–9784. doi: 10.1523/JNEUROSCI.0354-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Espinosa A, Alegret M, Valero S, et al. A longitudinal follow-up of 550 mild cognitive impairment patients: evidence for large conversion to dementia rates and detection of major risk factors involved. J Alzheimers Dis. 2013;34(3):769–780. doi: 10.3233/JAD-122002. [DOI] [PubMed] [Google Scholar]
- 30.Craft S, Peskind E, Schwartz MW, et al. Cerebrospinal fluid and plasma levels in Alzheimer’s disease, relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50(1):164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 31.De Felice FG. Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. J Clin Invest. 2013;123(2):531–539. doi: 10.1172/JCI64595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujisawa Y, Sasaki K, Akiyama K. Increased insulin levels after OGTT load in peripheral blood and cerebrospinal fluid of patients with dementia of Alzheimer type. Biol Psychiatry. 1991;30(12):1219–1228. doi: 10.1016/0006-3223(91)90158-i. [DOI] [PubMed] [Google Scholar]
- 33.Molina JA, Jiménez-Jiménez FJ, Vargas C, et al. Cerebrospinal fluid levels of insulin in patients with Alzheimer’s disease. Acta Neurol Scand. 2002;106(6):347–350. doi: 10.1034/j.1600-0404.2002.01326.x. [DOI] [PubMed] [Google Scholar]
- 34.Frölich L, Blum-Degen D, Bernstein H-G, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm. 1998;105(4–5):423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- 35.Ho L, Yemul S, Knable L, et al. Insulin receptor expression and activity in the brains of nondiabetic sporadic Alzheimer’s disease cases. Int J Alzheimers Dis. 2012;2012:321280. doi: 10.1155/2012/321280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jack CR, Jr, Vemuri P, Wiste HJ, et al. Evidence for ordering of Alzheimer disease biomarkers. Arch Neurol. 2011;68(12):1526–1535. doi: 10.1001/archneurol.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Straub JE, Thirumalai D. Toward a molecular theory of early and late events in monomer to amyloid fibril formation. Ann Rev Phys Chem. 2011;62:437–463. doi: 10.1146/annurev-physchem-032210-103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsumura S, Shinoda K, Yamada M, et al. Two distinct amyloid-β-protein (Aβ) assembly pathways leading to oligomers and fibrils identified by combined fluorescence correlation spectroscopy, morphology, and toxicity analyses. J Biol Chem. 2011;286(13):11555–11562. doi: 10.1074/jbc.M110.181313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferretti MT, Cuello AC. Does a pro-inflammatory process precede Alzheimer’s disease cognitive impairment. Curr Alzheimer Res. 2011;8(2):164–174. doi: 10.2174/156720511795255982. [DOI] [PubMed] [Google Scholar]
- 40.Heneka MT, O’Banion KO, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer’s disease. J Neural Transm. 2010;117(8):919–947. doi: 10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- 41▪.Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–678. doi: 10.1038/nature11729. Shows that amyloid-β (Aβ) effects on AD pathology and cognitive deficits in an animal model of AD are dependent on Aβ microglial activation, which results in release of cytokines that others found to activate IRS-1 serine kinases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Q-L, Yang F, Rosario ER, et al. β-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by fatty acids and curcumin. J Neurosci. 2009;29(28):9078–9089. doi: 10.1523/JNEUROSCI.1071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43▪▪.Bomfim TR, Forny-Germano L, Sathler LB, et al. An antidiabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Aβ oligomers. J Clin Invest. 2012;122(4):1339–1353. doi: 10.1172/JCI57256. Demonstrates that Aβ oligomers trigger elevated IRS-1 pS in hippocampal neuron cultures via the cytokine TNF-α and that this effect was blocked by the GLP-1 analog exenatide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. 2009;296(4):e581–e591. doi: 10.1152/ajpendo.90437.2008. [DOI] [PubMed] [Google Scholar]
- 45▪▪.Ho L, Qin W, Pompl PN, et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J. 2004;18(7):902–904. doi: 10.1096/fj.03-0978fje. Shows that peripheral insulin resistance can induce brain insulin resistance and exacerbate AD pathology and cognitive impairment in an animal model of AD. [DOI] [PubMed] [Google Scholar]
- 46.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67(6):505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118(9):2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akash MS, Rehman K, Chen S. Role of inflammatory mechanism in pathogenesis of Type 2 diabetes mellitus. J Cell Biochem. 2013;114(3):525–531. doi: 10.1002/jcb.24402. [DOI] [PubMed] [Google Scholar]
- 49.Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood–brain barrier. Neuroimmunomodulation. 2012;19(2):121–130. doi: 10.1159/000330247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamaki C, Ohtsuki S, Terasaki T. Insulin facilitates the hepatic clearance of plasma amyloid-β-peptide (1–40) by intracellular translocation of low-density lipoprotein receptor-related protein 1 (LRP-1) to the plasma membrane in hepatocytes. Mol Pharmacol. 2007;72(4):850–855. doi: 10.1124/mol.107.036913. [DOI] [PubMed] [Google Scholar]
- 51.Marques MA, Kulstad JJ, Savard CE. Peripheral amyloid-β levels regulate amyloid-β clearance from the central nervous system. J Alzheimers Dis. 2009;16(2):325–329. doi: 10.3233/JAD-2009-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bullard KM, Saydah SH, Imperatore G, et al. Secular changes in U.S. prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National Health and Nutrition Examination Surveys 1999–2010. Diabetes Care. 2013;36(8):2286–2293. doi: 10.2337/dc12-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng YJ, Imperatore G, Geiss LS. Secular changes in the age-specific prevalence of diabetes among U.S. adults, 1988–2010. Diabetes Care. 2013;36(9):2690–2696. doi: 10.2337/dc12-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Eng J Med. 2013;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 55▪.Talbot K. Brain insulin resistance in Alzheimer’s disease and its potential treatment with a Mediterranean diet and GLP-1 analogues. Psychiatr Times. 2013 Reviews the literature on dietary means of lowering peripheral insulin resistance, slowing age-related cognitive decline and protecting against Aβ pathology, including elevations in IRS-1 pS. [Google Scholar]
- 56.Balsomo S, Willardson JM, de Frederico SS, et al. Effectiveness of exercise on cognitive impairment and Alzheimer’s disease. Int J Gen Med. 2013;6:387–391. doi: 10.2147/IJGM.S35315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown BM, Peiffer JJ, Martins RN. Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol Psychiatry. 2013;18(8):864–874. doi: 10.1038/mp.2012.162. [DOI] [PubMed] [Google Scholar]
- 58.Solfrizzi V, Frisardi V, Seripa D, et al. Mediterranean diet in predementia and dementia syndromes. Curr Alzheimer Res. 2011;8(5):520–542. doi: 10.2174/156720511796391809. [DOI] [PubMed] [Google Scholar]
- 59.Cole GM, Ma Q-L, Frautschy SA. Omega-3 fatty acids and dementia. Prostoglandins Leukot Essent Fatty Acids. 2009;81(2–3):213–221. doi: 10.1016/j.plefa.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gillette-Guyonnet S, Secher M, Vellas B. Nutrition and neurodegeneration: epidemiological evidence and challenges for future research. Brit J Clin Pharmcol. 2013;75(3):738–755. doi: 10.1111/bcp.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61▪.Imfeld P, Bodmer M, Jick SS, et al. Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J Am Geriatr Soc. 2012;60(5):916–921. doi: 10.1111/j.1532-5415.2012.03916.x. Shows that long-term users of many antidiabetics (not including GLP-1 analogs) do not have a reduced risk of developing AD and that long-term use of metformin may actually raise such a risk. [DOI] [PubMed] [Google Scholar]
- 62▪.Miller BW, Willett KC, Desilets AR. Rosiglitazone and pioglitazone for the treatment of Alzheimer’s disease. Ann Pharmacother. 2011;45(11):1416–1424. doi: 10.1345/aph.1Q238. Reviews studies that tested the effects of the insulin sensitizers rosiglitazone and pioglitazone on AD, and concludes that they have no clear clinical benefit and that they pose safety concerns. [DOI] [PubMed] [Google Scholar]
- 63.Hernandez AV, Usmani A, Rajamanickam A, Moheet A. Thiazolidinediones and risk of heart failure in patients with or at high risk of Type 2 diabetes mellitus. Am J Cardiovasc Drugs. 2011;11(2):115–128. doi: 10.2165/11587580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Shemesh E, Rudich A, Harman-Boehm I, et al. Effect of intranasal insulin on cognitive function: a systematic review. J Clin Endocrinol Metab. 2012;97(2):366–376. doi: 10.1210/jc.2011-1802. [DOI] [PubMed] [Google Scholar]
- 65.Freiherr J, Hallschmid M, Frey WH, 2nd, et al. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs. 2013;27(7):505–514. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 67.Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas. Neuroscience. 2011;180:111–121. doi: 10.1016/j.neuroscience.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salcedo I, Tweedie D, Li Y, Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Brit J Pharmacol. 2012;166(5):1586–1599. doi: 10.1111/j.1476-5381.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duarte AI, Candeias E, Correia SC, et al. Crosstalk between diabetes and brain: glucagon-like peptide-1 mimetics as a promising therapy against neurodegeneration. Bicohim Biophys Acta. 2013;1832(4):527–541. doi: 10.1016/j.bbadis.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixesenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. doi: 10.1186/1471-2202-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao H, Wang X, Zhang Z, et al. GLP-1 amplifies insulin signaling by upregulation of IRβ, IRS-1, and GLUT4 in 3T3-L1 adipocytes. Endocrine. 2007;32(1):90–95. doi: 10.1007/s12020-007-9011-4. [DOI] [PubMed] [Google Scholar]
- 72▪.Li L, Yang G, Li Q. Exenatide prevents fat-induced insulin resistance and raises adiponectin expression and plasma levels. Diabetes Obes Metab. 2008;10(10):921–930. doi: 10.1111/j.1463-1326.2007.00832.x. Reports laboratory tests on obese rats demonstrating that exenatide significantly reduces peripheral insulin resistance. [DOI] [PubMed] [Google Scholar]
- 73▪.Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride for Type 2 diabetes (LEAD-3 Mono): randomized, 52-week, Phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–481. doi: 10.1016/S0140-6736(08)61246-5. Reports clinical trial results demonstrating that liraglutide significantly reduces peripheral insulin resistance in early Type 2 diabetics. [DOI] [PubMed] [Google Scholar]
- 74.Boland CL, DeGeeter M, Nuzum DS, Tzefos M. Evaluating second-line treatment options for Type 2 diabetes: focus on secondary effects of GLP-1 agonists and DPP-4 inhibitors. Ann Pharmacother. 2013;47(4):490–505. doi: 10.1345/aph.1R444. [DOI] [PubMed] [Google Scholar]
- 75.Peters KR. Liraglutide for the treatment of Type 2 diabetes: a clinical update. Am J Therap. 2013;20(2):178–188. doi: 10.1097/MJT.0b013e3182204c16. [DOI] [PubMed] [Google Scholar]
- 76.Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract. 2012;98(2):271–284. doi: 10.1016/j.diabres.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 77.Monami M, Dicembrini I, Marchionni N, et al. Effects of glucagon-like peptide-1 receptor agonists on body weight: a meta-analysis. Exp Diabetes Res. 2012;344:d7771. doi: 10.1155/2012/672658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Rel Disord. 2003;27(3):313–318. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- 79.Hamilton A, Hölscher C. Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system. NeuroReport. 2009;20(13):1161–1166. doi: 10.1097/WNR.0b013e32832fbf14. [DOI] [PubMed] [Google Scholar]
- 80▪▪.McClean PL, Parthsarathy V, Faivre E, Hölscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci. 2011;31(17):6587–6594. doi: 10.1523/JNEUROSCI.0529-11.2011. Demonstrates that liraglutide markedly improves many basic pathological features of AD in an animal model of that disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McLean PL, Hölscher C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology. 2014;76(Pt A):57–67. doi: 10.1016/j.neuropharm.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 82.Long-Smith CM, Manning S, McLean PL, et al. The diabetes drug liraglutide ameliorates aberrant insulin receptor localization and signalling in parallel with decreasing both amyloid-β plaque and glial pathology in a mouse model of Alzheimer’s disease. Neuromolecular Med. 2013;15(1):102–114. doi: 10.1007/s12017-012-8199-5. [DOI] [PubMed] [Google Scholar]
- 83.Wang H-Y, Stucky A, Kvasic J, et al. The diabetes drug liraglutide ameliorates insulin resistance in the hippocampal formation of the APP/PS1 model of Alzheimer’s disease (AD) Soc Neurosci. 2012:Abstract 749.29. [Google Scholar]
- 84.Villemagne VL, Rowe CC. Long night’s journey into the day: amyloid-β imaging in Alzheimer’s disease. J Alzheimers Dis. 2013;33(Suppl 1):S349–S359. doi: 10.3233/JAD-2012-129034. [DOI] [PubMed] [Google Scholar]
- 85.Hölscher C. Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection. CNS Drugs. 2012;26(10):871–882. doi: 10.2165/11635890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 86▪▪.Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123(6):2730–2736. doi: 10.1172/JCI68295. Provides the first clinical evidence that a GLP-1 analog improves cognition in a neurodegenerative disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bosco D, Plastino M, Cristiano D, et al. Dementia is associated with insulin resistance in patients with Parkinson’s disease. J Neurol Sci. 2012;315(1–2):39–43. doi: 10.1016/j.jns.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 88.Faivre E, Holscher C. Neuroprotective effects of D-Ala2GIP on Alzheimer’s disease biomarkers in an APP/PS1 mouse model. Alzheimer’s Res Ther. 2013;5:20. doi: 10.1186/alzrt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Finan B, Ma T, Ottaway N, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5:209ra151. doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- 90.Zhao W-Q, Lacor PN, Chen H, et al. insulin receptor dysfunction impairs clearance of neurotoxic oligomeric Aβ. J Biol Chem. 2009;284(28):18742–18753. doi: 10.1074/jbc.M109.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]