Abstract
Ozone is a commonly encountered environmental oxidant which has been linked to asthma exacerbation in epidemiological studies. Ozone induces airway inflammation and enhances response to inhaled allergen. It has been suggested that antioxidant therapy may minimize the adverse effects of ozone in asthma. We have previously shown that the antioxidant gamma-tocopherol (γT), an isoform of vitamin E, also has anti-inflammatory effects. We employed a Brown Norway rat model of ozone-enhanced allergic responses to test the therapeutic effects of γT on O3-induced airway inflammation. Ovalbumin (OVA) -sensitized rats were intranasally challenged with 0 or 0.5% OVA on Days 1 and 2, and exposed to 0 or 1 ppm ozone (8h/day) on Days 4 and 5. Rats were also given 0 or 100 mg/kg γT on Days 2 through 5. Pulmonary tissue and bronchoalveolar lavage fluid (BALF) were collected on Day 6. OVA challenge caused increased total cells (267% increase) and eosinophils (4000%) in BALF that was unaffected by ozone exposure. Morphometric evaluation of lung tissue revealed increases in intraepithelial mucosubstances (IM) (300%) and subepithelial eosinophils (400%) in main axial airways. Ozone exposure of allergic rats enhanced IM increases in proximal axial airways (200%), induced cys-leukotrienes, MCP-1 and IL-6 production in BALF, and upregulated expression of IL-5 and IL-13 mRNA. γT treatment had no effect on IM increases by allergen, but blocked enhancement by ozone. γT attenuated both OVA- or ozone –stimulated eosinophilic infiltration, and increases of BALF cys-leukotrienes, MCP-1 and IL-6, as well as IL-5 and IL-13 mRNA. These data demonstrate broad anti-inflammatory effects of a γT and suggest it may be an effective therapy of allergic airway inflammation.
Keywords: γ-tocopherol, vitamin E=, ozone, ovalbumin, mucous cell metaplasia, eosinophil, inflammation
INTRODUCTION
Epidemiological studies implicate a role for ozone, the most prominent oxidant pollutant in photochemical smog, in the exacerbation of allergic airway diseases. Increases in ambient ozone concentration are associated with hospital admissions and reported symptoms from individuals with asthma and allergic rhinitis [1-3]. In challenge studies of asthmatic subjects, our research group has shown ozone induces eosinophilic infiltration in both upper and lower airways, decrements in pulmonary function, and enhances response to allergen [4-6]. Even in the absence of provocation with an allergen, atopic asthmatics appear to mount greater airway inflammatory responses after ozone exposure than healthy subjects [7, 8].
Specific mechanisms by which ozone exacerbates allergic responses are incompletely understood. Asthma is a disease of chronic inflammation in which an imbalance of oxidant versus antioxidant species is thought to contribute to disease severity and incidence of symptoms. Decreases in antioxidant vitamins E and C, and increases in oxidized glutathione have been reported in airway fluids of asthmatics [9-11]. In addition, enzymatic anti-inflammatory capacity in the lung and plasma of asthmatics is depressed as indicated by lower activities of glutathione peroxidase, superoxide dismutase and catalase [12-14]. Deficient defenses in the airway microenvironment could allow for increased sensitivity to further oxidative insult by ozone exposure. Deficits in the antioxidant repertoire in allergic airways may be due to a lower dietary intake of antioxidants compounds such as from fruits and vegetables, especially in persons with genetically defined decreases in antioxidant defense. In case studies, intake of vitamins C, E and carotene by asthmatics was inversely correlated with symptom severity [15-17], and asthmatics tend to have lower amounts of antioxidant nutrients in their diets, especially of vitamins E and C [18].
Although α-tocopherol (αT), the major form of vitamin E in mammalian tissues is thought to neutralize lipid radicals, and inhibit cyclooxygenase (COX) and leukotriene synthesis [19], intervention with αT supplements failed to appreciably improve allergic airway symptoms in both human and animal studies [20-22]. However, γ-tocopherol (γT), the major form of vitamin E in US diets, has been demonstrated to be more powerful in trapping electrophiles such as reactive nitrogen species [23, 24] and displays a broader anti-inflammatory profile than αT [25]. In addition to neutralizing oxidized lipid radicals, γT also protects both lipids and proteins from nitrosative damage from nitric oxide-derived metabolites [26, 27] and is a more potent inhibitor of COX activity than αT [28]. Furthermore, in an animal model of inflammation, we have shown that γT is superior to αT for inhibition of key mediators of inflammation such as TNFα, nitric oxide, and inflammatory eicosanoid production [29].
We hypothesized that the γT form of vitamin E would inhibit pollutant and allergen induced exacerbation of allergic airway inflammation. To test this hypothesis, we used a rodent model of allergic airways disease to assess the ability of γT to prevent ozone and allergen-induced exacerbation of airway inflammation. As described herein, we observed that γT attenuated allergen-and ozone-induced inflammatory cell recruitment, cytokine gene expression, and overproduction of mucus that characterizes asthma.
MATERIALS AND METHODS
Animals
Fifty-six male Brown Norway rats (Harlan, Indianapolis, IN), 10-12 weeks of age, were randomly assigned to one of 8 experimental groups (n = 7/group). Rats were free of pathogens and respiratory disease, and used in accordance with guidelines set forth by the All-University Committee on Animal Use and Care at Michigan State University. Animals were housed two per cage in polycarbonate boxes on aspen chip bedding, covered with filter lids, and had free access to tap water and food (Tek Lad 22/5 Rodent Diet W 8649, Harlan Sprague Dawley, Indianapolis, IN). During the inhalation portion of the study, rats were housed individually in rack-mounted stainless steel wire cages in Hazelton whole-body inhalation exposure chambers (HC-100, Lab Products, Maywood, NJ). During ozone exposure, food was removed, but water was available.
γ-Tocopherol
γ-gamma-tocopherol (γT; R,R,R naturally occurring, 96% pure by HPLC and NMR) was obtained from Yasoo Health Inc (Johnson City, TN). Stock and diluted preparations were kept under nitrogen at 4°C and contained no other tocopherols/tocotrienols or oxidation products. Prior to treatment protocols γT was diluted in tocopherol-stripped corn oil (Dyets Inc., Bethlehem, PA).
Ozone Exposure
Ozone was generated with an OREC model O3V1-O ozonizer (Ozone Research and Equipment Corp., Phoenix, AZ) using compressed air as a source of oxygen. Total airflow through the exposure chambers was 250 L/min (15 chamber air changes/h). The concentration of ozone within the chambers was monitored throughout the exposure using two Dasibi 1003 AH ambient air ozone monitors (Dasibi Environmental Corp., Glendale, CA). Sampling probes were placed in the breathing zone of rats within the middle of the cage racks. The concentration of ozone during exposures was 0.994 ±0.012 ppm (mean ± SEM) for ozone chambers and less than 0.02 ppm for chambers receiving filtered air. Ozone concentrations of 1ppm were used to enhance allergic responses in a rat strain that is comparatively less sensitive to ozone than other rats or humans. Pulmonary ozone dosimetry studies demonstrate that humans have 4-5 times greater pulmonary deposition of inhaled ozone than Fisher F344 rats [30], and that rats required greater exposures for similar responses. Furthermore the Brown Norway rat is much less sensitive to ozone than F344 rats. As such, 1 ppm ozone in Brown Norway rats provides a reasonable exposure scenario to what might occur in humans.
Treatment and Exposure Protocols
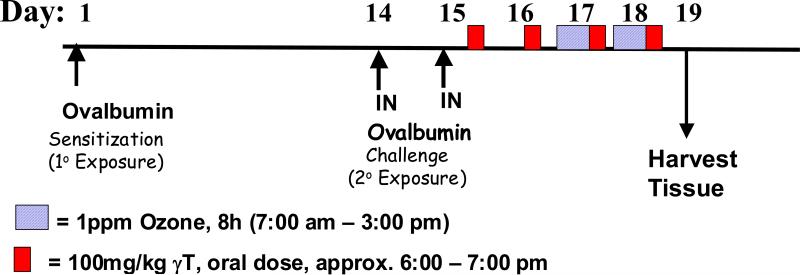
The time course for treatment protocols is summarized in Figure 1. On Day 1, rats were sensitized to chicken albumin (ovalbumin; OVA; Sigma Chemical Co., St. Louis, MO) by intraperitoneal injection with 2.5 ml sterile saline of a solution containing 1 mg OVA prepared with 10 mg alum (aluminum potassium sulfate) which acts as an adjuvant. Two weeks later rats were challenged to OVA or saline for two consecutive days (i.e., Days 14 and 15) under light anesthesia (4% halothane in oxygen) by intranasal instillation (IN) with 150 μl of 0 or 0.5% OVA in saline into each nasal passage (300 μl total volume). IN challenge procedures were conducted at 10:00am each day.
Figure 1. Experimental Protocol.
Ovalbumin-sensitized Brown Norway rats were challenged with intranasal ovalbumin (Days 14, 15) and then exposed to 0 or 1.0 ppm ozone (Days 17 & 18). Animals were given γT of vehicle beginning after challenge daily for the duration of the study (Days 15-18).
Beginning 8h after the last IN challenge, rats were administered by oral gavage, 100 mg/kg body weight γT prepared in tocopherol-stripped corn oil. Treatment with γT was repeated daily at 6:00pm for four consecutive days (Days 15-18). Beginning on Day 17, animals were exposed to 0 or 1.0 ppm ozone for 8h/day (7:30am – 3:30pm) for two consecutive days (Days 17 and 18). Animals were euthanized and tissues were collected on Day 19.
Necropsy, Lavage Collection and Tissue Preparation
Animals were anesthetized with sodium pentobarbital (50 mg/kg), a midline laparotomy was performed, 4 ml of blood was drawn into a vacutainer for separation of plasma, and animals were exsanguinated by cutting the abdominal aorta. Immediately after death, the trachea was exposed and cannulated, the heart and lung were excised en bloc. The bronchus to the left lung was temporarily closed with a hemostatic clamp, and 4 ml of sterile saline was instilled through the tracheal cannula and withdrawn to recover bronchoalveolar lavage fluid (BALF) from the right lung lobes. A second saline lavage was performed and combined with the first.
After lavage, the right lung lobes were ligated and removed. The axial conducting airway from the right caudal lobe was removed by microdissection and homogenized in 0.5 ml RNAlater (Qiagen Inc., Valencia, CA) using a post-mounted homogenizer with a 5-mm generator (Model 250, Pro-Scientific, Inc., Monroe, CT). The right cranial lobe was also excised and placed in RNAlater. Samples were kept at –80°C until further processing for RNA isolation. The clamp was removed from the left bronchus, and the left lobe was inflated under constant pressure (30 cm H2O) with neutral-buffered formalin for 2h, while immersed in a large volume of fixative. Twenty-four hours later, two sections were excised at the level of the 5th and 11th airway generation along the main axial airway (G5 and G11), to sample proximal and distal airways, respectively (Harkema and Hotchkiss, 1992).
Tissue blocks were then embedded in paraffin, and 5-6 μm thick sections were cut from the anterior surface. Lung sections were stained with hematoxylin and eosin (H&E) for routine histology, with Alcian Blue (pH 2.5)/Periodic Acid-Schiff (AB/PAS) to detect intraepithelial mucosubstances, or with May Grunwald stain to detect eosinophils.
Bronchoalveolar Lavage Fluid (BALF)
Cellularity
Total leukocytes in BALF were enumerated with a hemocytometer, and fractions of eosinophils, neutrophils, macrophages, and lymphocytes were determined in a cytospin sample stained with Diff-Quick (Dade Behring, Newark, DE).
Cytokine and Leukotriene Content
For leukotriene analysis, cell-free BAL fluid was first purified in 2mL methanol and 5mL hexane to precipitate proteins and lipid, respectively. The methanol layer containing leukotrienes was dried under N2 stream. The amount of LTB4 and total cysteinyl-leukotrienes (LTC4, LTD4 and LTE4) were measured using an EIA assay from Cayman Chemicals (Ann Arbor, MI). Cytokine content in BAL fluid was determined using a custom bead-based multiplex assay capable of detecting 15 different rat protein targets. Assays are conducted in 96-well MultiScreen plates (Millipore) by the same principle of standard ELISA. Bound protein:antibody conjugates on beads are detected using a Streptavidin-PE system and analyzed using the Luminex100 total system (Luminex Corp, Austin, TX).
Tocopherols
Plasma and BAL fluid αT and γT were extracted using a mixture of methanol/hexane (2:5, v/v) in the presence of 0.8 mM butylated hydroxytoluene (BHT). After brief centrifugation at 4°C, the top hexane layer was dried under N2 and the residue was resuspended in ethanol. Tocopherols were separated on a 150 × 4.6 mm, 5 μm Supelcosil™ LC-18-DB column (Supelco, Bellefonte, PA, USA) and eluted with 95:5 (v/v) methanol/0.1M lithium acetate (final 25 mM, pH 4.75) at a flow rate of 1.3 mL/min. Tocopherols were monitored by coulometric detection (Model Coulochem II, ESA Inc., Chelmsford, MA, USA) at 300 (upstream) and 500 mV (downstream electrode) using a Model 5011 analytical cell.
Real Time RT-PCR
Total RNA was extracted using Rneasy Mini Kit according to manufacturers instructions (Qiagen, Valencia, CA). The evaluation of relative expression levels of IL-5 and –13 mRNA was performed by two-step RT-PCR using manufacturer protocols (Applied Biosystems, Foster City, CA). Briefly, reverse transcription was performed using High Capacity cDNA archive Kit reagents. Quantitative mRNA expression analysis was performed using an ABI PRISM 7900 HT Sequence Detection System at Michigan State University's Research Technology Support Facility using Taqman Gene Expression Assay reagents (Applied Biosystems). Relative gene expression was normalized to 18S. Data is expressed as fold-increase in RNA expression compared to control animals, which are set at a value of 1.
Morphometry of Stored Intraepithelial Mucosubstances (IM)
To estimate the amount of the intraepithelial mucosubstances (IM) in respiratory epithelium lining pulmonary axial airways, the volume density (Vs) of AB/PAS-stained mucosubstances was quantified using computerized image analysis and standard morphometric techniques. The area of AB/PAS stained mucosubstance was calculated from the automatically circumscribed perimeter of stained material using the public domain NIH Image program (written by Wayne Rasband, U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). The length of the basal lamina underlying the surface epithelium was calculated from the contour length of the digitized image of the basal lamina. The volume density (Vs) of intraepithelial mucosubstances is expressed as nanoliters of intraepithelial mucosubstances per mm2 of basal lamina.
Morphometry of Tissue Eosinophil Numeric Density
The numeric density of eosinophils in the mucosa underlying the respiratory epithelium of conducting pulmonary airways, were determined by counting May-Grunwald-positive stained cells with distinct eosinophil morphology. The length of the basal lamina was calculated from its contour length in a digitized image as described above, and the data is expressed as the number of eosinophils divided by the length of the underlying basal lamina.
Statistical Analysis
Data are expressed as mean ± standard error of the mean (SEM). Data were analyzed using a completely randomized analysis of variance. Multiple comparisons were made by Student-Newman-Keuls post hoc test. Criterion for significance was taken to be p ≤ 0.05.
RESULTS
Tocopherol Content in Plasma and BALF
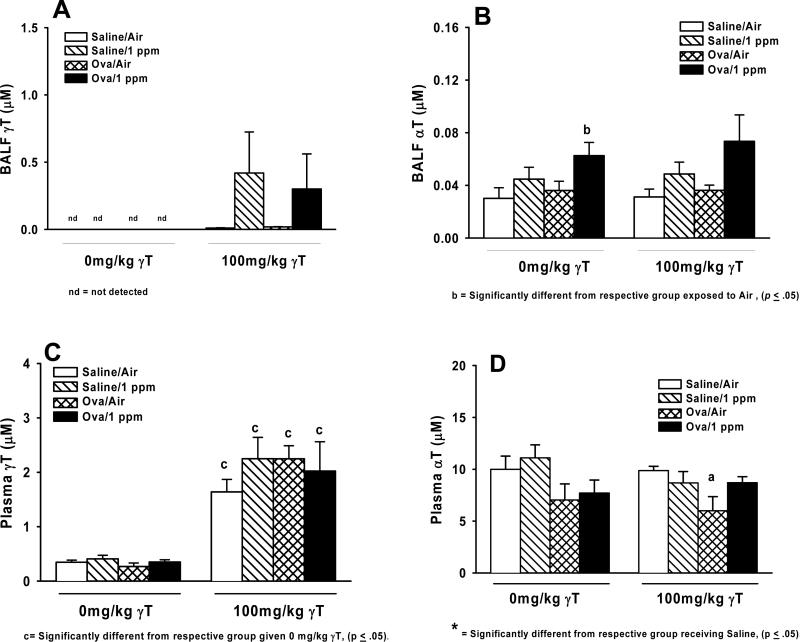
γT was not detected in BALF from corn oil vehicle treated rats regardless of ozone exposure or allergen challenge (Figure 2A). Administration with γT produced measurable levels of this tocopherol in all BALF samples, but there were no differences in any treatment group compared to saline/air controls. αT was detected in all BALF samples (Figure 2B). Treatment related changes were detected only in ozone-exposed allergic rats given 0 mg/kg γT (Figure 2B), while apparent increases in similarly ozone-treated rats given 100 mg/kg γT were not statistically significant (p=0.053).
Fig 2. Tissue tocopherol content.
Normal and allergic Brown Norway rats were exposed to 0 or 1.0 ppm ozone and treated orally with 0 or 100 mg/kg γT as described previously. Plasma and bronchoalveoler lavage fluid was collected and analyzed for content of α- and γ-tocopherol by HPLC as described in Materials and Methods. Data are expressed as mean ± SEM. a = significantly different from respective group challenged with intranasal saline; b = significantly different from respective group exposed to 0 ppm ozone; c = significantly different from respective group treated with 0 mg/kg γT. Criteria for significance, p ≤ 0.05.
Plasma γT was unaffected by any exposure or challenge regimens in control, corn oil-fed rats, but was increased in all groups 4 to 5-fold by administration with 100 mg/kg γT. No differences were detected between exposure or challenge groups (Figure 2C). Plasma αT was similar in all groups given 0mg/kg γT, with trends toward decreases in OVA-challenged rats (Figure 2D). Allergic animals dosed with 100mg/kg γT and exposed to filtered air had significantly less αT in plasma than saline-challenged, air-exposed rats.
Histopathology
Rats instilled with saline and exposed to ozone
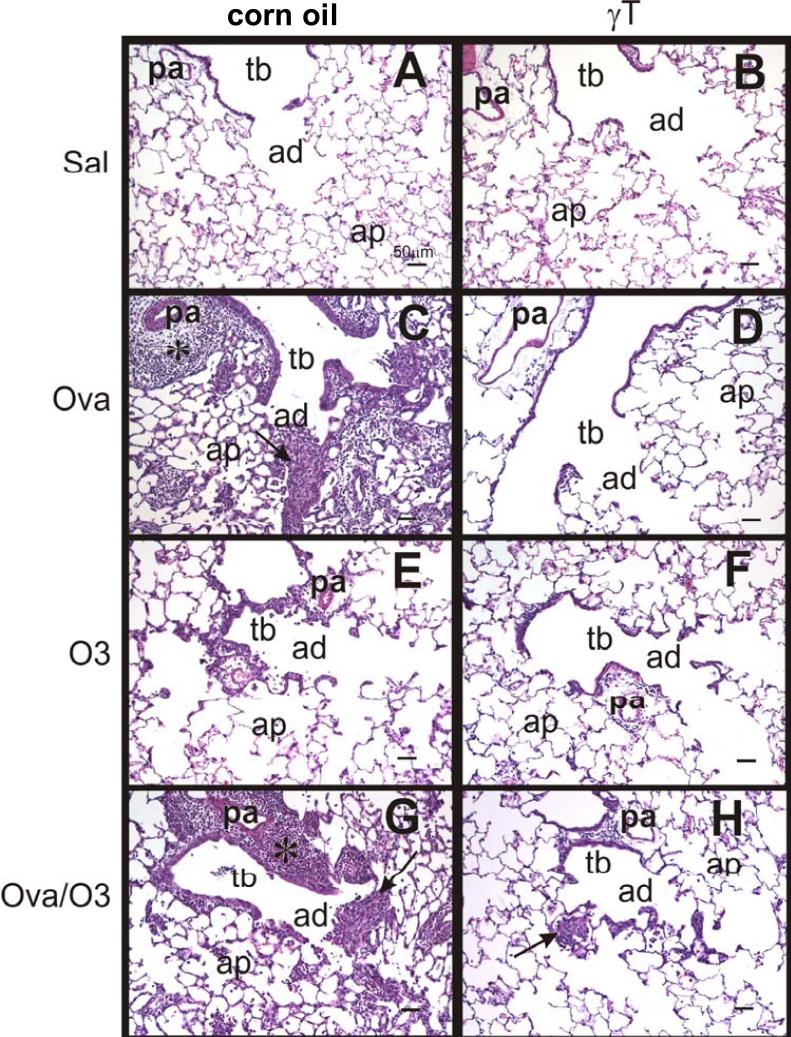
No exposure related lesions were found in the lungs of filtered air-exposed rats that were intranasally instilled with only saline. In contrast, repeated ozone exposures induced site-specific lung lesions that were restricted to centriacinar regions (junction of terminal bronchioles and proximal alveolar ducts) throughout the lung lobe (Figure 3E). These centriacinar lesions were characterized by a conspicuous thickening of the walls of terminal bronchioles and proximal alveolar ducts with associated small aggregates of alveolar macrophages/monocytes and lesser numbers of neutrophils, eosinophils and lymphocytes in the alveolar airspaces of the proximal alveolar ducts. Intramural thickening was mainly due to 1) a mild-moderate influx of mixed inflammatory cells consisting of neutrophils, eosinophils, and mononuclear inflammatory cells (lymphocytes and monocytes) and 2) an associated hypertrophy and hyperplasia of cuboidal epithelial cells lining the centriacinar airways (Figure 3E). Mucous cell hyperplasia/metaplasia, a principal feature in the conducting airway epithelium of ovalbumin-instilled rats, was not present in the large- and small-diameter conducting airways of these saline-instilled and ozone-exposed rats. The alveolar parenchyma outside of the centriacinar regions resembled that of the control rats instilled with saline and exposed only to filtered air (0 ppm ozone). Interestingly, treatment with γT did not alter the morphologic character or severity of the centriacinar lesions caused by the ozone exposures (Figure 3F).
Figure 3.
Photomicrographs of the epithelium of conducting airways in lungs of rats challenged with saline (Sal) and treated with either corn oil (A) or γT (B); challenged with Ova and treated with corn oil (C) or γT (D); exposed to ozone (O3) and treated with corn oil (E) or γT (F); or challenged with Ova and exposed to ozone (Ova/O3), and then treated with corn oil (G) or γT (H). Tissues were stained with H&E. pa = pulmonary artery, tb = terminal bronchiole, ad = alveolar duct, ap = alveolar parenchyma.
Rats instilled with ovalbumin (OVA) and exposed to air alone or ozone
Intranasal challenge of OVA in filtered air or ozone-exposed rats induced an allergic bronchiolitis of the intrapulmonary conducting airways involving both large-diameter, proximal, axial airways and small diameter, distal, preterminal and terminal bronchioles. The OVA-induced inflammatory and epithelial changes in the conducting airways within the proximal transverse section of the left lung lobe (axial airway level G5) were more marked than those in the distal lung lobe section (axial airway level G11), indicating a proximal-distal decrease in the lobar lesion severity and distribution. OVA-induced bronchiolitis was characterized by peribronchiolar edema associated with a mixed inflammatory cell influx of lymphocytes, plasma cells, eosinophils, and occasional neutrophils (Figure 3C). Peribronchiolar inflammation was principally located in the subepithelial interstitial tissues (e.g., lamina propria and submucosa) with markedly fewer inflammatory cells in the surface epithelium that lines these airways. Bronchiole-associated lymphoid tissues in these OVA-challenged airways were also enlarged due to lymphoid hyperplasia. Perivascular interstitial (i.e., surrounding pulmonary arteries/arterioles adjacent to bronchioles and pulmonary veins scattered throughout the alveolar parenchyma) accumulation of a similar mixture of eosinophils and mononuclear cells, along with perivascular edema, were also present in the lungs of OVA-challenged rats (Figure 3E).
In addition to the perivascular and peribronchiolar lesions, there were varying sized focal areas of allergic alveolitis in the parenchyma surrounding or adjacent to the affected large and small conducting airways. These alveolar lesions were characterized by accumulations of large numbers of alveolar macrophages, epithelioid cells, and eosinophils, with lesser numbers of lymphocytes, monocytes and plasma cells, in the alveolar airspace. Often the alveolar septa in these areas of alveolitis were thickened due to mild-to-moderate alveolar type II cell hyperplasia/hypertrophy, intracapillary accumulation of inflammatory cells, and capillary congestion.
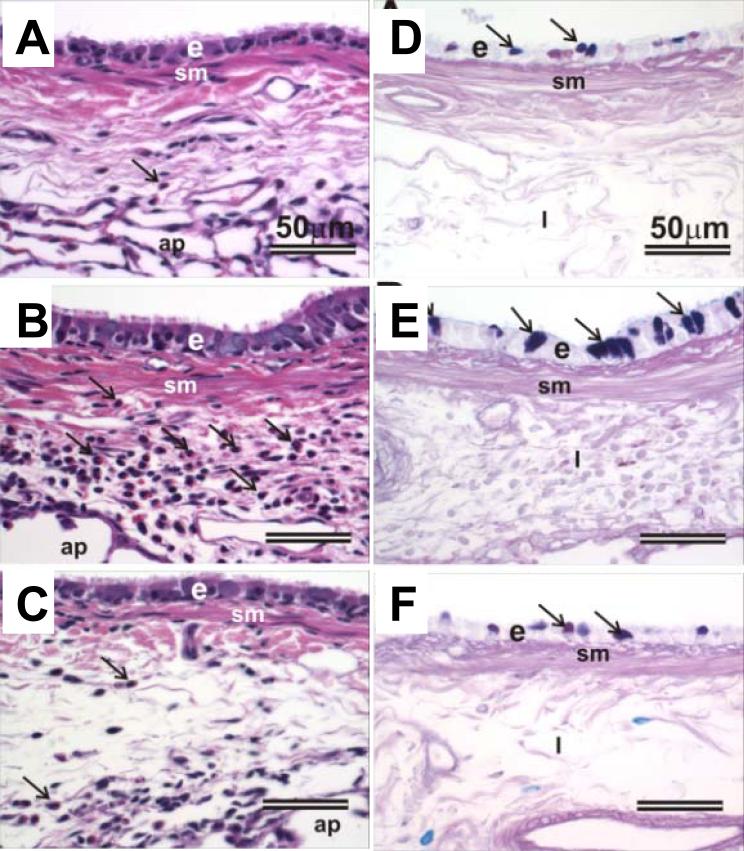
OVA-challenged rats exposed to either filtered air or ozone had conspicuous hypertrophy (tall columnar epithelial cells) and mucous cell metaplasia/hyperplasia (MCM) with increased amounts of AB/PAS-stained intraepithelial mucosubstances (IM) in the respiratory epithelium lining the large-diameter intrapulmonary airways, including the proximal axial airway (G5) and to a much lesser extent in the distal axial airway (G11) and the small-diameter preterminal and terminal bronchioles (Figure 4B, E). In contrast, saline-instilled rats exposed to either filtered air (Figure 4A, D) or ozone had significantly fewer mucous cells and IM in a much thinner, low columnar, respiratory epithelium lining large-diameter airways. Very few or no mucous cells with only scant amounts of IM were present in the respiratory epithelium lining small diameter preterminal bronchioles of these saline-instilled animals. Terminal bronchioles of saline-instilled rats contained no mucous cells. Interestingly, epithelial and inflammatory lesions observed in the centriacini of ozone-exposed rats intranasally instilled with only saline, described above, were not microscopically detectable in the ozone-exposed, OVA-challenged rats, even in those centriacinar regions with little or no OVA-induced lesions (Figure 3G).
Figure 4.
Photomicrographs of the respiratory airway epithelium lining the axial airway in lungs of rats (A,D) challenged with salineand treated corn oil respectively, (B,E) challenged with OVA, exposed to ozone and treated with corn oil, or (C,F) challenged with OVA, exposed to ozone and treated with γT. Tissues were stained with H&E (A,B,C) or AB/PAS (D,E,F) to identify intraepithelial mucosubstances (identified by arrows). e = epithelium; sm = smooth muscle; i = interstitium; arrowheads in H&E =inflammatory cells.
Treatment with γT markedly attenuated OVA-induced epithelial and inflammatory lesions in both the intrapulmonary proximal and distal conducting airways and in the alveolar parenchyma (Figure 3D). Airway epithelial thickening with MCM, peribronchial and perivascular inflammatory cell influx, and alveolitis were all markedly reduced both in severity and distribution in the lungs of OVA-challenged rats that were exposed either to filtered air alone or ozone (Figures 3,4). Surprisingly, centriacinar inflammatory and epithelial lesions similar to those observed in the saline-instilled rats exposed to ozone were also present in the OVA-challenged, ozone-exposed rats that received γT, indicating that γT treatment was effective in attenuating allergen- but not oxidant-induced pulmonary injury in Brown Norway rats (Figure 3H).
Bronchoalveolar Lavage Fluid (BALF)
Cellularity
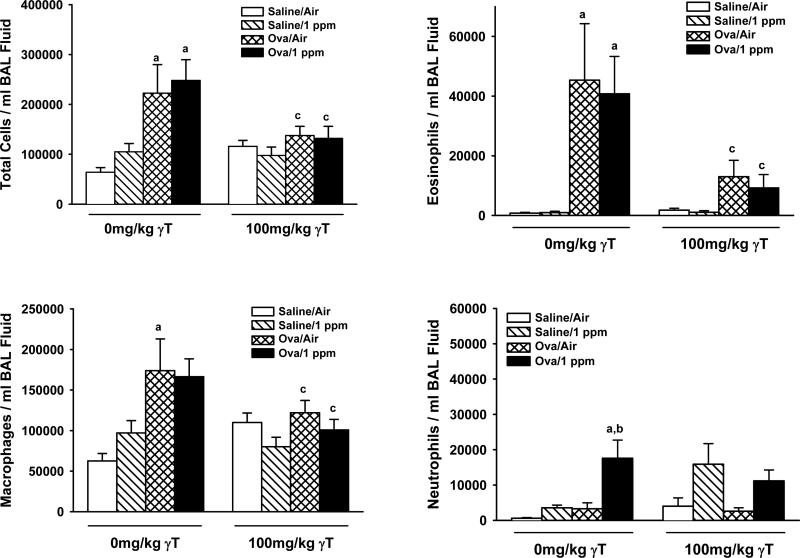
Ozone exposure of saline-challenged rats had no effect on lavage cellularity (Figure 5). Conversely, provocation of allergic rats with OVA induced significant accumulation of total cells, eosinophils, and macrophages in BALF, that was unaffected by ozone exposure. However ozone exposure of OVA-challenged animals caused increases in BALF neutrophils compared to OVA-challenged rats exposed to filtered air (Figure 5). γT supplementation led to a reduction of OVA- and ozone-induced increases in BALF total cells, eosinophils, and macrophages, but not neutrophils. Furthermore, neutrophils were significantly greater in saline-challenged, ozone-exposed animals given γT compared those dosed with corn oil.
Figure 5. Effect of γT on Inflammatory Cells Recovered in Bronchoalveolar Lavage Fluid.
Normal and allergic Brown Norway rats were exposed to 0 or 1.0 ppm ozone and treated orally with 0 or 100 mg/kg γT as described previously. Bronchoalveoler lavage fluid was collected and cell enumerated as described in Materials and Methods. Data are expressed as mean ± SEM. a = significantly different from respective group challenged with intranasal saline; b = significantly different from respective group exposed to 0 ppm ozone; c = significantly different from respective group treated with 0 mg/kg γT. Criteria for significance, p ≤ 0.05.
Leukotrienes and Cytokines
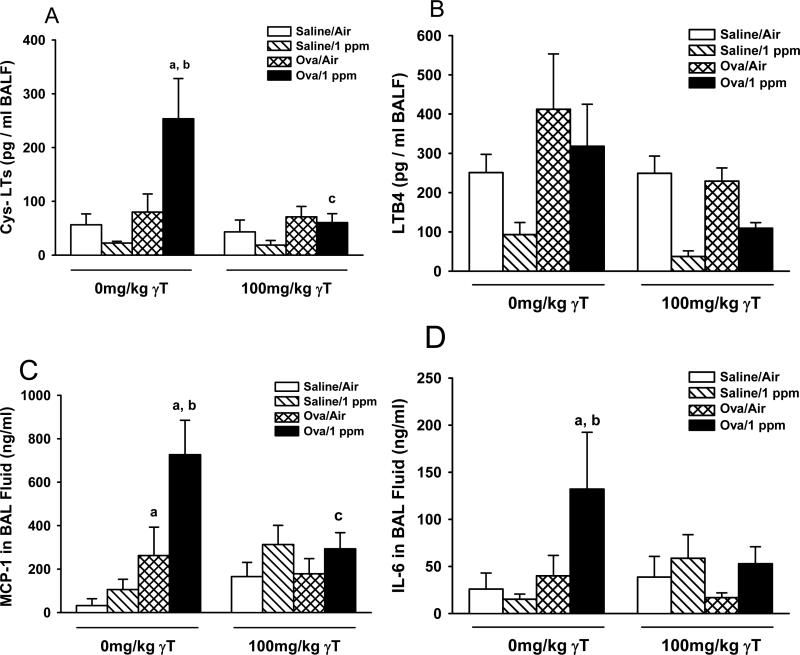
Ozone exposure or OVA challenge alone had no effect on cysteinyl leukotrienes (Cys-LTs) levels in BALF (Figure 6A). However ozone exposure to allergic rats increased Cys-LTs by 3-4 fold over controls, which was blocked completely by γT supplementation. Increases in BALF LTB4 in allergic rats were not statistically significant, irrespective of ozone exposure (Figure 6B). γT caused a reduction of LTB4 in allergic rats exposed to ozone, compared to similarly treated rats given corn oil.
Figure 6. Soluble Inflammatory Mediators in BALF.
Normal and allergic Brown Norway rats were exposed to 0 or 1.0 ppm ozone and treated orally with 0 or 100 mg/kg γT as described previously. Bronchoalveoler lavage fluid was collected and analyzed for cytokine and leukotrienes as described in Materials and Methods. Data are expressed as mean ± SEM. a = significantly different from respective group challenged with intranasal saline; b = significantly different from respective group exposed to 0 ppm ozone; c = significantly different from respective group treated with 0 mg/kg γT. Criteria for significance, p ≤ 0.05
BALF MCP-1 was unaffected by ozone exposure alone but was increased 4-fold in OVA-challenged rats (Figure 6C). Ozone exposure further enhanced MCP-1in allergic rats by another 3-fold. Only allergic rats exposed to ozone had significant amounts of IL-6 in BALF. Both ozone-induced responses in BALF MCP-1 and IL-6 were attenuated by supplementation with γT.
Morphometry
Mucous Cell Metaplasia and Eosinophilic Inflammation
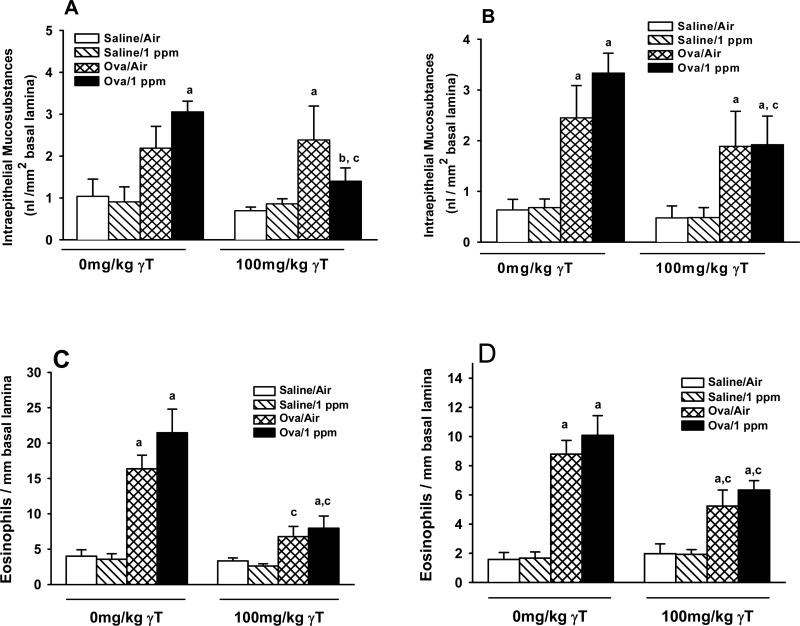
Two consecutive days of ozone exposure did not alter the amount of IM in pulmonary airways (Figure 7A & B). Challenge with OVA caused mucous cell metaplasia (i.e., increased IM) in the respiratory epithelium lining distal pulmonary axial airways (Figure 7B), while apparent increases in IM in proximal airways were not statistically significant (Figure 7A). However, ozone exposure caused IM levels to be significant in proximal airways of allergic rats, with a trend toward further increases in distal airways (p=0.21). Treatment with γT attenuated the increases in IM in both proximal and distal airways of ozone-exposed allergic rats. By contrast, OVA-induced increases in IM remained elevated in γT-treated, OVA-challenged rats exposed to filtered air.
Figure 7. Effect of γT on Ozone-Induced Enhancement of Allergic Mucous Cell Metaplasia and Eosinophilic Inflammation in Pulmonary Axial Airways.
Normal and allergic Brown Norway rats were exposed to 0 or 1.0 ppm ozone and treated orally with 0 or 100 mg/kg γT as described previously. Lungs sections from proximal (A) and distal (B) axial airways were stained with AB/PAS and intraepithelial mucosubstances were morphometrically analyzed as described in Materials and Methods. Lungs stained with May Grunwald were used to quantify eosinophils in proximal (C) and distal (D) axial airways. Data are expressed as mean ± SEM. a = significantly different from respective group challenged with intranasal saline; b = significantly different from respective group exposed to 0 ppm ozone; c = significantly different from respective group treated with 0 mg/kg γT. Criteria for significance, p ≤ 0.05.
OVA challenge induced a 4- to 5-fold increase in the number of eosinophils in the submuscosa surrounding proximal and distal axial airways (Figures 7C & D). Ozone exposures induced a non-significant trend for increased eosinophil accumulation in allergic rats. In both air- and ozone-exposed allergic rats given γT, eosinophil numbers were attenuated in both proximal and distal airways.
Gene Expression
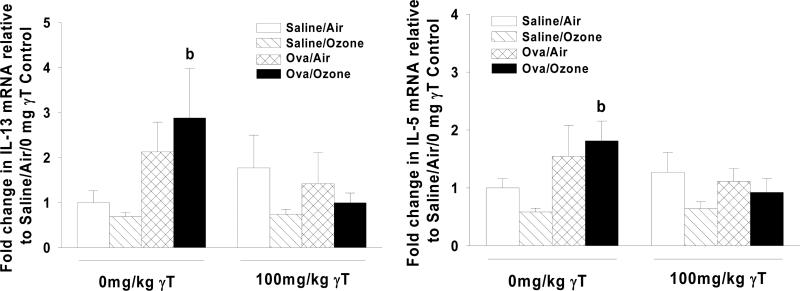
Allergic rats exposed to ozone were characterized by higher expression levels of inflammatory genes IL-5 (1.9-fold) and IL-13 (2.9-fold) in the cranial lung lobe (Figure 8). Changes induced by allergen alone were not statistically different from those detected in control animals. Supplementation with γT inhibited ozone-induced changes in gene expression for both IL-5 and IL-13.
Figure 8. Effect of γT on Inflammatory Gene Expression in Lung Tissue.
Normal and allergic Brown Norway rats were exposed to 0 or 1.0 ppm ozone and treated orally with 0 or 100 mg/kg γT as described previously. RNA was isolated from the cranial lung lobe and analyzed by RT-PCR as described in Materials and Methods. Data are expressed as mean ± SEM. a = significantly different from respective group challenged with intranasal saline; b = significantly different from respective group exposed to 0 ppm ozone; c = significantly different from respective group treated with 0 mg/kg γT. Criteria for significance, p ≤ 0.05.
DISCUSSION
This study was designed to determine if γT inhibited ozone-induced exacerbation of allergic inflammation in a rodent model of allergic inflammation. Our models using the allergic Brown Norway rat mimics the human clinical condition in which asthma exacerbations are induced by ozone exposure. In the present protocol, we generally found that ozone alone had little effect on airway inflammation of OVA-sensitized rats, but challenge with OVA alone or OVA plus ozone had remarkable effects on many elements of allergen-induced inflammation. Intriguingly, ozone did not appear to augment cellular responses to OVA (eosinophil influx), but dual exposure to both agents was necessary to induce increases in inflammatory mediators, gene expression and mucous cell metaplasia.
We found that short-term acute supplementation with γT inhibited inflammatory changes in airways of OVA-sensitized rats, whether they were challenged with OVA alone, or with OVA coupled with ozone exposure. Dosing with γT attenuated inflammatory cell recruitment, airway cytokine and leukotriene production, epithelial cell metaplasia, mucous storage, and gene expression. It is noteworthy that the anti-inflammatory effects of γT were observed with acute dosing that occurred after acute challenges with allergen. Thus, it was not necessary to supply γT for several days prior to challenge, suggesting that rapidly induced serum changes in γT, rather than chronic changes in tissue levels of γT or its metabolites were sufficient to protect against inflammatory changes due to ovalbumin or ozone. These novel effects suggest that γT might be an affective adjunct or complementary intervention for asthma.
We used pharmacological doses of 100mg/kg γT in rodents, which extrapolates to about 6-times the human recommended upper limit for the alpha-form of Vitamin E. However preliminary data in our laboratory suggests that antiallergic effects of γT may occur as low as 10mg/kg (data not shown). Although the doses appropriate to humans need to be established, the bioavailability of γT in human tissues are much higher than that in rodents; concentrations of γT in human skin, adipose and muscle tissue are 6-60 fold greater than those in laboratory rodents with similar γT intake [31, 32].
We designed our protocols for γT pretreatment to specifically target airway responses to ozone exposure by dosing with γT after initiating allergic inflammation, but before beginning exposures to ozone. One of our striking findings however, is that γT specifically inhibited allergen-induced responses, despite being administered 10 hours after the second of two airway provocations with allergen. OVA-induced increases in BALF eosinophils and macrophages (Figure 5), MCP-1 (Figure 6), and eosinophilic inflammation surrounding conducting airways (Figure 7), were all attenuated by γT supplementation in air-exposed animals. Furthermore the extent and severity of pulmonary lesions in allergic lungs, including interstitial edema and the involvement of both bronchiolar and alveolar airways, were markedly decreased in rats given γT. These data demonstrate that acute dosing with γT can arrest ongoing pulmonary inflammation that is downstream from the initial allergic stimulus and does not require weeks of dietary intake to produce therapeutic effects. By contrast, the present protocols did not test if γT could prevent responses from the initial interactions of airway allergen, such as uptake by dendritic cells or IgE-mediated activation of alveolar macrophages and mast cells. The possible prophylactic effects of γT on allergic responses are currently under investigation.
In addition to blocking responses engendered by OVA provocation, γT also attenuated all ozone-induced enhancements of allergic responses. Under the current experimental design, γT was administered twice by the time of the first ozone exposure, and administered immediately after each exposure (Figure 1). This acute supplementation regimen was apparently sufficient for pulmonary γT to block any acute oxidant or inflammatory stimuli induced by inhalation with ozone. We have previously described the requirement for neutrophils in the initiation and progression of ozone-induced MCM in a non-allergic model [33], as well as associations of eosinophils and neutrophils with MCM in allergic models [34]. In the present study, eosinophilic infiltrates that were dramatically increased in BALF by allergen were attenuated by supplementation with γT. We also saw a trend for ozone-induced enhancement of tissue eosinophils that was inhibited by γT. Although ozone did not dramatically enhance eosinophil numbers in allergic rats, ozone (or mediators generated after ozone exposure) may activate inflammatory cells that infiltrate tissues after allergen challenge to release factors that promote MCM. This idea is supported by our observation that ozone exposure coupled with OVA challenge (but neither stimulus alone) led to a significant increase in cysteinyl leukotrienes (cysLTs), MCP-1, and IL-6 as indicated by their increases in BALF (Figure 6). These mediators were not present in allergic animals exposed to filtered air, despite significant infiltration of inflammatory cells into lung tissue. Ozone promotes lipoxygenase activity in the lung [35], and cysLTs have been shown to induce MCM in allergic rodents [36, 37]. Eosinophils and IgE-activated mast cells are primary sources of cysLTs in allergic airways. That ozone can activate both mast cells [38] and eosinophils [39] in healthy airways, suggests it may promote allergic MCM by stimulating eosinophils, mast cells and other inflammatory or epithelial cells for enhanced production of inflammagens, including cysLTs. Complete blockade by γT of BALF cysLTs agrees with our previous finding of LOX inhibition by γT [29]. Taken together, the therapeutic effects of γT in ozone-exposed allergic rats may be due to inhibitions of OVA-initiated inflammatory cell recruitment and ozone-induced activation of inflammatory and epithelial airway cells.
Interestingly, γT was effective at resolving the ongoing responses of inflammatory cell infiltration (Figure 5), but had no effect on allergic mucous cell metaplasia (Figure 7). These differential inhibitory effects might be explained by the acute versus chronic stimuli elicited by 1) acute allergen exposure, and then 2) the cascade of cellular and soluble mediators engendered over a period of hours and days. Recurring signals for cell recruitment and activation persist well after the initial allergen stimulus, and are still present when γT dosing begins. In contrast, metaplastic changes to airway epithelium (e.g., mucous cell metaplasia) are a relatively long-lived phenotypic alteration that is initiated by signaling pathways present early after an allergen challenge (e.g., 6-48h). These early pathways of mucin gene expression and metaplasia have been shown to involve intracellular reactive oxygen species [40, 41], production of which during our protocol would likely occur well before supplementation with by γT. Thus, interruption by γT of allergic inflammation versus its lack of effect on allergic mucous cell metaplasia may be dependent on the timing of γT supplementation during allergic processes.
There may be multiple mechanisms that account for inhibition of elements of both allergen- and ozone-induced inflammation by γT, especially given that ozone and allergen-induced inflammation likely involve different mechanisms. Ozone is an oxidant gas, which induces epithelial cell injury and production of ozonated lipid mediators, followed by secondary responses of inflammatory cell recruitment. By comparison, allergic inflammation involves activation of mast cells, lymphocytes, epithelial cells and eosinophils. These multiple cell types are sources of various mediators, including eosinophil-derived granular proteins, peroxidases, leukotrienes and interleukins, and mast cell-derived tryptase, histamine and leukotrienes.
Using γT in models of non-allergic inflammation, we have previously described its ability to inhibit lipoxygenase (LOX) and cyclooxygenase (COX) activities [28, 29]. In the present study we present similar findings of decreased cysLT and a trend for lesser amounts of LTB4 in BAL fluid. Well known as a neutrophil chemoattractant, a critical role for LTB4 has recently been described for the recruitment and activation of IL-13-producing T cells [42]. Both IL-13 and cysLTs promote allergic inflammatory responses, including mucin gene expression [36, 43]. These data suggest that the anti-eicosanoid actions of γT likely account for a significant fraction of the anti-allergic effects we observed in this study.
There are other potential mechanisms by which γT may minimize inflammatory responses in the airway. One potential common inflammatory mechanism that may play a role in both ozone and allergen-induced inflammation is, production of reactive oxygen species (ROS) and formation of lipid peroxides, as these can be induced by either ozone or allergic stimuli. These oxidant mediators are likely targets for the antioxidant activities of tocopherols such as γT and αT, both which can directly neutralize superoxide anion, singlet oxygen and lipid peroxyl radicals, though γT is relatively less potent for quenching most ROS than is αT [27].
However, reactive nitrogen species, such as nitric oxide and peroxynitrite, which lead to the nitration of cellular lipids, proteins and nucleotides also cause significant inflammatory changes and injury in tissues during allergy. The protective effects of γT may be mediated by the neutralization of reactive nitrogen species, such as nitric oxide and peroxynitrite, which lead to the nitration of cellular lipids, proteins and nucleotides. Supporting this idea is the observations that γT (but not αT) is a highly efficient quencher of nitric oxide and peroxynitrite in vitro [26], and that γT can inhibit protein nitration during systemic inflammation in laboratory rodents [44]. This may be very relevant to allergen-induced inflammation, as nitric oxide production during allergic inflammation, primarily from eosinophils, has been implicated in the nitration and inactivation of key anti-inflammatory and protective enzymes, including catalase, superoxide dismutase, and glutathione transferases [14]. It is reasonable therefore to speculate that γT could provide protection from nitrosative stress that occurs in allergic airways with eosinophilia. Though we did not assess markers of ROS or RNS in these studies, this is clearly an important focus for subsequent studies of the actions of γT in asthma.
It has also been reported that γT can upregulate peroxisome proliferator activated receptor – gamma (PPARγ), [45, 46] a nuclear transcription factor that regulates many inflammatory genes including those expressed in the asthmatic tissues. Specifically, PPARγ can modulate eosinophil survival and activation [47], as well as epithelial mucin production [48]. It is possible that the relatively rapid effects of γT against allergen-induced inflammation could be mediated by an acute activation of PPARγ to inhibit signaling cascades that lead to airway inflammatory cell recruitment and activation. This is another mechanistic focus for subsequent studies of the effect of γT on airway inflammation.
In clinical studies, most vitamin E intervention studies directed against ozone-induced respiratory disease have used αT, which is the predominant form of vitamin E in mammalian tissues and for which the recommended dietary allowance (RDA) has been established. Intervention with combination therapy with αT and vitamin C protected from pulmonary function deficits in asthmatic children with decreased intake of dietary antioxidants during high ambient ozone episodes in Mexico City [49], though this effect was most pronounced in children with genetic mutations for glutathione-S-transferase M1, a key enzymatic mediator that opposes pathways of oxidative stress [50]. In a separate study of healthy subjects with limited dietary ascorbate, supplementation with αT, vitamin C and vegetable juice protected them from ozone-induced decrement in pulmonary function but not from airway inflammation [51]. These two studies are in contrast to a recent report by Mudway and colleagues [52], who used supplements with twice the concentrations of αT and vitamin C used above, but failed to inhibit lung function decrements and airway inflammation after acute 2h ozone exposures. Taken together, these studies suggest that αT is most effective in populations with a decreased intake of dietary antioxidants.
Compared to combination supplements, intervention with large doses of αT alone is much less effective against allergic airway responses. For example, daily supplements of 250mg αT (16 times the RDA of 15mg/d, for 6 weeks) provides no improvement in airway function or bronchodilator use in asthmatics [20]. Doses of 800mg αT /day (53 times RDA) in patients with allergic rhinitis fail to ameliorate symptom severity [22]. Similar results were recently reported in rodents with allergic airways where oral administration of 400 mg/kg/day αT for 10 days failed to reduce allergen-induced pulmonary lesions or airway hyperreactivity [21]. Using a very similar allergen sensitization and challenge protocol, we observed inhibition of ozone and allergen-induced allergic inflammation with only 4 days of treating with a much smaller dose of γT. This comparison is reminiscent of our findings in a non-immunogenic inflammation in rats, where γT was superior to αT in limiting inflammatory cell recruitment and cytokine production [29]. The role of γT in ozone-induce airway responses has not been extensively examined in either laboratory animals or humans. However, acute ozone exposure to BALB/c mice preferentially increased γT, ascorbate and uric acid in BAL fluid compared to αT [53], which is similar to the trend we detect for BALF γT from ozone-exposed rats in the present study. These results in laboratory rodents suggest that the γ-isoform of vitamin E may play a relatively more important role in regulating pulmonary inflammation than αT
Taken together, we have demonstrated broad and novel anti-inflammatory effects of γT after exposure to OVA allergen and/or ozone, two disparate stimuli. We furthermore described therapeutic effects with a relatively acute dosing regimen, and suggest that γT does not require prophylactic use or repeated doses to produce efficacy. On the basis of these observations in laboratory animals, we are presently developing a rodent study to examine the effect of γT on airway inflammation induced only by allergen as well as a series of phase I studies to test the safety and efficacy of γT in allergic people coexposed to allergens and environmental pollutants.
Acknowledgements
Research supported by NIH:NCCAM P01 AT002620
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tolbert PE, Mulholland JA, MacIntosh DL, Xu F, Daniels D, Devine OJ, et al. Air quality and pediatric emergency room visits for asthma in Atlanta, Georgia, USA. Am J Epidemiol. 2000;151:798–810. doi: 10.1093/oxfordjournals.aje.a010280. [DOI] [PubMed] [Google Scholar]
- 2.Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, et al. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. Jama. 2003;290:1859–1867. doi: 10.1001/jama.290.14.1859. [DOI] [PubMed] [Google Scholar]
- 3.Lewis TC, Robins TG, Dvonch JT, Keeler GJ, Yip FY, Mentz GB, et al. Air pollution-associated changes in lung function among asthmatic children in Detroit. Environ Health Perspect. 2005;113:1068–1075. doi: 10.1289/ehp.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kehrl HR, Peden DB, Ball B, Folinsbee LJ, Horstman D. Increased specific airway reactivity of persons with mild allergic asthma after 7.6 hours of exposure to 0.16 ppm ozone. J Allergy Clin Immunol. 1999;104:1198–1204. doi: 10.1016/s0091-6749(99)70013-8. [DOI] [PubMed] [Google Scholar]
- 5.Peden DB, Setzer RW, Jr., Devlin RB. Ozone exposure has both a priming effect on allergen-induced responses and an intrinsic inflammatory action in the nasal airways of perennially allergic asthmatics. Am J Respir Crit Care Med. 1995;151:1336–1345. doi: 10.1164/ajrccm.151.5.7735583. [DOI] [PubMed] [Google Scholar]
- 6.Peden DB, Boehlecke B, Horstman D, Devlin R. Prolonged acute exposure to 0.16 ppm ozone induces eosinophilic airway inflammation in asthmatic subjects with allergies. J Allergy Clin Immunol. 1997;100:802–808. doi: 10.1016/s0091-6749(97)70277-x. [DOI] [PubMed] [Google Scholar]
- 7.Scannell C, Chen L, Aris RM, Tager I, Christian D, Ferrando R, et al. Greater ozone-induced inflammatory responses in subjects with asthma. Am J Respir Crit Care Med. 1996;154:24–29. doi: 10.1164/ajrccm.154.1.8680687. [DOI] [PubMed] [Google Scholar]
- 8.Balmes JR, Aris RM, Chen LL, Scannell C, Tager IB, Finkbeiner W, et al. Effects of ozone on normal and potentially sensitive human subjects. Part I: Airway inflammation and responsiveness to ozone in normal and asthmatic subjects. Res Rep Health Eff Inst. 1997:1–37. discussion 81-99. [PubMed] [Google Scholar]
- 9.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet. 1999;354:482–483. doi: 10.1016/S0140-6736(99)01812-7. [DOI] [PubMed] [Google Scholar]
- 10.Ochs-Balcom HM, Grant BJ, Muti P, Sempos CT, Freudenheim JL, Browne RW, et al. Antioxidants, oxidative stress, and pulmonary function in individuals diagnosed with asthma or COPD. Eur J Clin Nutr. 2006 doi: 10.1038/sj.ejcn.1602410. [DOI] [PubMed] [Google Scholar]
- 11.Kongerud J, Crissman K, Hatch G, Alexis N. Ascorbic acid is decreased in induced sputum of mild asthmatics. Inhal Toxicol. 2003;15:101–109. doi: 10.1080/08958370304477. [DOI] [PubMed] [Google Scholar]
- 12.Mak JC, Leung HC, Ho SP, Law BK, Lam WK, Tsang KW, et al. Systemic oxidative and antioxidative status in Chinese patients with asthma. J Allergy Clin Immunol. 2004;114:260–264. doi: 10.1016/j.jaci.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Powell CV, Nash AA, Powers HJ, Primhak RA. Antioxidant status in asthma. Pediatr Pulmonol. 1994;18:34–38. doi: 10.1002/ppul.1950180109. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh S, Janocha AJ, Aronica MA, Swaidani S, Comhair SA, Xu W, et al. Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation. J Immunol. 2006;176:5587–5597. doi: 10.4049/jimmunol.176.9.5587. [DOI] [PubMed] [Google Scholar]
- 15.Misso NL, Brooks-Wildhaber J, Ray S, Vally H, Thompson PJ. Plasma concentrations of dietary and nondietary antioxidants are low in severe asthma. Eur Respir J. 2005;26:257–264. doi: 10.1183/09031936.05.00006705. [DOI] [PubMed] [Google Scholar]
- 16.Patel BD, Welch AA, Bingham SA, Luben RN, Day NE, Khaw KT, et al. Dietary antioxidants and asthma in adults. Thorax. 2006;61:388–393. doi: 10.1136/thx.2004.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Troisi RJ, Willett WC, Weiss ST, Trichopoulos D, Rosner B, Speizer FE. A prospective study of diet and adult-onset asthma. Am J Respir Crit Care Med. 1995;151:1401–1408. doi: 10.1164/ajrccm.151.5.7735592. [DOI] [PubMed] [Google Scholar]
- 18.de Luis DA, Armentia A, Aller R, Asensio A, Sedano E, Izaola O, et al. Dietary intake in patients with asthma: a case control study. Nutrition. 2005;21:320–324. doi: 10.1016/j.nut.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 19.Centanni S, Santus P, Di Marco F, Fumagalli F, Zarini S, Sala A. The potential role of tocopherol in asthma and allergies: modification of the leukotriene pathway. BioDrugs. 2001;15:81–86. doi: 10.2165/00063030-200115020-00002. [DOI] [PubMed] [Google Scholar]
- 20.Pearson PJ, Lewis SA, Britton J, Fogarty A. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax. 2004;59:652–656. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suchankova J, Voprsalova M, Kottova M, Semecky V, Visnovsky P. Effects of oral alpha-tocopherol on lung response in rat model of allergic asthma. Respirology. 2006;11:414–421. doi: 10.1111/j.1440-1843.2006.00864.x. [DOI] [PubMed] [Google Scholar]
- 22.Shahar E, Hassoun G, Pollack S. Effect of vitamin E supplementation on the regular treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2004;92:654–658. doi: 10.1016/S1081-1206(10)61432-9. [DOI] [PubMed] [Google Scholar]
- 23.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc Natl Acad Sci U S A. 1993;90:1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN. gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proc Natl Acad Sci U S A. 1997;94:3217–3222. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 26.Wolf G. gamma-Tocopherol: an efficient protector of lipids against nitric oxide- initiated peroxidative damage. Nutr Rev. 1997;55:376–378. doi: 10.1111/j.1753-4887.1997.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 27.Wagner KH, Kamal-Eldin A, Elmadfa I. Gamma-tocopherol--an underestimated vitamin? Ann Nutr Metab. 2004;48:169–188. doi: 10.1159/000079555. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. Faseb J. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 30.Hatch GE, Slade R, Harris LP, McDonnell WF, Devlin RB, Koren HS, et al. Ozone dose and effect in humans and rats. A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med. 1994;150:676–683. doi: 10.1164/ajrccm.150.3.8087337. [DOI] [PubMed] [Google Scholar]
- 31.Bieri JG, Evarts RP. Gamma tocopherol: metabolism, biological activity and significance in human vitamin E nutrition. Am J Clin Nutr. 1974;27:980–986. doi: 10.1093/ajcn/27.8.980. [DOI] [PubMed] [Google Scholar]
- 32.Weber C, Podda M, Rallis M, Thiele JJ, Traber MG, Packer L. Efficacy of topically applied tocopherols and tocotrienols in protection of murine skin from oxidative damage induced by UV-irradiation. Free Radic Biol Med. 1997;22:761–769. doi: 10.1016/s0891-5849(96)00346-2. [DOI] [PubMed] [Google Scholar]
- 33.Wagner JG, Hotchkiss JA, Harkema JR. Effects of ozone and endotoxin coexposure on rat airway epithelium: potentiation of toxicant-induced alterations. Environ Health Perspect. 2001;109(Suppl 4):591–598. doi: 10.1289/ehp.01109s4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner JG, Hotchkiss JA, Harkema JR. Enhancement of nasal inflammatory and epithelial responses after ozone and allergen coexposure in Brown Norway rats. Toxicol Sci. 2002;67:284–294. doi: 10.1093/toxsci/67.2.284. [DOI] [PubMed] [Google Scholar]
- 35.Coffey MJ, Wheeler CS, Gross KB, Eschenbacher WL, Sporn PH, Peters-Golden M. Increased 5-lipoxygenase metabolism in the lungs of human subjects exposed to ozone. Toxicology. 1996;114:187–197. doi: 10.1016/s0300-483x(96)03487-7. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu T, Hirano H, Majima Y, Sakakura Y. A mechanism of antigen- induced mucus production in nasal epithelium of sensitized rats. A comparison with lipopolysaccharide-induced mucus production. Am J Respir Crit Care Med. 2000;161:1648–1654. doi: 10.1164/ajrccm.161.5.9908101. [DOI] [PubMed] [Google Scholar]
- 37.Vargaftig BB, Singer M. Leukotrienes mediate murine bronchopulmonary hyperreactivity, inflammation, and part of mucosal metaplasia and tissue injury induced by recombinant murine interleukin-13. Am J Respir Cell Mol Biol. 2003;28:410–419. doi: 10.1165/rcmb.2002-0032OC. [DOI] [PubMed] [Google Scholar]
- 38.Longphre M, Zhang LY, Harkema JR, Kleeberger SR. Mast cells contribute to O3-induced epithelial damage and proliferation in nasal and bronchial airways of mice. J Appl Physiol. 1996;80:1322–1330. doi: 10.1152/jappl.1996.80.4.1322. [DOI] [PubMed] [Google Scholar]
- 39.Frischer T, Studnicka M, Halmerbauer G, Horak F, Jr., Gartner C, Tauber E, et al. Ambient ozone exposure is associated with eosinophil activation in healthy children. Clin Exp Allergy. 2001;31:1213–1219. doi: 10.1046/j.1365-2222.2001.01155.x. [DOI] [PubMed] [Google Scholar]
- 40.Fischer BM, Voynow JA. Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am J Respir Cell Mol Biol. 2002;26:447–452. doi: 10.1165/ajrcmb.26.4.4473. [DOI] [PubMed] [Google Scholar]
- 41.Shao MX, Nadel JA. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-alpha-converting enzyme. J Immunol. 2005;175:4009–4016. doi: 10.4049/jimmunol.175.6.4009. [DOI] [PubMed] [Google Scholar]
- 42.Miyahara N, Miyahara S, Takeda K, Gelfand EW. Role of the LTB4/BLT1 pathway in allergen-induced airway hyperresponsiveness and inflammation. Allergol Int. 2006;55:91–97. doi: 10.2332/allergolint.55.91. [DOI] [PubMed] [Google Scholar]
- 43.Busse W, Kraft M. Cysteinyl leukotrienes in allergic inflammation: strategic target for therapy. Chest. 2005;127:1312–1326. doi: 10.1378/chest.127.4.1312. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Q, Lykkesfeldt J, Shigenaga MK, Shigeno ET, Christen S, Ames BN. Gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic Biol Med. 2002;33:1534–1542. doi: 10.1016/s0891-5849(02)01091-2. [DOI] [PubMed] [Google Scholar]
- 45.Campbell SE, Stone WL, Whaley SG, Qui M, Krishnan K. Gamma (gamma) tocopherol upregulates peroxisome proliferator activated receptor (PPAR) gamma (gamma) expression in SW 480 human colon cancer cell lines. BMC Cancer. 2003;3:25. doi: 10.1186/1471-2407-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Pascale MC, Bassi AM, Patrone V, Villacorta L, Azzi A, Zingg JM. Increased expression of transglutaminase-1 and PPARgamma after vitamin E treatment in human keratinocytes. Arch Biochem Biophys. 2006;447:97–106. doi: 10.1016/j.abb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Ueki S, Matsuwaki Y, Kayaba H, Oyamada H, Kanda A, Usami A, et al. Peroxisome proliferator-activated receptor gamma regulates eosinophil functions: a new therapeutic target for allergic airway inflammation. Int Arch Allergy Immunol. 2004;134(Suppl 1):30–36. doi: 10.1159/000077790. [DOI] [PubMed] [Google Scholar]
- 48.Lee SY, Kang EJ, Hur GY, Jung KH, Jung HC, Lee SY, et al. Peroxisome proliferator-activated receptor-gamma inhibits cigarette smoke solution-induced mucin production in human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L84–90. doi: 10.1152/ajplung.00388.2005. [DOI] [PubMed] [Google Scholar]
- 49.Romieu I, Meneses F, Ramirez M, Ruiz S, Perez Padilla R, Sienra JJ, et al. Antioxidant supplementation and respiratory functions among workers exposed to high levels of ozone. Am J Respir Crit Care Med. 1998;158:226–232. doi: 10.1164/ajrccm.158.1.9712053. [DOI] [PubMed] [Google Scholar]
- 50.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Moreno-Macias H, Reyes- Ruiz NI, Estela del Rio-Navarro B, et al. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax. 2004;59:8–10. [PMC free article] [PubMed] [Google Scholar]
- 51.Samet JM, Hatch GE, Horstman D, Steck-Scott S, Arab L, Bromberg PA, et al. Effect of antioxidant supplementation on ozone-induced lung injury in human subjects. Am J Respir Crit Care Med. 2001;164:819–825. doi: 10.1164/ajrccm.164.5.2008003. [DOI] [PubMed] [Google Scholar]
- 52.Mudway IS, Behndig AF, Helleday R, Pourazar J, Frew AJ, Kelly FJ, et al. Vitamin supplementation does not protect against symptoms in ozone-responsive subjects. Free Radic Biol Med. 2006;40:1702–1712. doi: 10.1016/j.freeradbiomed.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 53.Jang AS, Choi IS, Yang SY, Kim YG, Lee JH, Park SW, et al. Antioxidant responsiveness in BALB/c mice exposed to ozone. Respiration. 2005;72:79–84. doi: 10.1159/000083405. [DOI] [PubMed] [Google Scholar]