Abstract
Background
Genotype-phenotype correlations are poorly characterized in arrhythmogenic right ventricular cardiomyopathy (ARVC). We investigated whether carriers of rare variants in desmosomal genes (DC) and titin gene (TTN) display different phenotypes and clinical outcomes, when compared to non-carriers (NT-ND).
Methods and Results
Thirty-nine ARVC families (173 subjects, 67 affected) with extensive follow up (mean 9 years), prospectively enrolled in the International Familial Cardiomyopathy Registry since 1991, were screened for rare variants in TTN and desmosomal genes (DSP, PKP2, DSG2, DSC2). Multiple clinical and outcome variables were compared between 3 genetic groups (TTN, DC, NT-ND) to define genotype-phenotype associations.
Of the 39 ARVC families, 13% (5/39) carried TTN rare variants (11 affected subjects), 13% (5/39) DC (8 affected), while 74% (29/39) were NT-ND (48 affected). Compared to NT-ND, DC had a higher prevalence of inverted T waves in V2-3 (75% vs. 31%, p=0.004), while TTN had more supraventricular arrhythmias (46% vs. 13%, p=0.013) and conduction disease (64% vs. 6% p<0.001). Compared to the NT-ND group, the DC group experienced a worse prognosis (67% vs. 11%, p=0.03) and exhibited a lower survival free from death or heart transplant (59% vs. 95% at 30 years, and 31% vs. 89% at 50 years, HR 9.66, p=0.006), while the TTN group showed an intermediate survival curve (HR 4.26, p=0.037).
Conclusions
TTN carriers display distinct phenotypic characteristics including a greater risk for supraventricular arrhythmias and conduction disease. Conversely, DC are characterized by negative T waves in anterior leads, severe prognosis, high mortality and morbidity.
Keywords: Arrhythmogenic right ventricular cardiomyopathy, human genome, sudden cardiac death, desmosome, titin
INTRODUCTION
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC, MIM107970) is an inherited cardiomyopathy characterized by fibro-fatty replacement of the right ventricular myocardium that predisposes patients to life-threatening ventricular arrhythmias and slowly progressive ventricular dysfunction, although biventricular and left-dominant forms are increasingly found [1]. The diagnostic criteria, established by an international task force in 1994 [2], and modified in 2010 [3], are based on fulfillment of major and minor clinical variables. ARVC is familial in 30 to 50% of patients [4–5], with most cases exhibiting autosomal dominant transmission with incomplete and age-related penetrance [6].
ARVC is currently considered to be a disease of myocyte adhesion caused by defects at the intercellular junction. Cardiac myocyte-to-myocyte adhesion is maintained by desmosomes, adherens junctions and gap junctions, which together comprise the intercalated disc [5,7]. The desmosomes have a complex structure that includes adhesion molecules of the cadherin (desmoglein-DSG and desmocollin-DSC), plakin (desmoplakin-DSP) and catenin (plakophilin-PKP, and plakoglobin-JUP) families, which link intermediate filaments of the cytoskeleton to the desmosomal cadherins [8,9]. Mutations in numerous genes encoding proteins of the desmosome have been identified in ARVC, the majority of which are in 5 genes: plakophilin-2 (PKP2), desmoplakin (DSP), desmoglein-2 (DSG2), desmocollin-2 (DSC2) and plakoglobin (JUP)
Recently, we identified rare variants in the gene encoding the sarcomeric protein titin (TTN), the largest gene in mammals [10], in our cohort of ARVC families. Titin filaments bridge the sarcomere along its longitudinal axis, overlapping end-to-end at the Z disc and M band at the amino and carboxyl ends, respectively, forming a contiguous filament along the myofibril. Titin is involved in cellular mechanics, specifically, the spring-like properties of the sarcomere that underlie passive and restorative forces occurring after sarcomere lengthening or shortening [11–13].
The objective of our study was to compare the phenotype of TTN rare variant carriers to that of desmosomal rare variant carriers (DC), and to a group of ARVC patients with no detectable mutation in either pathway (non-TTN/non-desmosomal: NT-ND). Unique features of our study are the length of clinical follow up of our ARVC cohort, up to 22 years with a mean of 9 years, and the analysis of the giant gene titin along with the most common desmosomal genes. Here we show that in ARVC, desmosomal gene rare variant carriers are characterized by specific clinical/electrocardiographic features and the worst prognosis, while titin gene carriers have an intermediate outcome and severe conduction defects. These findings have important implications in the clinical practice.
METHODS
Patient Population
Patients meeting ARVC consensus task force criteria [2,3] were enrolled at the University of Colorado Cardiovascular Institute (CU-CVI) and the Cardiovascular Department of the University Hospital of Trieste, Italy, as part of the International Familial Cardiomyopathy Registry. A total 67 affected subjects from 39 ARVC families (173 family members) with long-term follow up were clinically analyzed in this study Individual medical and family history, physical exam, electrocardiogram (ECG), Holter monitoring ECG, and echocardiogram were performed on all index patients and available family members. In appropriate cases, signal-averaged ECG, cardiac MRI and endomyocardial biopsy were performed. Subsequent follow-up examinations were conducted, as clinically indicated. Medical records from deceased subjects were reviewed when available. One proband was excluded from our analysis for the lack of detailed clinical information. Blood samples were collected for DNA analysis after obtaining informed consent. In two patients DNA was extracted from preserved tissue. The University of Colorado and Trieste local Institutional Review Boards approved the protocol.
Data points considered included presence of T wave inversion in V2-V3, epsilon waves, atrioventricular block, left and right bundle branch block (LBBB/RBBB), supraventricular and ventricular arrhythmias. Echocardiograms were assessed for chamber size and function, right ventricular aneurysmal bulging/dilatation and evidence of valvular disease. We evaluated two clinical endpoints: time to death or heart transplant (D/OHT), and time to malignant ventricular arrhythmia (MVA), defined as sudden cardiac death (SD), sustained ventricular tachycardia (S-VT) or appropriate implantable cardioverter defibrillator discharge (ICD).
Genetic Analysis
Desmosomal genes analysis
Consensus sequences for the cardiac isoforms of desmosomal genes were obtained: plakophilin-2 (PKP2) (GenBank accession X97675), desmoglein-2 (DSG2) (GenBank accession Z26317), desmoplakin (DSP) (GenBank accession J05211), and desmocollin-2 (DSC2) (GenBank accession X56807). The non-desmosomal gene, the phospholamban gene (PLN) (GenBank accession NM_002667), was also sequenced. Exons from all genes were sequenced using direct Sanger sequencing (primers available on request).
Criteria for classifying variants as disease-causing included change in predicted amino acid sequence, co-segregation of mutation with disease phenotype within the family, evolutionary conservation across species, absence in 150 ethnically similar controls using pyrosequencing (PSQ96MA, Biotage, Uppsala, Sweden) and from public databases (Accessed August 1st, 2013: 1,000 Genome Project, NHLBI Exome Variant Server, dbSNP,) [10,14] and the ARVD/C genetic Variant Database (http://www.arvcdatabase). A conservative allele frequency of <0.04% was used as cutoff according to Norton et al. [15]. We used PolyPhen2 HVAR (http://genetics.bwh.harvard.edu) to assess the potential effect of amino acid substitutions on the structure and function of proteins [16,17]. In families in which we found a variant of interest, all available relatives were screened. Co-segregation was suggested by the presence of the rare variant in 2 or more affected relatives and/or absence in unaffected relatives.
Titin gene analysis
As previously reported [10, 14], TTN DNA resequencing was provided by the University of Washington, Department of Genome Science (grant NHLBI N01-HV-48194). Exons and peri-exonic regions of titin isoform N2A along with additional exons unique to the principal cardiac isoform N2B (NM 003319) were Sanger sequenced. Rare variants detected were evaluated against known TTN single nucleotide polymorphisms (SNPs) in available databases and rare variants present in multiple other families in the entire cohort were considered common and unlikely to be pathogenic rare variants. Non-synonymous coding rare variants were evaluated for putative functional effects using SIFT and PolyPhen-2 analysis and scored as tolerant or intolerant; tolerant rare variants were considered unlikely to be pathogenic [18,19]. Variants were further filtered by public databases as described above. The recent guidelines of MacArthur et al. for implicating sequence variants in human disease were taken into account [20].
Statistical analysis
Summary statistics of clinical and instrumental variables at enrollment were expressed as means and standard deviations, or counts and percentages, as appropriate. Comparisons between groups were made by the ANOVA test on continuous variables using the Brown-Forsythe statistic when the assumption of equal variances did not hold. For the purpose of the analysis between groups, the PLN carrier subject was included in the NT-ND group. Post-hoc, the least significant difference or Tamhane tests were also applied. The Chi-square test was calculated for discrete variables both on the three groups simultaneously and by pairs, evaluating Fisher’s exact test p value when necessary. Event-free survival curves for D/OHT, MVA (SD/S-VT/ICD) and combined endpoints (D/OHT and MVA) were estimated and plotted using the Kaplan-Meier method, and the log-rank test was applied in order to investigate for differences in long-term survival. To take into account the clustered failure times (i.e. relatives within families cannot be taken as entirely independent) a survival regression Cox model was also estimated, with “group” (with levels: DC, TTN and NT-ND) as the unique covariate and the “family index” as a cluster indicator. Statistical analyses were performed with IBM SPSS Statistical Package 19.0 and the R statistical package version 2.14.1.
RESULTS
Molecular genetics of ARVC
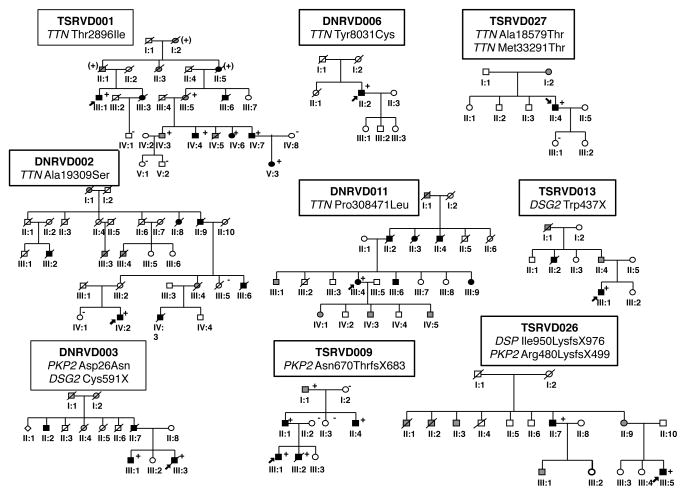
Eleven out of 39 ARVC families (28%) had rare variants associated with the disease: 5 in desmosomal genes (DC) and 5 in TTN (Table 1, Figure 1). One patient carried a rare variant in PLN. Within the DC group we identified 4 PKP2, 2 DSG2 and 1 DSP rare variants, including one proband with two PKP2 rare variants and one double heterozygote with rare variants in DSP and PKP2. One family harbored two TTN rare variants.
Table 1.
Summary of rare variants identified in our ARVC study population
| Gene | Family ID | DNA change | Predicted effect | References |
|---|---|---|---|---|
| TTN | TSRVD001 | 29453 C>T | Thr2896Ile | 10 |
| TSRVD027* | 281801 T>C | Ala18579Thr | 10 | |
| TSRVD027* | 221380A G>T | Met33291Thr | 10 | |
| DNRVD002 | 226177G>T | Ala19309Ser | 10 | |
| DNRVD006 | 97341 G>A | Tyr8031Cys | 10 | |
| DNRVD011 | 272848 C>T | Pro30847Leu | 10 | |
| PKP2 | TSRVD009 | 2009delC | Asn670ThrfsX683 | 25 |
| TSRVD016* | 148_151delACAG | Thr50SerfsX61 | 23 | |
| TSRVD016* | 1060G>C | Glu354Gln | Novel rare variant | |
| TSRVD026‡ | 1440_1444delTCCCA | Arg480LysfsX499 | Novel rare variant | |
| DNRVD003‡ | 76G>A * | Asp26Asn* | VUS† 4,5,25,27 |
|
| DSG2 | TSRVD013 | 1311G>A | Trp437X | Novel rare variant |
| DNRVD003‡ | 1773_1774delTG | Cys591X | 6 | |
| DSP | TSRVD026‡ | 2848delA | Ile950LysfsX976 | Novel rare variant† |
| PLN | DNRVD014 | 40_42delAGA | Arg14Del | 22 |
Legend: TTN= titin; PKP2 plakophillin-2; DSG2, desmoglein-2; PLN, phospholamban.
Compound mutation;
reported variant of unknown significance in ARVC database;
digenic mutation.
Figure 1. Pedigrees of ARVC families with desmosomal and TTN rare variants.
Squares and circles indicate male and female individuals, respectively. Black shading indicates individuals meeting full ARVC diagnostic criteria, gray shading a suspected cardiac history and/or history of sudden unexplained death, and white squares/circles unaffected individuals based on available family and/or medical history or, when carrier status is indicated, based on full clinical evaluation. Carrier rare variant status is indicated (+ present; − absent). Probands are identified by an arrow.
Of the 67 ARVC affected family members tested, 20 (30%) carried rare variants (11 in TTN, 8 in desmosomal genes, 1 in PLN) while 47 (70%) did not have identifiable rare variants. No rare variant suspected of causing the disease was found in DSC2. A DSC2 c.2686_2687dupGA (A897fsX900) variant previously described by Syrris et al. [21], was found in 5 families and considered a common variation according to the ARVD/C Database. Similarly, we identified a PKP2 SNP 76G>A (Asp26Asn) that has also been identified by multiple authors and currently appears to be a common variation.
Genotype-phenotype correlation
Individual patients were followed for a mean of 105 ± 87 months, with a median period of 77 months. One PKP2 carrier was excluded from our genotype/phenotype analysis due to insufficient clinical data, and the PLN carrier was included in the NT-ND group.
There was no difference between groups with respect to gender, age at onset of symptoms or age at diagnosis. However, compared to the TTN carriers, the DC group demonstrated a significantly younger age for each endpoint, D/OHT (31±12 years vs. 58±11 years, p=0.015) and MVA (SD, S-VT and ICD shock) (26±17 vs. 52±13, p=0.035), respectively (Table 2, Supplemental Table 2 and 3).
Table 2.
Summary of clinical features of the 67 ARVC study subjects
| DC (n=8) | TTN (n=11) | NT-ND (n=48)* | Total (n=67) | P value | |
|---|---|---|---|---|---|
| Age at onset, y | 24 ± 16 | 33 ± 13 | 35 ± 20 | 34 ± 18 | 0.448 |
| Age at diagnosis, y | 27 ± 15 | 42 ± 10 | 38 ± 19 | 38 ± 18 | 0.244 |
| Age at end-point1 (D/OHT), y | 31 ± 12 | 58 ± 11 | 49 ± 20 | 49 ± 19 | 0.015† |
| Age at end-point2 (SD/MVA/ICD), y | 26 ± 17 | 52 ± 13 | 45 ± 20 | 44 ± 20 | 0.035† |
| LVEDD (cm) | 4.93 ± 0.53 | 5.28 ± 0.54 | 5.23 ± 0.71 | 5.21 ± 0.67 | 0.566 |
| LV dilation, EDV (cm2) | 102.0 ± 31.6 | 93.8 ± 29.4 | 97.1 ± 32.7 | 97 ± 31.4 | 0.899 |
| LV EF% | 57 ± 9 | 55 ± 11 | 56 ± 13 | 56 ± 12 | 0.939 |
| RV dilation area (cm2) | 35.33 ± 4.16 | 37.43 ± 8.62 | 27.29 ±7.21 | 30.81 ± 8.53 | 0.013§ |
| RV FS% | 17.00 ± 1.41 | 20.50 ± 7.92 | 30.33 ± 12.20 | 26.61 ± 11.71 | 0.101 |
| LA dimension, (cm) | 3.18 ± 0.45 | 4.27 ± 0.59 | 3.25 ± 0.59 | 3.41 ± 0.69 | <0.001†‡ |
| LA f/u dimension (cm) | 3.37 ± 0.16 | 4.63 ± 0.64 | 3.47 ± 0.64 | 3.68 ± 0.76 | <0.001†‡ |
| LA area f/u, (cm2) | 22.00 ± 2.83 | 31.17 ± 6.88 | 21.33 ± 6.12 | 23.41 ± 7.20 | 0.007‡ |
Legend: DC, desmosomal carriers; TTN, titin carriers; NT-ND, non titin-non-desmosomal carriers.
includes NT-ND and one PLN carrier;
DC vs. TTN p<0.05;
TTN vs. NT-ND p<0.05;
DC vs. NT-ND p<0.05.
LVEDD, left ventricular end-diastolic dilation; LVEF, left ventricular ejection fraction; EDV, end-diastolic volume, LA, left atrium; MR, mitral regurgitation; LA f/u, left atrium at follow up; MR f/u, changing of severity at follow up. Values reported are calculated means and standard deviations.
Regarding clinical symptoms, compared with NT-ND, TTN patients were noted to have more exertional dyspnea/heart failure (64 vs. 23%, p= 0.022), supraventricular arrhythmias (atrial fibrillation, paroxysmal atrial fibrillation, paroxysmal atrial tachycardia: 46% vs. 13% p=0.013), and bradyarrhythmias (2° and 3° degree atrioventricular block) requiring pacemaker (64% vs. 6%, p< 0.001).
Inverted T waves in V2-3 (in patients older than 12 years, in absence of RBBB) were present in 6/8 DC carriers (75% vs. 31% in NT-ND, p=0.004), suggesting that this could be an indicator of desmosomal rare variants. There was no difference in the frequency of epsilon waves between the three groups (Figure 2, Supplemental Table 3). Late potentials on SAECG, non-sustained VT and premature ventricular complexes did not show any statistical difference between groups. There was no evidence of supraventricular arrhythmias, 2° or 3° degree atrioventricular blocks, or pacemakers in DC.
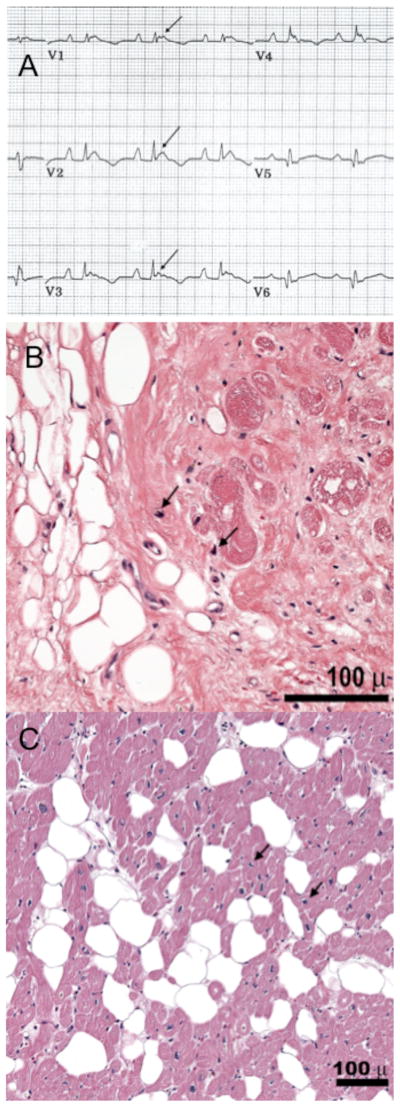
Figure 2. Clinical features of DC and TTN rare variant carriers.
(A) DNRVD003, III-3 (PKP2): the ECG shows an incomplete RBBB, epsilon waves (arrows), and negative T waves extending to V4. (B) Specimen from the RV wall in subject DNRVD006 II-2, showing patchy fibro-fatty infiltration (hematoxylin and eosin, 20X). (C) Specimen from the RV wall in subject DNRVD002-V: 2 (hematoxylin and eosin, 40X) showing massive fibro-fatty infiltration. In both, myocyte nuclei with abnormal morphologic characteristics (arrowheads) as previously described in DCM caused by TTN truncations [14].
There were no differences between groups regarding RV systolic function, bulging and tricuspid regurgitation by echocardiography (Figure 3). Similarly we did not detect any difference in LV involvement, including systolic function, dimension and diastolic function. TTN carriers vs. NT-ND had greater RV dilatation (35.33±4.16 vs.27.29±7.21 cm2, p=0.013). TTN carriers had larger left atria compared to DC and NT-ND at baseline (4.27±0.59, 3.18±0.45 vs. 3.25±0.59 cm, p< 0.001) and at follow up (4.63±0.64, 3.37±0.16 vs. 3.47±0.64 cm, p< 0.001). Figure 2, Table 2 and Supplemental Table 2 and 3 report the phenotypic characteristics of the patients carrying rare desmosomal or TTN variants.
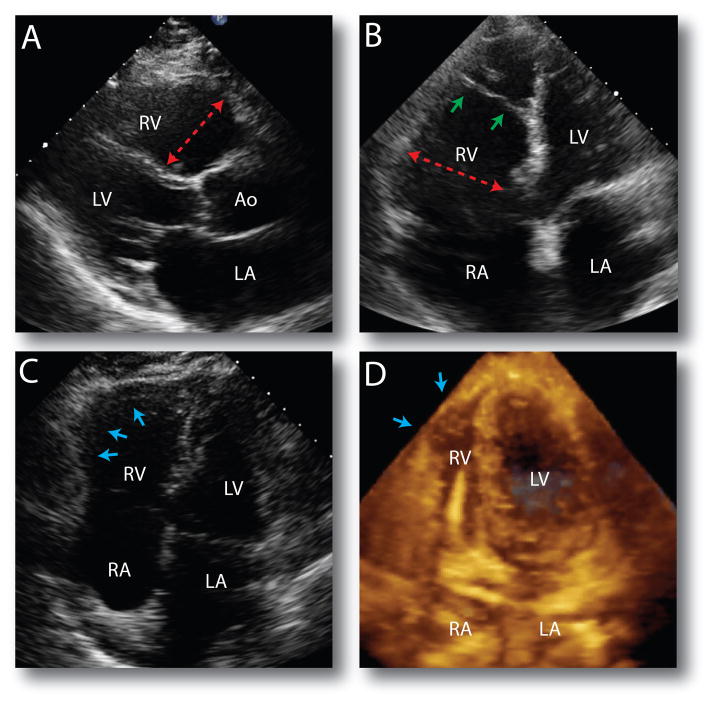
Figure 3. Echocardiographic features in ARVC patients.
(A) ND-NT carrier: parasternal long axis view demonstrating a dilated RV outflow tract (red arrow) and (B) apical four chamber view demonstrating a dilated RV inflow (red arrow). The small green arrows point to a stretched and fibrotic moderator band. (C) TTN family DNRVD002, subject V: 2. The blue arrows point to a large aneurysm on the RV free wall. (D) PNL Arg14Del variant carrier. 3D transthoracic echocardiogram where the blue arrows point to a small RV apical aneurysm. A defibrillator cable is seen entering the RV. LV: left ventricle; Ao: Aorta; LA: left atrium; RA: right atrium.
The penetrance in TTN variant carriers, measured in the large TSRVD001-Thr2896Ile family, was reduced (84%) and comparable to the penetrance previously reported in the ARVC population [4].
Natural history and prognosis of ARVC genes
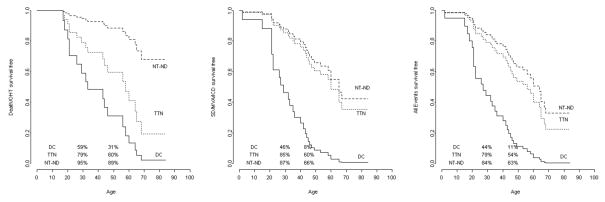
The outcome analysis showed differences between the 3 groups. DC and TTN carriers required more heart transplantations compared to NT-ND (50%, 54% vs. 10%, p<0.001). DC and TTN carriers showed a survival curve free from D/OHT-free that sharply decreased after 30 years of age compared to NT-ND: 59% and 79% vs. 95% at 30 years (p=0.006), 31% and 61% vs. 89% at 50 years (p=0.037) (Table 3 and Figure 4A). Likewise, the survival curve free from major arrhythmic events (SD/MVA/ICD) showed a significant difference in DC (Figure 4B), this group was characterized by a higher frequency of events compared to others two groups (DC vs. TTN p=0.005, and DC vs. NT-ND p=0.0002). Finally, the DC group maintained a worse survival curve when including all events (death, OHT, MVA, ICD), compared to the other two groups (Figure 4C: DC vs. TTN p=0.01, DC vs. NT-ND p=0.001). No significant change in the different outcomes was observed when we analyzed the survival of our study population without the double variant carriers (TSRVD026-III:5 and TSRVD027-III:5), as shown in Supplemental Table 4 and Supplemental Figure 1.
Table 3.
Hazard ratios estimated by Cox models.
| Death/OHT | SD/MVA/ICD | All events | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | p | |
| DC vs. NT-ND | 9.66 | 1.9 – 15 | 0.006 | 5.87 | 2.3 – 12 | 0.0002 | 4.74 | 1.9 – 12 | 0.001 |
| TTN vs. NT-ND | 4.26 | 1.4 – 13 | 0.037 | 1.21 | 0.57 – 3 | 0.21 | 1.35 | 0.8 – 2 | 0.19 |
| DC vs. TTN | 2.27 | 0.68 – 7 | 0.185 | 4.85 | 1.59 – 13 | 0.005 | 3.53 | 1.35 – 9 | 0.01 |
Legend: Levels: ‘DC’, ‘TTN’ and ‘NT-ND’ as the unique covariate; the “family index” was accounted for in the model as a cluster indicator. Estimated survival curves are shown in figure 4.
Figure 4. Comparison of long-term natural history between desmosomal genes (PKP2, DSP, DSG2), TTN rare variant carriers and NT-ND.
Follow up from birth to end point/last follow up evaluation. (a) Survival-free from D/OHT. (B). Survival-free from MVA (SD, S-VT or appropriate discharge of ICD). (C) Survival-free from the combined end-point A+B. Percentages in the figures are referred to the survival rate at the age 30 and 50 years, respectively.
Phenotype in multiple mutations
The proband III-5 of family TSRVD026 harbored two rare variants (Figure 1), one in DSP (fs c.2848delA) and the other in PKP2 (fs c.1440_1444delTCCCCA). The phenotype was characterized by biventricular arrhythmogenic cardiomyopathy, with severe and progressive left (LVEF 47% to 27% and LVEDD 5 to 6.2 during the follow-up) and right (mild dysfunction to RVFS 16 %) ventricular involvement, requiring orthotropic heart transplant at the age of 30 years. A maternal uncle (II-7) was also diagnosed with bi-ventricular arrhythmogenic cardiomyopathy (carrying same rare variants of the proband), and 2 maternal aunts (II-1 and II-2) died for heart failure: in these relatives, no genetic material was available for testing.
The proband of family TSRVD 027 (III-5) harbored 2 rare variants in TTN and showed a milder phenotype, with mild RV dysfunction, normal LVEF and a benign outcome with no endpoints. No detailed clinical data were available for family TSRVD016, as mentioned above.
DISCUSSION
Genotype-phenotype association of desmosomal and titin rare variants in ARVC
In this study, we evaluated a well-characterized cohort of ARVC patients followed for a mean of 8.8 years. Based on our initial case series, 12.8% of ARVC families had TTN rare variants (5/39), 12.8% had desmosomal genes rare variants (5/39) and 2% (1/39) a founder mutation in PLN [22]. Furthermore, our results suggest that TTN and desmosomal genes have distinct clinical phenotypes.
Individuals with desmosomal variants had the poorest prognosis of the three groups with high mortality and morbidity attributable to malignant ventricular arrhythmias (Figure 4B). The DC group was characterized by the presence of T-wave inversions in leads V2-V3 on ECG, suggesting that this finding may be more specific to DC. On echocardiography, the DC group had more severe RV dilation compared to the NT-ND group; however, there were no discernible differences in LV function between any of the groups (Supplementary Table 3). The DC group exhibited decreased survival free from the clinical endpoint D/OHT compared to the NT-ND group, HR 9.66 (1.9–15 95% CI, p=0.006). Similarly, DC had lower survival free from the MVA endpoint compared to the NT-ND group, HR 5.87 (2.3–12 95% CI, p=0.002) as well as all events (D/OHT/MVA) combined, HR 4.74 (1.9–12 CI 95%, p=0.001). These findings were evident at age 30; however, a more pronounced difference was noted at 50 years (Table 3, Figure 4).
In contrast, the TTN group phenotype was characterized by symptoms of heart failure and, as we previously reported [10], supraventricular arrhythmias and a higher incidence of high-degree (type II 2nd degree and 3rd degree), heart block necessitating pacemaker implantation (Supplementary Table 3). Echocardiographic differences include greater left atrial enlargement, mitral regurgitation, and RV dilation compared to the DC and NT-ND groups (Table 2). Regarding the two clinical endpoints, TTN carriers exhibited an intermediate survival curve demonstrating reduced survival compared to the NT-ND group for the D/OHT endpoint but not for the MVA endpoint. When comparing the DC to TTN groups directly, while the two groups do differ with respect to D/OHT, the DC group had reduced event-free survival for MVA and for all events combined (Figure 4A & 4B).
Similar to other reports, in our cohort PKP2 was the most common mutated desmosomal gene [23]. Likewise, according to other studies [4,5,24,25], the number of patients in which the genetic defect remains unknown remains still high. These data suggests that the ARVC population harbors unidentified mutations in desmosomal and as yet to be discovered pathways [22, 26–29].
Multiple mutations
Compound or double heterozygous, have been reported in ARVC, a phenomenon identified also in other forms of cardiomyopathies [24,27,30]. In our series, we found two compound heterozygous rare variants (PKP2 and TTN) and one digenic rare variant (Table 1). In this case, the phenotype of the proband carrying a DSP and a PKP2 truncating mutation was characterized by bi-ventricular severe and progressive cardiomyopathy, with heart transplantation at early age (30 years).
The role of titin in the pathogenesis of ARVC
While a large series of investigation have shown that defective desmosomal proteins can cause ARVC, more intriguing is the potential role of the giant sarcomeric protein, titin, in causing ARVC. The importance of titin in cardiomyopathies has only recently emerged likely due in part to the limitations of first-generation sequencing methods to easily analyze this enormous gene [10,11,14]. TTN is now considered the most common disease gene in dilated cardiomyopathy, accounting for up to 25% of patients [10]. Indeed, in our ARVC cohort, TTN rare variants accounted for 13% of families. However, the role of TTN rare missense variant, whose frequency in cardiomyopathies is far above the expected frequency of disease causing mutations, suggest that some of these variants could be either benign or could act as modifiers in genetically susceptible hosts [31]. These findings complicate the interpretation of detected TTN missense variants. However, as previously reported, in our ARVC patient population, the strict filtering criteria, the unique characteristics of the phenotype, the different natural history, the distribution of ARVC variants across the spring region and A-band and, finally, the functional studies on Thr2896Ile variant support a mechanistic role of TTN in ARVC [10,32]. Specifically, the Thr2896Ile variant found in family TSRVD001 decreases the force needed to unfold the immunoglobulin-like domain of titin spring region (Ig10) and increases its rate of unfolding 4-fold leading to a molecule more prone to degradation, presumably due to compromised local protein structure. The weakened structural integrity of titin and the proteolysis may lead to the mechanical dysfunction of the RV and trigger the apoptosis characteristic of ARVC.
Study limitations
A limitation of the present study is the relatively small number of study subjects in each group, which is common in many studies dealing with rare diseases. Although our detection rate of desmosomal rare variants was lower in probands (12%) compared to other reports, the overall detection rate of 28% in our cohort was similar to several other investigators, largely due to the significant number of TTN rare variants [24,25]. The lower detection rate may be attributable to use of updated and more stringent bioinformatic filtering criteria and the current availability of large control cohorts from public databases (e.g. 1000 Genome Project, Exome Sequence Variant, dbSNP) to filter common variants. Indeed, Kapplinger et al. reported that in ARVC, the genetic ‘noise’ of possible mutations can be as high as 16% in controls [25]. Therefore, it is not surprising that genetic variants considered causal mutations in the past, now have to be reclassified as benign genetic variants [26]. Rare causes of ARVC, such as LMNA, which can mimic ARVC [33], were not tested. As frequently occurring in the clinical practice, we could not prove the segregation rare variants with the phenotypes when only a single family member was available for genetic testing: in these cases, the possible pathogenicity was based on state-of-the-art bioinformatic approach. Finally, functional assays were limited to the Thr2896Ile spring variant [10, 32].
Conclusions
Our data demonstrate identifiable genetic subgroups within ARVC that display different phenotypic and prognostic characteristics based on long-term follow-up data. Our study is unique for the extended clinical follow-up of our ARVC cohort up to 22 years, the genotype-phenotype analysis comparing common desmosomal genes to giant gene titin carriers and the findings of gene-specific phenotypes and prognosis. We found that DC exhibit specific clinical and electrocardiographic features and poor clinical outcome, while TTN carriers have a distinct phenotype characterized by supraventricular arrhythmias and conduction disease and less severe clinical outcome relative to DC. Finally, ARVC patients lacking identifiable variants in the desmosomal or TTN pathways had a better prognosis in our cohort. The results on our initial case series have important clinical implications will be further expanded and replicated by the ongoing North American ARVC Registry [3].
Supplementary Material
Acknowledgments
The authors wish to thank the family members for their participation in these studies.
Funding Sources
This study was supported by the NIH grants UL1 RR025780, UL1 TR001082 N01-HV-48194, R01 HL69071, R01 116906 to LM, and K23 JL067915 and R01HL109209 to MRGT.
Footnotes
Conflict of Interest Disclosures:
No conflict of interest to disclose.
References
- 1.Elliott P, O’Mahony C, Syrris P, Evans A, Rivera Sorensen C, Sheppard MN, Carr-White G, Pantazis A, McKenna WJ. Prevalence of desmosomal protein gene mutations in patients with dilated cardiomyopathy. Circ Cardiovasc Genet. 2010;3:314–322. doi: 10.1161/CIRCGENETICS.110.937805. [DOI] [PubMed] [Google Scholar]
- 2.McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task force of the working group myocardial and pericardial disease of the european society of cardiology and of the scientific council on cardiomyopathies of the international society and federation of cardiology. Br Heart J. 1994;71:215–218. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox MG, van der Zwaag PA, van der Werf C, van der Smagt JJ, Noorman M, Bhuiyan ZA, Wiesfeld AC, Volders PG, van Langen IM, Atsma DE, Dooijes D, van den Wijngaard A, Houweling AC, Jongbloed JD, Jordaens L, Cramer MJ, Doevendans PA, de Bakker JM, Wilde AA, van Tintelen JP, Hauer RN. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: Pathogenic desmosome mutations in index-patients predict outcome of family screening: Dutch arrhythmogenic right ventricular dysplasia/cardiomyopathy genotype-phenotype follow-up study. Circulation. 2011;123:2690–2700. doi: 10.1161/CIRCULATIONAHA.110.988287. [DOI] [PubMed] [Google Scholar]
- 5.Fressart V, Duthoit G, Donal E, Probst V, Deharo JC, Chevalier P, Klug D, Dubourg O, Delacretaz E, Cosnay P, Scanu P, Extramiana F, Keller D, Hidden-Lucet F, Simon F, Bessirard V, Roux-Buisson N, Hebert JL, Azarine A, Casset-Senon D, Rouzet F, Lecarpentier Y, Fontaine G, Coirault C, Frank R, Hainque B, Charron P. Desmosomal gene analysis in arrhythmogenic right ventricular dysplasia/cardiomyopathy: Spectrum of mutations and clinical impact in practice. Europace. 2010;12:861–868. doi: 10.1093/europace/euq104. [DOI] [PubMed] [Google Scholar]
- 6.Quarta G, Muir A, Pantazis A, Syrris P, Gehmlich K, Garcia-Pavia P, Ward D, Sen-Chowdhry S, Elliott PM, McKenna WJ. Familial evaluation in arrhythmogenic right ventricular cardiomyopathy: Impact of genetics and revised task force criteria. Circulation. 2011;123:2701–2709. doi: 10.1161/CIRCULATIONAHA.110.976936. [DOI] [PubMed] [Google Scholar]
- 7.Sheikh F, Ross RS, Chen J. Cell-cell connection to cardiac disease. Trends Cardiovasc Med. 2009;19:182–190. doi: 10.1016/j.tcm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters NS, Green CR, Poole-Wilson PA, Severs NJ. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation. 1993;88:864–875. doi: 10.1161/01.cir.88.3.864. [DOI] [PubMed] [Google Scholar]
- 9.Smith JH, Green CR, Peters NS, Rothery S, Severs NJ. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. Am J Pathol. 1991;139:801–821. [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor M, Graw S, Sinagra G, Barnes C, Slavov D, Brun F, Pinamonti B, Salcedo EE, Sauer W, Pyxaras S, Anderson B, Simon B, Bogomolovas J, Labeit S, Granzier H, Mestroni L. Genetic variation in titin in arrhythmogenic right ventricular cardiomyopathy-overlap syndromes. Circulation. 2011;124:876–885. doi: 10.1161/CIRCULATIONAHA.110.005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeWinter MM, Granzier HL. Titin is a major human disease gene. Circulation. 2013;127:938–944. doi: 10.1161/CIRCULATIONAHA.112.139717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung CS, Hutchinson KR, Methawasin M, Saripalli C, Smith JE, 3rd, Hidalgo CG, Luo X, Labeit S, Guo C, Granzier HL. Shortening of the elastic tandem immunoglobulin segment of titin leads to diastolic dysfunction. Circulation. 2013;128:19–28. doi: 10.1161/CIRCULATIONAHA.112.001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson BR, Granzier HL. Titin-based tension in the cardiac sarcomere: Molecular origin and physiological adaptations. Prog Biophys Mol Biol. 2012;110:204–217. doi: 10.1016/j.pbiomolbio.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, Teodorescu DL, Cirino AL, Banner NR, Pennell DJ, Graw S, Merlo M, Di Lenarda A, Sinagra G, Bos JM, Ackerman MJ, Mitchell RN, Murry CE, Lakdawala NK, Ho CY, Barton PJ, Cook SA, Mestroni L, Seidman JG, Seidman CE. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366:619–628. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norton N, Robertson PD, Rieder MJ, Zuchner S, Rampersaud E, Martin E, Li D, Nickerson DA, Hershberger RE. Evaluating pathogenicity of rare variants from dilated cardiomyopathy in the exome era. Circ Cardiovasc Genet. 2012;5:167–174. doi: 10.1161/CIRCGENETICS.111.961805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reese MG, Eeckman FH, Kulp D, Haussler D. Improved splice site detection in genie. J Comput Biol. 1997;4:311–323. doi: 10.1089/cmb.1997.4.311. [DOI] [PubMed] [Google Scholar]
- 18.Ng PC, Henikoff S. Accounting for human polymorphisms predicted to affect protein function. Genome Res. 2002;12:436–446. doi: 10.1101/gr.212802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramensky V, Bork P, Sunyaev S. Human non-synonymous snps: Server and survey. Nucleic Acids Res. 2002;30:3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, Adams DR, Altman RB, Antonarakis SE, Ashley EA, Barrett JC, Biesecker LG, Conrad DF, Cooper GM, Cox NJ, Daly MJ, Gerstein MB, Goldstein DB, Hirschhorn JN, Leal SM, Pennacchio LA, Stamatoyannopoulos JA, Sunyaev SR, Valle D, Voight BF, Winckler W, Gunter C. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508:469–76. doi: 10.1038/nature13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Syrris P, Ward D, Evans A, Asimaki A, Gandjbakhch E, Sen-Chowdhry S, McKenna WJ. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am J Hum Genet. 2006;79:978–984. doi: 10.1086/509122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Zwaag PA, van Rijsingen IA, Asimaki A, Jongbloed JD, van Veldhuisen DJ, Wiesfeld AC, Cox MG, van Lochem LT, de Boer RA, Hofstra RM, Christiaans I, van Spaendonck-Zwarts KY, Lekanne dit Deprez RH, Judge DP, Calkins H, Suurmeijer AJ, Hauer RN, Saffitz JE, Wilde AA, van den Berg MP, van Tintelen JP. Phospholamban r14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: Evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail. 2012;14:1199–1207. doi: 10.1093/eurjhf/hfs119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Tintelen JP, Entius MM, Bhuiyan ZA, Jongbloed R, Wiesfeld AC, Wilde AA, van der Smagt J, Boven LG, Mannens MM, van Langen IM, Hofstra RM, Otterspoor LC, Doevendans PA, Rodriguez LM, van Gelder IC, Hauer RN. Plakophilin-2 Mutations Are the Major Determinant of Familial Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Circulation. 2006;113:1650–1658. doi: 10.1161/CIRCULATIONAHA.105.609719. [DOI] [PubMed] [Google Scholar]
- 24.Xu T, Yang Z, Vatta M, Rampazzo A, Beffagna G, Pilichou K, Scherer SE, Saffitz J, Kravitz J, Zareba W, Danieli GA, Lorenzon A, Nava A, Bauce B, Thiene G, Basso C, Calkins H, Gear K, Marcus F, Towbin JA. Compound and digenic heterozygosity contributes to arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2010;55:587–597. doi: 10.1016/j.jacc.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapplinger JD, Landstrom AP, Salisbury BA, Callis TE, Pollevick GD, Tester DJ, Cox MG, Bhuiyan Z, Bikker H, Wiesfeld AC, Hauer RN, van Tintelen JP, Jongbloed JD, Calkins H, Judge DP, Wilde AA, Ackerman MJ. Distinguishing arrhythmogenic right ventricular cardiomyopathy/dysplasia-associated mutations from background genetic noise. J Am Coll Cardiol. 2011;57:2317–2327. doi: 10.1016/j.jacc.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mestroni L, Taylor MR. Pharmacogenomics, personalized medicine, and heart failure. Discov Med. 2011;11:551–561. [PubMed] [Google Scholar]
- 27.Lorenzon A, Beffagna G, Bauce B, De Bortoli M, Li Mura IE, Calore M, Dazzo E, Basso C, Nava A, Thiene G, Rampazzo A. Desmin mutations and arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2013;111:400–405. doi: 10.1016/j.amjcard.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandjbakhch E, Vite A, Gary F, Fressart V, Donal E, Simon F, Hidden-Lucet F, Komajda M, Charron P, Villard E. Screening of genes encoding junctional candidates in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Europace. 2013;15:1522–1525. doi: 10.1093/europace/eut224. [DOI] [PubMed] [Google Scholar]
- 29.Marcus FI, Abidov A. Arrhythmogenic right ventricular cardiomyopathy 2012: Diagnostic challenges and treatment. J Cardiovasc Electrophysiol. 2012;23:1149–1153. doi: 10.1111/j.1540-8167.2012.02412.x. [DOI] [PubMed] [Google Scholar]
- 30.den Haan AD, Tan BY, Zikusoka MN, Llado LI, Jain R, Daly A, Tichnell C, James C, Amat-Alarcon N, Abraham T, Russell SD, Bluemke DA, Calkins H, Dalal D, Judge DP. Comprehensive desmosome mutation analysis in north americans with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Cardiovasc Genet. 2009;2:428–435. doi: 10.1161/CIRCGENETICS.109.858217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golbus JR, Puckelwartz MJ, Fahrenbach JP, Dellefave-Castillo LM, Wolfgeher D, McNally EM. Population-based variation in cardiomyopathy genes. Circ Cardiovasc Genet. 2012;5(4):391–9. doi: 10.1161/CIRCGENETICS.112.962928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson BR, Bogomolovas J, Labeit S, Granzier H. Single molecule force spectroscopy on titin implicates immunoglobulin domain stability as a cardiac disease mechanism. J Biol Chem. 2013;288(8):5303–15. doi: 10.1074/jbc.M112.401372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quarta G, Syrris P, Ashworth M, Jenkins S, Zuborne Alapi K, Morgan J, Muir A, Pantazis A, McKenna WJ, Elliott PM. Mutations in the lamin a/c gene mimic arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2012;33:1128–1136. doi: 10.1093/eurheartj/ehr451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.